Abstract
Atrial arrhythmias are being increasingly recognized in inherited arrhythmogenic disorders particularly in patients with Brugada syndrome and short QT syndrome. Atrial arrhythmias in inherited arrhythmogenic disorders have significant epidemiologic, clinical, and prognostic implications. There has been progress in the understanding of underlying genetic characteristics and the mechanistic link between atrial arrhythmias and inherited arrhythmogenic disorders. Appropriate management of these patients is of paramount importance.
Keywords: Brugada syndrome, Short QT syndrome, Atrial arrhythmias, Atrial fibrillation, Atrioventricular nodal reentrant tachycardia
1. Introduction
The inherited arrhythmogenic disorders include J wave syndromes, consisting of Brugada (BrS) and early repolarization syndrome (ERS), long QT syndrome (LQTS), short QT syndrome (SQTS), and catecholaminergic polymorphic ventricular tachycardia (CPVT). Atrial arrhythmias including atrial fibrillation (AF), atrial flutter (AFL), and paroxysmal supraventricular tachycardias (atrioventricular nodal reentrant tachycardia [AVNRT], atrioventricular reentrant tachycardia [AVRT], and atrial tachycardia [AT]) frequently coexist with inherited arrhythmogenic disorders. Atrial arrhythmias are being increasingly recognized particularly in patients with BrS and SQTS [1], [2].
Atrial arrhythmias in inherited arrhythmogenic disorders have important epidemiologic, clinical, and prognostic implications. There has been progress in the understanding of underlying genetic characteristics and the mechanistic link between atrial arrhythmias and inherited arrhythmogenic disorders. Appropriate management of these patients is of paramount importance.
2. Prevalence of atrial arrhythmias
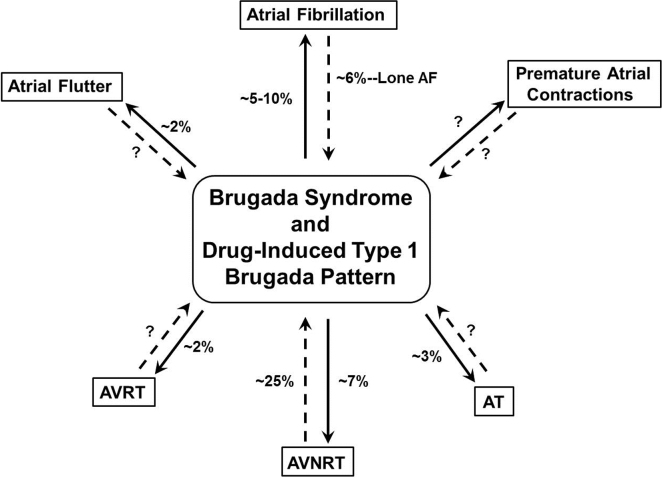
The prevalence of atrial arrhythmias in inherited arrhythmogenic disorders varies depending on the type of arrhythmia, mode of detection (12-lead electrocardiogram [ECG], Holter monitoring, or implantable cardioverter defibrillator [ICD] monitoring), and clinical presentation of inherited arrhythmogenic disorders, which can be manifested, suspected, or concealed (drug-induced type 1 Brugada pattern) (Fig. 1).
Fig. 1.
Prevalence of atrial arrhythmias in patients with Brugada syndrome and drug-induced type 1 Brugada pattern. Patients presenting with manifest type 1 or suspected type 2 or 3 Brugada pattern and atrial arrhythmias are shown with a straight line. Patients with concealed BrS in which the type 1 Brugada pattern is unmasked for the first time after administration of class IC agents given for the termination of AF or after the ajmaline challenge for screening purposes are shown with a dashed line. AT=atrial tachycardia, AVNRT=atrioventricular nodal reentrant tachycardia, AVRT=atrioventricular reentrant tachycardia.
Atrial fibrillation is the most common atrial arrhythmia studied in BrS [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14]. The prevalence of AF has been reported to be higher in patients with BrS than in the general population of the same age [15], [16]. Earlier studies reported an approximately 10–50% prevalence of spontaneous, clinical AF in patients with BrS. The most recent studies with larger cohorts reported a prevalence of approximately 5–10% [13], [14]. The prevalence of concealed BrS after administration of class IC agents administered for the termination of new-onset AF was reported to be 3.2% overall and 5.8% in patients with AF alone [10]. The prevalence of spontaneous, clinical AVNRT, AVRT, and AT among patients with BrS has been reported to be approximately 7%, 2%, and 3%, respectively [8]. The prevalence of drug-induced type 1 Brugada pattern among patients with spontaneous, clinical AVNRT had been studied by our group and was found to be 27.1% [17] (Fig. 1).
The most common mode of detection of atrial arrhythmias in the majority of studies was 12-lead ECG and/or Holter monitoring. The incidence of atrial arrhythmias detected by ICD monitoring because of inappropriate shocks during long-term follow-up has been reported to be 4–8.5% [7], [18].
The clinical presentation of the J wave syndrome is of paramount importance in determining the true prevalence of atrial arrhythmias. The majority of studies have reported on the prevalence of atrial arrhythmias in BrS cohorts. These patients usually present with symptoms (palpitations, syncope, or cardiac arrest) along with manifested type 1 or suspected type 2 or 3 Brugada pattern and develop type 1 Brugada pattern after the drug challenge test. In contrast, in patients with concealed BrS, type 1 Brugada pattern is unmasked for the first time after administration of class IC agents for the termination of AF [10], [13], [14]. Another group of patients with concealed BrS presenting with clinical, spontaneous AVNRT or AT/AF and without any signs of Brugada pattern on baseline 12-lead ECG, develop type 1 Brugada pattern with the administration of ajmaline for screening purposes (Fig. 2, Fig. 3).
Fig. 2.
