Abstract
Sudden cardiac death (SCD) is a rare but devastating complication of a number of underlying cardiovascular diseases. While coronary artery disease and acute myocardial infarction are the most common causes of SCD in older populations, inherited cardiac disorders comprise a substantial proportion of SCD cases aged less than 40 years. Inherited cardiac disorders include primary inherited arrhythmogenic disorders such as familial long QT syndrome (LQTS), Brugada syndrome (BrS), catecholaminergic polymorphic ventricular tachycardia (CPVT), and inherited cardiomyopathies, most commonly hypertrophic cardiomyopathy (HCM). In up to 40% of young SCD victims (defined as 1–40 years old, excluding sudden unexplained death in infancy from 0 to 1 years, referred to as SIDS), no cause of death is identified at postmortem [so-called “autopsy negative” or “sudden arrhythmic death syndrome” (SADS)]. Management of families following a SCD includes the identification of the cause of death, based either on premorbid clinical details or the pathological findings at the postmortem. When no cause of death is identified, genetic testing of DNA extracted from postmortem tissue (the molecular autopsy) may identify a cause of death in up to 30% of SADS cases. Targeted clinical testing in a specialized multidisciplinary clinic in surviving family members combined with the results from genetic testing, provide the optimal setting for the identification of relatives who may be at risk of having the same inherited heart disease and are therefore also predisposed to an increased risk of SCD.
Keywords: Sudden cardiac death, Postmortem, Molecular autopsy, Genetics, Specialized multidisciplinary clinic
1. Introduction
Sudden cardiac death (SCD) is a tragic complication of a number of cardiovascular diseases. The death can occur at all ages, and in the young it is often unexpected when it occurs in a previously healthy and asymptomatic person [1], [2], [3]. SCD is defined as a death occurring within an hour of the onset of symptoms, due to an underlying cardiac disease. The prevalence of SCD is significant, with at least 3 million people worldwide dying suddenly each year. In the United States, SCD occurs in up to 450,000 people each year, translating to over 1000 deaths per day, or one SCD every 1.5 min [2], [4].
SCD is significantly more common in older age groups, and the incidence in young people aged less than 40 years is generally low. The best estimate of the incidence of SCD in the general population aged 20–75 years is 1 in every 1000 individuals, accounting for 18.5% of all deaths [5]. In the 1–40 age group, the incidence is up to 8.5 per 100,000 person years, including competitive athletes [4], [5], [6], [7], [8], [9]. While relatively uncommon, the SCD of a young person is a devastating event. In addition, the public health burden of premature death for men and women is greater for SCD than for all individual cancers and most other leading causes of death [10].
2. Causes of sudden cardiac death in the young
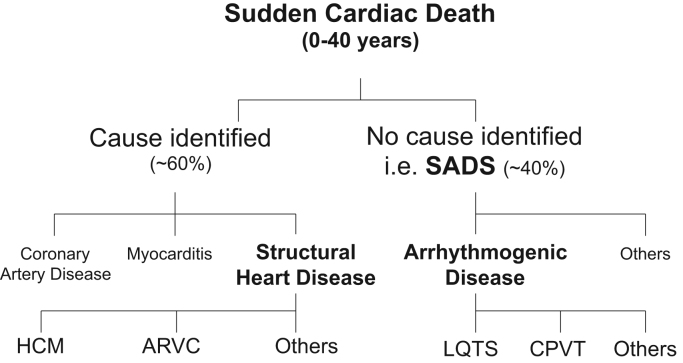
The causes of SCD can be broadly divided into structural and arrhythmogenic etiologies. In subjects over the age of 40 years, coronary artery disease and acute myocardial infarction account for over 90% of SCD cases [2], [11]. Fig. 1 summarizes the causes of SCD in the young (aged 0–40), illustrating the lower prevalence of coronary artery disease and higher proportion of inherited heart diseases. Structural causes of SCD in the young include inherited cardiomyopathies, such as hypertrophic cardiomyopathy (HCM), dilated and restrictive cardiomyopathies, arrhythmogenic right ventricular cardiomyopathy (ARVC), and left ventricular non-compaction. Other structural causes of SCD in the young include myocarditis, congenital heart diseases, and coronary artery disease [12], [13]. HCM is the most common inherited heart disease with a prevalence of 1 in every 200 people [14]. HCM remains the most common structural cause of SCD in the young, including competitive athletes [15]. Of note, in all structural causes of SCD in the young, the postmortem examination has a high probability of identifying the cause of death.
Fig. 1.
Causes of sudden cardiac death in the young (0–40 years) based on postmortem findings. SADS=sudden arrhythmic death syndrome, LQTS=long QT syndrome, CPVT1=catecholaminergic polymorphic ventricular tachycardia type 1, HCM=hypertrophic cardiomyopathy, ARVC=arrhythmogenic right ventricular cardiomyopathy.
The main inherited arrhythmogenic causes of SCD in the young are summarized in Fig. 1. These arrhythmogenic disorders include familial long QT syndrome (LQTS), catecholaminergic polymorphic ventricular tachycardia (CPVT), Brugada syndrome (BrS), idiopathic ventricular fibrillation, early repolarization syndromes, and short QT syndrome. Since these disorders rarely cause any structural changes to the heart, the postmortem is often “negative” (no cause of death is identified), including normal histopathology and toxicology analyses (key features are summarized in Table 1). Such cases in which no abnormalities are found at the postmortem examination occur in up to 40% of SCD cases in young populations [7], [8], [12], [16], [17], [18]. This strongly suggests an underlying arrhythmogenic cause (Fig. 1). Sudden unexplained deaths are often referred to as sudden arrhythmic death syndrome (SADS) with “young” defined as 1–40 years old (excluding sudden unexplained death in infancy from 0 to 1 years, referred to as SIDS) [19]. The true overall incidence of SCD is likely to be an underestimate, since primary arrhythmogenic disorders can predispose people to more overt causes of death, such as drowning and motor vehicle accidents. Such overt causes may be caused by ventricular arrhythmias in patients with LQTS and CPVT [20], [21], [22].
