Abstract
Background
The human ether-a-go-go-related gene (HERG) encodes the α-subunit of rapidly activating delayed-rectifier potassium channels. Mutations in this gene cause long QT syndrome type 2 (LQT2). In most cases, mutations reduce the stability of the channel protein, which can be restored by heat shock (HS).
Methods
We identified the novel mutant A78T-HERG in a patient with LQT2. The purpose of the current study was to characterize this mutant protein and test whether HS and heat shock factors (HSFs) could stabilize the mutant protein. A78T-HERG and wild-type HERG (WT-HERG) were expressed in HEK293 cells and analyzed by immunoblotting, immunoprecipitation, immunofluorescence, and whole-cell patch clamping.
Results
When expressed in HEK293 cells, WT-HERG gave rise to immature and mature forms of the protein at 135 and 155 kDa, respectively. A78T-HERG gave rise only to the immature form, which was heavily ubiquitinated. The proteasome inhibitor MG132 increased the expression of immature A78T-HERG and increased both the immature and mature forms of WT-HERG. WT-HERG, but not A78T-HERG, was expressed on the plasma membrane. In whole-cell patch clamping experiments, depolarizing pulses evoked E4031-sensitive HERG channel currents in cells transfected with WT-HERG, but not in cells transfected with A78T-HERG. The A78V mutant, but not A78G mutant, remained in the immature form similarly to A78T. Maturation of the A78T-HERG protein was facilitated by HS, expression of HSF-1, or exposure to geranyl geranyl acetone.
Conclusions
A78T-HERG was characterized by protein instability and reduced expression on the plasma membrane. The stability of the mutant was partially restored by HSF-1, indicating that HSF-1 is a target for the treatment for LQT2 caused by the A78T mutation in HERG.
Abbreviations: LQTS, long QT syndrome; HERG, human-ether-a-go-go-related gene; HS, heat shock; HSP, heat shock protein; HSF, heat shock factor; GGA, geranyl geranyl acetone
Keywords: Long QT syndrome, Human ether-a-go-go-related gene, Heat shock factor
1. Introduction
Long QT syndrome (LQTS) is characterized by QT intervals longer than 450 ms that lead to a torsades de pointes ventricular tachycardia and sudden death. LQTS is attributed to mutations in genes that encode ion channels or their regulatory proteins responsible for the proper repolarization of cardiomyocytes [1], [2], [3], and is classified into 13 types based on the mutated genes. LQTS types 1, 2, and 3 account for 90% of LQTS cases [4].
Human-ether-a-go-go-related gene (HERG) encodes the α-subunit of rapidly-activating delayed-rectifier potassium channels that generate IKr. This outward potassium current is elicited during the plateau phase of action potentials and is required for repolarization [4]. After being translated in the endoplasmic reticulum (ER), the HERG protein is transported to the Golgi apparatus where it undergoes glycosylation [5]. This modification transforms the protein from the premature form into the mature form. Eventually, HERG is transported to the plasma membrane [6]. LQTS type 2 (LQT2) is caused by mutations in HERG [7]. In most LQT2 cases, the mutations destabilize the HERG protein and impair its maturation and intracellular transport [8]. Sorted by the ER quality control system, the mutant HERG protein is reverse-transported from the ER to the cytoplasm where it is degraded by the ubiquitin–proteasome system, resulting in reduced HERG channel currents and impaired repolarization of ventricular action potentials [9].
It has been reported that heat shock (HS) assists both in the folding of newly synthesized proteins and the refolding of denatured proteins [10]. Molecular chaperones such as heat shock proteins (HSPs), including Hsp90 and Hsp70, induced by HS play an important role in the maturation of HERG [11], [12]. In mammals, the heat shock factor (HSF) family consists of 4 subtypes and increases in response to HS to activate the transcription of molecular chaperones.
In the present study, we characterized a novel mutant, A78T-HERG, found in a patient with LQT2. The A78T-HERG protein showed reduced stability and failed to transfer to the plasma membrane. Its stability was partially restored by HSF-1, indicating that HSF-1 is a target for the treatment for LQT2 caused by the A78T mutation in HERG.
2. Materials and methods
2.1. Cell culture and transfection
HEK293 cells were cultured in DMEM (Sigma, St. Louis, MO) supplemented with 10% fetal bovine serum (JRH Biosciences, Inc., St. Louis, MO) and penicillin/streptomycin/geneticin at 37 °C, in a 5% CO2 atmosphere. Missense mutations were introduced into pcDNA3/HERG-FLAG by site-directed mutagenesis. pcDNA3 expression plasmids for HSF-1, 2, and 4 were provided by A. Nakai. Cells were transfected using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. The total amount of cDNA was adjusted using vector cDNA.
