Abstract
Cytosolic phospholipase A2α (cPLA2α) mediates agonist-induced release of arachidonic acid from membrane phospholipid for production of eicosanoids. The activation of cPLA2α involves increases in intracellular calcium, which binds to the C2 domain and promotes cPLA2α translocation from the cytosol to membrane to access substrate. The cell permeable pyrrolidine-containing cPLA2α inhibitors including pyrrophenone have been useful to understand cPLA2α function. Although this serine hydrolase inhibitor does not inhibit other PLA2s or downstream enzymes that metabolize arachidonic acid, we reported that it blocks increases in mitochondrial calcium and cell death in lung fibroblasts. In this study we used the calcium indicators G-CEPIA1er and CEPIA2mt to compare the effect of pyrrophenone in regulating calcium levels in the endoplasmic reticulum (ER) and mitochondria in response to A23187 and receptor stimulation. Pyrrophenone blocked calcium release from the ER and concomitant increases in mitochondrial calcium in response to stimulation by ATP, serum and A23187. In contrast, ER calcium release induced by the sarco/endoplasmic reticulum Ca2+-ATPase inhibitor thapsigargin was not blocked by pyrrophenone suggesting specificity for the calcium release pathway. As a consequence of blocking calcium mobilization, pyrrophenone inhibited serum-stimulated translocation of the cPLA2α C2 domain to Golgi. The ability of pyrrophenone to block ER calcium release is an off-target effect since it occurs in fibroblasts lacking cPLA2α. The results implicate a serine hydrolase in regulating ER calcium release and highlight the importance of careful dose-response studies with pyrrophenone to study cPLA2α function.
Keywords: cPLA2α, calcium, pyrrophenone, endoplasmic reticulum, mitochondria
1. Introduction
The Group IVA cytosolic phospholipase A2 (cPLA2α) is a widely expressed enzyme that releases arachidonic acid for production of eicosanoids [1]. Arachidonic acid is metabolized by cyclooxygenases to prostaglandins and thromboxane A2, and by 5-lipoxygenase to generate leukotrienes [2, 3]. cPLA2α is composed of two domains, an N-terminal calcium and phospholipid binding C2 domain, and a globular catalytic domain that contains the active site Ser/Asp catalytic dyad and phosphorylation sites [4]. cPLA2α is activated in cells by diverse stimuli that trigger common signaling pathways including calcium mobilization and activation of mitogen-activated protein kinases [1]. When calcium binds to the cPLA2α C2 domain, the affinity of cPLA2α for membrane increases, and it translocates from the cytosol to the Golgi apparatus and endoplasmic reticulum/nuclear envelope for accessing phospholipid substrate [5]. The phosphorylation of cPLA2α on Ser505 by mitogen-activated protein kinases enhances its catalytic activity [6, 7].
cPLA2α is the only mammalian PLA2 that exhibits preference for sn-2 arachidonic acid and its role in initiating eicosanoid production is well established [8, 9]. However, mammalian cells contain a number of PLA2 enzymes that can potentially release arachidonic acid for lipid mediator production [10]. A common approach to study PLA2 enzymes involves the use small molecule cell permeable inhibitors [11]. Potent cPLA2α inhibitors containing 1,2,4-trisubstituted pyrrolidine have been generated and are used widely to study the role of cPLA2α in cells [12, 13]. The pyrrolidine inhibitors, such as pyrrophenone, are more potent than other commonly used cPLA2α inhibitors such as arachidonyl trifluoromethyl ketone and methyl arachidonyl fluorophosphonate [12–14]. They are also more selective and do not inhibit Group VI PLA2s or downstream enzymes that metabolize arachidonic acid [12–15]. Small molecule inhibitors are important for probing the cellular function of PLA2 enzymes, however, there is the potential for concentration-dependent off-target effects.
In a recent study we investigated the role of cPLA2α in regulating cell death in lung fibroblasts by using the pyrrolidine derivative pyrrophenone, and by comparing fibroblasts from cPLA2α wild type and knockout mice [16]. Cell death was induced in lung fibroblasts with the calcium ionophore A23187, a known inducer of necrotic cell death due to mitochondrial calcium overload and cyclophilin D-dependent opening of the mitochondrial permeability transition pore (MPTP) [16–18]. Cell death was induced to a similar extent in A23187 treated cPLA2α+/+ and cPLA2α−/− lung fibroblasts indicating no role for cPLA2α [16]. However, cell death in response to A23187 was blocked by pyrrophenone in both cPLA2α+/+ and cPLA2α−/− lung fibroblasts by inhibiting mitochondrial calcium uptake and MPTP [16]. The ability of pyrrophenone to block cell death in cells lacking cPLA2α represents an off-target effect suggesting that it may target a novel pathway involving a serine hydrolase that regulates mitochondrial calcium uptake. Calcium is transferred from the ER to mitochondria through specialized contact sites, a process that is important for regulating mitochondrial function but that also promotes cell death when not properly controlled [19]. In this study we specifically addressed whether pyrrophenone inhibits the release of calcium from the ER thereby preventing calcium transfer to mitochondria. We monitored the effect of pyrrophenone on regulating agonist-stimulated ER and mitochondrial calcium levels by using the recently developed calcium-measuring organelle-entrapped protein indicators (CEPIA) that can be targeted to specific organelles for evaluating intra-organelle calcium levels [20].