Table 1.
Postmortem criteria for a “negative autopsy”.
| • Structurally normal heart |
| • No abnormal histopathological findings in the heart |
| • No other cause of death identified at postmortem (e.g. pulmonary embolus or cerebral aneurysm) |
| • Normal blood toxicology screen |
| • No pre-death clinical features to suggest other causes of sudden death (e.g. epilepsy or asthma) |
3. Role of the postmortem in the investigation of sudden cardiac death in the young
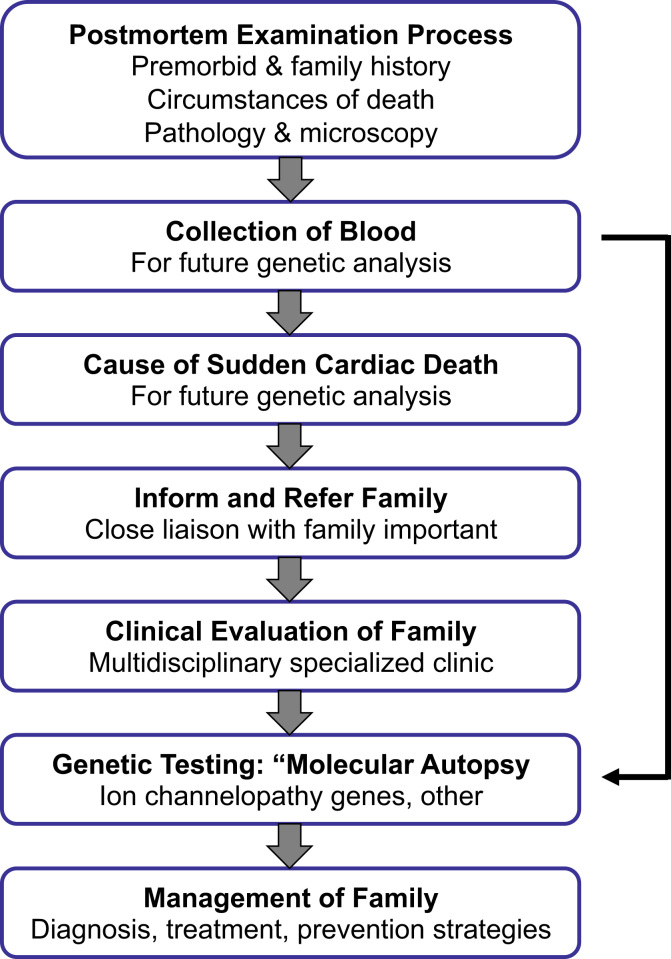
Establishing a definitive cause of death in a young SCD case is of major importance, and so the postmortem evaluation is a critical first step in all cases (Fig. 2). Important aspects of the postmortem include the proper and detailed conduct of the postmortem examination itself, collection of appropriate samples for subsequent analysis (including DNA analysis), careful evaluation of the findings, and a precise and accurate final conclusion are important aspects of the role of the postmortem. A comprehensive and detailed postmortem process can not only define the exact cause of death in a young SCD case, but the results also have far-reaching effects in identifying at-risk relatives of the decedent. This information can thereby provide a therapeutic window for disease and sudden death prevention in at-risk relatives (Fig. 2).
Fig. 2.
Evaluation of families in the setting of a sudden cardiac death in a young family member. Genetic testing is often performed concurrently with clinical evaluation of family members. This figure is modified from Semsarian et al. [34].
The key aspects of the postmortem evaluation are summarized in Table 2, and are modified from the Best Practice Guidelines of the Royal Australasian College of Pathologists implemented in Australia in 2008 [23]. A comprehensive postmortem by an experienced forensic pathologist should be performed in all SCD cases in the young (0–40 years) [19], [23], [24]. A complete premorbid medical history should be sought, including a history of syncopal episodes, exertional symptoms, intercurrent illnesses, recent pharmacological therapies, previous ECGs, and other relevant studies. The investigation should also include a comprehensive, 3-generation family pedigree focused on identifying any family history of cardiac disease, premature sudden death, or suspicious deaths (e.g. SIDS cases or drowning). Other relevant family history information can include family members with epilepsy, identifying “fainters”, and any other unusual symptoms or clinical presentations. The circumstances of SCD need to be established when possible, including activity at the time of death, the level of physical activity, and the symptoms immediately preceding the death. This often relies on obtaining information from available ambulance and police reports, as well as talking to witnesses or those who found the deceased.
Table 2.
Key aspects of the postmortem process in SCD cases.
| • Performed in all cases of sudden unexplained death in the young (0–40 years) |
| • Detailed premorbid clinical history |
| • Comprehensive family history |
| • Skilled macroscopic and microscopic examination of all organs, particularly the heart and brain |
| • Adequate collection of histological material for review or referral if necessary |
| • Collection of 5–10 mL of whole blood, and frozen sections of highly cellular tissues (e.g. liver or spleen), where possible, for future DNA extraction and analysis |
| • Early liaison with a multidisciplinary specialized cardiac genetics service |
The postmortem examination should include a detailed macroscopic and histological evaluation of the heart, as well as other key organs such as the brain, with the purpose of identifying any non-cardiac causes of death (e.g. pulmonary embolism or cerebral aneurysm), before focusing on specific cardiac pathologies. In all SCD cases in the young, a 5–10 mL blood sample should be collected for subsequent DNA extraction and analysis. In addition, frozen sections of the liver or spleen, which are highly cellular and therefore rich in DNA, should also be collected and stored when possible. Of note, paraffin-embedded tissues are not suitable for DNA studies, as the quality of the DNA is significantly diminished [25], [26]. Obtaining a postmortem blood sample in young SCD cases is now recommended by the HRS/EHRA guidelines [24], and is mandated in several countries, including Australia and New Zealand [23]. A representative best practice document entitled “Postmortem in sudden unexpected death in the young: guidelines on autopsy practice” and endorsed by the Royal College of Pathologists of Australasia is detailed in Supplementary File 1.