12-lead electrocardiogram at baseline and after the ajmaline challenge. The patient was an 18-year-old female adolescent who presented with a 2-year history of paroxysmal supraventricular tachycardia. The electrophysiological study reveals slow/fast atrioventricular nodal reentrant tachycardia. She underwent successful slow pathway ablation. Genetic analysis revealed a missense mutation in KCNE2 (p.Thr8Ala). The ajmaline challenge results in prolongation of the PR interval (202 ms), QRS duration (114 ms) with a QRS axis of 8°, and type 1 Brugada pattern in V1 and V2. ICS=intercostal space.
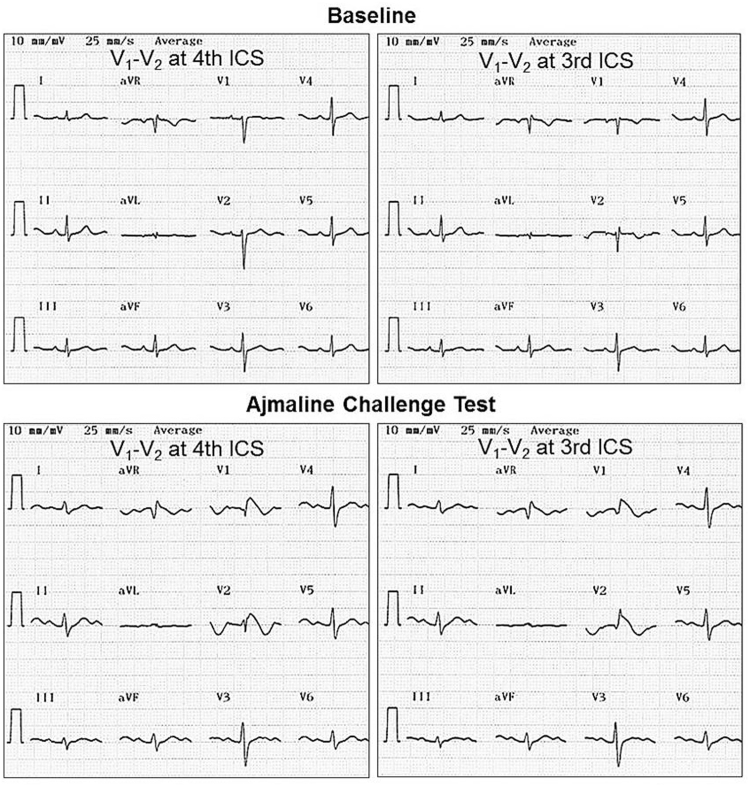
Fig. 3.
A Rhythm strip during palpitation. The patient is a 48-year-old man who presented with a 1-year history of palpitations. Several 12-lead electrocardiograms (ECGs) demonstrate atrial fibrillation with fast ventricular rate. He has a structurally normal heart. His final diagnosis is lone atrial fibrillation. B/C 12-lead ECGs at baseline with V1–V2 recorded at the 4th and 3rd intercostal spaces (ICS), respectively. D/E 12-lead ECGs after the ajmaline challenge with V1–V2 recorded at the 4th and 3rd intercostal spaces, respectively. The ajmaline challenge results in prolongation of the PR interval (196 ms), QRS duration (132 ms) with a QRS axis of 28°, and type 1 Brugada pattern in V1 and V2.
In terms of ERS, the strongest relationship exists among patients with Wolff–Parkinson–White syndrome (WPWS) [19], [20], [21], [22], [23], [24], [25]. The prevalence of inferolateral early repolarization (ER) pattern has been reported to be approximately 40–50% in patients with WPWS prior to and after catheter ablation [22], [23]. Following catheter ablation, the ER pattern persists in 25% of patients, disappears in 18% of patients, and newly appears in 10–15% of patients. ER was always observed in leads with positive deflection of the initial part of the delta wave [22]. The inferolateral ER pattern in the general population is not associated with increased risk of AF [26]. In contrast, the prevalence of type 2 Brugada pattern has been demonstrated to be significantly higher in patients with lone AF than in control subjects [27].
The prevalence of AF and/or AFL has been reported to be approximately 11–16% among patients with SQTS [28], [29]. The incidence of AF among family members with SQTS ranges from 26 to 70% [2], [30]. AF was more common in patients with type 2 SQTS (63% versus 21%, p=0.012) than in those with other types [30].
The prevalence of AF and/or AFL has been reported to be approximately 1.7% among patients with LQTS [31]. AF was more common in type 1 (2.4%) than in patients with type 2 LQTS (none). The incidence of atrial arrhythmias (AF and/or AT) detected by ICD monitoring in patients with LQTS during long-term follow-up has been reported to be 33% [32].
There are anecdotal reports of AF in patients with CPVT [33], [34]. In the largest cohort of patients with CPVT, the prevalence of clinical AF and/or AFL has been reported to be 38% [35].
3. Epidemiologic implications of atrial arrhythmias
The prevalence of diagnosed AF in the general population is closer to 2% [16]. This prevalence increases with age, from <0.5% at 40–50 years, to 5–15% at 80 years. In one recent study, the prevalence of concealed BrS among patients presenting with new-onset AF was found to be 5.8% [10]. All patients had lone AF. If these results could be extrapolated to the entire population, we estimate that nearly 20,000 new cases of AF would be found per year among 1,000,000 persons. Lone AF comprises 5–10% of all AF cases. If the reported prevalence of concealed BrS in patients with new-onset lone AF is confirmed by further studies, the prevalence of concealed BrS may reach approximately 1500 new cases per year among 1,000,000 persons.
The prevalence of drug-induced type 1 Brugada pattern among patients with clinical AVNRT was found to be 27.1% by our group [17]. This high prevalence has important implications in terms of the epidemiology of BrS. The prevalence of paroxysmal supraventricular tachycardia (PSVT) (AVNRT and AVRT) in the general population in the US has been reported to be approximately 2.25/1000 persons [36]. If these results are extrapolated to the entire US population, we estimate nearly 140,000 new cases of PSVT per year. AVNRT comprises 60% of all PSVT cases. If a high prevalence of drug-induced type 1 Brugada pattern in patients with spontaneous AVNRT is confirmed by further studies, the prevalence of drug-induced type 1 Brugada pattern may reach approximately 21,000 new cases per year.
