Abstract
Recent data show shifts in genetic and environmental influences on emotional eating across the menstrual cycle, with significant shared environmental influences during pre-ovulation, and primarily genetic effects during post-ovulation. Factors driving differential effects are unknown, although increased estradiol during pre-ovulation and increased progesterone during post-ovulation are thought to play a role. We indirectly investigated this possibility by examining whether overall levels of estradiol and progesterone differentially impact genetic and environmental risk for emotional eating in adult female twins (N = 571) drawn from the MSU Twin Registry. Emotional eating, estradiol levels, and progesterone levels were assessed daily and then averaged to create aggregate measures for analysis. As predicted, shared environmental influences were significantly greater in twins with high estradiol levels, whereas additive genetic effects increased substantially across low versus high progesterone groups. Results highlight significant and differential effects of ovarian hormones on etiologic risk for emotional eating in adulthood.
Keywords: Emotional eating, genetic, environmental, menstrual cycle, estrogen, progesterone
Previous studies have provided suggestive evidence for ovarian hormone effects on genetic risk for disordered eating. The earliest studies showed that the heritability of overall disordered eating (e.g., measures tapping weight preoccupation, body dissatisfaction, binge eating, and compensatory behavior) and individual eating disorder symptoms (e.g., binge eating only) increased dramatically across puberty in girls, from accounting for essentially 0% of the variance in pre-pubertal female twins to over 50% in post-pubertal female twins (Klump, Burt, McGue, & Iacono, 2007; Klump, McGue, & Iacono, 2003). This same pubertal effect was not observed in boys, highlighting potential sex-specific effects of puberty on genetic risk (Culbert, Burt, McGue, Iacono, & Klump, 2009; Klump et al., 2012).
Theories regarding potential mechanisms of effects focused on ovarian hormone activation during puberty in girls, most notably estrogen. Estrogen increases linearly throughout puberty and is known to directly regulate gene transcription in several neurotransmitter systems that are disrupted in eating disorders (e.g., serotonin, dopamine; Becker, 2009; Craft, 2008; Hildebrandt, Alfano, Tricamo, & Pfaff, 2010; Ostlund, Keller, & Hurd, 2003). One small twin study provided preliminary support for an effect of estrogen on genetic risk, as the degree of genetic influence on overall disordered eating symptoms, body dissatisfaction, weight preoccupation, and binge eating was significantly greater in twins with higher versus lower estradiol levels during puberty (Klump, Keel, Sisk, & Burt, 2010). Although sample sizes were small in this pilot study, the effects of estrogen were found to be independent of age, body mass index (BMI), and the physical changes of puberty, suggesting specific effects of estrogen on genetic risk for a range of disordered eating symptoms.
To date, there have been no follow-up studies of the effects of estrogen on genetic risk during puberty, although large-scale replications are currently underway (Klump et al., NIMH R01MH092377). Nonetheless, the initial findings described above sparked interest in using alternative designs and examining differential developmental time periods to help confirm/disconfirm a role for ovarian hormones in genetic risk for disordered eating symptoms. One notable alternative design is examining changes in genetic risk for disordered eating symptoms across the menstrual cycle. Like puberty, the menstrual cycle is marked by hormonal activation and de-activation that provides a natural test of the effects of hormones on genetic risk for disordered eating. If ovarian hormones impact genetic effects on disordered eating, then changes in heritability should be observed across the menstrual cycle, and these changes should track with the known changes in ovarian hormones that are dictated by the female reproductive system.
A recent study from our group examined this possibility in a sample of female twins assessed daily across one menstrual cycle (Klump et al., 2015). Results suggested rather robust changes in genetic effects, as the heritability of emotional eating (i.e., overeating in response to negative emotions) was 2–4x higher in the post-ovulatory phases (39–46% in the luteal and premenstrual phases) as compared to the pre-ovulatory phases (12–17% in the follicular and ovulatory phases; Klump et al., 2015) of the cycle. This pattern of genetic activation mimicked phenotypic expression of emotional eating, as significantly higher rates of emotional eating (and binge eating) have been consistently observed during post-ovulation (Edler, Lipson, & Keel, 2007; Klump, Keel, Culbert, & Edler, 2008; Klump et al., 2013; Klump et al., 2014; Lester, Keel, & Lipson, 2003). Higher levels of estradiol and progesterone during post-ovulation appear to contribute to these elevated rates (Klump et al., 2013; Klump et al., 2014), raising the possibility that one or both of these hormones may be responsible for the increased heritability of emotional eating symptoms during post-ovulation.
Interestingly, there were also novel shifts in the type of environmental risk factors that were important across the cycle (Klump et al., 2015). Although nonshared environmental risk factors (i.e., those that are unique to twins growing up in the same family) were prominent across all cycle phases, shared environmental factors (i.e., those that are common to twins raised in the same family) were two times higher in pre- as opposed to post-ovulation – in fact, all of the models showed essentially no shared environmental effects in post-ovulation, but significant (32%) shared environmental influences in pre-ovulation. Notably, the pre-ovulatory phases of the cycle are characterized by rising estradiol levels and essentially the absence of progesterone. Estrogen is known to decrease food intake (Asarian & Geary, 2013) and appears to be protective against emotional eating and binge eating during pre-ovulation (Edler et al., 2007; Klump et al., 2008; Klump et al., 2013). Taken together, results suggest that higher levels of estradiol (in the absence of progesterone) during pre-ovulation may increase shared environmental influences on emotional eating and somehow protect against the development of these eating disorder symptoms. Such findings would be counter to those observed during puberty in girls showing estrogen activation of genetic (but not shared environmental) effects (Culbert et al., 2009; Klump et al., 2007; Klump et al., 2003) and suggest differential mechanisms of action for estrogen in adulthood versus adolescence – a theory that has been put forth in past work (Klump, 2013; Klump et al., 2015) but never directly tested.
Unfortunately, past studies have been unable to examine whether changes in estradiol and progesterone across the menstrual cycle drive changes in genetic and environmental risk. Methodological constraints have prohibited direct examination of these theories/hypotheses, as the twin models that can be used to test hormone effects on etiologic risk (Purcell, 2002; van der Sluis, Posthuma, & Dolan, 2012) do not have repeated measures extensions that could model a large number of daily hormone levels and binge eating/emotional eating scores (see Klump et al., 2015 for a brief discussion of these methodological constraints). However, we can begin to answer these questions by using single or aggregate (i.e., averages over several days) measures to ask whether general hormone levels impact genetic and environmental risk for general levels of emotional eating. Although these single/aggregate assessments index risk at a between-subject level (i.e., do women with higher/lower levels of hormones have differential genetic/environmental risk?) rather than a within-subject level (i.e., when do women experience greater genetic/environmental risk across their cycle?), they mirror between-subject analyses conducted during puberty (i.e., do girls with higher/lower estradiol levels during puberty have differential genetic/environmental risk?) (Klump et al., 2010) and provide an important and constructive test of overall hormone effects as well as potential differences across development. For example, it may be that estrogen shows disparate effects across puberty and the menstrual cycle because different levels of analysis (e.g., between-subject effects for puberty, within-subject effects for the menstrual cycle) produced different results. Focusing on between-subject effects of hormones in adulthood allows us to clarify estrogen’s role and answer the important question of whether and how estrogen and progesterone impact between-subject, etiologic risk for emotional eating in adulthood. Moreover, analyzing single, between-subject measures is quite straightforward using traditional gene x “environment” (in this case, hormones) interaction models (Purcell, 2002; van der Sluis et al., 2012) that can examine whether genetic and environmental influences on emotional eating differ across levels of estradiol and/or progesterone.