2.2. Immunoblotting and immunoprecipitation
Cells were harvested 48 h after transfection and lysed by sonication in lysis buffer (PBS supplemented with 1% polyoxyethylene octylphenyl ether, 0.5% sodium deoxycholate, 0.1% SDS, 1.5 mM aprotinin, 21 mM leupeptine, 15 mM pepstain, and 1 mM phenylmethylsulfonylfluoride). After removal of insoluble materials by centrifugation, protein concentrations were determined by the bicinchoninic acid protein assay using a commercially available kit (Pierce Biotechnology, Rockford, IL). Proteins were separated by SDS-PAGE and electrotransferred to PVDF membranes. The membranes were blocked with 5% non-fat dry milk in PBS containing 0.1% Tween and probed with the primary antibody. Antibodies against the following proteins were used: FLAG epitope (Cosmo Bio USA, Carlsbad, CA), β-actin (Nuclea Diagnostic Laboratories, LLC/Oncogene Science, Cambridge, MA), and green fluorescent protein (GFP, Molecular Probes, Thermo Fisher Scientific, Waltham, MA). The blots were developed using an ECL system (Amersham, Biosciences, Amersham, UK). Immunoprecipitation was carried out at 4 °C overnight in PBS supplemented with 1% Triton X-100, 0.5% SDS, 0.25% sodium deoxycholate, 1 mM EDTA, and protease inhibitors. Immune complexes were collected using protein G agarose (Pharmacia, Uppsala, Sweden) and bound proteins were analyzed by SDS-PAGE followed by immunoblotting. Band densities were quantified using NIH image J software (Bethesda, MD) and normalized to the densities of β-actin.
2.3. Immunofluorescence
HEK293 cells on gelatin-coated coverslips were transfected with pcDNA3/HERG-FLAG and pAcGFP-Mem constructs (Clontech Laboratories, Inc., Mountain View, CA). Twenty-four hours after transfection, the cells were fixed in 4% paraformaldehyde/PBS and then permeabilized with 0.5% Triton X-100. Next, the cells were incubated for 1 h at room temperature with the primary antibody (FLAG, 1:1000). After blocking in 3% albumin, bound antibodies were visualized with Alexa Fluor 546-conjugated mouse secondary antibody (1:2000), and images were obtained using a Bio-Rad MRC 1024 confocal microscope (Bio-Rad, Hercules, CA).
2.4. Electrophysiological recordings
HEK293 cells were transfected with pcDNA3/HERG-FLAG (0.5 μg) and either pcDNA3/HSF-1, HSF-2 or HSF-4 (0.5 μg) together with pEGFP (0.2 μg). The total amount of cDNA was adjusted using vector cDNA (0.5 μg) for the dominant negative suppression experiments. Twenty-four hours after transfection, the cells were visualized by EGFP fluorescence and HERG channel currents corresponding to IKr were measured at 37 °C using whole-cell patch-clamping techniques with an Axopatch-200 amplifier (Axon Instruments, Inc., Union City, CA). The extracellular solution contained (mM) NaCl 140, KCl 4, CaCl2 1.8, MgCl2 0.53, NaH2PO4 0.33, glucose 5.5, and HEPES 5 (pH 7.4 with NaOH). The internal pipette solution contained (mM) K-aspartate 100, KCl 20, CaCl2 1, Mg-ATP 5, EGTA 5, HEPES 5, and creatine phosphate dipotassium 5 (pH 7.2 with KOH). The membrane potential was held at −50 mV, depolarized by 1-s test pulses (from −40 to +40 mV in 10-mV increments), and then repolarized back to the holding potential. E4031, an anti-arrhythmic drug and blocker selective for HERG, was added at 10 μM. E4031-sensitive currents were used to characterize HERG channel currents as precisely as possible.
2.5. Statistical analysis
All data are presented as the mean±SEM. For statistical analysis, Student’s t-test and repeated measures analysis of variance (two-way ANOVA) were used, with P<0.05 considered statistically significant.
3. Results
3.1. Characterization of the LQT2 patient
A 7-year-old boy was diagnosed with LQT2. The QT interval was 535 ms and the QT interval corrected for heart rate was 496 ms (Fig. 1A). There were no abnormal findings on the chest X-ray film, ultrasonic cardiogram, or treadmill exercise test. Direct sequencing of HERG (KCNH2) alleles identified the mutation G232A (A78T) in one allele (Fig. 1B). A78 is in the N-terminal intracellular domain of the HERG channels (Fig. 1C). No other mutations were detected.