2. Material and methods
2.1 Cells
Mouse lung fibroblasts were isolated from cPLA2α+/+ and cPLA2α−/− mice and immortalized with SV40 to generate immortalized mouse lung fibroblasts (IMLFα+/+ and IMLFα−/−) as previously described [21, 22]. Unless otherwise noted all experiments were carried out with IMLFα−/−.
2.2 Transfection protocol
IMLFα (5 × 103 cells) were plated on the glass insert of MatTek dishes (MatTek Corp.) and cultured in Dulbecco's Modified Eagle's Medium (DMEM) containing 10% fetal bovine serum (growth media) for 24 hr as previously described [6]. The cells were transfected using Lipofectamine 3000 (ThermoFisher Scientific) according to the manufacturer's protocol with pCMV G-CEPIAer and pCMV CEPIA2mt (gifts from Masamitsu Iino) (Addgene plasmids #58215 and #58218, respectively) and mCherry-ER-3 (a gift from Michael Davidson) (Addgene plasmid # 55041) [20]. After 24 hr the medium was replaced with serum-free DMEM containing 0.1% bovine serum albumin and the cells incubated for an additional 24 hr.
The monomeric (A206K) enhanced yellow fluorescent protein (EYFP)-C2 domain of cPLA2α was cloned into pVQAd5CMVK-NpA shuttle plasmid (ViraQuest, Inc) and recombinant adenoviruses generated by ViraQuest. IMLFα plated on the glass insert of MatTek dishes were cultured in growth media as described above for 18 hr. The medium was replaced with serum- and antibiotic-free DMEM containing 0.1% bovine serum albumin and cells incubated with recombinant adenovirus for 26 hr as previously described [6].
2.2 Live-cell imaging
For imaging cytosolic Ca2+, IMLFα were loaded with Fura Red-AM for 15 min as previously described [16]. Imaging of ER and mitochondrial calcium was carried out in IMLFα expressing G-CEPIAer or CEPIA2mt, respectively. Cells were pre-incubated with the indicted concentration of pyrrophenone (Cayman Chemical) or DMSO vehicle for 30 min at 37°C in serum-free phenol red-free DMEM containing 25 mM Hepes, pH 7.4 and 0.1% BSA. Calcium transients were induced by stimulation with A23187 (1 µg/ml), mouse serum (5%), ATP (200 µM) or thapsigargin (3 µM) and cells imaged on an inverted Zeiss 200M microscope using Intelligent Imaging Innovations Inc. (3I) software (Slidebook 6). In some experiments the cells were incubated in media containing 3 mM EGTA. Fluorescence intensities of Fura Red excited at 403/490 nm for bound/unbound calcium were used for cytosolic calcium analysis as described [16]. The ratio of fluorescence at each time point (Rt) after cell stimulation was determined after correcting for background fluorescence. Fluorescence data (Rt/R0) represents the fold-increase at Rt relative to the ratio of fluorescence at time 0 (R0) set at 1. For imaging ER calcium, fluorescence (488nm/525nm excitation/emission) of G-CEPIA1er was recorded every 5 sec for ATP and A23187, and every 15 sec after thapsigargin stimulation. For imaging mitochondrial calcium, fluorescence (488nm/525nm excitation/emission) of CEPIA2mt was recorded every 5 sec after ATP, A23187, and thapsigargin stimulation.
2.3 EYFP-C2 domain translocation
IMLFα−/− expressing EYFP-C2 domain were pre-incubated with the indicted concentration of pyrrophenone or DMSO vehicle for 30 min and then stimulated with 5% mouse serum to induce EYFP-C2 domain translocation to the Golgi as previously described [6]. Images were collected every 5 sec using a YFP filter with the microscopy system described above. Translocation data were calculated based on average fluorescence intensity of EYFP-C2 domain on the Golgi in each cell. Values were corrected for background fluorescence and differential bleaching at each wavelength through the duration of the imaging and expressed relative to time 0 (Ft/F0).
