Abstract
Background
Given concern regarding the abuse liability of hypnotics this study assessed hyperarousal in people with insomnia and its relation to hypnotic self-administration over 12 months of nightly hypnotic use.
Methods
Ninety-five subjects with insomnia, 32–64 yrs old, received a screening nocturnal polysomnogram (NPSG) and Multiple Sleep Latency Test (MSLT) the following day and were randomized to take zolpidem 10 mg or placebo nightly for 12 months. During months 1 and 8, while taking their prescribed medications, NPSGs and MSLTs were conducted and urine was collected (0700–1500 hr) and analyzed for norepinephrine (NE) levels. A subset (n=54) underwent hypnotic self-administration assessments in months 1, 4, and 12.
Results
Mean daily sleep latency on the screening MSLT was distributed across the full range of MSLT latencies (2–20 min). Those with the highest screening MSLT latencies had the higher NE levels compared to those with the lowest MSLT latencies. In the subset undergoing the self-administration assessment those with the highest MSLT latencies chose more capsules (placebo and zolpidem) and increased the number of capsules chosen in months 4 and 12 relative to month 1 compared to those with the lowest MSLT latencies.
Conclusions
These data show that some insomniacs are hyperaroused with high MSLT/NE levels and compared to low MSLT/NE insomniacs, they increase the number of capsules (zolpidem and placebo) self-administered on months 4 and 12 relative to month 1.
Keywords: insomnia, MSLT, NE, hypnotic self-administration
INTRODUCTION
Insomnia is generally considered a disorder of hyperarousal.1 One of the reported signs of hyperarousal is an elevated Multiple Sleep Latency Test (MSLT) average daily sleep latency. In a large sample (N=95) of people with insomnia average daily sleep latency on the MSLT was significantly elevated compared to an age- and sex-matched general population-based representative control sample and these MSLT findings in insomnia were stable over eight months.2 Yet, some smaller sample studies have failed to show MSLT elevations in people with insomnia.3–5 In part, the discrepancy may be due to the fact not all insomniacs show MSLT elevation, which was revealed in the larger sample study.2 Among the 95 people with insomnia in the previously cited study, the MSLT latencies ranged from 2–20 min with approximately 30% having latencies of 10 min or less.2
Hyperarousal in insomnia has been shown on a number of other physiological measures, including elevated levels of circulating catecholamines6, increased basal metabolic rate7, increased body temperature8, altered heart rate9,10, elevated beta EEG frequency11,12, and cortical activation on functional neuroimaging.13 However, to date only a single study has shown “hyperarousal” on concurrent physiological measures, that being increased metabolic rate in conjunction with elevated MSLTs relative to age-match controls.7 But no study has shown differences on concurrent physiological measures among insomniacs, that is between insomniacs with and without MSLT-defined hyperarousal. Thus, we sought in this study to determine whether or not daytime urinary norepinephrine (NE) levels would differ among insomniacs as a function of their MSLT average daily sleep latency. We hypothesized that insomniacs with MSLT-defined hyperarousal would also show concurrent elevated daytime NE levels compared to those without MSLTdefined hyperarousal.
Hyperarousal in insomnia potentially reflects an activated stress system. Many theories of addiction hypothesize that stress increases vulnerability to drug abuse.14 Animal literature and human neuroimaging studies have identified brain circuits involved in stress that include release of CRF from the paraventicular nucleus and NE activation initiated in locus coeruleus.14 This CRF/NE activation also activates dopaminergic brain motivational pathways known to be engaged by drugs of abuse including the ventral tegmental area and nucleus accumbens. Thus stress co-activates brain stress and motivational circuits simultaneously.14
The acknowledged drugs of choice for the pharmacological treatment of insomnia are the benzodiazepine receptor ligand hypnotics (BzRL). Studies have shown that with therapeutic doses used either short-term or chronically, the abuse liability of BzRLs in insomnia is relatively low.15 Yet case reports and retrospective studies continue to report BzRL dependence and for the majority of these cases the abuse developed through initial therapeutic use.16 In this study we sought to determine whether elevated MSLT average daily sleep latency (i.e. hyperarousal) and elevated NE levels in insomniacs would be predictive of increasing rates of self-administration during 12 months of nightly use of zolpidem 10 mg or placebo. We hypothesized that insomniacs with elevated MSLT latencies and NE levels would show increasing rates of hypnotic self-administration.