Fig. 1.
Characterization of the LQT2 patient. (A) Electrocardiogram of the patient with LQT2. (B) Novel HERG (KCNH2) mutation, G232A (A78T), in the patient. The patient was heterozygous for the missense mutation. (C) Schematic representation of the HERG (KCNH2) channel, indicating the A to T substitution at position 78.
3.2. Impaired maturation of A78T-HERG protein
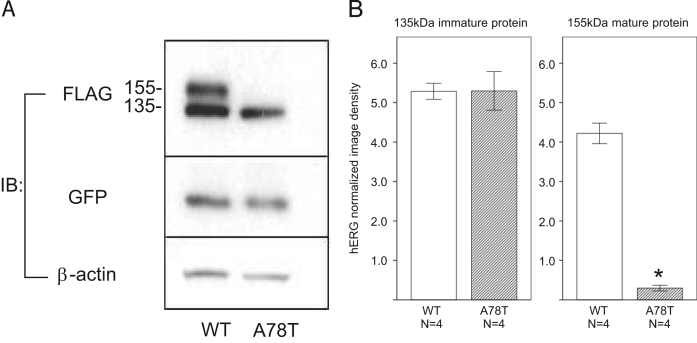
When expressed in HEK293 cells, WT-HERG-FLAG gave rise to immature and mature proteins of 135 kDa and 155 kDa, respectively. In contrast, A78T-HERG gave rise to an immature protein only (Fig. 2A). There was a significant reduction in the mature protein in cells transfected with A78T-HERG compared to transfection with WT-HERG (Fig. 2B). Cotransfection of the A78-HERG and WT-HERG plasmids (0.5 μg each) reduced the mature band of 155 kDa, while transfection with the same amount of WT-HERG and vector plasmid (0.5 μg) yielded the mature band, indicating a dominant-negative effect of A78T-HERG (Supplementary Fig. 1A).
Fig. 2.
Expression of wild-type HERG (WT-HERG) and mutant (A78T-HERG). (A) Western blots showing WT-HERG (lane 1: WT) and A78T-HERG (lane 2: A78T) expressed in HEK293 cells. Cell lysates were subjected to immunoblotting (IB) with indicated antibodies. (B) Quantification of immature and mature HERG proteins. Bars indicate the levels of 155 kDa mature and 135 kDa immature forms of WT-HERG and A78T-HERG (n=4 each) normalized to the levels of β-actin. *P<0.01 vs. WT.
3.3. Ubiquitination of A78T-HERG proteins and effects of proteasome inhibitor
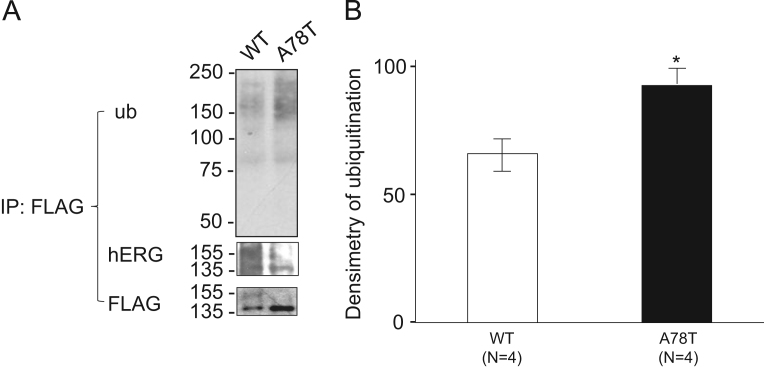
Representative ubiquitination of the A78T-HERG and WT-HERG proteins is shown in Fig. 3A. Ubiquitination of A78T-HERG proteins was much more abundant than that of WT-HERG proteins. Quantification of ubiquitination, obtained from 4 different experiments, is shown in Fig. 3B. The A78T-HERG protein was significantly ubiquitinated compared to the WT-HERG protein. Treatment with the proteasome inhibitor MG132 increased the mature form of WT-HERG proteins but not that of A78T-HERG, whereas the immature forms of both WT-HERG and A78T-HERG were increased (Fig. 4A and B).
Fig. 3.