Given the above, the purpose of our study was to examine whether aggregate measures of ovarian hormones impact genetic and environmental risk for emotional eating in a population-based sample of adult female twins. We investigated this question in the same sample of twins used in the Klump et al. (2015) menstrual cycle study, but this time, we averaged daily levels of hormones and daily levels of emotional eating scores across the data collection period to obtain single, aggregate measures of the phenotypes. We focused on emotional eating in order to extend results from our past menstrual cycle study (Klump et al., 2015) and increase understanding of this key dysregulated eating behavior that is present across a range of eating disorders (e.g., bulimia nervosa, binge eating disorder, anorexia nervosa binge/purge type; Deaver, Miltenberger, Symth, Meidinger, & Crosby, 2003; Ricca et al., 2012; Ricca et al., 2009; Wardle, 1987) and health conditions (e.g., obesity; Goldschmidt et al., 2014)
Methods
Participants
Participants were 571 same-sex female twins (334 (59%) monozygotic (MZ), 237 (41%) dizygotic (DZ)) ages 16–25 years (M = 17.69, SD = 1.77) from 286 pairs (157 MZ, 129 DZ) who participated in the Twin Study of Hormones and Behavior across the Menstrual Cycle (HBMC) project (Klump et al., 2013) within the Michigan State University Twin Registry (MSUTR; for MSUTR details, see Burt & Klump, 2013; Klump & Burt, 2006). The primary aim of the HBMC study was to examine phenotypic and genetic associations between changes in ovarian hormones and changes in binge eating across the menstrual cycle. Study inclusion/exclusion criteria were: 1) menarche before the age of 15; 2) regular menstrual cycles every 22–32 days for the past 6 months; 3) no hormonal contraceptive use within the past 3 months; 4) no psychotropic or steroid medications within the past 4 weeks; 5) no pregnancy or lactation within the past 6 months; and 6) no history of genetic or medical conditions known to influence hormone functioning or appetite/weight. Despite these criteria, the HBMC twins are representative of the recruitment region (79.5% White, 14.3% Black, 0.6% Asian, 0.3% Native American, 5.3% more than one race; 7.8% Hispanic) (see Table S1 in the Supplemental Material available online for additional sample demographics), and they do not differ from other MSUTR twins on measures of emotional eating, binge eating or other symptoms (e.g., weight preoccupation) (average d = 0.12).
In order to maximize sample sizes for our twin models, we did not exclude HBMC twins who were missing some of the daily emotional eating or hormone data across the 45-day collection period. These twins were excluded from previous HBMC papers examining daily changes in hormones/emotional eating (e.g., Klump et al., 2015), but given our focus on aggregate/average measures of hormones and emotional eating, these twins were appropriate for inclusion in the current study. Notably, all of our included twins had at least 6 days of available data, with the vast majority (over 90%) having 20 days or more. Inspection of the mean number of missing days further suggests that our aggregate scores were based on several days worth of data for each twin (mean number of missing days: emotional eating scores M = 3.49 days, SD = 4.64 days; estradiol levels M = 10.99, SD = 5.90; progesterone levels M = 10.63, SD = 5.65), although more twins were missing hormone values due to our strategy for assaying hormone samples (see Ovarian Hormone section below).
Given that our 45-day collection period spans 1.5 menstrual cycles, we were concerned that variability in the days with available data for each twin, and/or the inclusion of twins with unexpected, anovulatory cycles, could have unduly influenced results. Thus, we conducted all of our analyses including only those twins who ovulated during the study period and who had data available for one full menstrual cycle (N = 460 twins). Ovulation was determined by plotting daily estradiol and progesterone values (see more on hormone data collection and assays below) across the cycle for each twin. Two independent raters then coded menstrual cycle phase and ovulation status (i.e. ovulatory versus anovulatory) based on changes in hormones across days (for more information on phase and ovulation coding, see Klump et al. (2015)).
Notably, there were no significant differences in average emotional eating scores, estradiol levels, or progesterone levels (all p’s > .05) between twins included versus excluded from these subsample analyses (d’s = .01–.13). Moreover, the pattern of twin correlations and model fitting results in the subsample of twins with complete data (see Tables S2 and S3, and Figure S2, in the Supplemental Material available online) was very similar to that of the full sample of twins reported below.
Procedures
Participants collected data for 45 consecutive days. Ratings of emotional eating were made each evening after 5:00 pm. Participants completed three in-person visits either in our laboratory or in their homes: one at the start of data collection, one mid-way through (about day 23) and one at the end (about day 45). Each visit included an assessment of height and weight (see methods below), re-assessment of eligibility, and collection of samples. Staff also contacted participants once per week to answer questions and confirm adherence.
Measures
Emotional eating
We used the emotional eating scale of the Dutch Eating Behavior Questionnaire (DEBQ; Van Strien, Frijters, Bergers, & Defares, 1986) to assess eating in response to negative emotions (e.g., “Did you have desire to eat when you were depressed?”) on a 5-point scale (i.e., “not at all” to “very often”). Eating in response to negative emotions is thought to be a core feature of binge eating (McManus & Waller, 1995), and the emotional eating scale has demonstrated validity by differentiating between individuals who binge eat versus those who are overweight and/or those drawn from a general community sample of women (e.g., college students) (Deaver et al., 2003; Wardle, 1987). Emotional eating scores correlate with established measures of binge eating (r’s = .55–.69) (Racine, Culbert, Larson, & Klump, 2009; Van Strien, 2000) as well as with palatable food intake (i.e., ice cream; Van Strien, 2000) in adults as well as in adolescents (Laghi, Pompili, Baumgartner, & Baiocco, 2015; Lluch, Herbert, Mejean, & Siest, 2000; Nguyen-Michel, Unger, & Spruijt-Metz, 2007; Snoek, Van Strien, Janssens, & Engels, 2007; Van Strien, 2010). The instructions for the scale were modified with permission to ask about emotional eating over the current day (45-day average α = .90) (Klump et al., 2008).
Ovarian hormones
Estradiol and progesterone were assayed from daily saliva samples. Saliva samples are preferred over other methods (e.g., blood spots) because they represent a less invasive collection method, particularly if repeated samples are needed. Previous research has shown that saliva samples are associated with higher compliance and more robust hormone-behavior associations than blood-spot sampling (Edler et al., 2007).
Saliva samples were processed by Salimetrics, LLC (State College, PA) using enzyme immunoassay kits designed specifically for analyzing saliva. These assays show excellent intra- and interassay coefficients of variation (estradiol = 7.1% and 7.5%; progesterone = 6.2% and 7.6%), as well as assay sensitivity (measured by interpolating the mean optical density minus 2 SDs of 10–20 replicates at the 0-pg/ml level; estradiol = 0.10 pg/ml; progesterone = 5 pg/ml) and method accuracy (determined by spike recovery and linearity; estradiol = 104.2% and 99.4%; progesterone = 99.6% and 91.8%). To conserve resources, we assayed samples from every other day during menstrual bleeding and the early follicular phase when hormones are expected to be low and stable. This process ensured that we captured periods of significant hormonal change across the menstrual cycle (e.g., the mid-follicular through premenstrual phase) while, in turn, maximizing the number of participant samples assayed.
Body Mass Index (BMI)
BMI was calculated (weight in kg/height in m2) using height and weight measurements taken during all three in-person study visits. These measurements were made using a wall-mounted ruler and digital scale for in-lab assessments, and a measuring tape and digital scale for home assessments.
Statistical Analyses
Data preparation
Daily emotional eating scores, daily estradiol levels, daily progesterone levels, and the three BMI values were averaged to create overall measures of each phenotype over the study period. Average emotional eating scores and average BMIs were then log transformed prior to analysis to account for positive skew. The log transformations brought skewness values for these measures to within acceptable limits (1.68 and 1.09, respectively).
Although continuous data are typically preferred over categorical data for most statistical analyses, the twin moderation models (see details on the models below) require several cases per level of the moderator to obtain robust and interpretable estimates, particularly when sample sizes are more modest. The range and variability of hormone values in our sample was quite large (see Table S1 in the Supplemental Material available online), with only 1–4 twins having each hormone value. To account for this variability and our more modest sample sizes, we followed the approach of previous work (e.g., Burt, Klahr, Neale, & Klump, 2013; Culbert et al., 2009; Suisman et al., 2014) and “binned” our subjects into the two, non-exclusive estrogen and progesterone groups in order to maximize statistical power and our ability to detect significant moderation effects for each hormone.1 Using median splits for the average hormone levels, we created a low/high estrogen group (cut-off value = 2.58 pg/ml; high estrogen N = 285; low estrogen N = 286) and a low/high progesterone group (cut-off value = 105.80 pg/ml; high progesterone N = 285; low progesterone N = 286) and examined each set of hormone groups separately in analyses. Although this meant that our analyses of estrogen and progesterone were unable to account for possible redundancy of effects across the two steroids, the comparisons provided a first pass at understanding the extent to which high/low levels of estrogen and high/low levels of progesterone influence genetic and environmental risk for emotional eating.