METHODS
Participants
Persons 21–65 yrs old with difficulty falling asleep and/or staying asleep were recruited. Ninety-five participants (42 men and 53 women), aged 32–65 yrs, meeting Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM IV-TR) criteria for primary insomnia entered a trial of the efficacy and abuse liability of chronic hypnotic use (see Table 1 for sample demographics). All were in good physical and psychiatric health based on an extensive screening as previously described.17
Table 1.
History of Insomnia Groups
| PLACEBO | ZOLPIDEM | |
|---|---|---|
| N | 45 | 50 |
| Average Age (range) | 49.44 (30–70) | 49.58 (23–68) |
| Gender | Males: 16 | Males: 26 |
| Females: 29 | Females: 24 | |
| INSOMNIA MEASURES | ||
| Reported Sleep Time (hrs) ± SD; median | 5.58 ± 1.04 5.50 |
5.17 ± 1.22 5.25 |
| NPSG Sleep Time (hrs) mean ± SD; median | 5.90 ± 0.74 6.02 |
6.02 ± 0.78 6.12 |
| SL NPSG (min) ± SD median; range | 44.26 ± 38.35 | 35.46 ± 29.32 |
| 33; 2–169 | 26; 5–141 | |
| WASO NPSG (min) ± SD median; range | 98.44 ± 40.67 | 95.15 ± 42.26 |
| 91.5; 22–185.5 | 92.0; 24.5–218.5 | |
| Age of insomnia onset median; range | 38.60 40; 10.5–65 |
36.46 38.5; 7–58 |
| Duration of insomnia median; range | 10.93 7; 1–31 |
12.92 8; 1–54 |
| SELF REPORTED ALCOHOL AND DRUG CONSUMPTION | ||
| Alcohol (drinks/week: N) | 0–1: 31 | 0–1: 31 |
| 2–6: 6 | 2–6: 16 | |
| 7–14: 7 | 7–14: 3 | |
| 16: 1 | 16: 0 | |
| Caffeinated Beverages (drinks/week: N) | 0–1: 9 | 0–1: 13 |
| 2–6: 7 | 2–6: 16 | |
| 7–14: 16 | 7–14: 12 | |
| 16 or more: 13 | 16 or more: 9 | |
| Previous Illicit Drug History | No drug history: 31 | No drug history: 41 |
| Marijuana use ≥ 2 yrs ago: 3 | Marijuana use ≥ 2yrs ago: 5 | |
| Marijuana use >10 yrs ago: 10 | Marijuana use >10 yrs ago: 4 | |
| Cocaine use 20 yrs ago: 1 | Cocaine use: 0 | |
| Current Nicotine Usage (Cigarettes smoked per day) | Non smokers: 37 | Non smokers: 41 |
| 1–2: 3 | 1–2: 3 | |
| 4–5: 2 | 3–5: 4 | |
| >6: 3 | >6: 2 | |
The Institutional Review Board of the Henry Ford Health System approved the study protocol. All participants provided informed consent and were paid for participation. The study was posted on clinicaltrials.gov.
Study Design
The trial was a mixed design, double-blind, placebo-controlled, study with a between group comparison of insomniacs randomly assigned to use zolpidem 10 mg or placebo nightly for 12 months. After the screening NPSG and MSLT, subjects received a single NPSG and next-day MSLT on week one of month 1 and again after 8 months of nightly use of their assigned medication. The NPSG-defined hypnotic efficacy data for month 1 and 8 were previously published.17 Concurrent with the MSLT done on month 1 and 8, urine was collected (0700–1500 hrs) for NE analyses, which along with the MSLT data, are one focus of this report.
Procedures
The general health, psychiatric and sleep disorders screening of the participants has been described previously17 The standard Rechtschaffen and Kales methods for recording of sleep were used18 and the MSLT was performed according to the standard protocol20, 21. The screening MSLT was used to define insomnia associated with “hyperarousal”. A previous report from this clinical trial showed that MSLT results are highly repeatable over 8 months2.