Despite our best efforts, the cause of SCD in young individuals is sometimes not established; no cause is identified in up to 40% of cases. Importantly, whether a cause of death is established or not, the possibility of an underlying inherited cardiac disorder remains in many cases, including the possibility of a primary arrhythmogenic disease. Recent studies also suggest that in addition to true SADS cases, postmortem examinations may reveal some non-specific changes of uncertain clinical significance, such as unclassified “cardiomegaly” or minor cardiac histopathological changes. Importantly, the chance of finding an underlying primary arrhythmogenic syndrome in these uncertain “borderline” cases is as high as in those with true SADS [27].
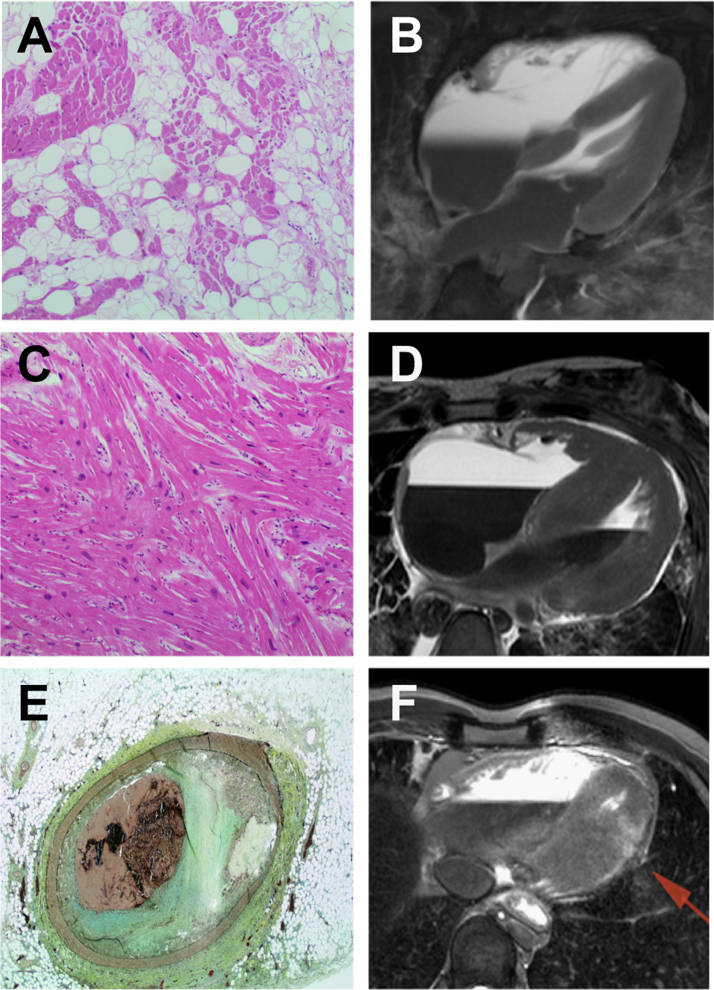
While the conventional postmortem remains the cornerstone of the investigation of young SCD cases, there is emerging evidence that other imaging modalities may be helpful in diagnosing structural causes of SCD. Such non-invasive approaches may overcome some of the reservations that families have in proceeding with the traditional postmortem process for religious, logistic, personal, or cultural reasons. These modalities include computer tomography (CT) scanning and cardiovascular magnetic resonance (CMR) imaging [28]. The majority of the studies to date examining the accuracy of postmortem CMR imaging have focused on fetal and neonatal deaths [29], [30]. Two recent studies have shown great promise in terms of utilizing CT and CMR imaging in determining the cause of SCD in young adults [31], [32]. Fig. 3 shows young SCD cases in which postmortem imaging identified the causes of death due to ARVC, HCM, and acute coronary occlusion [32]. While the studies represent a small number of young SCD cases, the possibility of additional diagnostic tools to elucidate the cause of SCD at the postmortem now exists.
Fig. 3.
Role of CT scanning and cardiac magnetic resonance imaging postmortem. Diagnosis of arrhythmogenic right ventricular cardiomyopathy (A, B), hypertrophic cardiomyopathy (C, D), acute coronary occlusion, and myocardial infarction (E, F) based on histological and imaging analyses. This figure was modified from Puranik et al. [32].
4. Genetic testing and the “molecular autopsy”
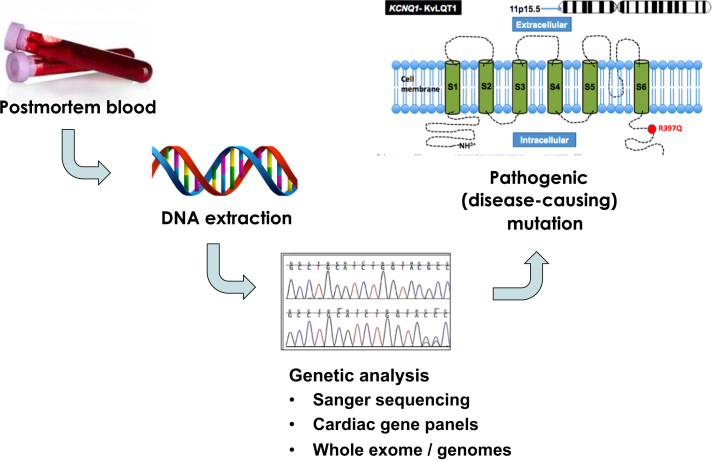
The use of genetic testing in the setting of SCD cases was initiated over a decade ago [33]. In SADS, where no cause of death is identified after a comprehensive postmortem examination, genetic testing of the decedent’s blood sample collected at postmortem may identify an underlying genetic cause of sudden death. This genetic testing process has been termed the “molecular autopsy”, and involves DNA extraction from postmortem blood, followed by DNA analysis of selected candidate genes responsible for the main inherited arrhythmogenic diseases (Fig. 4) [34], [35].