Ubiquitination of WT-HERG and A78T-HERG proteins. (A) Representative blots of ubiquitinated WT-HERG and A78T-HERG proteins. Cell lysates were subjected to anti-FLAG immunoprecipitation (IP). 80% of the IP products were subjected to anti-ubiquitin immunoblotting; the other 20% were subjected to anti-FLAG and anti-HERG antibody immunoblotting. (B) Quantification of ubiquitinated proteins. Bar graphs indicate the levels of ubiquitinated WT-HERG or A78T-HERG proteins normalized to those of β-actin (n=4 each). *P<0.001 vs. WT-HERG.
Fig. 4.
Effects of proteasome inhibitor on HERG expression. (A) Representative western blots showing the levels of mature and immature WT-HERG and A78T-HERG proteins. Cells were transfected with the WT-HERG or A78T-HERG-FLAG plasmids and cultured overnight in the absence or presence of MG132 (10 μM). (B) Quantification of immature and mature HERG proteins in the absence (CTRL) and presence of MG132. Band densities are expressed as the ratios to those of β-actin (n=4 each).
3.4. Cell-surface expression of A78T-HERG and its channel activity
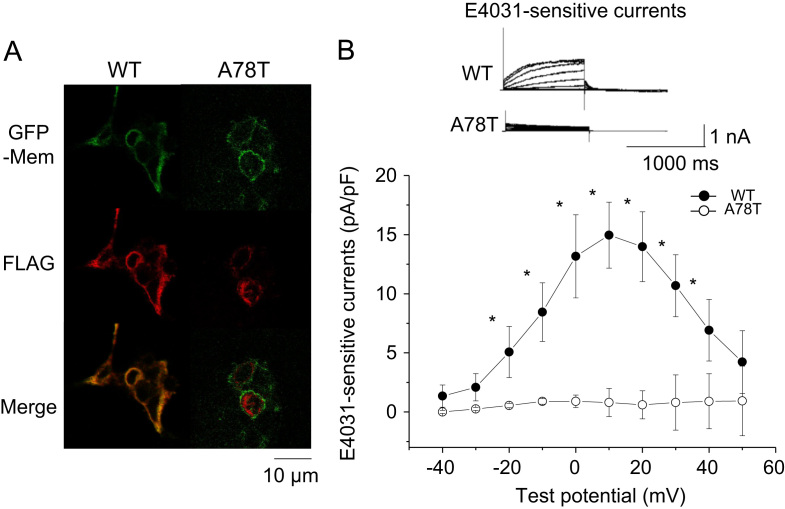
Representative immunofluorescence images of cells expressing WT-HERG or A78T-HERG are shown in Fig. 5A. The signal from the WT-HERG protein, but not that of the A78T-HERG protein, co-localized with that from AcGFP-Mem. Representative original traces of HERG channel currents in HEK293 cells expressing WT-HERG or A78T-HERG are shown in Fig. 5B. The E4031-sensitive outward currents and tail currents were largely time-dependent and observed in cells expressing WT-HERG, whereas there were only small E4031-sensitive outward currents with no detectable tail current in cells expressing A78T-HERG. The current–voltage relationship of HERG channel currents in cells expressing WT-HERG or A78T-HERG (Fig. 5B, lower panel; n=10) shows that there was a significant reduction in peak outward HERG currents in cells expressing A78T-HERG. As shown in Supplementary Fig. 1B, cells transfected with both the WT-HERG and A78T-HERG plasmids gave rise to reduced E4031-sensitive currents, indicating a dominant negative effect of the mutant.
Fig. 5.
Intracellular localization of HERG proteins and channel currents. (A) Immunofluoresence of WT-HERG and A78T-HERG expressed in HEK293 cells. All cells were stained with anti-FLAG and Alexa Fluor 546-conjugated secondary antibody. Shown are representative images obtained by confocal microscopy. HEK293 cells expressing WT-HERG or A78T-HERG-FLAG (red) together with AcGFP-Mem (green). Bar, 10 μm. (B) E4031-sensitive HERG currents recorded by whole-cell patch clamp. The upper panel shows representative original current traces recorded from HEK293 cells expressing WT-HERG or A78T-HERG-FLAG. Current–voltage relationships of peak currents are shown in the lower panel. *P<0.001 vs. WT.
3.5. Effects of amino acid substitutions on HERG expression
The type and position of a mutation in a gene influences protein structure and stability [13]. To clarify whether the key factor for instability of the A78T mutant is the change in molecular size or hydrophobicity (or both) at position 78, we investigated the effect of substituting the amino acid alanine (A) at position 78 with valine (V), which is similar in molecular radius to a threonine residue (larger than an alanine residue), but is a non-polar amino acid similarly to alanine. For A78T, A78V failed to give rise to the mature form of HERG. Next, in order to evaluate the importance of the methyl group, we constructed another mutant, A78G-HERG, in which the alanine at position 78 was substituted with glycine (G), which has no methyl group and is smaller than alanine. The mature form of the HERG protein was detected when A78G-HERG was expressed, suggesting a tolerance for deletion of the methyl group, i.e., a decrease in size and hydrophobicity (Fig. 6).