Table 2 presents the timing of assessments over the 12 month study. During week 4 of month 1 and week 1 of month 8 subjects entered the laboratory for a NPSG and MSLT while taking their assigned nightly hypnotic treatment (zolpidem 10 mg or placebo). Urine was collected in an 8 hr aliquot (0700–1500 hrs) over the time period of the MSLT assessments. Assays were performed for NE by Warde Laboratories (Ann Arbor, MI) using HPLC.
Table 2.
Timing of Assessments and Study Group Ns
| Assessment by Month | ||||
|---|---|---|---|---|
| Screen | Month 1 | Month 4 | Month 8 | Month 12 |
| NPSG | NPSG | Self Admin | NPSG | Self Admin |
| MSLT | MSLT | MSLT | ||
| Urinary NE | Urinary NE | |||
| Self Admin | ||||
| Study Ns | ||||
| Whole Grp | Self Admin Grp | |||
| Hi MSLT | 42 | 27 | ||
| Lo MSLT | 27 | 13 | ||
| Inter MSLT1 | 26 | 14 | ||
| TOTAL | 95 | 54 | ||
Inter MSLT not used in the study comparisons
NPSG = nocturnal polysomonography; MSLT = Multiple Sleep Latency Test; Urinary NE = urinary collection (07–1500 hr) for norepinephrine levels; Self Admin = one week hypnotic self administration assessment
A randomly selected subsample of the subjects (n=54) underwent a hypnotic self-administration assessment in months 1, 4, and 12. The other half of the subjects underwent a rebound insomnia assessment, the results of which have been previously reported22. The hypnotic self-administration assessment involved a 7 night protocol in which the first two nights were sampling nights and the next five nights were choice nights. For the zolpidem group the color assigned to placebo and zolpidem capsules differed and on sampling nights one of each color-coded capsule was administered each night, so that both zolpidem and placebo were sampled. For the placebo group both capsules were placebo, but the colors administered differed each sampling night. Subjects were instructed on sampling nights to attend to the capsule color because on choice nights they would select which medication they wanted for a given night based on the capsule color of the sampling nights. On the following 5 choice nights, based on their sampling experience, the subjects were instructed to choose a capsule color and then were given the option on that night of taking up to three capsules of their chosen color. For the zolpidem group capsules were 5 mg each for a total possible nightly dose of 15 mg. For the placebo group the capsules were placebo, up to three nightly, regardless of color choice. All capsules were taken 30 min before lights out.
Statistical Analyses
To produce separation for the NE and hypnotic dose escalation comparisons and maintain sufficient “n” in the subgroups, the MSLT distribution (2–20 min) of the current study was divided at approximately one standard deviation on either side of the mean for MSLT latencies shown in a representative population sample drawn from southeastern Michigan where the mean was approximately 11.2 min with a deviation of 3 min21. Those showing elevated MSLT latencies (i.e., ≥ 15 min) (n= 42: Hi MSLT group) were compared to those showing lower MSLT latencies (i.e., ≤ 10 min) (n= 27: Lo MSLT group). Subjects with MSLT latencies of 11–14 min (n= 26) were not included. In the subset undergoing the self-administration assessment (N=54), 27 had Hi MSLT latencies and 13 had Lo MSLT latencies. The remaining 14 were intermediate and were not included in these analyses. Table 2 outlines the Ns for the MSLT groups.
Daytime NE levels in months 1 and 8 and total number of capsules chosen (both placebo and zolpidem) in the placebo and zolpidem groups on months 1, 4 and 12 were compared between the two MSLT groups. To assess possible dose escalation change scores were calculated to compare the change in number of capsules chosen from month 1 to month 4 and to month 12.
Mixed design MANOVAs were used to compare the two MSLT groups on NE levels in month 1 and 8 and on number of capsules self-administered in months 1, 4, and 12 and on the change from month 1 to months 4 and 12. Similarly, the zolpidem vs placebo comparison on NE levels in months 1 and 8 was done using mixed design MANOVAs.