Twin Correlations
Twin intraclass correlations were used as initial indicators of genetic and environmental effects on emotional eating across hormone groups. Because MZ twins share approximately 100% of their segregating genes while DZ twins share, on average, 50%, significantly greater MZ relative to DZ twin correlations indicate the presence of additive genetic effects (i.e., effects that add or sum across genes). By contrast, MZ and DZ twin correlations that are similar in magnitude and are significantly greater than 0 signify a lack of genetic effects, but significant shared environmental influences (i.e., environmental factors that are common to siblings growing up in the same family and contribute to their behavioral similarity). Finally, MZ twin correlations that are less than 1.00 indicate the presence of nonshared environmental factors (i.e., factors that are unique to siblings growing up in the same family and contribute to behavioral differences) and measurement error.
We present twin correlations for pairs who were concordant for estrogen group or progesterone group (e.g., both co-twins were in the high estrogen group), as well as those who were discordant for their hormone group (e.g., one twin was in the low estrogen group, while the other was in the high estrogen group), as both types of pairs are included in the twin moderation models (see below). Notably, twins from concordant and discordant pairs did not differ significantly in their emotional eating scores (all ps > .45 for estrogen and progesterone groups).
Twin Moderation Models
We used extended, univariate twin moderation models (see Figure S1 in the Supplemental Material available online; van der Sluis et al., 2012) to directly test the effects of estrogen and progesterone groups on additive genetic (A), shared environmental (C), and nonshared environmental (E) influences on emotional eating. These models use concordant and discordant twin pairs to estimate two sets of parameters that index whether genetic and environmental influences on emotional eating differ across estrogen group or progesterone group. The first set of parameters contains the “paths” or intercepts (i.e., a, c, e) and estimate the degree of genetic and environmental influences on emotional eating at the lowest level of the moderator (e.g., low estradiol or low progesterone levels). The second set of parameters are the hormone moderators (i.e., βXE, βYE, βZE for estrogen and βXP, βYP, βZP for progesterone) which assess whether genetic and environmental influences differ between levels of the moderator (i.e., low versus high hormone levels).
Separate models were fit with estrogen and then progesterone as the moderator, and we coded low values on both hormones as 0 and high values as 1. We tested the significance of each moderator by fitting a series of nested models. We first fit the “full” ACE model, which freely estimated additive genetic, shared environmental, and nonshared environmental effects and moderators (e.g., for additive genetic effects, this model included: a + βXEME). In the remaining models, we tested the significance of each moderator by constraining the moderator coefficient (e.g., βXE) to 0 and comparing the fit of the reduced models to the full model. Comparisons of model fit were made by taking the difference in minus twice the log-likelihood (−2lnL) between the “full” and reduced models, which is chi-squared distributed under the null hypothesis implied by the reduced model. Large (statistically significant) differences led to a rejection of the nested model in favor of the full model, as this suggests that dropping the parameter resulted in a significantly worse fit. Akaike’s information criterion (AIC; χ2−2*Δdf) (Akaike, 1987) was also used to select the best fitting model, where models that minimize the AIC were preferred.
All models were fit to the raw data using the maximum likelihood option in Mx (Neale, Boker, Xie, & Maes, 1999). This option treats missing data as missing-at-random and is expected to produce less biased and more consistent estimates than other techniques (e.g., listwise deletion). Following previous recommendations (Purcell, 2002), we report unstandardized (or absolute) parameter estimates. Unstandardized estimates are generally preferred as they more accurately depict absolute differences in genetic and environmental influences than standardized estimates, which represent differences as proportions of the total variance. Nonetheless, we also report standardized estimates in the text (where appropriate), as these estimates allow for a direct comparison of our findings to previous twin studies that focused on standardized estimates of genetic/environmental effects.
Notably, all of the twin moderation models described above rest on one important assumption – that the moderators (i.e., ovarian hormones) are genetically independent of the dependent variable (i.e., emotional eating). If there is not independence, then genetic mediation (i.e., termed gene-environment correlations or rGE) may be present for hormone levels and emotional eating, such that the same genes influence hormone levels and emotional eating. These types of rGE are troublesome in the context of twin moderation models, as rGE could conceivably “masquerade” as hormone moderation effects. For example, if emotional eating stems, in part, from genes common to hormone levels, then increases in genetic influences at high levels of hormones could be a reflection of shared genes and/or environments, rather than an independent effect of the hormone levels on genetic influences on emotional eating. To avoid this interpretive difficulty, twin researchers are advised to use the “gene x environment (GxE) in the presence of rGE” model (Purcell, 2002) that tests the significance of genetic and/or environmental mediation by comparing the fit of models that allow for genetic/environmental covariance between the moderator and the dependent variable to models that constrain the covariance to be zero.
Consequently, before fitting the models described above, we fit a series of “GxE in the presence of rGE” models separately for estrogen and progesterone and in both cases, the moderation of the covariance between hormones and emotional eating scores was non-significant (i.e., the parameter could be constrained to 0 without a worsening of model fit for both estrogen (comparison with full model: χ2Δ (3) = .72, p = .69) and progesterone (comparison with full model: χ2Δ (3) = .38, p = 94)). Moreover, the best fitting models retained the parameter that tests for the unique/independent moderating effects of the hormones on emotional eating, outside of any rGE processes. These findings provide the first indication that our extended, univariate twin models would show significant effects of the hormones on genetic and/or environmental risk for emotional eating scores that reflect independent hormone effects rather than rGE.
Results
Descriptive Statistics
There were minimal differences between the low/high estrogen or progesterone groups in terms of race or ethnicity (see Table S1 in the Supplemental Material available online). However, twins with high hormone levels tended to be older in both the estrogen and progesterone groups, and BMI differed across the low/high estrogen groups. Nonetheless, group differences on these variables tended to be modest and of small effect (Cohen’s d = .18–.35).2
A somewhat unexpected finding was that twins in the high estrogen and high progesterone groups had significantly higher levels of both hormones (see Table S1 in the Supplemental Material available online), rather than just the hormone that was used to define the group. These findings suggest that hormone levels tend to track together, and that there may be general mechanisms that contribute to twins being generally high or generally low on both hormones. Nonetheless, low/high group differences were largest within groups defined by that hormone (e.g., differences in estradiol were larger between the low/high estrogen groups than between the low/high progesterone groups), and roughly 30% of our sample was categorized in the high group for one hormone and the low group for the other.
Finally, there were no significant low/high group differences in mean emotional eating scores (or variances – all ps > .45) for either estrogen or progesterone (see Table S1 in the Supplemental Material available online). Importantly, the lack of differences in emotional eating by low/high estradiol and progesterone levels does not negate a moderating effect of the hormones on genetic/environmental risk, as the presence of etiologic moderation would be expected to attenuate phenotypic associations. This pattern of results has been observed previously, where estradiol (Klump et al., 2010) and puberty (Culbert et al., 2009; Klump et al., 2003) were found to be significant moderators of genetic effects despite negligible phenotypic associations with disordered eating.