Fig. 6.
Effects of amino acid substitutions at position 78 on the expression level of immature and mature HERG proteins. Shown are representative western blots showing the levels of WT, A78T, A78G, and A78V-HERG expressed in HEK293 cells. Cell lysates were subjected to immunoblotting with indicated antibodies.
3.6. Effects of HS, HSF-1, and a HSF-1 inducer on the maturation of A78T-HERG protein
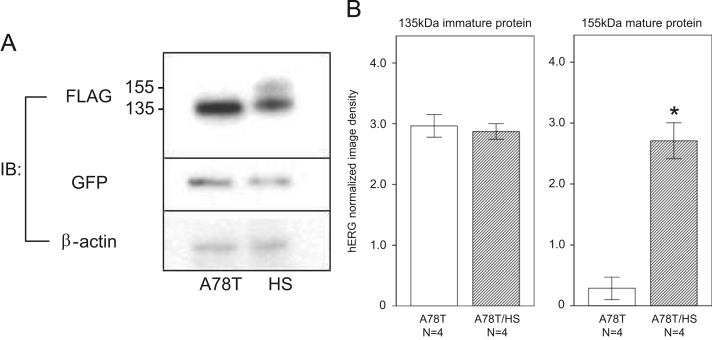
Heat shock increases HSF levels and facilitates the maturation of WT-HERG proteins [11]. HS at 42 °C for 1 h increased the expression of the mature 155 kDa protein of A78T-HERG (Fig. 7A), which was confirmed in four different experiments (B). HSP expression is regulated by HSF-1, 2, and 4. HSF-1 increased the mature form of A78T-HERG, whereas neither HSF-2 nor 4 influenced the maturation of A78T-HERG (Fig. 8A, left panel), which was confirmed in three different experiments (A, right panel). Geranyl geranyl acetone (GGA) at 200 nM, which is an inducer of HSF-1, also facilitated the maturation of A78T-HERG.
Fig. 7.
Effect of heat shock (HS) on A78T-HERG expression. (A) Western blots showing the levels of immature and mature A78T-HERG proteins with and without HS treatment. (B) Quantification of immature and mature HERG proteins to determine the effect of HS (n=4 each). *P<0.01 vs. A78T without HS.
Fig. 8.
Effects of heat shock factor (HSF) family protein expression and GGA on the maturation of A78T-HERG proteins. (A) Western blots showing the levels of A78T-HERG proteins. HEK293 cells were transfected with pcDNA3/A78T-HERG-FLAG and pEGFP, together with pcDNA3/HSF-1, HSF-2, or HSF-4. The HSF-1 activator GGA at 200 nM was added to the culture medium at 36 h after transfection and the cells were further cultured for 12 h. Right panel shows quantification of the 155 kDa mature protein (n=4 each). *P<0.001 vs. control (A78T). (B) Representative HERG current traces (left) and current–voltage relationships (right). Currents were recorded 24 h after HS treatment at 42 °C for 1 h (middle) or 24 h after transfection with pcDNA3/A78T-HERG and HSF-1 (bottom). Current–voltage relationships of E4031-sensitive peak currents showed significant increases in A78T-HERG currents by HS or co-expression with HSF-1 (n=10 each). *P<0.001 vs. control (A78T).
Representative original current traces recorded from cells expressing A78T-HERG after treatment with HS are shown in Fig. 8B (left panel). A significant increase in the E4031-sensitive mutant channel current was observed in cells treated with HS. The current–voltage relationships of A78T-HERG currents in transfected cells with or without HS treatment, obtained from ten experiments, are also shown in Fig. 8B (right panel). The E4031-sensitive peak outward A78T-HERG currents significantly increased in cells treated with HS. This effect of HS was mimicked by co-expression with HSF-1.