RESULTS
The average daily MSLT in the Hi MSLT group was 17.6±1.7 min and in the Lo MSLT group it was 7.1±2.8 min. Those with Hi MSLT latencies compared to those with Lo MSLT latencies had significantly higher daytime urinary NE levels in month 1 (23.2±2.9 vs 21.6±1.9 ug/L) and in month 8 (28.2±4.0 vs 17.6±2.29 ug/L) (F=4.67, p<0.03; main effect of MSLT groups). There also was a months by MSLT group interaction (F=6.93, p<.01). In the Hi MSLT group, NE levels increased from month 1 to 8, while in the Lo MSLT group they declined slightly. Table 3 presents NE levels in the two MSLT groups at month 1 and 8. Daytime NE levels did not differ as a function of nightly hypnotic use (placebo vs zolpidem) over the 8 months (F=0.69, NS) (see Table 3). Figure 1 presents the average of month 1 and 8 NE levels in the Hi versus Lo MSLT groups.
Table 3.
Daytime (0700–1500 hrs) Urinary Norepinephrine Levels as a Function of MSLT and Nightly Drug
| Lo MSLT | Hi MSLT | |
|---|---|---|
| Whole Sample | ||
| Number Subjects | 27 | 42 |
| Month 1 | 21.6 (1.9) | 23.2 (2.9) |
| Month 8 | 17.6 (2.2) | 28.2 (4.0) |
| Self-Administration Sub Sample | ||
| Number Subjects | 13 | 27 |
| Month 1 | 18.8 (2.6) | 17.6 (4.0) |
| Month 8 | 27.3 (4.5) | 30.5 (6.2) |
| Drug Effects | ||
| Placebo | Zolpidem | |
| Number Subjects | 45 | 50 |
| Month 1 | 24.5 (2.7) | 19.8 (1.6) |
| Month 8 | 23.2 (2.7) | 23.1 (3.1) |
Data are Means (SEM) ug/L
Lo MSLT vs Hi MSLT: p<.03 and .04 in both samples
Zolpidem vs Placebo: NS
Figure 1.
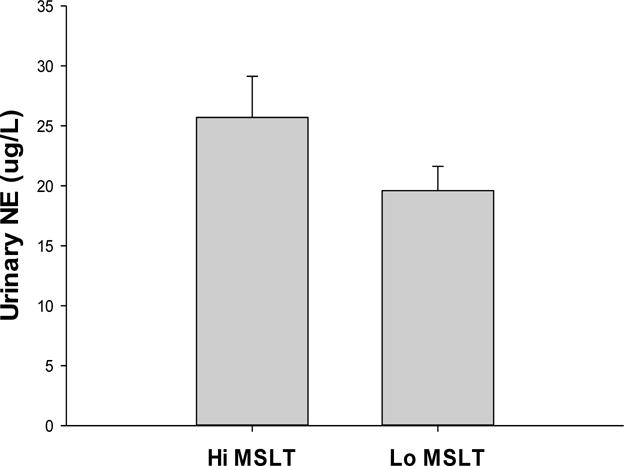
Daytime (0700–1500 hrs) urinary norepinephrine (NE) in people with insomnia and Hi MSLT (≥15 min) latencies vs Lo MSLT (≤ 10 min) latencies (p <.03)
The NE levels in the subset of subjects undergoing the dose escalation assessment were higher in the Hi MSLT group in month 1 (27.3±4.5 vs 18.9±2.6 ug/L) and month 8 (30.5±6.2 vs 17.6±4.1 ug/L) (F=4.78, p<.03) than those in the Lo MSLT group (see Table 3); unlike the full data set, the MSLT group by month interaction was not significant.
As to capsule choice, a total of 15 possible capsules could be chosen each month over the five choice nights. The placebo group increased the average number of placebo capsules taken over months (M1: 8.2, M4: 9.9, M12: 9.7) (F=4.38, p<.004), while the zolpidem group did not (M1: 8.7, M4: 8.9, M12: 9.0) (NS). The average number of capsule choices in the zolpidem group includes both placebo and zolpidem capsules. Of note, the zolpidem group did chose zolpidem on more of the nights than placebo (80%), but did not differentially chose a greater number of placebo capsules on nights placebo was chosen compared to zolpidem capsules on nights zolpidem was chosen.