Fig. 4.
Steps in the molecular autopsy process. DNA is extracted from blood collected at postmortem. Subsequent DNA analysis of selected genes is done by Sanger sequencing or newer parallel next generation sequencing platforms. This figure was modified from Semsarian et al. [35].
4.1. The traditional “molecular autopsy”
The traditional molecular autopsy has focused on direct DNA sequencing of the protein coding exons of 4 genes, including the three major LQTS genes (KCNQ1, KCNH2, SCN5A) and the CPVT gene (RYR2, Table 3) [24], [36], [37]. Mutations in the SCN5A gene cause LQTS3 and also BrS. Initial studies in highly-selected SADS populations reported detection rates for a disease-causing (pathogenic) mutation of up to 34% [33], [38]. However, more recent studies suggest the detection rate with the 4-gene molecular autopsy is more likely to be up to 15–20% [39] (Table 3), ranging from 0% to 35% [25], [33], [39], [40], [41], [42], [43], [44]. This largely reflects a range of clinical and methodological issues relating to the type of DNA obtained, selection bias of the populations studied, the definition of sudden death, and variation in the stringency and interpretation of DNA variants in terms of pathogenicity.
Table 3.
Current 4-gene molecular autopsy.
| Gene name | Encoded protein | Disease | % of disease | % of SADSa |
|---|---|---|---|---|
| KCNQ1 | IKs K+ channel α-subunit | LQTS1 | 35–40 | 10–15 |
| KCNH2 | IKr K+ channel α-subunit | LQTS2 | 30–35 | 1–5 |
| SCN5A | INa Na+ channel α-subunit | LQTS3 | 5–10 | <1 |
| BrS | 15–25 | <1 | ||
| RYR2 | Ryanodine receptor | CPVT1 | 60–65 | 10–20 |
LQTS=long QT syndrome, BrS=Brugada syndrome, CPVT1=catecholaminergic polymorphic ventricular tachycardia type 1, SADS=sudden arrhythmic death syndrome (normal postmortem).
Pick-up rate for molecular autopsy.
4.2. The “exome-wide molecular autopsy”
Recent advances in next generation sequencing technologies have allowed ever expanding panels of genes (cardiac gene panels with up to 200 genes) to be re-sequenced from comparatively small quantities of DNA, with excellent throughput capabilities, and in a cost-efficient manner. This includes sequencing the protein coding exons of all ~22,000 genes (the “exome”). Next generation sequencing technologies thus offer the technology for an “exome-wide molecular autopsy”, and potentially allow genetic testing of all major disease-associated genes, as well as genes less frequently involved in any given disease. We recently used an exome-wide approach in a series of SADS cases in a proof-of-principle study [42]. Using stringent pathogenicity criteria, a likely pathogenic variant was identified in 32% of cases. Moreover, the exome data provided information on a number of gene variants currently being evaluated for novel disease associations, and an archive of genotypes that can be mined in the future as new SADS-associated genes are characterized.
4.3. Determining pathogenicity of the identified genetic variants
The major Achilles’ heel of genetic testing and the molecular autopsy is establishing genetic causality or pathogenicity. While newer genetic technologies provide excitement and hope of identifying more genetic causes of SCD in the young, the uncertainty surrounding the pathogenicity of the identified genetic variants is an important drawback to consider. In addition, the emerging understanding of multigenic models of disease where several variants may cumulatively contribute to disease [45] and the inevitable clinical implications of incidental and secondary findings [46] are all important considerations in the setting of the molecular autopsy.
In brief, determining the pathogenicity of the variants identified in the postmortem setting is complicated by the absence of a true “phenotype” in the deceased. In general, many factors need to be considered in determining pathogenicity including the type of mutation, the frequency of the variation in genetic population databases, the type of amino acid change and its conservation, the predicted damaging effect using in silico tools, supportive functional data, and evidence of co-segregation of the variant within a family [46], [47]. Taking such factors into account, the genetic variant is classified as pathogenic (disease-causing), benign, or of uncertain significance [so-called “variant of uncertain significance” (VUS)] [46], [47], [48], [49]. Generally speaking, pathogenic and likely pathogenic variations can be used for cascade testing of surviving family members, while a VUS and benign findings do not impact clinical care [46]. The key consideration for the clinician seeing the surviving family of the decedent is that cardiac genetic results are “probabilistic.” As such, they are on a gradation or continuum from benign (clinically insignificant), to VUS (may become clinically significant), to pathogenic (clinically highly significant). Importantly, genetic testing to determine the underlying genetic cause of disease in a family is not a binary “yes/no” outcome. Therefore, efforts to gather evidence of causation are important, as variants with little certainty of their pathogenicity could lead to potential danger if used as a screening tool for the remaining family members. The worst-case scenario would be releasing a family member from clinical surveillance based on an incorrect gene result. This is an important consideration in family management and highlights the need to consider the genetic findings of the molecular autopsy with caution, and in conjunction with clinical findings derived from screening family relatives.
These complexities highlight both the need for ongoing collaborative efforts to improve the ways we determine pathogenicity and the key role of the specialized multidisciplinary model of care for SCD and families with genetic heart diseases [50]. This is especially important and relevant in the setting of SADS, where there is no phenotype in the decedent in up to 40% of cases. Correlation between the pathogenicity of a genetic finding at the molecular autopsy and the absence of a phenotype in the decedent becomes very challenging. In this situation, we rely heavily on the characteristics of the variant, rarity in populations, previous reports, any available functional data, and overall predictions from in silico tools. Finding a relevant clinical cardiac phenotype in family relatives of the decedent may significantly help in the determination of pathogenicity of genetic variants identified in young SADS cases.