4. Clinical characteristics
The initiation of AF is more likely to occur during night time in patients with BrS [9]. Studies wherein an association between AF and BrS has been evaluated have involved mostly male patients. Therefore, the involvement of a sex predilection for the development of AF in patients with BrS is not clear [1]. Clinical AFL has been anecdotally reported either in young patients (<20 years old) as an isolated arrhythmia, or coexisting with AF in adults, or as a part of atrial myopathy and progressive cardiac conduction disorder in patients with BrS [37], [38], [39], [40], [41], [42], [43], [44], [45].
Our group recently showed that patients with AVNRT and drug-induced type 1 Brugada pattern were predominantly women (88.5% versus 62.9%, p=0.015) and had higher prevalence of chest pain (38.5% versus 18.6%, p=0.042) and migraine headaches (38.4% versus 14.2%, p=0.008) than patients without concealed BrS. Drug-induced initiation and/or worsening of duration and/or frequency of palpitations (along with documented AVNRT) were observed more frequently in patients with concealed BrS than in patients without concealed BrS (15.4% versus 1.4%, p=0.006) [17].
AF can be the first mode of presentation in SQTS particularly in patients with lone AF [2]. Sinus node dysfunction coexisting with AF can be observed particularly in patients with type 2 SQTS [30]. Sinus node dysfunction can be a clinical manifestation in patients with CPVT either as a part of a primary genetic defect or atrial myopathy coexisting with AF and/or AFL [46].
5. Genetics of atrial arrhythmias
In BrS, a decrease in cardiac sodium (INa) or calcium (ICa) channel current or augmentation of any one of a number of outward cardiac potassium channel currents, including IKr, IKs, and Ito, can cause preferential abbreviation of the right ventricular epicardial action potential secondary to all-or-none repolarization of the action potential at the end of phase 1 [47]. The substrate responsible for the development of ventricular arrhythmias also may contribute to arrhythmogenesis in the atria of the heart [1]. Two cardiac potassium channel currents, Ito and IKur are known to be major repolarizing currents in the human atrium. Nav1.5 has been demonstrated as the predominant channel followed by Nav1.1, Nav1.3, and Nav1.6 in humal atrial cells [48].
Loss-of-function mutations in the INa channel α-subunit (SCN5A) is the most common (~20%) etiology of BrS [49]. Recently, SCN10A, a neuronal sodium channel gene encoding Nav1.8 has been identified as a major susceptibility gene (~16%) for BrS [50]. In addition, loss-of-function mutations in the ICa,L channel α- and β-subunits cause BrS in a significant number of patients (~10%) [51].
Loss-of-function mutations in the INa channel α- and β-subunits (SCN5A, SCN1B, SCN2B, and SCN3B) and ICa,L channel α- and β-subunits (CACNA1C and CACNB2) have been identified in patients with both BrS and AF [9], [12], [52], [53], [54], [55]. A missense mutation (S422L) in the cardiac KATP channel (KCNJ8) has been identified as the cause of ERS associated with AF [56], [57].
The role of SCN5A mutations in the development of AF in patients with BrS remains unclear. Two studies showed that the prevalence of AF is not different in patients with BrS with or without SCN5A mutation [9], [12]. The presence of SCN5A mutations have been associated with lower number of premature atrial contractions on Holter monitoring, longer intra-atrial conduction time, and structural remodeling (higher left atrial volume index) but not with AF episodes [11]. A reduced number of potentially triggering premature atrial contractions in the presence of a more extensive substrate in SCN5A mutation carriers hypothetically account for AF not being more prevalent in patients with SCN5A mutations than in those without them [12], [58].
Common and rare SCN10A variants may contribute to AF susceptibility [59], [60]. SCN10A mutations (R14L and R1268Q) cause a loss of function of Nav1.5 current, which is expected to reduce excitability and lead to the development of the arrhythmogenic substrate responsible for BrS and AF. A common variant in SCN10A (A1073) is associated with increased susceptibility to AF [60].
Genetic mutations, particularly, loss-of-function mutations and/or deletions in INa channel α-subunit (SCN5A), have been identified in patients with both BrS and AFL [37], [38], [39], [40], [41], [42], [43], [44], [45].
Genetic screening in the group of patients studied (n=17) with both AVNRT and drug-induced type 1 Brugada pattern identified 19 mutations or rare variants in 13 different genes in 13 of 17 patients (yield=76.5%). Ten of these 13 genotype-positive patients (76.9%) harbored genetic variants known or suspected to cause a loss of function of cardiac sodium channel current (INa) (SCN5A, SCN10A, SCN1B, GPD1L, PKP2, and HEY2) [17].
In SQTS, gain-of-function mutations in potassium channels (IKr [KCNH2], IKs [KCNQ1], and IK1 [KCNJ2]) and loss-of-function mutations in ICa,L channel α- and β-subunits (CACNA1C, CACNB2, and CACNA2D1) cause shortening of the action potential duration and QT interval [55], [61], [62].
In CPVT, loss-of-function mutations in the gene encoding the sarcoplasmic reticulum calcium release channel (RyR2), or in genes encoding the RyR2-binding proteins, such as cardiac calsequestrin (CASQ2), triadin, and calmodulin, cause dysregulation in intracellular calcium handling [46].
6. Mechanistic link between atrial arrhythmias and inherited arrhythmogenic disorders
Atrial arrhythmias can be maintained by reentry (AF, AFL, and AVNRT) and/or rapid focal ectopic firing (AF) [63]. AF requires triggers (most commonly pulmonary veins) for the initiation of reentrant circuits and a vulnerable substrate that enables reentrant circuits to remain in the atria. Both triggers and the atrial substrate are modulated by electrical, structural/anatomical remodeling, and the autonomic nervous system.