Across both treatment groups those with Hi MSLT latencies (≥15 min) took a greater number of capsules (placebo or zolpidem) in months 1, 4, and 12 than those with Lo MSLT latencies [(M1: 9.4 vs 8.4, M4: 10.4 vs 8.7, M12: 10.7 vs 8.0; (F= 4.19, p<.05)]. Within the placebo group alone, those with Hi MSLT latencies choose more capsules than those with Lo MSLT latencies [(M1: 9.9 vs 5.5, M4: 11.1 vs 7.5, M12: 10.7 vs 7.0; (F=3.88, p<. 06)]. Finally, there was a trend showing those with Hi MSLT latencies were more likely to increase (as reflected in a positive change score) the number of capsules taken from month 1 to that in months 4 (1.0 vs 0.3) and 12 (1.4 vs −0.5) compared to those with Lo MSLT latencies (p<.08). Figure 2 presents the escalation of number of capsules chosen from month 1 to month 4 and 12.
Figure 2.
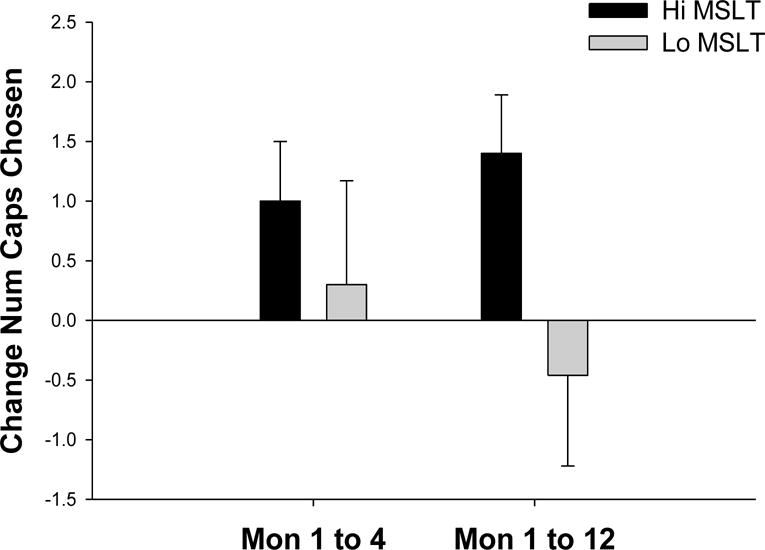
Change in number of capsules chosen (placebo and zolpidem) from month 1 to month 4 and month 12 in Hi and Lo MSLT groups (p<.08).
DISCUSSION
These are the first data in individuals with insomnia that differentiates those with hyperarousal from those without using two concurrent physiological measures, daytime urinary NE and MSLT. All subjects met DSM-IV-TR criteria for insomnia with the additional criteria of having a sleep efficiency of ≤ 85% on a screening NPSG. MSLT criteria were not imposed and the MSLT average daily sleep latencies ranged from 2–20 min. Approximately 30% had MSLT latencies of 10 min or less, while 50% had latencies of 15 min or more.
Of significance is the absence of a placebo vs zolpidem drug effect on NE or MSLT. In month 1 after three weeks of nightly use of their assigned hypnotic and further after 7 months of nightly use, MSLT and NE levels remained elevated in the hyperaroused vs non-hyperaroused insomniacs. As previously reported, PSG measures of sleep were improved in the zolpidem compared to placebo groups; sleep time was increased by about 45 min22. The absence of a reduction in MSLT/NE levels, despite improved nightly sleep over seven months, suggests the hyperarousal of insomnia is a trait characteristic in these individuals.
The dose escalation in the aroused insomniacs with Hi MSLT/NE levels compared to the non–aroused insomniacs suggests a heightened risk for hypnotic abuse. The fact that it was primarily the placebo group that escalated the number of capsules chosen is further informative. Sleep was not improved in the placebo group over months; there was no placebo effect. The insomnia in this group remained severe. In other words, given an ineffective “hypnotic” the hyperaroused insomniac is at risk for dose escalation. But, the dose escalation only occurred in the hyperaroused insomniacs. An alternative explanation is that the hyperaroused insomniacs require higher doses of active drug. We intend to analyze PSGs collected during month 1 and 8 of the study to determine whether basal PSG measures are predictive of dose escalation in the hyperaroused insomniacs. Furthermore, we will assess whether or not there is a differential zolpidem versus placebo effect in the hyperaroused versus non-hyperaroused insomnia or whether there is a reduction of drug effects from month 1 to month 8.