3. Results
3.1 Pyrrophenone blocks receptor-mediated release of calcium from intracellular stores
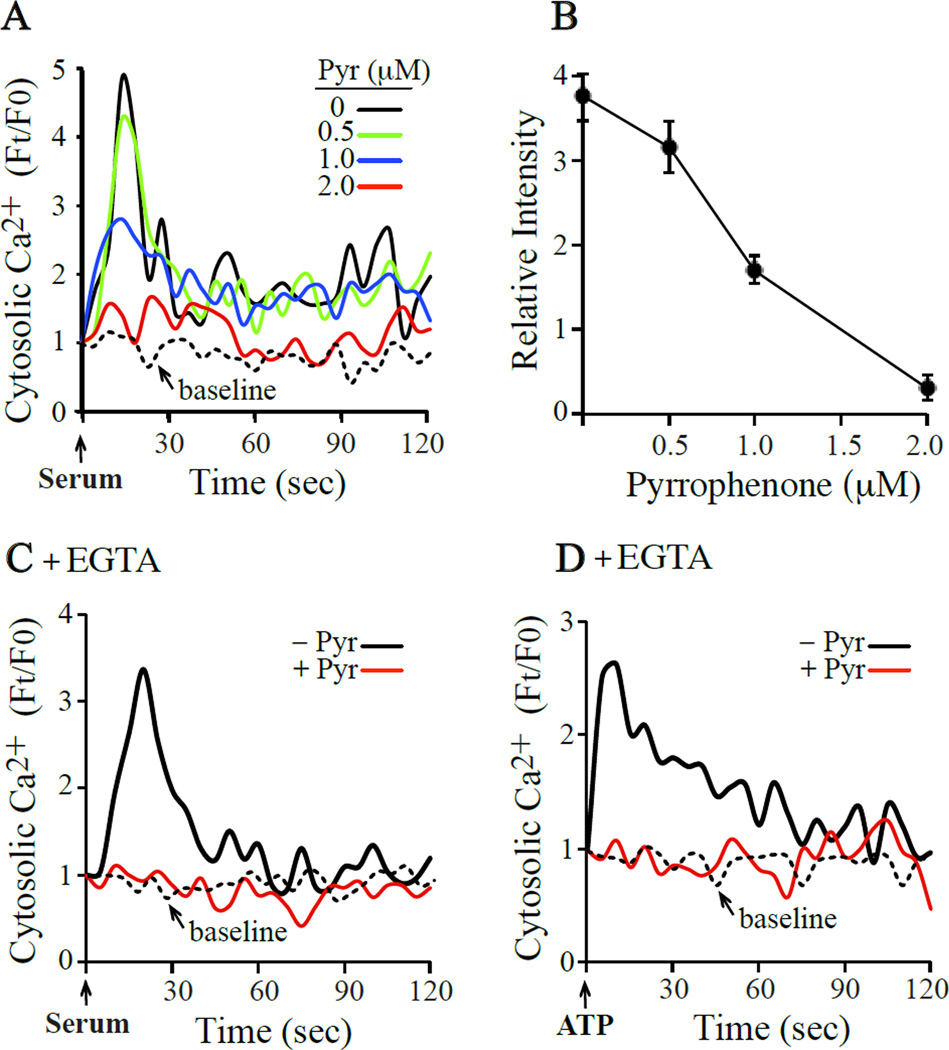
We previously reported that pyrrophenone blocked A23187-induced increase in mitochondial calcium [Ca2+]mt and partially suppressed cytosolic calcium [Ca2+]c increases [16]. To determine if pyrrophenone inhibited receptor-mediated increases in [Ca2+]c, fibroblasts were treated with serum or ATP. For these experiments, IMLFα−/− were used to avoid any potential effects of cPLA2α activation and arachidonic acid release in influencing calcium mobilization. We previously reported that serum stimulates a rapid increase in [Ca2+]c in IMLFα with an initial peak at 15 sec followed by lower amplitude oscillations, a pattern typical of release from intracellular stores followed by capacitative influx of extracellular calcium [6]. As shown in Fig. 1A, serum-stimulated calcium mobilization was dose-dependently inhibited with pyrrophenone that was almost completely blocked at a concentration of 2 µM. Pyrrophenone inhibited the peak of [Ca2+]c increase, which occurred 15 sec after serum addition, with an IC50 of ~1 µM (Fig. 1B). The increase in [Ca2+]c induced by ATP was also inhibited by pyrrophenone. Serum and ATP stimulated the release of calcium from intracellular stores since they trigger [Ca2+]c increase in fibroblasts incubated in media containing EGTA to chelate extracellular calcium (Fig. 1C and Fig. 1D). Pyrrophenone inhibited serum and ATP-stimulated release of calcium from intracellular stores to basal levels. The results are not unique to fibroblasts lacking cPLA2α since similar effects of pyrrophenone were observed using IMLF+/+ (data not shown).
Fig. 1. Pyrrophenone blocks calcium mobilization induced by serum, ATP and A23187.