Twin Correlations
Twin correlations for emotional eating scores in each of the hormone groups are presented in Table 1. An interesting divergence of results was observed between the estrogen and progesterone groups, with differences in shared environmental effects between estrogen groups, and differences in genetic effects between progesterone groups. Specifically, in the low estrogen and discordant estrogen groups, the MZ twin correlations for emotional eating were significantly greater than the DZ twin correlations, suggesting the presence of genetic effects. By contrast, in the high estrogen group, the MZ and DZ twin correlations for emotional eating were relatively similar and were not significantly different from each other – a situation that strongly implicates shared environmental, but no genetic, influences. Interestingly, in all of the progesterone groups, the MZ twin correlations were at least double the DZ twin correlations, suggesting genetic effects. However, the MZ/DZ twin difference was much greater in the high progesterone group - in this group, the MZ twin correlation was almost triple the DZ twin correlation, suggesting the possible presence of much stronger genetic effects3 on emotional eating scores in twins with higher progesterone levels. Overall, these findings suggest that high estradiol levels and high progesterone levels may have specific etiologic effects on emotional eating scores that are not present in pairs with lower levels of these hormones or pairs that are discordant for hormone levels.
Table 1.
Twin Correlations for Emotional Eating Scores by Estrogen and Progesterone Groups.
| Scales | MZ Pairs | DZ Pairs | Z Test of Independence | p |
|---|---|---|---|---|
| Estrogen Groups: | ||||
| Low Estradiol (MZ n = 65 pairs; DZ n = 35 pairs) | .49*** | −.13 | 3.04 | .001 |
| High Estradiol (MZ n = 47 pairs; DZ n = 44 pairs) | .63*** | .56*** | 0.52 | .30 |
| Discordant Pairs (MZ n = 45 pairs; DZ n = 39 pairs) | .29* | .05 | 1.09 | .14 |
| Progesterone Groups: | ||||
| Low Progesterone (MZ n = 60 pairs; DZ n = 33 pairs) | .44*** | .22 | 1.36 | .13 |
| High Progesterone (MZ n = 56 pairs; DZ n = 38 pairs) | .62*** | .24 | 2.20 | .01 |
| Discordant Pairs (MZ n = 51 pairs; DZ n = 47 pairs) | .47*** | .21 | 1.42 | .08 |
Note. MZ = monozygotic; DZ = dizygotic; Z Test of Independence = a z test of significant differences between the MZ and DZ twin correlations; Discordant Pairs = pairs in which one twin had high levels of the hormone while the co-twin had low levels. Emotional eating scores were log transformed prior to analysis. All p values are one-sided, as MZ twin correlations would be expected to be larger than DZ twin correlations.
p < .05,
p < .001.
The twin correlation is significantly different from zero.
Twin Models
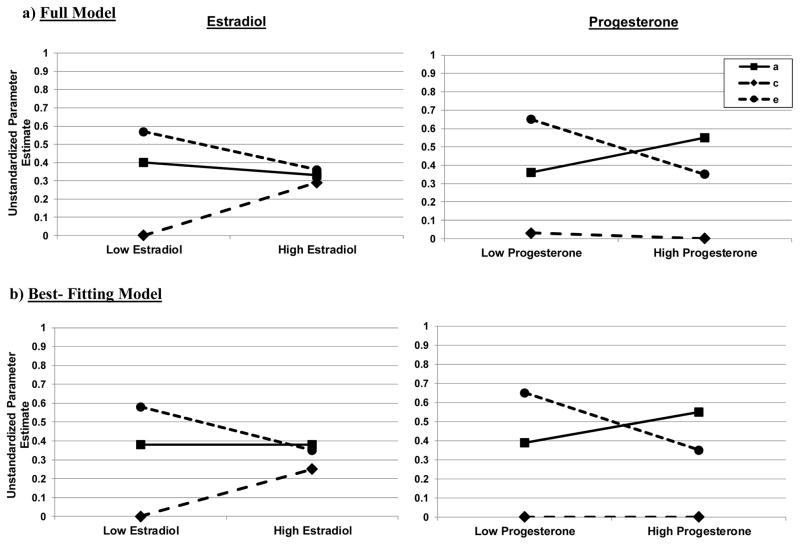
Biometric twin models confirmed the presence of differential effects across estrogen and progesterone groups. Model fit statistics and unstandardized parameter estimates are presented in Table 2, while unstandardized estimates from the full and best fitting models are shown in Figure 1.
Table 2.
Unstandardized Parameter Estimates and Model Fit Comparisons for the Hormone Moderation Models.
| Paths | Moderators | Model Fit Statistics | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Model | a | c | e | βX | βY | βZ | −2lnL (df) | χ2Δ (df) | p | AIC |
| Estrogen | ||||||||||
| Full Model | −.63 (−.79,−.28) | −.001 (−.46,.45) | .76 (.65,.89) | .06 (−.42,.52) | .54 (−.84,.84) | −.16 (−.33,.01) | 1541.03 (558) | -- | -- | -- |
| Constrain A Mod | .62 (.37,.74) | .03 (−.41, .33) | −.76 (−.88,−.65) | -- | −.53 (−.84,−.15) | .17 (.02,.31) | 1541.11 (559) | 0.08 (1) | .78 | −1.92 |
| Constrain C Mod | −.51 (−.72,.28) | −.21 (−.64,.64) | −.81 (−.93,−.70) | −.25 (−.81,−.002) | -- | .22 (.04,.38) | 1544.19 (559) | 3.16 (1) | .07 | 1.16 |
| Constrain E Mod | .69 (.47,.83) | −.08 (−.46,.33) | −.68 (−.77,−.61) | −.27 (−.70,−.10) | −.52 (−.81,−.15) | -- | 1544.41 (559) | 3.38 (1) | .07 | 1.38 |
| Constrain All Mods | .67 (.26,.78) | −.12 (−.59,.59) | −.71 (−.79,−.65) | -- | -- | -- | 1549.72 (561) | 8.69 (3) | .03 | 2.69 |
| Progesterone | ||||||||||
| Full Model | −.60 (−.78,−.07) | −.19 (−.65,.65) | .81 (.70,.93) | −.14 (−.56,.28) | .17 (−.66,.66) | −.21 (−.37,−05) | 1544.30 (558) | -- | -- | -- |
| Constrain A Mod | .65 (.35,.78) | −.04 (−.53,.53) | −.79 (−.90,−.69) | -- | −.27 (−.68,.68) | .19 (.04,.33) | 1544.74 (559) | 0.44 (1) | .51 | −1.56 |
| Constrain C Mod | −.62 (−.77,−.13) | .00 (−.57,.57) | −.80 (−.92,−.71) | −.12 (−.41,.09) | -- | .21 (.05,.37) | 1544.34 (559) | 0.04 (1) | .84 | −1.96 |
| Constrain E Mod | .70 (.28,.84) | −.08 (−.63,.63) | −.71 (−.79,−.64) | −.11 (−.52,.27) | −.18 (−.65,.65) | -- | 1550.56 (559) | 6.26 (1) | .01 | 4.26 |
| Constrain All Mods | .68 (.29,.78) | .00 (−.58,.58) | −.71 (−.79,−.65) | -- | -- | -- | 1551.13 (561) | 6.83 (3) | .07 | 0.83 |
Note. a = main effects of genes; c = main effect of shared environment; e = main effect of nonshared environment; βX = additive genetic moderator; βY = shared environmental moderator; βZ = nonshared environmental moderator; χ2Δ = chi-square change; AIC = Akaike information criterion; Full Model = model with all paths and moderators; Constrain A Mod = model that constrained the additive genetic moderator to be 0; Constrain C Mod = model that constrained the shared environmental moderator to be 0; Constrain E Mod = model that constrained the nonshared environmental moderator to be 0; Constrain All Mods = model that constrained all of the moderators to be 0. Values are unstandardized parameter estimates with 95% confidence intervals in parentheses. Each nested, “constrain” model is compared to the full model when calculating the χ2Δ and the degrees of freedom. The AICs were calculated using this formula (χ2−2*Δdf), where the chi-square (i.e., χ2) value was the difference in −2lnL values between the full model and the nested model, and the change in the degrees of freedom (i.e., Δdf) was the difference between the degrees of freedom from the full model versus the degrees of freedom from the nested model. The best-fitting models are noted in bolded and shaded text.
Figure 1.
Unstandardized Parameter Estimates for Additive Genetic (a), Shared Environmental (c), and Nonshared Environmental (e) Effects across the Levels of the Moderator (i.e., Low/High Estradiol and Progesterone Levels). Findings from the Full Moderation Models (i.e., with a, c, and e moderators estimated) and the Best-Fitting Models (i.e., the “Constrain A Moderation Model” for estradiol, and the “Constrain C Moderation Model” for progesterone) are depicted.