Prolonged intra-atrial conduction time determined by surface ECG (P-wave duration and PR interval) or intra-cardiac electrograms (time interval from the stimulus at the high right atrium to atrial deflection at the distal coronary sinus) has been demonstrated in patients with BrS with or without AF [11], [12]. Signal-averaged ECG has been used to assess the vulnerability to AF [64]. The filtered P-wave duration is prolonged in patients with BrS. Atrial structural remodeling (increased left atrial volume index) assessed by transthoracic echocardiography has been also demonstrated in these patients [11].
The onset of AF is often preceded by fluctuations in autonomic tone, consistent with most AF cases occurring at night in patients with BrS [9]. Vagal stimulation reduces atrial conduction velocities and shortens the effective refractory period facilitating the induction of AF. Previous studies demonstrated the expression of SCN5A and SCN10A in intracardiac ganglia [65], [66]. Therefore, genetic variants SCN5A and/or SCN10A may generate an imbalance in the intracardiac ganglia activity and increase vagal tone.
Genetic variants impairing the INa channel function along with structural remodeling promote AF by prolonging refractoriness and slowing conduction velocity. Reduced levels of INa are known to depress INa-dependent parameters such as excitability and conduction leading to heterogeneous prolongation of refractoriness, thus facilitating the development of unidirectional block and reentry, giving rise to AF, AFL, and AVNRT. Loss of function of INa is also known to cause an outward shift in the balance of currents in the right ventricular epicardium. This shift can accentuate the epicardial action potential notch, thus giving rise to repolarization and depolarization abnormalities that result in the BrS phenotype, including the development of phase-2 reentry and polymorphic ventricular tachycardia [67].
Most forms of AVNRT are created by reentry between two (or more) atrial connections to the AV node. The fast AV nodal pathway (shortest conduction time) is formed by transitional cells crossing the tendon of Todaro superiorly. Two slow AV nodal pathways are formed by the rightward and leftward inferior extensions of the AV node [68]. Atrial tissue surrounding Koch’s triangle is involved in all types of AVNRT. Optical mapping data obtained from isolated rabbit and adult mongrel dog AV nodal preparations suggested that AV nodal pathways are located outside the compact AV node, and atrial and transitional cells are involved in the reentrant circuit of AVNRT [69]. In light of these findings, we hypothesize that a loss of function of INa secondary to a mutation in sodium channel related genes (e.g., SCN5A, SCN1B, SCN10A) may cause reduced excitability, thus leading to block in one of the AV nodal pathways and the development of reentrant re-excitation.
Action potential duration shortening in patients with SQTS leads to transmural dispersion of action potentials and subsequently spiral wave reentry resulting in AF [70]. Prolonged atrial action potential durations as demonstrated by monophasic action potentials in humans with LQTS can cause polymorphic AT potentially as a result of atrial early after depolarizations. These arrhythmias have been reported to be a specific arrhythmia of LQTS reminiscent of an atrial form of “torsades de pointes” [71].
The loss of CASQ2 causes abnormal sarcoplasmic reticulum Ca2+ release and selective interstitial fibrosis in the atrial pacemaker complex, which disrupts sinoatrial node pacemaking but enhances latent pacemaker activity, creates conduction abnormalities, and increases susceptibility to AF. These functional and extensive structural alterations could contribute to sinoatrial node dysfunction as well as AF in patients with CPVT [72].
7. Prognostic implications of atrial arrhythmias
Several clinical and electrocardiographic/electrophysiological variables have been demonstrated to predict a worse outcome in patients with BrS, including aborted sudden death, presence of syncopal episodes in patients with a spontaneous type 1 Brugada pattern at baseline, fragmented QRS, and short (<200 ms) ventricular effective refractory period [73], [74]. Spontaneous AF in patients with BrS has been reported to be associated with syncope, documented ventricular fibrillation, aborted sudden death, and appropriate ICD shocks. This may represent a more advanced electrophysiological and structural remodeling in atrial as well as ventricular tissue [9], [11]. Whether documentation of spontaneous AF in patients with BrS is an independent risk factor for a worse outcome remains unknown.
The prognostic value of spontaneous, clinical atrial arrhythmias, particularly AF and/or AFL, in patients with SQTS, LQTS, and CPVT is currently unknown.
8. Management of atrial arrhythmias
The management of atrial arrhythmias in the condition of BrS might be challenging for the following reasons. First, many anti-arrhythmic agents with sodium channel blocking properties might expose the patients to the development of ventricular arrhythmias [75], [76], and second, patients with BrS might experience inappropriate ICD shocks because of atrial arrhythmias in approximately 5–10% of patients during an average 7-year follow-up period [18], [77].
The use of AV node blocking agents (verapamil, diltiazem, and propranolol) for heart rate control and class IC anti-arrhythmic agents (propafenone and flecainide) in patients with paroxysmal and/or persistent AF is known to exacerbate the Brugada phenotype [75], [76]. The safety of amiodarone and sotalol is currently unknown. Quinidine sulfate, a class IA anti-arrhythmic agent with strong vagolytic and Ito channel blocking properties is known to be a safe and effective treatment for patients with BrS and AF [14].
Pulmonary vein isolation (without any additional lesions) by radiofrequency energy or cyroablation can be performed safely and effectively in patients with BrS and drug-resistant AF with or without inappropriate ICD shocks [78], [79]. Catheter ablation should be considered as a first choice of therapy for patients with AFL.
Current treatment options for paroxysmal supraventricular tachycardias include AV node blocking agents (verapamil, diltiazem, propranolol, and metoprolol), class IC anti-arrhythmic agents (propafenone and flecainide), and catheter ablation. The identification of frequent coexistence of clinical, spontaneous AVNRT and drug-induced type 1 Brugada pattern calls for greater vigilance in the use of certain anti-arrhythmic agents that are known to exacerbate the Brugada phenotype (verapamil, diltiazem, propranolol, and class IC agents), avoidance of Brugada pattern-inducing non-cardiac drugs (certain selective serotonin reuptake inhibitors, tricyclic antidepressants, and antipsychotic and antiepileptic agents), and possible need for standard preventive measures such as use of antipyretics during fever [75], [76].