4. Discussion
The novel HERG mutation A78T, found in a patient with LQT2, was located at the N-terminus of HERG proteins. The A78T-HERG protein failed to mature and was expressed as a highly ubiquitinated 135-kDa immature protein. Since ubiquitination of the protein to be degraded by the proteasome is known to occur in the core-glycosylated immature form at the ER [11], [12], this finding indicates the impaired stability of A78T-HERG proteins to be degraded via the ubiquitin proteasome system. Protein maturation is facilitated by the accumulation of immature proteins (mass effect) as well as by posttranslational modifications including glycosylation. In the present study, MG132 increased the expression of the 135-kDa immature form of A78T-HERG, but not that of the 155-kDa mature form, suggesting that the accumulation of immature A78T-HERG did not facilitate its maturation. We previously demonstrated that MG132 increased the expression of the mature form of Kv1.5, a voltage-gated potassium channel, indicating that accumulated immature wild-type Kv1.5 protein could be converted to its mature form as a result of the mass effect [14]. Since a substantial portion of Kv1.5 is degraded via the ubiquitin–proteasome system, proteasomal inhibition results in the accumulation of a large amount of immature Kv1.5, facilitating its maturation as a mass effect. In contrast, during the synthesis of WT-HERG, most of the core-glycosylated immature protein is converted to the fully-glycosylated mature form [6]. Thus, accumulation of the immature form of HERG protein in a setting of proteasomal inhibition is lower than that of Kv1.5. The failure of MG132 to increase the expression of mature A78T-HERG suggests that accumulation of immature A78T-HERG is not sufficient to exert a mass effect, while accumulation of immature A78T-HERG may not increase its mature form because of severe dysfunction of its maturation process.
Since the type and position of a mutation in the gene influence protein structure and stability [13], the A78T mutation may destabilize the HERG protein because of an alteration in the molecular radius or hydrophobicity of the amino acid at position 78. A78G-HERG yielded the mature protein, whereas A78V-HERG, similarly to A78T-HERG, did not. The former finding excludes the possibility that deletion of the methyl group by substitution for alanine destabilizes the A78T-HERG protein. The latter finding suggests that the larger molecular radius but not hydrophilicity of the threonine residue destabilizes A78T-HERG.
Heat shock facilitates the folding of various proteins as mediated by the HSP family and can stabilize mutant proteins. HS has been reported to facilitate maturation of WT-HERG as well as mutant HERG by activating the HSF family [15]. HSF-1, 2, and 4 were reported to be expressed in humans. HSF-1 is a master regulator of Hsp70 and Hsp90. HSF-2 plays roles in the development of the brain and reproductive organs and acts as a major regulator of the proteostasis capacity against febrile-range thermal stress to prevent the accumulation of misfolded proteins [16]. HSF-4, which possesses transcriptional repressor properties in vivo, inhibits basal transcription of Hsp27 and Hsp90 in cultured cells [17]. In the present study, we found that HSF-1, but not HSF- 2 or 4, increased the expression of mature A78T-HERG protein. These results suggest that HSF-1 stabilizes A78T-HERG protein, promotes its transport to the plasma membrane, and increases the membrane current IKr.
The ubiquitin proteasome system in cardiomyocytes, including HL-1 cells and neonatal rat cardiomyocytes, involves three activities: chymotrypsin-like, trypsin-like, and caspase-like activities. The degradation of several ion channel proteins showed similar time courses between cardiomyocytes and HEK293 cells, indicating no difference in ubiquitin–proteasome system activities in these cells. Thus, mutant HERG proteins such as A78T-HERG may be degraded through the ubiquitin–proteasome system in cardiomyocytes as well [18]. Accordingly, the clinical implications of our results are as follows. To date, many studies have shown that maturation of mutant HERG proteins can be improved by low temperature or HS and by application of E-4031 or a gastroprokinetic agent such as cisapride [19], [20], [21]. GGA is a non-toxic acyclic isoprenoid compound with a retinoid skeleton that increases Hsp70 expression by activating HSF-1 [22]. In the present study, GGA at the clinical concentration of 200 nM facilitated maturation of the A78T-HERG protein, suggesting that GGA is of therapeutic value for patients with LQT2 caused by the A78T mutation in HERG.
5. Conclusions
The A78T-HERG protein showed reduced stability and failed to transfer to the plasma membrane. Its stability was partially restored by HSF-1, indicating that HSF-1 is a potential target for the treatment for LQT2 caused by the A78T mutation in HERG.
Conflict of interest
All authors declare no conflict of interest related to this study.
Financial support
This study was supported by a Grant in-Aid for scientific research from the Ministry of Education, Culture, Science, Sports and Technology of Japan (25670110 to I. H.).
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.joa.2015.10.005.