For the estrogen groups, the full model suggested the presence of shared and nonshared environmental moderation, as shared environmental influences appeared to increase across low to high estradiol groups, whereas nonshared environmental effects decreased (see Figure 1). Differences in genetic effects were very minimal across groups. Not surprisingly, the best-fitting model for estrogen was the model that constrained the additive genetic moderator to 0. This model evidenced a non-significant change in −2lnL value as compared to the full model, and it had the lowest AIC value (see Table 2). Unstandardized estimates from this model (see Figure 1) showed rather dramatic increases in shared environmental influences across estrogen groups, and more modest decreases in nonshared environment. Standardized estimates indicated that genetic effects accounted for 39% of the variance in both estrogen groups, whereas shared environmental factors increased from 0 to 25% of the variance from low-to-high estrogen groups, and nonshared environment decreased from 60% to 36% across the same groups.
Progesterone showed a very different pattern of results. Findings from the full model indicated the possible presence of genetic and nonshared environmental moderation, as genetic effects appeared to be much higher in the high than the low progesterone group, whereas nonshared environmental factors seemed to decrease across the groups (see Figure 1). Shared environmental influences were minimal in both groups. Again, not surprisingly, the best fitting model (i.e., non-significant change in −2lnL values and lowest AIC value) was the model that constrained shared environmental moderation to 0. Unstandardized (see Table 2 and Figure 1) estimates suggested differences in both additive genetic and nonshared environmental factors across the progesterone groups, although group differences were more modest than those for estrogen. For example, genetic effects increased across progesterone groups (from accounting for 37% of the variance in the low group to 61% in the high group), but the additive genetic moderator just missed reaching statistical significance (see 95% confidence intervals in Table 2). The nonshared environmental moderator did reach significance, with decreasing nonshared environmental effects across groups (63% in the low group to 39% in the high group).
Discussion
Our study was the first to examine hormone moderation of genetic and environmental influences on a behavioral or psychiatric phenotype in adulthood. Results showed significant and differential effects of estrogen and progesterone on etiologic risk for emotional eating. Estrogen appeared to primarily impact environmental influences, with much larger shared environmental effects on emotional eating scores at higher estradiol levels. By contrast, progesterone impacted genetic influences, with more substantial genetic effects on emotional eating scores at higher progesterone levels. Overall, these results confirm and extend previous twin studies in adolescence (Klump et al., 2007; Klump et al., 2010) and across the menstrual cycle (Klump et al., 2015) by showing direct and substantial effects of ovarian hormones on etiologic influences for a key and transdiagnostic disordered eating symptom in adulthood.
Our findings preliminarily confirm hypotheses put forth in our previous paper (Klump et al., 2015) about the potential effects of ovarian hormones on etiologic risk. That study examined changes in heritability across the menstrual cycle and showed substantial shared environmental (and minimal genetic) influences on emotional eating during pre-ovulation, and significant genetic (and minimal shared environmental) influences in post-ovulation. We had proposed that estrogen likely contributed to the pre-ovulatory, shared environmental effects (Klump et al., 2015), given the predominance of estrogen during the first half of the menstrual cycle. Although the current study examined between-subject differences in overall levels of hormones, rather than changes per se, our results provide preliminary support for these predictions by showing that estrogen primarily impacts shared environmental influences on emotional eating. Likewise, we had proposed that progesterone contributed to increased genetic effects in post-ovulation (Klump et al., 2015). In the current study, we observed substantial increases in genetic influences across progesterone groups, where unstandardized and standardized genetic estimates were 2x higher in twins with high versus low progesterone levels. Nonshared environmental effects were found to be important in low and high groups for both hormones, with decreasing effects when moving from low to high hormone groups. These results mimic what we observed in our menstrual cycle study where nonshared environmental factors were present across all phases of the cycle, yet they decreased somewhat when moving from pre- to post-ovulation (Klump et al., 2015).
Overall, the similar pattern of results across our past menstrual cycle study (Klump et al., 2015) and current analyses of general hormone/emotional eating levels is striking. These data lend credence to the idea that ovarian hormones, regardless of the measurement time frame or level of analysis (i.e., between- versus within-subjects), have specific and differential etiologic effects on emotional eating in adulthood. These effects may change over the course of a menstrual cycle, but they are also present at a more general level across time.
Nonetheless, there was one effect that did not replicate across the menstrual cycle studies and the current analysis - the phenotypic effects of hormones on emotional eating scores. Whereas previous studies found significant, within-subject effects of estrogen and progesterone on changes in emotional eating scores across the menstrual cycle (Klump et al., 2013; Klump et al., 2014) we did not find significant, between-group differences in emotional eating scores in low versus high hormone groups. Thus, despite similar etiologic influences of hormones on within- versus between-subject risk, the translation of these etiologic effects into the phenotypic expression of emotional eating may differ across levels of analysis.
There at least two possible reasons for discrepant results. The first is that our computational/analytic approach obscured significant between-group and between-subject phenotypic differences. Namely, that by taking the mathematical average of emotional eating scores across very low risk (i.e., pre-ovulation) and high-risk (i.e., post-ovulation) phases, we may have cancelled out the nuanced, between-subject effects of the hormones on emotional eating scores. One way to partially rule out measurement issues is to conduct more extensive between-subject analyses of phenotypic effects, such that average levels of hormones in each phase are examined in relation to the average emotional eating scores in that phase. These analyses would avoid the pitfalls of averaging emotional eating scores from low versus high-risk phases and could possibly reveal significant phenotypic effects of overall hormone levels on emotional eating scores within menstrual cycle phases. Although these more nuanced follow-up studies of phenotypic effects are beyond the scope of the present study, they are analyses that we will pursue in future work.
The second possibility is more substantive – namely, that ovarian hormones have different phenotypic effects on emotional eating when examining effects within- versus between-subjects. It may be that within-person changes in ovarian hormones significantly influence a woman’s level of emotional eating relative to her own baseline levels (as observed in Hildebrandt et al., 2015; Klump et al., 2013; Klump et al., 2014), but that these hormone changes do not alter her between-subject, rank ordering in emotional eating scores relative to other women. In other words, regardless of peaks and valleys in daily rates of emotional eating, a woman is still relatively high or low on emotional eating, relative to other women. Interestingly, there are animal data suggesting that this could be the case, as the removal of ovarian hormones in adulthood in female rats does result in predictable changes in binge eating in all rats, but it does not change the rank ordering of rats in terms of their categorization as binge eating resistant (rats with very low levels of binge eating) or binge eating prone (rats with consistently high rates of binge eating) (Klump et al., 2011). Rats that were categorized as binge eating prone remained higher in their binge eating levels than binge eating resistant rats, even though rates of binge eating changed with ovariectomy in both groups.
Importantly, the categorization of binge eating resistant versus binge prone rats appears to become stable during puberty; despite malleable rates of binge eating in adulthood (i.e., increasing and decreasing with changing levels of hormones), the binge eating resistant/prone phenotype itself is relatively stable from mid-puberty on. This finding raises an additional point for consideration in our paper - developmental and pubertal differences in the effects of ovarian hormones on binge eating. Early data suggested that estrogen may activate genetic (but not shared environmental) risk for disordered eating symptoms during puberty, and that progesterone has little-to-no effect on genetic or environmental influences (Klump, 2013; Klump et al., 2010). These earlier results contradict our current findings in adulthood and provide preliminary evidence in support of novel shifts in the role of ovarian hormones in disordered eating across development. Indeed, our use of similar assessments in the current study (i.e., between-subject effects in women with low/high estradiol levels) and past studies of puberty (i.e., between-subject effects in girls with low/high estradiol levels) provides additional support for etiologic shifts that are indicative of differential mechanisms rather than differential measurement/levels of analysis.