IMLFα−/− loaded with FuraRed-AM were pre-incubated with (A,B) the indicated concentrations of pyrrophenone followed by stimulation with serum, or with 2 µM pyrrophenone (red line) or DMSO (black line) followed by stimulation with (C) serum or (D) ATP in media containing EGTA. Line traces show the fold changes in cytosolic calcium over time. The hatched lines show baseline calcium over-time in untreated, un-stimulated cells. The data represents the average of three independent experiments from analysis of at least 5 cells/experiment. From the data in (A), the effect of pyrrophenone dose on cytosolic calcium levels 15 sec after adding serum is shown (B).
3.2 Use of the CEPIA calcium indicators to monitor changes in [Ca2+]er in IMLF
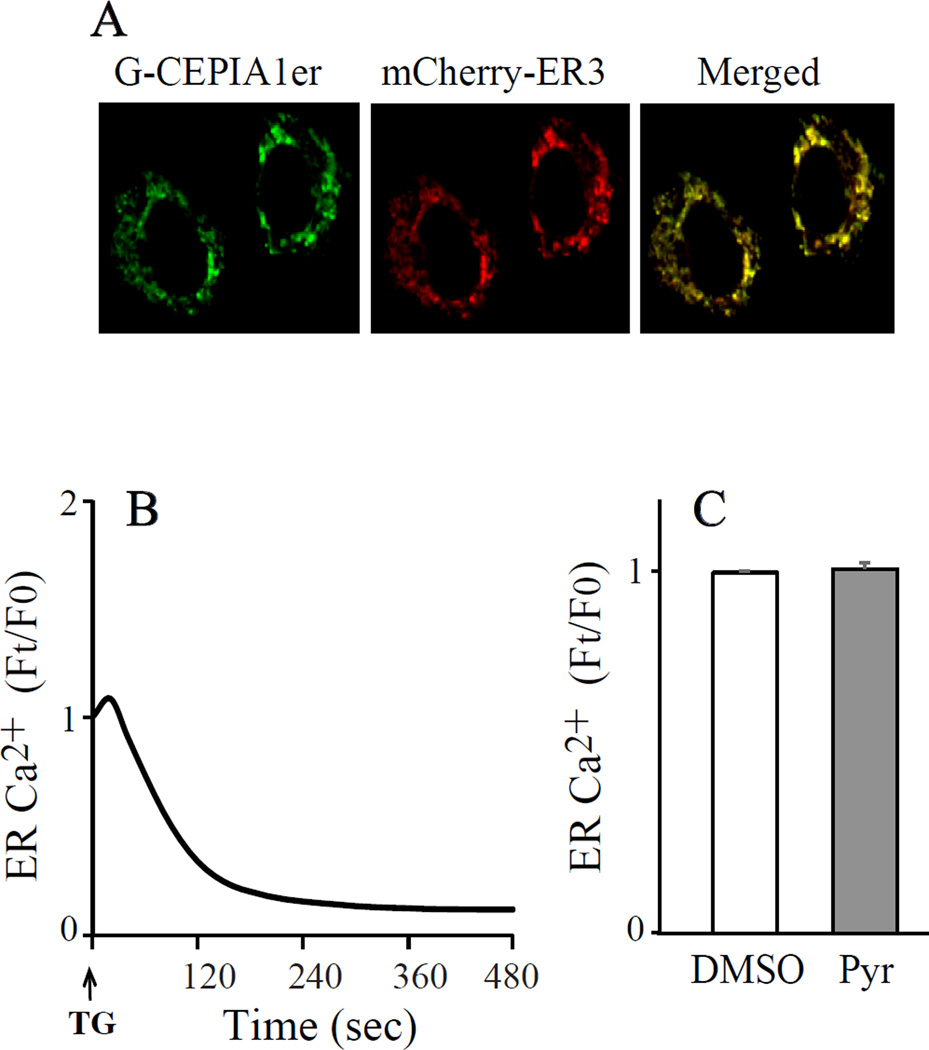
Since the ER is the main intracellular store of Ca2+ that is released in response to cell stimulation, we tested the ability of pyrrophenone to block calcium release from the ER by imaging ER calcium in IMLFα−/− expressing G-CEPIA1er. This calcium indicator contains an enhanced green fluorescent protein (EGFP) tag, and an ER localization and retention sequence [20]. It was designed to have low Ca2+ affinity (672 µM) and high dynamic range (Fmax/Fmin=4.7) in order to measure calcium changes in the ER lumen, which contains high levels of calcium. We first confirmed that G-CEPIA1er localized to the ER in IMLF. G-CEPIA1er co-localized with the mCherry-tagged ER marker (calreticulin + KDEL) when co-expressed in IMLF as previously reported in HeLa cells (Fig. 2A) [20]. To evaluate the ability of G-CEPIA1er to monitor changes in [Ca2+]er IMLF were treated with the sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) inhibitor thapsigargin, which promotes calcium release from the ER [23]. Thapsigargin induced a decrease in fluorescence of G-CEPIA1er consistent with release of calcium from the ER (Fig. 2B). Our protocol for testing the effect of pyrrophenone involved pre-treating cells for 30 min with the inhibitor or DMSO prior to cell stimulation. It was important to determine if pyrrophenone affected the steady state concentration of [Ca2+]er during the pre-incubation time. There was no difference in the fluorescence intensity of G-CEPIA1er in IMLF 30 min after incubation with either pyrrophenone (2 µM) or DMSO suggesting that the inhibitor does not influence steady state [Ca2+]er (Fig. 2C). In additional control experiments we also found that pyrrophenone had no effect on [Ca2+]c when monitored for 5 min after its addition to IMLF (data not shown).