5. Management of families following sudden cardiac death in the young
Given the possibility of an inherited cardiac disease as a cause of SCD, appropriate evaluation and management of the surviving family is essential. Overall clinical management is guided by the goal of establishing a cause of death (the victim), and the clinical screening and management of the surviving family members (the family). The ultimate goal is prevention of SCD in any other family relatives. In SADS cases, underpinning the clinical evaluation and screening of family members is the presumption that the underlying cause was an inherited arrhythmogenic disorder such as LQTS, CPVT, or BrS. By definition, these SCD cases are unexplained at postmortem. Importantly however, over 95% of cardiac genetic disorders are inherited as an autosomal dominant trait such that first-degree relatives have a 1 in 2 (50%) chance of inheriting the same gene mutation [36], [37]. Therefore, standard approaches for clinical evaluation of first-degree relatives are important, and may reveal disease in the family.
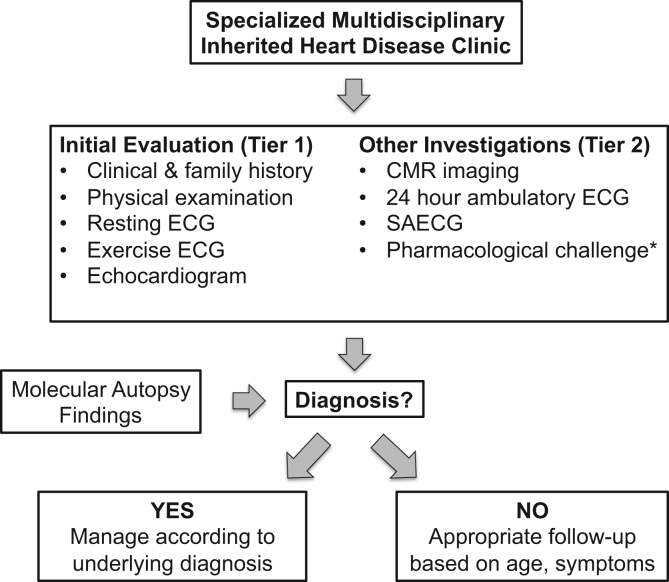
Fig. 5 summarizes the basic clinical investigation of the family, including first-degree relatives, obligate carriers, and symptomatic relatives. This is largely based on the recent HRS/EHRA consensus document [19]. Clinical investigation broadly involves two tiers of evaluation. All relatives should have a comprehensive medical and family history, physical examination, resting and exercise ECGs, and a standard transthoracic echocardiogram. Depending on the clinical situation, further second tier investigations may include CMR imaging, 24-h ECG monitoring and signal averaged ECG, and pharmacological challenge tests, such as an ajmaline challenge in suspected BrS patients. Clinical evaluation alone in families with a sudden unexplained death may identify an underlying cause in up to 50% of selected and comprehensively evaluated families in tertiary centers [51], [52], [53].
Fig. 5.
Clinical investigation pathway of surviving family members when no cause of death is identified. CMR=cardiac magnetic resonance, ECG=electrocardiogram, SAECG=signal averaged ECG. This figure was modified from Priori et al. [19].
Concurrent with the clinical evaluation of the family, the genetic findings from a molecular autopsy may help to elucidate the cause of death in the decedent, as well as provide a basis for cascade genetic testing of at-risk family members. Offering cascade genetic testing to asymptomatic relatives should always be performed in conjunction with clinical evaluation, and only alongside comprehensive pre- and post-test genetic counseling [47]. Cascade genetic testing may also reveal de novo cases, where the parents of the decedent do not carry the disease-causing mutation. In this case, the only at-risk relatives would be the offspring of the decedent.
If an underlying diagnosis is made using both clinical and genetic evaluation of the family, subsequent management and follow-up depend on the disease in question. This will often trigger more specific family cascade clinical screening, and, if available, genetic testing [24]. If no diagnosis is made after a comprehensive clinical (±genetic) review, then asymptomatic adult relatives can be discharged from care on the proviso that new symptoms or family information should be reported immediately. More commonly, the relative being screened is a child, in which case regular follow-up is indicated until adulthood, with the knowledge that many genetic heart diseases most commonly manifest as clinical disease in the second decade of life [54], [55]. Some diseases such as ARVC or HCM may occasionally present later in life; the clinical follow-up duration may therefore need to be extended.
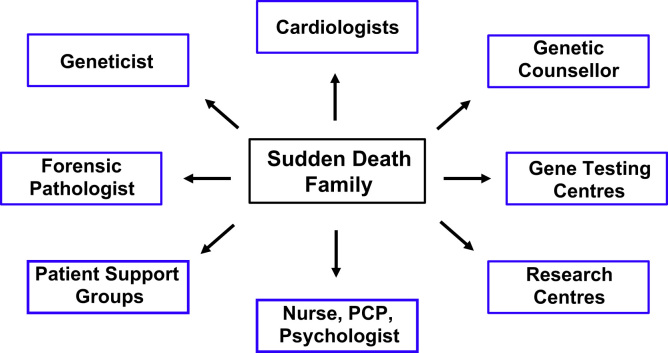
Family management in the setting of SCD of a young person is complex and ideally suited for a multidisciplinary specialized approach (Fig. 6). The range of management issues is diverse, including clinical cardiovascular care, genetic evaluation and tests, interpretation of results, conveying the information to the surviving family, and managing the ongoing psychosocial wellbeing of the families [56]. The specialized multidisciplinary cardiac genetic clinic is a model used globally, and is dedicated to cardiac genetics with appropriately trained staff [19], [24], [43], [57], [58]. The multidisciplinary clinic provides expertize not only in the clinical and genetic aspects of disease, but also in the integration of key links with other critical members of the team in addition to the cardiologist. These team members may include cardiac genetic counselors, geneticists, forensic pathologists, primary care physicians, nurses, clinical psychologists, and patient support groups (Fig. 6). The role of the cardiac genetic counselor is especially important in providing education, normalizing grief responses, arranging genetic testing, and developing a strong bond with the family as a key support person [59].