We propose that the shifting roles of estrogen across development likely reflect the organizational versus activational effects of gonadal hormones on brain and behavior. Gonadal hormone organizational effects are those that cause permanent changes in brain structure/function that set the stage for the brain to respond to the activational effects of hormones in adulthood (Sisk & Zehr, 2005). These organizational effects were originally proposed to occur during the prenatal period only, but it is now well-recognized that puberty is a second period of hormone-mediated re-organization as well (Sisk & Zehr, 2005). By contrast, activational effects are transient hormone effects that activate behavior in adulthood via circulating levels of both estrogen and progesterone (Sisk & Zehr, 2005). Pulling together data from puberty and adulthood, we have proposed (see Klump et al., 2015) that estrogen has organizational effects on eating disorder risk during puberty, such that increases in estrogen lead to genetically mediated, organizational changes in risk that are then differentially activated in adulthood by changes in both estrogen and progesterone – changes that are environmentally and genetically mediated. Interestingly, this theory of shifting etiologic effects of hormones across development fits well with data for phenotypic effects described above. It may be that increases in estrogen during puberty organize between-subject differences in binge eating phenotypes, and that changes in estrogen and progesterone in adulthood result in within-subject shifts in the degree of emotional eating across time. Clearly, additional data are needed to confirm these hypotheses, but they provide one framework for conceptualizing the differential effects of ovarian hormones on phenotypic and etiologic risk across development.
In addition to further clarifying between-subject effects of hormones in adulthood, an important next step will be to identify the specific genetic and shared environmental mechanisms underlying hormone effects across development. We continue to believe that estrogen’s genetic effects during puberty are likely due to its traditional, genomic effects within the central nervous system. Extant data indicate that estrogen regulates gene transcription (i.e., estrogen turns genes on and off) within several neural systems (e.g., serotonin, dopamine, opioids; Becker, 2009; Craft, 2008; Culbert et al., 2009; Hildebrandt et al., 2010; Klump et al., 2003; Ostlund et al., 2003) that are disrupted in binge related phenotypes and eating disorders. Estrogen activation at puberty could lead to differential production of these important neurotransmitters, their receptors, or their signal transduction mechanisms. Moreover, variants of estrogen receptor genes (e.g., estrogen receptor beta; Nilsson et al., 2004) may increase risk for emotional eating by causing different patterns of gene regulation by estrogen, leading to distinct cellular and behavioral responses. In these scenarios, individual differences in the production of estrogen at puberty are not of consequence – the key individual difference variable is the presence or absence of susceptibility alleles that are regulated by estrogen. These individual differences would become evident after estrogen activation during puberty and would lead to between-subject differences in risk for binge eating in women. Phenotypic expression of binge eating would then be most evident in post-puberty when circulating hormones differentially trigger genetic and environmental influences and within-subject differences in binge eating behavior.
Originally, we proposed that estrogen would continue to be the primary driver of genetic effects in adulthood (Klump et al., 2013; Klump et al., 2014), but data from the current study have led us to revise this hypothesis. In adulthood, progesterone appears to play a stronger role in genetic effects and is likely the primary contributor to increased genetic effects for emotional eating during the second half of the menstrual cycle (see Klump et al., 2015). Unlike estrogen, there are not extensive data documenting the specific neural systems regulated by progesterone, so it is difficult to propose neurobiological pathways. However, promising data suggest that progesterone may play an important regulatory role in gene expression within several systems (e.g., serotonin, Gamma-Amino Butyric acid) that are thought to be important in anxiety and/or depression (Arbo, Andrade, Osterkamp, Gomez, & Ribeiro, 2014; Bethea & Centeno, 2008; Bethea & Reddy, 2015; Lu, Eshleman, Janowsky, & Bethea, 2003; Quast et al., 2014; Schüle, Nothdurfterb, & Rupprecht, 2014). It is likely that progesterone activates genetic effects via the same mechanisms as those proposed for estrogen during puberty (e.g., differential production of neurotransmitters and/or their receptors), although this possibility awaits investigation.
Estrogen’s effects on environmental influences in adulthood are an important area for future research as well. The shared environmental effects of estrogen in adulthood may reflect a shift toward non-genomic, membrane estrogen receptor activation that would impact emotional eating in all women, regardless of the level of genetic risk (Klump et al., 2015). Membrane receptor activation produces molecular signals that change the excitability of neurons via processes that do not require changes in gene expression (Santollo, Marshall, & Daniels, 2012). Thus, membrane receptor activation would result in increased shared environmental, but not genetic effects, since the simple presence of high levels of estradiol (during pre-ovulation or in women with higher estradiol levels) would result in receptor activation and downstream behavioral effects. Within-subjects, the downstream behavioral effects are likely to decrease emotional eating, as higher within-subject levels of estradiol (in the absence of progesterone) are associated with decreased emotional eating. Between-subjects, the effects are more difficult to specify, given the need for more detailed analyses of hormone effects on between-subject risk (see above). Nonetheless, regardless of the direction of phenotypic effects, more research is needed to understand the role of nuclear and membrane hormone receptors on emotional eating and binge-related phenotypes across levels of analysis (i.e., within- versus between-subjects) and development.
Before ending, we should note a few key study limitations. First, sample sizes were somewhat modest for a study of gene x hormone effects (see Purcell, 2002). Our use of aggregated measures of both hormones and emotional eating likely decreased measurement error and contributed to more robust effects in the models. Nonetheless, our smaller sample sizes resulted in parameter estimates that just missed reaching significance (e.g., progesterone moderation of genetic effects). Likewise, our smaller sample sizes prohibited examination of more complex, two-moderator models that could examine whether estrogen x progesterone interactions significantly influence etiologic risk. These twin models require even larger samples for detection of significant interactive effects, as they require the inclusion of three additional moderators in the model (i.e., estrogen x progesterone moderation of additive genetic effects (βXExP), shared environmental effects (βYExP) and nonshared environmental effects (βZExP)) and four hormone groups (i.e., low levels of both hormones; high estrogen but low progesterone; low estrogen but high progesterone; high levels of both hormones). Given prior data showing significant phenotypic effects of estrogen x progesterone interactions on within-subject risk for emotional eating, larger twin studies are needed to examine the etiologic effects of estrogen x progesterone interactions on genetic and environmental risk for emotional eating.
Second, like most psychopathological constructs, our emotional eating scores were positively skewed. Skewed distributions for the dependent variable can sometimes lead to spurious moderator effects within gene x hormone models (Purcell, 2002). We believe this issue was unlikely to have unduly influenced our results, given that log transformations resulted in skewness values within acceptable limits (i.e., between −2 and 2), and our pattern of moderating effects (i.e., differentially significant moderators that were often in opposing directions) is not in line with what would be expected if skewed data were driving our results (i.e., similar moderation effects in the same direction across genetic, shared environmental, and nonshared environmental factors) (Purcell, 2002). Nonetheless, some caution is warranted, and replication studies are needed to confirm that non-normality of data did not unduly influence our results.
Finally, our sample was community-based, and we focused on a continuous measure of emotional eating rather than clinically defined binge eating episodes. Confirmation of hormone effects on genetic and environmental risk for binge eating in clinical samples awaits additional research. However, it is important to note that past data strongly suggest that phenotypic effects of ovarian hormones are similar across community and clinical samples, and results are nearly identical for emotional eating and clinically-diagnosed binge episodes (Edler et al., 2007; Klump et al., 2008; Klump et al., 2013; Klump et al., 2014). This similarity in findings is perhaps not surprising, given extensive data in the broader psychopathology field showing the superiority of dimensional models over categorical frameworks for understanding the underlying structure of psychiatric disorders (e.g., see Wright et al., 2013). Although eating disorders are rarely included in these studies of psychopathology, emerging data suggest that eating disorders may be dimensional in nature (Holm-Denoma, Richey, & Joiner, 2010; Keel, Brown, Holland, & Bodell, 2012; Luo, Donnellan, Burt, & Klump, submitted; Olatunji et al., 2012; Tylka & Subich, 2003). Consequently, although additional research is needed, findings thus far suggest that our studies of emotional eating will advance our understanding of more basic behaviors/phenotypes that have underlying genetic and biological dimensions that likely contribute to risk for clinical eating pathology as well.