Quinidine sulfate is known to be a safe and effective treatment for patients with SQTS and AF [28]. Inappropriate ICD shocks due to T-wave oversensing and/or AF are common in patients with STQS. Careful ICD programming and use of quinidine sulfate in those cases are recommended [28].
There is an anecdotal report of dramatic suppression of AF by mexiletine as a relatively selective blocker of late Na current in a patient with type 1 LQTS [80].
Pulmonary vein isolation can be performed safely and effectively in patients with CPVT and AF with or without inappropriate ICD shocks [33], [34].
Disclosures
All authors declare no conflict of interest related to this study.
References
- 1.Francis J., Antzelevitch C. Atrial fibrillation and Brugada syndrome. J Am Coll Cardiol. 2008;51:1149–1153. doi: 10.1016/j.jacc.2007.10.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borggrefe M., Wolpert C., Antzelevitch C. Short QT syndrome. Genotype-phenotype correlations. J Electrocardiol. 2005;38:75–80. doi: 10.1016/j.jelectrocard.2005.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brugada P., Brugada J. Right bundle branch block, persistent ST segment elevation and sudden cardiac death: a distinct clinical and electrocardiographic syndrome. A multicenter report. J Am Coll Cardiol. 1992;20:1391–1396. doi: 10.1016/0735-1097(92)90253-j. [DOI] [PubMed] [Google Scholar]
- 4.Itoh H., Shimizu M., Ino H. Brugada Study Group. Arrhythmias in patients with Brugada-type electrocardiographic findings. Jpn Circ J. 2001;65:483–486. doi: 10.1253/jcj.65.483. [DOI] [PubMed] [Google Scholar]
- 5.Morita H., Kusano-Fukushima K., Nagase S. Atrial fibrillation and atrial vulnerability in patients with Brugada syndrome. J Am Coll Cardiol. 2002;40:1437–1444. doi: 10.1016/s0735-1097(02)02167-8. [DOI] [PubMed] [Google Scholar]
- 6.Bordachar P., Reuter S., Garrigue S. Incidence, clinical implications and prognosis of atrial arrhythmias in Brugada syndrome. Eur Heart J. 2004;25:879–884. doi: 10.1016/j.ehj.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 7.Sacher F., Probst V., Iesaka Y. Outcome after implantation of a cardioverter-defibrillator in patients with Brugada syndrome: a multicenter study. Circulation. 2006;114:2317–2324. doi: 10.1161/CIRCULATIONAHA.106.628537. [DOI] [PubMed] [Google Scholar]
- 8.Schimpf R., Giustetto C., Eckardt L. Prevalence of supraventricular tachyarrhythmias in a cohort of 115 patients with Brugada syndrome. Ann Noninvasive Electrocardiol. 2008;13:266–269. doi: 10.1111/j.1542-474X.2008.00230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kusano K.F., Taniyama M., Nakamura K. Atrial fibrillation in patients with Brugada syndrome relationships of gene mutation, electrophysiology, and clinical backgrounds. J Am Coll Cardiol. 2008;51:1169–1175. doi: 10.1016/j.jacc.2007.10.060. [DOI] [PubMed] [Google Scholar]
- 10.Pappone C., Radinovic A., Manguso F. New-onset atrial fibrillation as first clinical manifestation of latent Brugada syndrome: prevalence and clinical significance. Eur Heart J. 2009;30:2985–2992. doi: 10.1093/eurheartj/ehp326. [DOI] [PubMed] [Google Scholar]
- 11.Toh N., Morita H., Nagase S. Atrial electrophysiological and structural remodeling in high-risk patients with Brugada syndrome: assessment with electrophysiology and echocardiography. Heart Rhythm. 2010;7:218–224. doi: 10.1016/j.hrthm.2009.10.035. [DOI] [PubMed] [Google Scholar]
- 12.Amin A.S., Boink G.J., Atrafi F. Facilitatory and inhibitory effects of SCN5A mutations on atrial fibrillation in Brugada syndrome. Europace. 2011;13:968–975. doi: 10.1093/europace/eur011. [DOI] [PubMed] [Google Scholar]
- 13.Rodríguez-Mañero M., Namdar M., Sarkozy A. Prevalence, clinical characteristics and management of atrial fibrillation in patients with Brugada syndrome. Am J Cardiol. 2013;111:362–367. doi: 10.1016/j.amjcard.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 14.Giustetto C., Cerrato N., Gribaudo E. Atrial fibrillation in a large population with Brugada electrocardiographic pattern: prevalence, management, and correlation with prognosis. Heart Rhythm. 2014;11:259–265. doi: 10.1016/j.hrthm.2013.10.043. [DOI] [PubMed] [Google Scholar]
- 15.Go A.S., Hylek E.M., Phillips K.A. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370–2375. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 16.Camm A.J., Kirchhof P., Lip G.Y. ESC Committee for Practice Guidelines. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC) Europace. 2010;12:1360–1420. doi: 10.1093/europace/euq350. [DOI] [PubMed] [Google Scholar]
- 17.Hasdemir C., Payzin S., Kocabas U. High prevalence of concealed Brugada syndrome in patients with atrioventricular nodal reentrant tachycardia. Heart Rhythm. 2015;12:1584–1594. doi: 10.1016/j.hrthm.2015.03.015. [DOI] [PubMed] [Google Scholar]
- 18.Conte G., Sieira J., Ciconte G. Implantable cardioverter–defibrillator therapy in Brugada syndrome: a 20-year single-center experience. J Am Coll Cardiol. 2015;65:879–888. doi: 10.1016/j.jacc.2014.12.031. [DOI] [PubMed] [Google Scholar]