Fig. 6.
Specialized multidisciplinary approach to caring for families with a sudden cardiac death in a young family member. PCP=primary care physician. This figure was modified from Ingles et al. [59].
6. Conclusions
SCD is a rare but tragic complication in a number of cardiovascular diseases. In the young, genetic heart diseases are an important cause of SCD, and have major implications for surviving at-risk family members. Therefore, a detailed evaluation of the premorbid history and comprehensive and expert postmortem examination are essential. In SADS cases where no cause of death is identified at the postmortem, targeted clinical evaluation coupled with molecular autopsy genetic testing in the setting of a specialized multidisciplinary clinic are key, with the ultimate goal to prevent future adverse clinical outcomes and SCD events in surviving relatives.
Conflict of interest
All authors declare that they have no conflicts of interest related to this study.
Acknowledgments
CS is the recipient of a National Health and Medical Research Council (NHMRC) Practitioner Fellowship (#1059156). JI is the recipient of an NHMRC and National Heart Foundation of Australia Early Career Fellowship (#1036756).
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.joa.2015.09.010.
Appendix A. Supplementary material
Supplementary material
References
- 1.Adabag A.S., Luepker R.V., Roger V.L. Sudden cardiac death: epidemiology and risk factors. Nat Rev Cardiol. 2010;7:216–225. doi: 10.1038/nrcardio.2010.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zipes D.P., Wellens H.J. Sudden cardiac death. Circulation. 1998;98:2334–2351. doi: 10.1161/01.cir.98.21.2334. [DOI] [PubMed] [Google Scholar]
- 3.Wellens H.J., Schwartz P.J., Lindemans F.W. Risk stratification for sudden cardiac death: current status and challenges for the future. Eur Heart J. 2014;35:1642–1651. doi: 10.1093/eurheartj/ehu176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liberthson R.R. Sudden death from cardiac causes in children and young adults. N Engl J Med. 1996;334:1039–1044. doi: 10.1056/NEJM199604183341607. [DOI] [PubMed] [Google Scholar]
- 5.Semsarian C., Sweeting J., Ackerman M.J. Sudden cardiac death in athletes. Br Med J. 2015;350:h1218. doi: 10.1136/bmj.h1218. [DOI] [PubMed] [Google Scholar]
- 6.Eckart R.E., Scoville S.L., Campbell C.L. Sudden death in young adults: a 25-year review of autopsies in military recruits. Ann Intern Med. 2004;141:829–834. doi: 10.7326/0003-4819-141-11-200412070-00005. [DOI] [PubMed] [Google Scholar]
- 7.Margey R., Roy A., Tobin S. Sudden cardiac death in 14- to 35-year olds in Ireland from 2005 to 2007: a retrospective registry. Europace. 2011;13:1411–1418. doi: 10.1093/europace/eur161. [DOI] [PubMed] [Google Scholar]
- 8.Winkel B.G., Holst A.G., Theilade J. Nationwide study of sudden cardiac death in persons aged 1–35 years. Eur Heart J. 2011;32:983–990. doi: 10.1093/eurheartj/ehq428. [DOI] [PubMed] [Google Scholar]
- 9.Shen W.K., Edwards W.D., Hammill S.C. Sudden unexpected nontraumatic death in 54 young adults: a 30-year population-based study. Am J Cardiol. 1995;76:148–152. doi: 10.1016/s0002-9149(99)80047-2. [DOI] [PubMed] [Google Scholar]
- 10.Stecker E.C., Reinier K., Marijon E. Public health burden of sudden cardiac death in the United States. Circ Arrhythm Electrophysiol. 2014;7:212–217. doi: 10.1161/CIRCEP.113.001034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eckart R.E., Shry E.A., Burke A.P. Sudden death in young adults: an autopsy-based series of a population undergoing active surveillance. J Am Coll Cardiol. 2011;58:1254–1261. doi: 10.1016/j.jacc.2011.01.049. [DOI] [PubMed] [Google Scholar]
- 12.Doolan A., Langlois N., Semsarian C. Causes of sudden cardiac death in young Australians. Med J Aust. 2004;180:110–112. doi: 10.5694/j.1326-5377.2004.tb05830.x. [DOI] [PubMed] [Google Scholar]
- 13.Behr E.R., Casey A., Sheppard M. Sudden arrhythmic death syndrome: a national survey of sudden unexplained cardiac death. Heart. 2007;93:601–605. doi: 10.1136/hrt.2006.099598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Semsarian C., Ingles J., Maron M.S. New perspectives on the prevalence of hypertrophic cardiomyopathy. J Am Coll Cardiol. 2015;65:1249–1254. doi: 10.1016/j.jacc.2015.01.019. [DOI] [PubMed] [Google Scholar]
- 15.Maron B.J. Sudden death in young athletes. N Engl J Med. 2003;349:1064–1075. doi: 10.1056/NEJMra022783. [DOI] [PubMed] [Google Scholar]
- 16.Corrado D., Basso C., Thiene G. Sudden cardiac death in young people with apparently normal heart. Cardiovasc Res. 2001;50:399–408. doi: 10.1016/s0008-6363(01)00254-1. [DOI] [PubMed] [Google Scholar]
- 17.Puranik R., Chow C.K., Duflou J.A. Sudden death in the young. Heart Rhythm. 2005;2:1277–1282. doi: 10.1016/j.hrthm.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 18.de Noronha S.V., Sharma S., Papadakis M. Aetiology of sudden cardiac death in athletes in the United Kingdom: a pathological study. Heart. 2009;95:1409–1414. doi: 10.1136/hrt.2009.168369. [DOI] [PubMed] [Google Scholar]