A study limitation is the relatively small “n” within the four-way subgroups (Hi vs Lo MSLT and placebo vs zolpidem) among those subjects undergoing the dose escalation assessment. Within the placebo group the “n” for Hi MSLT was 14 and for Lo MSLT it was 4. In the zolpidem group it was Hi MSLT = 13 and Lo MSLT = 9. Consequently, total number of capsules chosen and change in number of capsules chosen from month 1 to month 4 or 12 within a particular subgroup comparison (e. g., within the placebo group a comparison of Hi vs Lo MSLT choices) did not differ significantly. Only trends were observed for these interactions. This large study was originally designed with the expectation that all insomniacs would show hyperarousal and the primary comparison would only involve a placebo vs zolpidem comparison across months. But not all insomniacs were hyperaroused.
CONCLUSION
In conclusion, this study has shown that 1) not all people with insomnia are hyperaroused as shown by MSLT, 2) hyperarousal in insomnia as defined by MSLT is also seen in elevation of daytime NE, 3) Hi daytime MSLT/NE is associated with enhanced risk of hypnotic dose escalation, and 4) the dose escalation is expressed primarily in insomniacs receiving placebo.
Highlights.
not all people with insomnia are hyperaroused as shown by MSLT.
hyperarousal in insomnia as defined by MSLT is also seen in elevation of daytime NE. 3) Hi daytime MSLT/NE is associated with enhanced risk of hypnotic dose escalation.
the dose escalation is expressed primarily in insomniacs receiving placebo.
Acknowledgments
NIDA grant # R01DA17355 awarded to TAR. We wish to thank the Henry Ford Sleep Center technical staff for nocturnal recordings, as well as G. Koshorek, S. Cameron, and A. Rojas and D. Ditri for their meticulous scoring of PSG records.
DISCLOSURE STATEMENT: This was not an industry supported study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts:
T. Roehrs: consultant – Lundbeck, Pfizer; speaker – Pernix; grantee – Merck
T. Roth: consultant – Actelion, Addrenex, Cephalon, Eisai, Intec, Merck, Pfizer, Sanofi, Sepracor, Shire, Somaxon, TransOral; speaker – Cephalon, Sanofi; grantee – Merck
ClinicalTrials.gov Identifier: NCT01006525; Trial Name: Safety and Efficacy of Chronic Hypnotic Use; http://clinicaltrials.gov/ct2/show/NCT01006525
References
- 1.Roehrs T, Gumenyuk V, Drake C, et al. Physiological correlates of insomnia. Current Topics in Behavioral Neuroscience. 2014;21:277–290. doi: 10.1007/7854_2014_324. [DOI] [PubMed] [Google Scholar]
- 2.Roehrs TA, Randall S, Harris E, et al. MSLT in primary insomnia: Stability and relation to nocturnal sleep. Sleep. 2012;34:1647–1652. doi: 10.5665/sleep.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seidel WF, Ball S, Cohen S, et al. Daytime alertness in relation to mood, performance and nocturnal sleep in chronic insomniacs and no complaining sleepers. Sleep. 1984;7:230–238. doi: 10.1093/sleep/7.3.230. [DOI] [PubMed] [Google Scholar]
- 4.Lichstein KL, Wilson NM, Noe SL, et al. Daytime sleepiness in insomnia: Behavioral, biological and subjective indices. Sleep. 1994;17:683–702. doi: 10.1093/sleep/17.8.693. [DOI] [PubMed] [Google Scholar]
- 5.Edinger JD, Fins AI, Sullivan RJ. et: Do our methods lead to insomniacs’ madness?: Daytime testing after laboratory and home-based polysomnographic studies. Sleep. 1997;20:1127–1134. [PubMed] [Google Scholar]