Appendix A. Supplementary material
Supplementary material Dominant negative effects of A78T-HERG on HERG expression and HERG channel currents. (A) Western blots showing the levels of HERG-FLAG proteins in cells cotransfected pcDNA3/A78T-HERG-FLAG with WT-HERG-FLAG compared to that in cells transfected with pcDNA3/WT-HERG-FLAG alone. HEK293 cells were co-transfected with pcDNA3/A78T-HERG-FLAG (0.25 or 0.5 μg) and pcDNA3/WT-HERG-FLAG or pcDNA3/WT-HERG-FLAG (1 or 0.5 μg) and pcDNA3 vector (0.25 or 0.5 μg) together with the EGFP plasmid. (B) Representative HERG current traces (left) and current–voltage relationships (right). Left: Currents were recorded in cells cotransfected with pcDNA3/A78T-HERG-FLAG (0.5 μg) and WT-HERG-FLAG (0.5 μg) compared to that in cells transfected with pcDNA3/WT-HERG-FLAG (0.5 μg) and pcDNA3 vector (0.5 μg) together with the EGFP plasmid (0.2 μg). Right: Current–voltage relationships of E4031-sensitive peak currents showed marked reduction of currents in cells cotransfected with pcDNA3/A78T-HERG-FLAG (0.5 μg) and pcDNA3/WT-HERG-FLAG compared with cells co-transfected pcDNA3/WT-HERG-FLAG (0.5 μg) and pcDNA3 vector (0.5 μg) (n=10 each). *P<0.001 vs. WT.
References
- 1.Mizusawa Y., Horie M., Wilde A.A. Genetic and clinical advances in congenital long QT syndrome. Circ J. 2014;78:2827–2833. doi: 10.1253/circj.cj-14-0905. [DOI] [PubMed] [Google Scholar]
- 2.Nakano Y., Shimizu W. Genetics of long-QT syndrome. J Hum Genet. 2015 doi: 10.1038/jhg.2015.74. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 3.Nakajima T., Kaneko Y., Kurabayashi M. Unveiling specific triggers and precipitating factors for fatal cardiac events in inherited arrhythmia syndromes. Circ J. 2015;79:1185–1192. doi: 10.1253/circj.CJ-15-0322. [DOI] [PubMed] [Google Scholar]
- 4.Robin N.H., Tabereaux P.B., Benza R. Genetic testing in cardiovascular disease. J Am Coll Cardiol. 2007;50:727–737. doi: 10.1016/j.jacc.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 5.Sanguinetti M.C., Tristani-Firouzi M. hERG potassium channels and cardiac arrhythmia. Nature. 2006;440:463–469. doi: 10.1038/nature04710. [DOI] [PubMed] [Google Scholar]
- 6.Gong Q., Anderson C.L., January C.T. Role of glycosylation in cell surface expression and stability of HERG potassium channels. Am J Physiol. 2002;283:77–84. doi: 10.1152/ajpheart.00008.2002. [DOI] [PubMed] [Google Scholar]
- 7.Gong Q., Keeney D.R., Molinari M. Degradation of trafficking-defective long QT syndrome type II mutant channels by the ubiquitin–proteasome pathway. J Biol Chem. 2005;280:19419–19425. doi: 10.1074/jbc.M502327200. [DOI] [PubMed] [Google Scholar]
- 8.Curran M.E., Splawski I., Timothy K.W. A molecular basis for cardiac arrhythmia: HERG mutations cause long QT syndrome. Cell. 1995;80:795–803. doi: 10.1016/0092-8674(95)90358-5. [DOI] [PubMed] [Google Scholar]
- 9.Gong Q., Jones M.A., Zhou Z. Mechanisms of pharmacological rescue of trafficking-defective hERG mutant channels in human long QT syndrome. J Biol Chem. 2006;281:4069–4074. doi: 10.1074/jbc.M511765200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Young J.C. The role of the cytosolic HSP70 chaperone system in disease caused by misfolding and aberrant trafficking of ion channels. Dis Model Mech. 2014;7:319–329. doi: 10.1242/dmm.014001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Peili, Ninomiya H., Kurata Y. Reciprocal Control of hERG Stability by Hsp70 and Hsc70 with implication for restoration of LQT2 mutant stability. Circ Res. 2011;108:458–468. doi: 10.1161/CIRCRESAHA.110.227835. [DOI] [PubMed] [Google Scholar]
- 12.Ficker E., Dennis A.T., Wang L. Role of the cytosolic chaperones Hsp70 and Hsp90 in maturation of the cardiac potassium channel HERG. Circ Res. 2003;92:87–100. doi: 10.1161/01.RES.0000079028.31393.15. [DOI] [PubMed] [Google Scholar]