Fig. 2. Use of G-CEPIA1er for imaging ER calcium in IMLF.
(A) Live-cell images of G-CEPIA1er and the ER marker mCherry-ER3 co-expressed in IMLFα−/− are shown along with the merged image. (B) Live-cell imaging of G-CEPIA1er fluorescence in IMLFα−/− stimulated with thapsigargin is shown. (C) Live-cell imaging of G-CEPIA1er fluorescence in IMLFα−/− treated with 2 µM pyrrophenone or vehicle (DMSO) for 30 min. The data (B,C) show the relative levels of ER calcium in three independent experiments from analysis of at least 5 cells/experiment.
3.3 Pyrrophenone blocks ER calcium release and mitochondrial calcium uptake in response to ATP and A23187 but not to thapsigargin
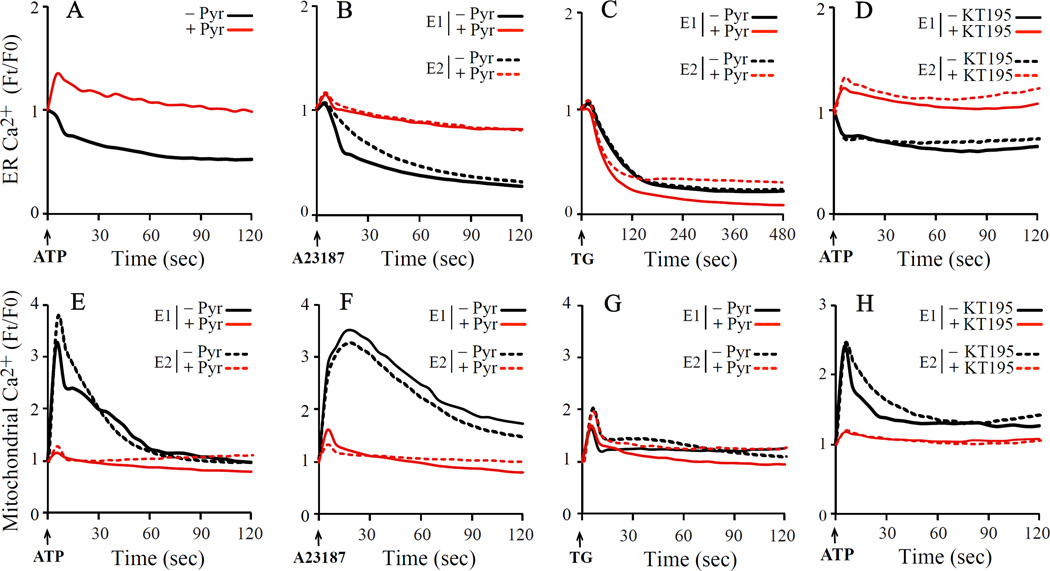
The effect of pyrrophenone on the release of calcium from the ER was investigated in IMLF stimulated with a variety of calcium mobilizing agonists. Stimulation of IMLF with ATP, which triggers receptor-mediated release of calcium from the ER, resulted in a decrease in G-CEPIA1er fluorescence that was inhibited by pyrrophenone (Fig. 3A). A23187, which is known to induce release of calcium from intracellular ER stores, also triggered a decrease in fluorescence of G-CEPIA1er that was inhibited by pyrrophenone (Fig. 3B) [24]. However, the release of calcium from the ER induced by thapsigargin was not inhibited by pyrrophenone suggesting that the serine hydrolase inhibitor targets specific pathways for calcium release (Fig. 3C).
Fig 3. Pyrrophenone inhibits calcium release from ER and calcium increase in mitochondria triggered by ATP and A23187 but not by thapsigargin.
IMLFα−/− expressing G-CEPIA1er or CEPIA2mt were pre-treated with 2 µM pyrrophenone (Pyr), 2 µM KT195 or vehicle (DMSO) for 30 min and then stimulated with ATP, A23187 or thapsigargin (TG). The relative changes in ER (upper panels) or mitochondrial (lower panels) calcium were determined by live-cell fluorescent imaging. The data are the average of three independent experiments (A), or from two independent experiments (E1, E2) from analysis of at least 5 cells/experiment.