- 19.Chia B.L., Teo W.S. Right praecordial early repolarization pattern occurring with the Wolff–Parkinson–White pattern. Int J Cardiol. 1995;50:163–165. doi: 10.1016/0167-5273(95)93685-l. [DOI] [PubMed] [Google Scholar]
- 20.Poh K.K., Low A., Tan H.C. Early repolarization pattern occurring with the Wolff–Parkinson–White syndrome. Asian Cardiovasc Thorac Ann. 2003;11:263–265. doi: 10.1177/021849230301100319. [DOI] [PubMed] [Google Scholar]
- 21.Nagao S., Hayashi Y., Yagihara N. Preexcitation unmasks J waves: 2 cases. J Electrocardiol. 2011;44:359–362. doi: 10.1016/j.jelectrocard.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 22.Mizumaki K., Nishida K., Iwamoto J. Early repolarization in Wolff–Parkinson–White syndrome: prevalence and clinical significance. Europace. 2011;13:1195–1200. doi: 10.1093/europace/eur144. [DOI] [PubMed] [Google Scholar]
- 23.Yagihara N., Sato A., Iijima K. The prevalence of early repolarization in Wolff–Parkinson–White syndrome with a special reference to J waves and the effects of catheter ablation. J Electrocardiol. 2012;45:36–42. doi: 10.1016/j.jelectrocard.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 24.Hasegawa K., Sato A., Watanabe H. Early repolarization and its modification by preexcitation in two patients with intermittent Wolff–Parkinson–White syndrome. Pacing Clin Electrophysiol. 2012;35:e231–e233. doi: 10.1111/j.1540-8159.2012.03360.x. [DOI] [PubMed] [Google Scholar]
- 25.Takahashi N., Shinohara T., Hara M. Wolff–Parkinson–White syndrome concomitant with idiopathic ventricular fibrillation associated with inferior early repolarization. Intern Med. 2012;51:1861–1864. doi: 10.2169/internalmedicine.51.7353. [DOI] [PubMed] [Google Scholar]
- 26.Junttila M.J., Tikkanen J.T., Kenttä T. Early repolarization as a predictor of arrhythmic and nonarrhythmic cardiac events in middle-aged subjects. Heart Rhythm. 2014;11:1701–1706. doi: 10.1016/j.hrthm.2014.05.024. [DOI] [PubMed] [Google Scholar]
- 27.Junttila M.J., Raatikainen M.J., Perkiömäki J.S. Familial clustering of lone atrial fibrillation in patients with saddleback-type ST-segment elevation in right precordial leads. Eur Heart J. 2007;28:463–468. doi: 10.1093/eurheartj/ehl474. [DOI] [PubMed] [Google Scholar]
- 28.Giustetto C., Schimpf R., Mazzanti A. Long-term follow-up of patients with short QT syndrome. J Am Coll Cardiol. 2011;58:587–595. doi: 10.1016/j.jacc.2011.03.038. [DOI] [PubMed] [Google Scholar]
- 29.Villafañe J., Atallah J., Gollob M.H. Long-term follow-up of a pediatric cohort with short QT syndrome. J Am Coll Cardiol. 2013;61:1183–1191. doi: 10.1016/j.jacc.2012.12.025. [DOI] [PubMed] [Google Scholar]
- 30.Harrell D.T., Ashihara T., Ishikawa T. Genotype-dependent differences in age of manifestation and arrhythmia complications in short QT syndrome. Int J Cardiol. 2015;190:393–402. doi: 10.1016/j.ijcard.2015.04.090. [DOI] [PubMed] [Google Scholar]
- 31.Johnson J.N., Tester D.J., Perry J. Prevalence of early-onset atrial fibrillation in congenital long QT syndrome. Heart Rhythm. 2008;5:704–709. doi: 10.1016/j.hrthm.2008.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zellerhoff S., Pistulli R., Mönnig G. Atrial Arrhythmias in long-QT syndrome under daily life conditions: a nested case control study. J Cardiovasc Electrophysiol. 2009;20:401–407. doi: 10.1111/j.1540-8167.2008.01339.x. [DOI] [PubMed] [Google Scholar]
- 33.Sugiyasu A., Oginosawa Y., Nogami A. A case with catecholaminergic polymorphic ventricular tachycardia unmasked after successful ablation of atrial tachycardias from pulmonary veins. Pacing Clin Electrophysiol. 2009;32:e21–e24. doi: 10.1111/j.1540-8159.2009.02519.x. [DOI] [PubMed] [Google Scholar]
- 34.Sumitomo N., Nakamura T., Fukuhara J. Clinical effectiveness of pulmonary vein isolation for arrhythmic events in a patient with catecholaminergic polymorphic ventricular tachycardia. Heart Vessel. 2010;25:448–452. doi: 10.1007/s00380-009-1214-6. [DOI] [PubMed] [Google Scholar]
- 35.Sumitomo N., Sakurada H., Taniguchi K. Association of atrial arrhythmia and sinus node dysfunction in patients with catecholaminergic polymorphic ventricular tachycardia. Circ J. 2007;71:1606–1609. doi: 10.1253/circj.71.1606. [DOI] [PubMed] [Google Scholar]
- 36.Orejarena L.A., Vidaillet H., Jr, DeStefano F. Paroxysmal supraventricular tachycardia in the general population. J Am Coll Cardiol. 1998;31:150–157. doi: 10.1016/s0735-1097(97)00422-1. [DOI] [PubMed] [Google Scholar]
- 37.Rossenbacker T., Carroll S.J., Liu H. Novel pore mutation in SCN5A manifests as a spectrum of phenotypes ranging from atrial flutter, conduction disease, and Brugada syndrome to sudden cardiac death. Heart Rhythm. 2004;1:610–615. doi: 10.1016/j.hrthm.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 38.Probst V., Denjoy I., Meregalli P.G. Clinical aspects and prognosis of Brugada syndrome in children. Circulation. 2007;115:2042–2048. doi: 10.1161/CIRCULATIONAHA.106.664219. [DOI] [PubMed] [Google Scholar]
- 39.Holst A.G., Liang B., Jespersen T. Sick sinus syndrome, progressive cardiac conduction disease, atrial flutter and ventricular tachycardia caused by a novel SCN5A mutation. Cardiology. 2010;115:311–316. doi: 10.1159/000312747. [DOI] [PubMed] [Google Scholar]