Supplementary Material
Acknowledgments
This work was supported by the National Institute of Mental Health (KLK, PKK, NM, CLS, SAB, R01 MH082054).
Footnotes
Notably, we also conducted all analyses using 3-groups for estradiol and progesterone levels (i.e., low, medium, and high). The pattern of results remained unchanged from those presented herein, although not unexpectedly, some moderation estimates did not reach significance, likely due to the much smaller sample sizes in each hormone group.
To ensure that our results were not unduly influenced by these modest group differences in age and BMI, we ran a second set of twin models in which we regressed age and BMI out of each twins’ score prior to analysis. Results for both estrogen and progesterone were nearly identical to those presented herein (data not shown).
This pattern of effects also could indicate the presence of dominant genetic effects, as these types of genetic effects make MZ twins more than twice as similar as DZ twins. We tested this possibility by including dominant genetic effects in the models for progesterone. The best-fitting model dropped the dominant genetic parameter, and the pattern of results for the remaining parameters and moderators in the model were identical to those presented herein. Thus, for simplicity and consistency of models across estrogen and progesterone groups, we only report findings for the models containing additive genetic, shared environmental, and nonshared environmental factors (although results from the dominant genetic effects models are available upon request).
Authorship
K.L.K. and P.K.K. developed the study concept. All authors contributed to the study design. Testing and data collection were performed by K.L.K., S.M.O., and B.A.H., and K.L.K., S.M.O., and B.A.H. performed the data analysis and interpretation. K.L.K., S.M.O., and B.A.H. drafted the paper, and P.K.K., C.L.S., and S.A.B. provided critical revisions. All authors approved the final version of the paper for submission.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIMH.
References
- Akaike H. Factor analysis and AIC. Psychometrika. 1987;52:317–332. [Google Scholar]
- Arbo BD, Andrade S, Osterkamp G, Gomez R, Ribeiro MF. Asymmetric effects of low doses of progesterone on GABA (A) receptor α4 subunit protein expression in the olfactory bulb of female rats. Canadian Journal of Physiology and Pharmacology. 2014;92:1045–1049. doi: 10.1139/cjpp-2014-0307. [DOI] [PubMed] [Google Scholar]
- Asarian L, Geary N. Sex differences in the physiology of eating. American Journal of Physiology: Regulatory, Integrative, and Comparative Physiology. 2013;305 doi: 10.1152/ajpregu.00446.02012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB. Sexual differentiation of motivation: A novel mechanism? Hormones and Behavior. 2009;55:646–654. doi: 10.1016/j.yhbeh.2009.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethea CL, Centeno ML. Ovarian steroid treatment decreases corticotropin-relesasing hormone (CRH) mRNA and protein in the hypothalamic paraventricular nucleus of ovariectomized monkeys. Neuropsychopharmacology. 2008;33:546–556. doi: 10.1038/sj.npp.1301442. [DOI] [PubMed] [Google Scholar]
- Bethea CL, Reddy AP. Ovarian steroids regulate gene expression related to DNA repair and neurodegenerative diseases in serotonin neurons of macaques. Molecular Psychiatry. 2015;20:1565–1578. doi: 10.1038/mp.2014.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt SA, Klahr A, Neale M, Klump KL. Maternal warmth and directiveness jointly moderate the etiology of conduct problems. Journal of Child Psychology and Psychiatry. 2013;54:1030–1037. doi: 10.1111/jcpp.12095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt SA, Klump KL. The Michigan State University Twin Registry (MSUTR): An Update. Twin Research and Human Genetics. 2013;16:344–350. doi: 10.1017/thg.2012.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craft RM. Sex differences in analgesic, reinforcing, discriminative, and motoric effects of opioids. Experimental and Clinical Psychopharmacology. 2008;16:376–385. doi: 10.1037/a0012931. [DOI] [PubMed] [Google Scholar]
- Culbert KM, Burt SA, McGue M, Iacono WG, Klump KL. Puberty and the genetic diathesis of disordered eating attitudes and behaviors. Journal of Abnormal Psychology. 2009;118:788–796. doi: 10.1037/a0017207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deaver CM, Miltenberger RG, Symth J, Meidinger A, Crosby R. An evaluation of affect and binge eating. Behavior Modification. 2003;27 doi: 10.1177/0145445503255571. [DOI] [PubMed] [Google Scholar]
- Edler C, Lipson SF, Keel PK. Ovarian hormones and binge eating in bulimia nervosa. Psychological Medicine. 2007;37:131–141. doi: 10.1017/S0033291706008956. [DOI] [PubMed] [Google Scholar]
- Goldschmidt AB, Crosby RD, Cao L, Engel SG, Durkin N, Beach HM, … Peterson CB. Ecological momentary assessment of eating episodes in obese adults. Psychosomatic Medicine. 2014;76:747–752. doi: 10.1097/PSY.0000000000000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrandt BA, Racine SE, Keel PK, Burt SA, Neale M, Boker S, … Klump KL. The effects of ovarian hormones and emotional eating on changes in weight preoccupation across the menstrual cycle. International Journal of Eating Disorders. 2015;48:477–486. doi: 10.1002/eat.22326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrandt T, Alfano L, Tricamo M, Pfaff DW. Conceptualizing the role of estrogens and serotonin in the development and maintenance of bulimia nervosa. Clinical Psychology Review. 2010;30:655–668. doi: 10.1016/j.cpr.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm-Denoma JM, Richey JA, Joiner TE. The latent structure of dietary restraint, body dissatisfaction, and drive for thinness: A series of taxometric analyses. Psychological Assessment. 2010;22:788–797. doi: 10.1037/a0020132. [DOI] [PubMed] [Google Scholar]
- Keel PK, Brown TA, Holland LA, Bodell LP. Empirical classification of eating diosrders. Annual Review of Clinical Psychology. 2012;8:381–404. doi: 10.1146/annurev-clinpsy-032511-143111. [DOI] [PubMed] [Google Scholar]
- Klump KL. Puberty as a critical risk period for eating disorders: A review of human and animal studies. Hormones and Behavior. 2013;64:399–410. doi: 10.1016/j.yhbeh.2013.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klump KL, Burt SA. The Michigan State University Twin Registry (MSUTR): Genetic, environmental and neurobiological influences on behavior across development. Twin Research and Human Genetics. 2006;9:971–977. doi: 10.1375/183242706779462868. [DOI] [PubMed] [Google Scholar]
- Klump KL, Burt SA, McGue M, Iacono WG. Changes in genetic and environmental influences on disordered eating across adolescence: A longitudinal twin study. Archives of General Psychiatry. 2007;64:1409–1415. doi: 10.1001/archpsyc.64.12.1409. [DOI] [PubMed] [Google Scholar]
- Klump KL, Culbert KM, Slane JD, Burt SA, Sisk CL, Nigg JT. The effects of puberty on genetic risk for disordered eating: Evidence for sex difference. Psychological Medicine. 2012;42:627–638. doi: 10.1017/S0033291711001541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klump KL, Hidebrandt BA, O’Connor SM, Keel PK, Neale M, Sisk CL, … Burt SA. Changes in genetic risk for emotional eating across the menstrual cycle: A longitudinal study. Psychological Medicine. 2015;45:3227–3237. doi: 10.1017/S0033291715001221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klump KL, Keel PK, Culbert KM, Edler C. Ovarian hormones and binge eating: Exploring associations in community samples. Psychological Medicine. 2008;38:1749–1757. doi: 10.1017/S0033291708002997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klump KL, Keel PK, Racine SE, Burt SA, Neale M, Sisk CL, … Hu JY. The interactive effects of estrogen and progesterone on changes in emotional eating across the menstrual cycle. Journal of Abnormal Psychology. 2013;122:131–137. doi: 10.1037/a0029524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klump KL, Keel PK, Sisk CL, Burt SA. Preliminary evidence that estradiol moderates genetic influences on disordered eating attitudes and behaviors during puberty. Psychological Medicine. 2010;40:1745–1753. doi: 10.1017/S0033291709992236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klump KL, McGue M, Iacono WG. Differential heritability of eating attitudes and behaviors in prepubertal versus pubertal twins. International Journal of Eating Disorders. 2003;33:287–292. doi: 10.1002/eat.10151. [DOI] [PubMed] [Google Scholar]