Since calcium is transferred from the ER to mitochondria, we investigated the effect of pyrrophenone on agonist-induced increases in [Ca2+]mt in IMLF−/− expressing CEPIA2mt. This indicator contains mitochondrial localization sequences in tandem, a GFP fluorescent tag and has a relatively high affinity for calcium (Kd 160 nM) [20]. The ability of ATP and A23187 to stimulate ER calcium release was accompanied by an increase in [Ca2+]mt, which was inhibited by pyrrophenone (Fig. 1E and 1F, respectively). Although thapsigargin treatment promoted release of Ca2+ from the ER, this resulted in only a small increase in [Ca2+]mt that was not inhibited by pyrophenone (Fig. 3G). We previously reported that another serine hydrolase inhibitor, the triazole urea compound KT195, also inhibited A23187-stimulated cell death by blocking mitochondrial calcium uptake [16]. Similar to the effect of pyrophenone, KT195 inhibited ER calcium release (Fig. 3D) and mitochondrial calcium uptake (Fig. 3H) in cells stimulated with ATP.
3.4 Pyrrophenone inhibits serum-stimulated translocation of the cPLA2α C2 domain to Golgi
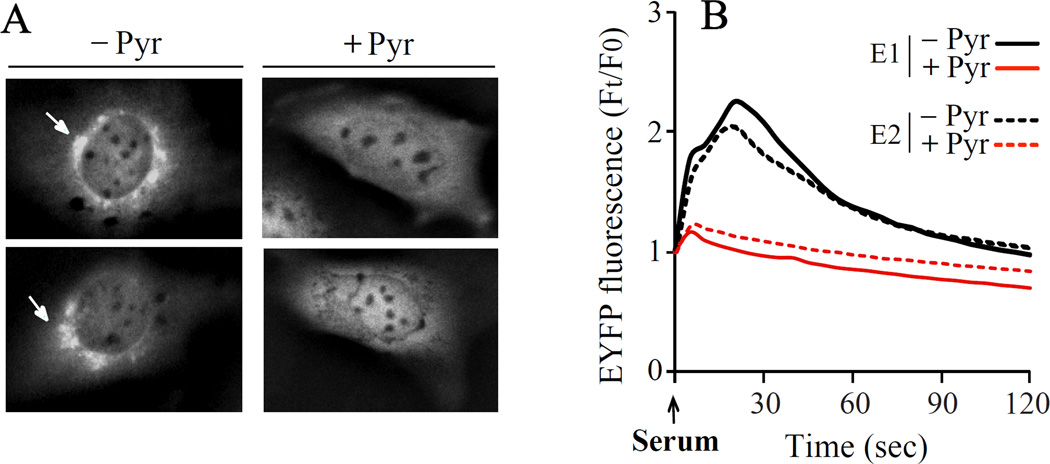
The ability of pyrrophenone to block ER calcium release suggested that it would inhibit the translocation of cPLA2α to Golgi mediated by the C2 domain, which requires an increase in [Ca2+]c [25]. We have previously reported that the cPLA2α C2 domain translocates to Golgi in proportion to the increase in [Ca2+]c indicating that it functions as a calcium sensor [26]. To investigate the effect of pyrrophenone on translocation, the EYFP-C2 domain was expressed in IMLF−/− and translocation induced by serum stimulation monitored by live-cell imaging (Fig. 4). Serum stimulated the rapid translocation of ECFP-C2 domain to Golgi seen as intense juxta-nuclear fluorescence that was completely blocked by pyrrophenone (2 µM). The results suggest that pyrrophenone blocks serum-induced increase in [Ca2+]c to levels below the threshold required for membrane binding of the C2 domain.
Fig. 4. Pyrrophenone inhibits translocation of the cPLA2α C2 domain in serum-stimulated IMLFα.
IMLFα−/− expressing the cPLA2α C2 domain were pre-treated with 2 µM pyrrophenone or vehicle (DMSO) for 30 min and then stimulated with serum. (A) Representative images show (left panel) translocation of EYFP-C2 domain to Golgi (arrow) 30 sec after stimulation with serum. (B) Graphical representation showing EYFP-C2 domain translocation to Golgi over time after stimulation with serum in two independent experiments (E1, E2) from analysis of at least 5 cells/experiment.