- 19.Priori S.G., Wilde A.A., Horie M. HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes: document endorsed by HRS, EHRA, and APHRS in May 2013 and by ACCF, AHA, PACES, and AEPC in June 2013. Heart Rhythm. 2013;10:1932–1963. doi: 10.1016/j.hrthm.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 20.Tester D.J., Ackerman M.J. Drowning. N Engl J Med. 2012;367:777. doi: 10.1056/NEJMc1207798. (author reply-8) [DOI] [PubMed] [Google Scholar]
- 21.Tester D.J., Kopplin L.J., Creighton W. Pathogenesis of unexplained drowning: new insights from a molecular autopsy. Mayo Clin Proc. 2005;80:596–600. doi: 10.4065/80.5.596. [DOI] [PubMed] [Google Scholar]
- 22.Hayashi M., Denjoy I., Extramiana F. Incidence and risk factors of arrhythmic events in catecholaminergic polymorphic ventricular tachycardia. Circulation. 2009;119:2426–2434. doi: 10.1161/CIRCULATIONAHA.108.829267. [DOI] [PubMed] [Google Scholar]
- 23.Skinner J.R., Duflou J.A., Semsarian C. Reducing sudden death in young people in Australia and New Zealand: the TRAGADY initiative. Med J Aust. 2008;189:539–540. doi: 10.5694/j.1326-5377.2008.tb02173.x. [DOI] [PubMed] [Google Scholar]
- 24.Ackerman M.J., Priori S.G., Willems S. HRS/EHRA expert consensus statement on the state of genetic testing for the channelopathies and cardiomyopathies this document was developed as a partnership between the Heart Rhythm Society (HRS) and the European Heart Rhythm Association (EHRA) Heart Rhythm. 2011;8:1308–1339. doi: 10.1016/j.hrthm.2011.05.020. [DOI] [PubMed] [Google Scholar]
- 25.Doolan A., Langlois N., Chiu C. Postmortem molecular analysis of KCNQ1 and SCN5A genes in sudden unexplained death in young Australians. Int J Cardiol. 2008;127:138–141. doi: 10.1016/j.ijcard.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 26.Carturan E., Tester D.J., Brost B.C. Postmortem genetic testing for conventional autopsy-negative sudden unexplained death: an evaluation of different DNA extraction protocols and the feasibility of mutational analysis from archival paraffin-embedded heart tissue. Am J Clin Pathol. 2008;129:391–397. doi: 10.1309/VLA7TT9EQ05FFVN4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Papadakis M., Raju H., Behr E.R. Sudden cardiac death with autopsy findings of uncertain significance: potential for erroneous interpretation. Circ Arrhythm Electrophysiol. 2013;6:588–596. doi: 10.1161/CIRCEP.113.000111. [DOI] [PubMed] [Google Scholar]
- 28.Burton J.L., Underwood J. Clinical, educational, and epidemiological value of autopsy. Lancet. 2007;369:1471–1480. doi: 10.1016/S0140-6736(07)60376-6. [DOI] [PubMed] [Google Scholar]
- 29.Brookes J.A., Hall-Craggs M.A., Sams V.R. Non-invasive perinatal necropsy by magnetic resonance imaging. Lancet. 1996;348:1139–1141. doi: 10.1016/S0140-6736(96)02287-8. [DOI] [PubMed] [Google Scholar]
- 30.Taylor A.M., Sebire N.J., Ashworth M.T. Postmortem cardiovascular magnetic resonance imaging in fetuses and children: a masked comparison study with conventional autopsy. Circulation. 2014;129:1937–1944. doi: 10.1161/CIRCULATIONAHA.113.005641. [DOI] [PubMed] [Google Scholar]
- 31.Roberts I.S., Benamore R.E., Benbow E.W. Post-mortem imaging as an alternative to autopsy in the diagnosis of adult deaths: a validation study. Lancet. 2012;379:136–142. doi: 10.1016/S0140-6736(11)61483-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Puranik R., Gray B., Lackey H. Comparison of conventional autopsy and magnetic resonance imaging in determining the cause of sudden death in the young. J Cardiovasc Magn Reson. 2014;16:44. doi: 10.1186/1532-429X-16-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tester D.J., Spoon D.B., Valdivia H.H. Targeted mutational analysis of the RyR2-encoded cardiac ryanodine receptor in sudden unexplained death: a molecular autopsy of 49 medical examiner/coroner׳s cases. Mayo Clin Proc. 2004;79:1380–1384. doi: 10.4065/79.11.1380. [DOI] [PubMed] [Google Scholar]
- 34.Semsarian C., Hamilton R.M. Key role of the molecular autopsy in sudden unexpected death. Heart Rhythm. 2012;9:145–150. doi: 10.1016/j.hrthm.2011.07.034. [DOI] [PubMed] [Google Scholar]
- 35.Semsarian C., Ingles J., Wilde A.A. Sudden cardiac death in the young: the molecular autopsy and a practical approach to surviving relatives. Eur Heart J. 2015;36:1290–1296. doi: 10.1093/eurheartj/ehv063. [DOI] [PubMed] [Google Scholar]
- 36.Cerrone M., Priori S.G. Genetics of sudden death: focus on inherited channelopathies. Eur Heart J. 2011;32:2109–2118. doi: 10.1093/eurheartj/ehr082. [DOI] [PubMed] [Google Scholar]
- 37.Wilde A.A., Behr E.R. Genetic testing for inherited cardiac disease. Nat Rev Cardiol. 2013;10:571–583. doi: 10.1038/nrcardio.2013.108. [DOI] [PubMed] [Google Scholar]
- 38.Tester D.J., Ackerman M.J. Postmortem long QT syndrome genetic testing for sudden unexplained death in the young. J Am Coll Cardiol. 2007;49:240–246. doi: 10.1016/j.jacc.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 39.Skinner J.R., Crawford J., Smith W. Prospective, population-based long QT molecular autopsy study of postmortem negative sudden death in 1 to 40 year olds. Heart Rhythm. 2011;8:412–419. doi: 10.1016/j.hrthm.2010.11.016. [DOI] [PubMed] [Google Scholar]