- 6.Vgontzas AN, Bixler EO, Lin HM, et al. Chronic insomnia is associated with nyctohemeral activation of the hypothalamic-pituitary-adrenal axis: clinical implications. J Clin Endocrinol Metab. 2001;86:3787–3794. doi: 10.1210/jcem.86.8.7778. [DOI] [PubMed] [Google Scholar]
- 7.Bonnet MH, Arand DL. 24-Hour metabolic rate in insomniacs and matched normal sleepers. Sleep. 1995;18:581–588. doi: 10.1093/sleep/18.7.581. [DOI] [PubMed] [Google Scholar]
- 8.Lushington K, Dawson D, Lack L. Core body temperature is elevated during constant wakefulness in elderly poor sleepers. Sleep. 2000;23:504–510. [PubMed] [Google Scholar]
- 9.Lichstein KL, Wilson NM, Noe SL, et al. Daytime sleepiness in insomnia: behavioral, biological and subjective indices. Sleep. 1994;17:693–702. doi: 10.1093/sleep/17.8.693. [DOI] [PubMed] [Google Scholar]
- 10.Bonnet MH, Arand DL. Heart rate variability in insomniacs and matched normal sleepers. Psychosom Med. 1998;60:610–615. doi: 10.1097/00006842-199809000-00017. [DOI] [PubMed] [Google Scholar]
- 11.Nofzinger E, Nowell P, Buysse D. Towards a neurobiology of sleep disturbance in primary insomnia and depression: a comparison of subjective, visually scored and periodic amplitude, and power spectral density sleep measures. Sleep. 1999;22:S99. [Google Scholar]
- 12.Perlis ML, Smith MT, Andrews PJ, et al. Beta/Gamma EEG activity in patients with primary and secondary insomnia and good sleeper controls. Sleep. 2001;24:110–117. doi: 10.1093/sleep/24.1.110. [DOI] [PubMed] [Google Scholar]
- 13.Nofzinger EA, Buysee DJ, Germain A, et al. Functional neuroimaging evidence for hyperarousal in insomnia. Am J Psychiatry. 2004;161:2126–2128. doi: 10.1176/appi.ajp.161.11.2126. [DOI] [PubMed] [Google Scholar]
- 14.Sinha R. Chronic stress, drug use, and vulnerability to addiction. Ann NY Acad Sci. 2008;1141:105–130. doi: 10.1196/annals.1441.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roehrs T, Roth T. Medication and substance abuse. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. 5th. St Louis, Mo: Elsevier Saunders; 2010. pp. 1512–1523. [Google Scholar]
- 16.Griffiths RR, Johnson MW. Relative abuse liability of hypnotic drugs: A conceptual framework and algorithm for differentiating among compounds. J Clin Psychiatry. 2005;66:31–41. [PubMed] [Google Scholar]
- 17.Randall S, Roehrs TA, Roth T. Efficacy of eight months of nightly zolpidem: A prospective placebo controlled study. Sleep. 2012;35:1551–1557. doi: 10.5665/sleep.2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rechtschaffen A, Kales A. A manual of standardized, techniques and scoring system for sleep stages of human sleep. Los Angeles: Brain Information Service/Brain Research Institute, University of California at Los Angeles; 1968. [Google Scholar]
- 19.American Academy of Sleep Medicine. The AASM manual for the scoring of sleep and associated events, Version 2.1. Darien IL: American Academy of Sleep Medicine; 2014. [Google Scholar]
- 20.Carskadon MA, Dement WC, Mitler MM. Guidelines for the Multiple Sleep Latency Test (MSLT): A standard measure of sleepiness. Sleep. 1986;9:519–524. doi: 10.1093/sleep/9.4.519. [DOI] [PubMed] [Google Scholar]
- 21.Roehrs T, Carskadon M, Dement WC, et al. Daytime sleepiness and alertness. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. 5th. St Louis, Mo: Elsevier Saunders; 2010. pp. 42–53. [Google Scholar]
- 22.Roehrs TA, Randall S, Harris E, et al. Twelve months of nightly zolpidem does not lead to rebound insomnia or withdrawal symptoms: A prospective placebo-controlled study. J Psychopharmacol. 2012;26:1088–1095. doi: 10.1177/0269881111424455. [DOI] [PMC free article] [PubMed] [Google Scholar]