4. Discussion
The serine hydrolase inhibitor pyrrophenone has been used extensively to understand the role of cPLA2α in mediating lipid mediator production and cell function. Pyrrophenone inhibits cPLA2α-mediated arachidonic acid release from serum-stimulated IMLF+/+ with an IC50 of ~0.05 µM with 80–90% inhibition occurring at 0.3–1 µM [16]. We previously reported that pyrrophenone inhibits mitochondrial calcium uptake and necrotic cell death due to MPTP formation [16]. This is an off-target effect since it occurs in IMLF lacking cPLA2α. We now show that pyrrophenone blocks calcium release from the ER triggered by receptor stimulation and to A23187, and the concomitant transfer of calcium to the mitochondria. The off-target effects of pyrrophenone occur at an IC50 of ~0.5–1 µM [16]. These results highlight the importance of careful dose response studies with pyrrophenone to study the role of cPLA2α in specific cell types. Pyrrophenone is a potent cPLA2α inhibitor that can be used at concentrations sufficient to inhibit cPLA2α catalytic activity in cells (<0.2 µM) that will limit off-target effects we described previously and in this study [16]. The results demonstrate that with concentrations exceeding ~0.5 µM, pyrrophenone can affect cPLA2α function by blocking calcium-dependent translocation as well as catalytic activity. In addition pyrrophenone has the potential of blocking cellular processes that require calcium mobilization.
The catalytic activity of cPLA2α is likely inhibited by pyrrophenone through formation of a hemiketal between its ketone carbonyl and the active site serine of the enzyme based on the ability of polarized ketones to form stable hemiketals with serine proteases and esterases [27–29]. In support of this mechanism, when the ketone of pyrrophenone is reduced to the secondary alcohol, it poorly inhibits serum-stimulated arachidonic acid release from IMLF+/+ [16]. The reduced form of pyrrophenone also does not effectively block cell death due to calcium overload in A23187-stimulated IMLF supporting involvement of a serine hydrolase that is inhibited through interaction with the ketone carbonyl group of pyrrophenone [16]. This off-target effect of pyrrophenone has uncovered a novel pathway implicating a serine hydrolase in regulating the release of calcium from the ER.
Calcium release from the ER in response to receptor stimulation with agonists such as ATP and serum is mediated by the production of IP3 that acts through receptors in the ER [30]. Calcium is transferred from the ER to mitochondria through sites where the membranes are in close contact, the mitochondrial-associated membranes [31, 32]. Mitochondrial calcium uptake is important for regulating mitochondrial function and cell survival, but when there is calcium overload in mitochondria that occurs with A23187 there is opening of MPTP and cell death [18, 33]. In addition to ATP, pyrrophenone also inhibited calcium release from the ER in response to A23187. Studies have shown that the calcium ionophore increases IP3 levels in cells that may play a role in inducing the release of calcium from the ER [34–36]. In contrast, the release of calcium from the ER in response to the SERCA inhibitor thapsigargin, which has been reported not to promote IP3 production, was not inhibited by pyrrophenone [23, 37]. Thapsigargin does not generate the formation of high-concentration Ca2+ micro-domains that are required for mitochondrial calcium uptake consistent with studies showing that it is not as effective in increasing mitochondrial calcium as the IP3-mediated pathway [31, 38, 39]. Steady state ER calcium levels are maintained by the continuous cycling of calcium in and out of the ER through SERCA-mediated uptake and passive leakage through basal ER calcium-leak channels [40]. Our results suggest that pyrrophenone targets a serine hydrolase in the IP3-mediated ER calcium release pathway that couples to mitochondrial calcium uptake and not pathways that control basal calcium release.
Supplementary Material
Highlights.
cPLA2α inhibitor pyrrophenone blocks calcium increase and C2 domain translocation.
Pyrrophenone inhibits ATP stimulated transfer of calcium from ER to mitochondria.
Effects of pyrrophenone are off-target occurring in cPLA2α-deficient cells.
Pyrrophenone targets a novel serine hydrolase that regulates calcium mobilization.
Acknowledgments
The work was funded by the National Institutes of Health Grant ES025015 to CCL.