- Klump KL, Racine SE, Hildebrandt B, Burt SA, Neale M, Sisk CL, … Keel PK. Ovarian hormone influences on dysregulated eating: A comparison of associations in women with versus without binge episodes. Clinical Psychological Science. 2014;2:545–559. doi: 10.1177/2167702614521794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klump KL, Suisman JL, Culbert KM, Kashy DA, Keel PK, Sisk CL. The effects of ovariectomy on binge eating proneness in adult female rats. Hormones and Behavior. 2011;59:585–593. doi: 10.1016/j.yhbeh.2011.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laghi F, Pompili S, Baumgartner E, Baiocco R. The role of sensation seeking and motivations for eating in female and male adolescents who binge eat. Eating Behaviors. 2015;17:119–124. doi: 10.1016/j.eatbeh.2015.01.011. [DOI] [PubMed] [Google Scholar]
- Lester NA, Keel PK, Lipson SF. Symptom fluctuation in bulimia nervosa: Relation to menstrual-cycle phase and cortisol levels. Psychological Medicine. 2003;33:51–60. doi: 10.1017/s0033291702006815. [DOI] [PubMed] [Google Scholar]
- Lluch A, Herbert B, Mejean L, Siest G. Dietary intakes, eating style and overweight in Stanilas Family Study. International journal of Obesity. 2000;24:1493–1499. doi: 10.1038/sj.ijo.0801425. [DOI] [PubMed] [Google Scholar]
- Lu NZ, Eshleman AJ, Janowsky A, Bethea CL. Ovarian steroid regulation of serotonin reuptake transporter (SERT) binding, distribution, and function in female macaques. Molecular Psychiatry. 2003;8:353–360. doi: 10.1038/sj.mp.4001243. [DOI] [PubMed] [Google Scholar]
- Luo X, Donnellan MB, Burt SA, Klump KL. The dimensional nature of eating pathology: Evidence from a direct comparison of categorical, dimensional, and hybrid models. doi: 10.1037/abn0000174. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McManus F, Waller GI. A functional analysis of binge-eating. Clinical Psychology Review. 1995;15:845–863. [Google Scholar]
- Neale MC, Boker SM, Xie G, Maes HH. Mx: Statistical Modeling. 5. Richmond, VA: Department of Psychiatry; 1999. [Google Scholar]
- Nguyen-Michel ST, Unger JB, Spruijt-Metz D. Dietary correlates of emotional eating in adolescence. Appetite. 2007;49:494–499. doi: 10.1016/j.appet.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson M, Naessén S, Dahlman I, Hirschberg AL, Gustafsson JÅ, Dahlman-Wright K. Association of estrogen receptor β gene polymorphisms with bulimic disease in women. Molecular Psychiatry. 2004;9:28–34. doi: 10.1038/sj.mp.4001402. [DOI] [PubMed] [Google Scholar]
- Olatunji BO, Broman-Fulks JJ, Ciesielski BG, Zawilinski LL, Shewmaker S, Wall D. A taxometric investigation of the latent structure of eating disorders. Psychiatry Research. 2012;197:97–102. doi: 10.1016/j.psychres.2011.12.016. [DOI] [PubMed] [Google Scholar]
- Ostlund H, Keller E, Hurd YL. Estrogen receptor gene expression in relation to neuropsychiatric disorders. Annals of the New York Academy of Sciences. 2003;1007:54–63. doi: 10.1196/annals.1286.006. [DOI] [PubMed] [Google Scholar]
- Purcell S. Variance components models for gene-environment interaction in twin analysis. Twin Research. 2002;5:554–571. doi: 10.1375/136905202762342026. [DOI] [PubMed] [Google Scholar]
- Quast C, Reif A, Brückl T, Pfister H, Weber H, Mattheisen M, … Erhardt A. Gender-specific association of variants in the AKR1C1 gene with dimensional anxiety in patients with panic disorder: Additional evidence for the importance of neurosteroids in anxiety? Depression and Anxiety. 2014;31:843–850. doi: 10.1002/da.22229. [DOI] [PubMed] [Google Scholar]
- Racine SE, Culbert KM, Larson CL, Klump KL. The possible influences of impulsivity and dietary restraint on associations between serotonin genes and binge eating. Journal of Psychiatric Research. 2009;43:1278–1286. doi: 10.1016/j.jpsychires.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricca V, Castellini G, Fioravanti G, Sauro CL, Rotella F, Ravaldi C, … Faravelli C. Emotional eating in anorexia nervosa and bulimia nervosa. Comprehensive Psychiatry. 2012;53:245–251. doi: 10.1016/j.comppsych.2011.04.062. [DOI] [PubMed] [Google Scholar]
- Ricca V, Castellini G, Sauro CL, Ravaldi C, Lapi F, Mannucci E, … Faravelli C. Corrrelations between binge eating and emotional eating in a sample of overweight subjects. Appetite. 2009;53:418–421. doi: 10.1016/j.appet.2009.07.008. [DOI] [PubMed] [Google Scholar]
- Santollo J, Marshall A, Daniels D. Activation of membrane associated estrogen receptors decreases food and water intake in ovariectomized rats. Endocrinology. 2012;154:320–329. doi: 10.1210/en.2012-1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schüle C, Nothdurfterb C, Rupprecht R. The role of allopregnanolone in depression and anxiety. Progress in Neurobiology. 2014;113:79–87. doi: 10.1016/j.pneurobio.2013.09.003. [DOI] [PubMed] [Google Scholar]
- Sisk CL, Zehr JL. Puberty hormones organize the adolescent brain and behavior. Frontiers in Neuroendocrinology. 2005;26:163–174. doi: 10.1016/j.yfrne.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Snoek HM, Van Strien T, Janssens JMAM, Engels RCME. Emotional, external, restrained eating and overweight in Dutch adolescents. Scandinavian Journal of Psychology. 2007;48:23–32. doi: 10.1111/j.1467-9450.2006.00568.x. [DOI] [PubMed] [Google Scholar]
- Suisman JL, Thompson JK, Keel PK, Burt SA, Neale M, Boker S, … Klump KL. Genetic and environmental influences in thin-ideal internalization across puberty and pre-adolescent, adolescent, and young adult development. International Journal of Eating Disorders. 2014;47:773–783. doi: 10.1002/eat.22321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tylka TL, Subich LM. Revisiting the latent structure of eating disorders: Taxometric analyses with nonbehavioral indicators. Journal of Counseling Psychology. 2003;50:276. [Google Scholar]
- van der Sluis S, Posthuma D, Dolan CV. A note on false positives and power in GxE modeling of twin data. Behavior Genetics. 2012;42:170–186. doi: 10.1007/s10519-011-9480-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Strien T. Ice-cream consumption, tendency toward overeating, and personality. Appetite. 2000;52:380–387. doi: 10.1002/1098-108x(200012)28:4<460::aid-eat16>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Van Strien T. Predicting distress-induced eating with self-reports: Mission impossible or a piece of cake? Health Psychology. 2010;29:343. doi: 10.1037/a0020329. [DOI] [PubMed] [Google Scholar]
- Van Strien T, Frijters JE, Bergers GP, Defares PB. The Dutch Eating Behavior Questionnaire (DEBQ) for assessment of restrained, emotional, and external eating behavior. International Journal of Eating Disorders. 1986;5:295–315. [Google Scholar]
- Wardle J. Eating style: A validation study of the Dutch Eating Behaviour Questionnaire in normal subjects and women with eating disorders. Journal of Psychosomatic Research. 1987;31:161–169. doi: 10.1016/0022-3999(87)90072-9. [DOI] [PubMed] [Google Scholar]
- Wright AGC, Krueger RF, Hobbs MJ, Markon KE, Eaton NR, Slade T. The structure of psychopathology: Toward an expanded quantitative empirical model. Journal of Abnormal Psychology. 2013;122:281–294. doi: 10.1037/a0030133. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.