- 40.Eastaugh L.J., James P.A., Phelan D.G. Brugada syndrome caused by a large deletion in SCN5A only detected by multiplex ligation-dependent probe amplification. J Cardiovasc Electrophysiol. 2011;22:1073–1076. doi: 10.1111/j.1540-8167.2010.02003.x. [DOI] [PubMed] [Google Scholar]
- 41.De Asmundis C., Sorgente A., Brugada P. Atrial flutter in normal heart could be first manifestation of Brugada syndrome. Acta Cardiol. 2012;67:97–100. doi: 10.1080/ac.67.1.2146571. [DOI] [PubMed] [Google Scholar]
- 42.Pagourelias E.D., Fragakis N., Koskinas K.C. Brugada syndrome masked by ibutilide treatment in a patient with atrial flutter. Cardiology. 2012;122:89–92. doi: 10.1159/000338735. [DOI] [PubMed] [Google Scholar]
- 43.Alampay M.M., Haigney M.C., Flanagan M.C. Transcranial magnetic stimulation as an antidepressant alternative in a patient with Brugada syndrome and recurrent syncope. Mayo Clin Proc. 2014;89:1584–1587. doi: 10.1016/j.mayocp.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 44.Hothi S.S., Ara F., Timperley J. p.Y1449C SCN5A mutation associated with overlap disorder comprising conduction disease, Brugada syndrome, and atrial flutter. J Cardiovasc Electrophysiol. 2015;26:93–97. doi: 10.1111/jce.12470. [DOI] [PubMed] [Google Scholar]
- 45.Park D.S., Cerrone M., Morley G. Genetically engineered SCN5A mutant pig hearts exhibit conduction defects and arrhythmias. J Clin Invest. 2015;125:403–412. doi: 10.1172/JCI76919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Faggioni M., van der Werf C., Knollmann B.C. Sinus node dysfunction in catecholaminergic polymorphic ventricular tachycardia: risk factor and potential therapeutic target? Trends Cardiovasc Med. 2014;24:273–278. doi: 10.1016/j.tcm.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Antzelevitch C., Yan G.X. J wave syndromes. Heart Rhythm. 2010;7:549–558. doi: 10.1016/j.hrthm.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kaufmann S.G., Westenbroek R.E., Maass A.H. Distribution and function of sodium channel subtypes in human atrial myocardium. J Mol Cell Cardiol. 2013;61:133–141. doi: 10.1016/j.yjmcc.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kapplinger J.D., Tester D.J., Alders M. An international compendium of mutations in the SCN5A-encoded cardiac sodium channel in patients referred for Brugada syndrome genetic testing. Heart Rhythm. 2010;7:33–46. doi: 10.1016/j.hrthm.2009.09.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hu D., Barajas-Martínez H., Pfeiffer R. Mutations in SCN10A are responsible for a large fraction of cases of Brugada syndrome. J Am Coll Cardiol. 2014;64:66–79. doi: 10.1016/j.jacc.2014.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Burashnikov E., Pfeiffer R., Barajas-Martinez H. Mutations in the cardiac L-type calcium channel associated with inherited J-wave syndromes and sudden cardiac death. Heart Rhythm. 2010;7:1872–1882. doi: 10.1016/j.hrthm.2010.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Watanabe H., Darbar D., Kaiser D.W. Mutations in sodium channel β1- and β2-subunits associated with atrial fibrillation. Circ Arrhythm Electrophysiol. 2009;2:268–275. doi: 10.1161/CIRCEP.108.779181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Olesen M.S., Jespersen T., Nielsen J.B. Mutations in sodium channel β-subunit SCN3B are associated with early-onset lone atrial fibrillation. Cardiovasc Res. 2011;89:786–793. doi: 10.1093/cvr/cvq348. [DOI] [PubMed] [Google Scholar]
- 54.Hu D., Barajas-Martinez H., Burashnikov E. A mutation in the beta 3 subunit of the cardiac sodium channel associated with Brugada ECG phenotype. Circ Cardiovasc Genet. 2009;2:270–278. doi: 10.1161/CIRCGENETICS.108.829192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Antzelevitch C., Pollevick G.D., Cordeiro J.M. Loss-of-function mutations in the cardiac calcium channel underlie a new clinical entity characterized by ST-segment elevation, short QT intervals, and sudden cardiac death. Circulation. 2007;115:442–449. doi: 10.1161/CIRCULATIONAHA.106.668392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Delaney J.T., Muhammad R., Blair M.A. A KCNJ8 mutation associated with early repolarization and atrial fibrillation. Europace. 2012;14:1428–1432. doi: 10.1093/europace/eus150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Barajas-Martínez H., Hu D., Ferrer T. Molecular genetic and functional association of Brugada and early repolarization syndromes with S422L missense mutation in KCNJ8. Heart Rhythm. 2012;9:548–555. doi: 10.1016/j.hrthm.2011.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Muggenthaler M., Behr E.R. Brugada syndrome and atrial fibrillation: pathophysiology and genetics. Europace. 2011;13:913–915. doi: 10.1093/europace/eur094. [DOI] [PubMed] [Google Scholar]