- 13.Idowu S.M., Gautel M., Perkins S.J. Structure, stability and dynamics of the central domain of cardiac myosin binding protein C (MyBP-C): implications for multidomain assembly and cause for cardiomyopathy. J Mol Biol. 2003;329:745–761. doi: 10.1016/s0022-2836(03)00425-x. [DOI] [PubMed] [Google Scholar]
- 14.Kato M., Ogura K., Miake J. Evidence for proteasome degradation of Kv1.5 channel protein. Biochem Biophys Res Commun. 2005;337:343–348. doi: 10.1016/j.bbrc.2005.09.053. [DOI] [PubMed] [Google Scholar]
- 15.Fujimoto M., Nakai A. The heat shock factor family and adaptation to proteotoxic stress. FEBS J. 2010;277:4112–4125. doi: 10.1111/j.1742-4658.2010.07827.x. [DOI] [PubMed] [Google Scholar]
- 16.Shinkawa T., Tan K., Fujimoto M. Heat shock factor 2 is required for maintaining proteostasis against febrile-range thermal stress and polyglutamine aggregation. Mol Biol Cell. 2011;22:3571–3583. doi: 10.1091/mbc.E11-04-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frejtag W., Zhang Y., Dai R. Heat shock factor-4 (HSF-4a) represses basal transcription through interaction with TFIIF. J Biol Chem. 2001;276:14685–14694. doi: 10.1074/jbc.M009224200. [DOI] [PubMed] [Google Scholar]
- 18.Bahrudin U., Morikawa K., Takeuchi A. Impairment of ubiquitin–proteasome system by E334K cMyBPC modifies channel proteins, leading to electrophysiological dysfunction. J Mol Biol. 2011;413:857–878. doi: 10.1016/j.jmb.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 19.Anderson C.L., Delisle B.P., Anson B.D. Most LQT2 mutations reduce Kv11.1 (hERG) current by a class 2 (trafficking-deficient) mechanisms. Circulation. 2006;113:365–373. doi: 10.1161/CIRCULATIONAHA.105.570200. [DOI] [PubMed] [Google Scholar]
- 20.Paulussen A., Raes A., Matthijs G. A novel mutation (T65P) in the PAS domain of the human potassium channel HERG results in the long QT syndrome by trafficking deficiency. J Biol Chem. 2002;277:48610–48616. doi: 10.1074/jbc.M206569200. [DOI] [PubMed] [Google Scholar]
- 21.Zhou Z., Gong Q., January C.T. Correction of defective protein trafficking of a mutant HERG potassium channel in human long QT syndrome. Pharmacological and temperature effects. J Biol Chem. 1999;274:31123–31126. doi: 10.1074/jbc.274.44.31123. [DOI] [PubMed] [Google Scholar]
- 22.Ooie T., Takahashi N., Saikawa T. Single oral dose of geranylgeranylacetone induces heat-shock protein 72 and renders protection against ischemia/reperfusion injury in rat heart. Circulation. 2001;104:1837–1843. doi: 10.1161/hc3901.095771. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material Dominant negative effects of A78T-HERG on HERG expression and HERG channel currents. (A) Western blots showing the levels of HERG-FLAG proteins in cells cotransfected pcDNA3/A78T-HERG-FLAG with WT-HERG-FLAG compared to that in cells transfected with pcDNA3/WT-HERG-FLAG alone. HEK293 cells were co-transfected with pcDNA3/A78T-HERG-FLAG (0.25 or 0.5 μg) and pcDNA3/WT-HERG-FLAG or pcDNA3/WT-HERG-FLAG (1 or 0.5 μg) and pcDNA3 vector (0.25 or 0.5 μg) together with the EGFP plasmid. (B) Representative HERG current traces (left) and current–voltage relationships (right). Left: Currents were recorded in cells cotransfected with pcDNA3/A78T-HERG-FLAG (0.5 μg) and WT-HERG-FLAG (0.5 μg) compared to that in cells transfected with pcDNA3/WT-HERG-FLAG (0.5 μg) and pcDNA3 vector (0.5 μg) together with the EGFP plasmid (0.2 μg). Right: Current–voltage relationships of E4031-sensitive peak currents showed marked reduction of currents in cells cotransfected with pcDNA3/A78T-HERG-FLAG (0.5 μg) and pcDNA3/WT-HERG-FLAG compared with cells co-transfected pcDNA3/WT-HERG-FLAG (0.5 μg) and pcDNA3 vector (0.5 μg) (n=10 each). *P<0.001 vs. WT.