- 40.Tester D.J., Medeiros-Domingo A., Will M.L. Cardiac channel molecular autopsy: insights from 173 consecutive cases of autopsy-negative sudden unexplained death referred for postmortem genetic testing. Mayo Clin Proc. 2012;87:524–539. doi: 10.1016/j.mayocp.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chugh S.S., Senashova O., Watts A. Postmortem molecular screening in unexplained sudden death. J Am Coll Cardiol. 2004;43:1625–1629. doi: 10.1016/j.jacc.2003.11.052. [DOI] [PubMed] [Google Scholar]
- 42.Bagnall R.D., Das K.J., Duflou J. Exome analysis-based molecular autopsy in cases of sudden unexplained death in the young. Heart Rhythm. 2014;11:655–662. doi: 10.1016/j.hrthm.2014.01.017. [DOI] [PubMed] [Google Scholar]
- 43.Hofman N., Tan H.L., Alders M. Yield of molecular and clinical testing for arrhythmia syndromes: report of 15 years׳ experience. Circulation. 2013;128:1513–1521. doi: 10.1161/CIRCULATIONAHA.112.000091. [DOI] [PubMed] [Google Scholar]
- 44.Winkel B.G., Larsen M.K., Berge K.E. The prevalence of mutations in KCNQ1, KCNH2, and SCN5A in an unselected national cohort of young sudden unexplained death cases. J Cardiovasc Electrophysiol. 2012;23:1092–1098. doi: 10.1111/j.1540-8167.2012.02371.x. [DOI] [PubMed] [Google Scholar]
- 45.Bezzina C.R., Barc J., Mizusawa Y. Common variants at SCN5A–SCN10A and HEY2 are associated with Brugada syndrome, a rare disease with high risk of sudden cardiac death. Nat Genet. 2013;45:1044–1049. doi: 10.1038/ng.2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ingles J., Semsarian C. Conveying a probabilistic genetic test result to families with an inherited heart disease. Heart Rhythm. 2014;11:1073–1078. doi: 10.1016/j.hrthm.2014.03.017. [DOI] [PubMed] [Google Scholar]
- 47.Ingles J., Semsarian C. The value of cardiac genetic testing. Trends Cardiovasc Med. 2014;24:217–224. doi: 10.1016/j.tcm.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 48.Maron B.J., Maron M.S., Semsarian C. Genetics of hypertrophic cardiomyopathy after 20 years: clinical perspectives. J Am Coll Cardiol. 2012;60:705–715. doi: 10.1016/j.jacc.2012.02.068. [DOI] [PubMed] [Google Scholar]
- 49.Semsarian C., Ingles J. Determining pathogenicity in cardiac genetic testing: filling in the blank spaces. Trends Cardiovasc Med. 2015 doi: 10.1016/j.tcm.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 50.Ingles J., Semsarian C. Sudden cardiac death in the young: a clinical genetic approach. Intern Med J. 2007;37:32–37. doi: 10.1111/j.1445-5994.2006.01241.x. [DOI] [PubMed] [Google Scholar]
- 51.Behr E.R., Dalageorgou C., Christiansen M. Sudden arrhythmic death syndrome: familial evaluation identifies inheritable heart disease in the majority of families. Eur Heart J. 2008;29:1670–1680. doi: 10.1093/eurheartj/ehn219. [DOI] [PubMed] [Google Scholar]
- 52.Behr E., Wood D.A., Wright M. Cardiological assessment of first-degree relatives in sudden arrhythmic death syndrome. Lancet. 2003;362:1457–1459. doi: 10.1016/s0140-6736(03)14692-2. [DOI] [PubMed] [Google Scholar]
- 53.Tan H.L., Hofman N., van Langen I.M. Sudden unexplained death: heritability and diagnostic yield of cardiological and genetic examination in surviving relatives. Circulation. 2005;112:207–213. doi: 10.1161/CIRCULATIONAHA.104.522581. [DOI] [PubMed] [Google Scholar]
- 54.Maron B.J., Ommen S.R., Semsarian C. Hypertrophic cardiomyopathy: present and future, with translation into contemporary cardiovascular medicine. J Am Coll Cardiol. 2014;64:83–99. doi: 10.1016/j.jacc.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 55.Hershberger R.E., Morales A., Siegfried J.D. Clinical and genetic issues in dilated cardiomyopathy: a review for genetics professionals. Genet Med. 2010;12:655–667. doi: 10.1097/GIM.0b013e3181f2481f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yeates L., Hunt L., Saleh M. Poor psychological wellbeing particularly in mothers following sudden cardiac death in the young. Eur J Cardiovasc Nurs. 2013;12:484–491. doi: 10.1177/1474515113485510. [DOI] [PubMed] [Google Scholar]
- 57.Ingles J., Lind J.M., Phongsavan P. Psychosocial impact of specialized cardiac genetic clinics for hypertrophic cardiomyopathy. Genet Med. 2008;10:117–120. doi: 10.1097/GIM.0b013e3181612cc7. [DOI] [PubMed] [Google Scholar]
- 58.van der Werf C., Onderwater A.T., van Langen I.M. Experiences, considerations and emotions relating to cardiogenetic evaluation in relatives of young sudden cardiac death victims. Eur J Hum Genet. 2014;22:192–196. doi: 10.1038/ejhg.2013.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ingles J., Yeates L., Semsarian C. The emerging role of the cardiac genetic counselor. Heart Rhythm. 2011;8:1958–1962. doi: 10.1016/j.hrthm.2011.07.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material