- 59.Savio-Galimberti E., Weeke P., Muhammad R. SCN10A/Nav1.8 modulation of peak and late sodium currents in patients with early onset atrial fibrillation. Cardiovasc Res. 2014;104:355–363. doi: 10.1093/cvr/cvu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jabbari J., Olesen M.S., Yuan L. Common and rare variants in SCN10A modulate the risk of atrial fibrillation. Circ Cardiovasc Genet. 2015;8:64–73. doi: 10.1161/HCG.0000000000000022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shimizu W., Horie M. Phenotypic manifestations of mutations in genes encoding subunits of cardiac potassium channels. Circ Res. 2011;109:97–109. doi: 10.1161/CIRCRESAHA.110.224600. [DOI] [PubMed] [Google Scholar]
- 62.Templin C., Ghadri J.R., Rougier J.S. Identification of a novel loss-of-function calcium channel gene mutation in short QT syndrome (SQTS6) Eur Heart J. 2011;32:1077–1088. doi: 10.1093/eurheartj/ehr076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Iwasaki Y.K., Nishida K., Kato T. Atrial fibrillation pathophysiology: implications for management. Circulation. 2011;124:2264–2274. doi: 10.1161/CIRCULATIONAHA.111.019893. [DOI] [PubMed] [Google Scholar]
- 64.Furukawa Y., Yamada T., Okuyama Y. Increased intraatrial conduction abnormality assessed by P-wave signal-averaged electrocardiogram in patients with Brugada syndrome. Pacing Clin Electrophysiol. 2011;34:1138–1146. doi: 10.1111/j.1540-8159.2011.03122.x. [DOI] [PubMed] [Google Scholar]
- 65.Scornik F.S., Desai M., Brugada R. Functional expression of "cardiac-type" Nav1.5 sodium channel in canine intracardiac ganglia. Heart Rhythm. 2006;3:842–850. doi: 10.1016/j.hrthm.2006.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Catterall W.A. Voltage-gated sodium channels at 60: structure, function and pathophysiology. J Physiol. 2012;590:2577–2589. doi: 10.1113/jphysiol.2011.224204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Antzelevitch C. Genetic, molecular and cellular mechanisms underlying the J wave syndromes. Circ J. 2012;76:1054–1065. doi: 10.1253/circj.cj-12-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nakagawa H., Jackman W.M. Catheter ablation of paroxysmal supraventricular tachycardia. Circulation. 2007;116:2465–2478. doi: 10.1161/CIRCULATIONAHA.106.655746. [DOI] [PubMed] [Google Scholar]
- 69.Wu J., Zipes D.P. Mechanisms underlying atrioventricular nodal conduction and the reentrant circuit of atrioventricular nodal reentrant tachycardia using optical mapping. J Cardiovasc Electrophysiol. 2002;13:831–834. doi: 10.1046/j.1540-8167.2002.00831.x. [DOI] [PubMed] [Google Scholar]
- 70.Deo M., Ruan Y., Pandit S.V. KCNJ2 mutation in short QT syndrome 3 results in atrial fibrillation and ventricular proarrhythmia. Proc Natl Acad Sci S A. 2013;110:4291–4296. doi: 10.1073/pnas.1218154110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kirchhof P., Eckardt L., Franz M.R. Prolonged atrial action potential durations and polymorphic atrial tachyarrhythmias in patients with long QT syndrome. J Cardiovasc Electrophysiol. 2003;14:1027–1033. doi: 10.1046/j.1540-8167.2003.03165.x. [DOI] [PubMed] [Google Scholar]
- 72.Glukhov A.V., Kalyanasundaram A., Lou Q. Calsequestrin 2 deletion causes sinoatrial node dysfunction and atrial arrhythmias associated with altered sarcoplasmic reticulum calcium cycling and degenerative fibrosis within the mouse atrial pacemaker complex. Eur Heart J. 2015;36:686–697. doi: 10.1093/eurheartj/eht452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Antzelevitch C., Brugada P., Borggrefe M. Brugada syndrome: report of the second consensus conference: endorsed by the Heart Rhythm Society and the European Heart Rhythm Association. Circulation. 2005;111:659–670. doi: 10.1161/01.CIR.0000152479.54298.51. [DOI] [PubMed] [Google Scholar]
- 74.Priori S.G., Wilde A.A., Horie M. Executive summary: HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes. Heart Rhythm. 2013;10:e85–e108. doi: 10.1016/j.hrthm.2013.07.021. [DOI] [PubMed] [Google Scholar]
- 75.Yap Y.G., Behr E.R., Camm A.J. Drug-induced Brugada syndrome. Europace. 2009;11:989–994. doi: 10.1093/europace/eup114. [DOI] [PubMed] [Google Scholar]
- 76.Postema P.G., Wolpert C., Amin A.S. Drugs and Brugada syndrome patients: review of the literature, recommendations, and an up-to-date website. Heart Rhythm. 2009;6:1335–1341. doi: 10.1016/j.hrthm.2009.07.002. 〈www.brugadadrugs.org〉 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sacher F., Probst V., Maury P. Outcome after implantation of a cardioverter-defibrillator in patients with Brugada syndrome: a multicenter study – Part 2. Circulation. 2013;128:1739–1747. doi: 10.1161/CIRCULATIONAHA.113.001941. [DOI] [PubMed] [Google Scholar]
- 78.Yamada T., Yoshida Y., Tsuboi N. Efficacy of pulmonary vein isolation in paroxysmal atrial fibrillation patients with a Brugada electrocardiogram. Circ J. 2008;72:281–286. doi: 10.1253/circj.72.281. [DOI] [PubMed] [Google Scholar]
- 79.Conte G., Chierchia G.B., Wauters K. Pulmonary vein isolation in patients with Brugada syndrome and atrial fibrillation: a 2-year follow-up. Europace. 2014;16:528–532. doi: 10.1093/europace/eut309. [DOI] [PubMed] [Google Scholar]
- 80.El Yaman M., Perry J., Makielski J.C. Suppression of atrial fibrillation with mexiletine pharmacotherapy in a young woman with type 1 long QT syndrome. Heart Rhythm. 2008;5:472–474. doi: 10.1016/j.hrthm.2007.10.029. [DOI] [PubMed] [Google Scholar]