Abbreviations
- cPLA2α
cytosolic phospholipase A2α
- ER
endoplasmic reticulum
- DMEM
Dulbecco's Modified Eagle's Medium
- MPTP
mitochondrial permeability transition pore
- IMLF
immortalized mouse lung fibroblasts
- EYFP
enhanced yellow fluorescent protein
- CEPIA
calcium-measuring organelle-entrapped protein indicators
- SERCA
sarco/endoplasmic reticulum Ca2+-ATPase.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Leslie CC. Cytosolic phospholipase A2: physiological function and role in disease. J. Lipid Res. 2015;56:1386–1402. doi: 10.1194/jlr.R057588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith WL, Urade Y, Jakobsson PJ. Enzymes of the cyclooxygenase pathways of prostanoid biosynthesis. Chemical Rev. 2011;111:5821–5865. doi: 10.1021/cr2002992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haeggstrom JZ, Funk CD. Lipoxygenase and leukotriene pathways: biochemistry, biology, and roles in disease. Chemical Rev. 2011;111:5866–5898. doi: 10.1021/cr200246d. [DOI] [PubMed] [Google Scholar]
- 4.Dessen A, Tang J, Schmidt H, Stahl M, Clark JD, Seehra J, Somers WS. Crystal structure of human cytosolic phospholipase A2 reveals a novel topology and catalytic mechanism. Cell. 1999;97:349–360. doi: 10.1016/s0092-8674(00)80744-8. [DOI] [PubMed] [Google Scholar]
- 5.Leslie CC, Gangelhoff TA, Gelb MH. Localization and function of cytosolic phospholipase A2α at the Golgi. Biochimie. 2010;92:620–626. doi: 10.1016/j.biochi.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tucker DE, Ghosh M, Ghomashchi F, Loper R, Suram S, St. John B, Girotti M, Bollinger JG, Gelb MH, Leslie CC. Role of phosphorylation and basic residues in the catalytic domain of cytosolic phospholipase A2α in regulating interacial kinetics and binding and cellular function. J. Biol. Chem. 2009;284:9596–9611. doi: 10.1074/jbc.M807299200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin L-L, Wartmann M, Lin AY, Knopf JL, Seth A, Davis RJ. cPLA2 is phosphorylated and activated by MAP kinase. Cell. 1993;72:269–278. doi: 10.1016/0092-8674(93)90666-e. [DOI] [PubMed] [Google Scholar]
- 8.Hanel AM, Schüttel S, Gelb MH. Processive interfacial catalysis by mammalian 85-kilodalton phospholipase A2 enzymes on product-containing vesicles: application to the determination of substrate preferences. Biochemistry. 1993;32:5949–5958. doi: 10.1021/bi00074a005. [DOI] [PubMed] [Google Scholar]
- 9.Leslie CC. Regulation of the specific release of arachidonic acid by cytosolic phospholipase A2 . Prostagl. Leukotr. Essen. Fatty Acids. 2004;70:373–376. doi: 10.1016/j.plefa.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 10.Murakami M, Taketomi Y, Miki Y, Sato H, Hirabayashi T, Yamamoto K. Recent progress in phospholipase A2 research: from cells to animals to humans. Prog. Lipid Res. 2011;50:152–192. doi: 10.1016/j.plipres.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 11.Magrioti V, Kokotos G. Phospholipase A2 inhibitors as potential therapeutic agents for the treatment of inflammatory diseases. Expert Opin. Ther. Pat. 2010;20:1–18. doi: 10.1517/13543770903463905. [DOI] [PubMed] [Google Scholar]
- 12.Ono T, Yamada K, Chikazawa Y, Ueno M, Nakamoto S, Okuno T, Seno K. Characterization of a novel inhibitor of cytosolic phospholipase A2α, pyrrophenone. Biochem. J. 2002;363:727–735. doi: 10.1042/0264-6021:3630727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seno K, Okuno T, Nishi K, Murakami Y, Watanabe F, Matsuura T, Wada M, Fujii Y, Yamada M, Ogawa T, Okada T, Hashizume H, Kii M, Hara S, Hagishita S, Nakamoto S, Yamada K, Chikazawa Y, Ueno M, Teshirogi I, Ono T, Ohtani M. Pyrrolidine inhibitors of human cytosolic phospholipase A2 . J. Med. Chem. 2000;43:1041–1044. doi: 10.1021/jm9905155. [DOI] [PubMed] [Google Scholar]
- 14.Flamand N, Picard S, Lemieux L, Pouliot M, Bourgoin SG, Borgeat P. Effects of pyrrophenone, an inhibitor of group IVA phospholipase A2, on eicosanoid and PAF biosynthesis in human neutrophils. British J. Pharmacol. 2006;149:385–392. doi: 10.1038/sj.bjp.0706879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghomashchi F, Stewart A, Hefner Y, Ramanadham S, Turk J, Leslie CC, Gelb MH. A pyrrolidine-based specific inhibitor of cytosolic phospholipase A2α blocks arachidonic acid release in a variety of mammalian cells. Biochim. Biophys. Acta. 2001;1513:160–166. doi: 10.1016/s0005-2736(01)00349-2. [DOI] [PubMed] [Google Scholar]
- 16.Yun B, Lee H, Ghosh M, Cravatt BF, Hsu KL, Bonventre JV, Ewing H, Gelb MH, Leslie CC. Serine hydrolase inhibitors block necrotic cell death by preventing calcium overload of the mitochondria and permeability transition pore formation. J. Biol. Chem. 2014;289:1491–1504. doi: 10.1074/jbc.M113.497651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baines CP. The mitochondrial permeability transition pore and the cardiac necrotic program. Pediatric Cardiol. 2011;32:258–262. doi: 10.1007/s00246-010-9880-9. [DOI] [PubMed] [Google Scholar]
- 18.Lemasters JJ, Theruvath TP, Zhong Z, Nieminen AL. Mitochondrial calcium and the permeability transition in cell death. Biochim. Biophys. Acta. 2009;1787:1395–1401. doi: 10.1016/j.bbabio.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Naon D, Scorrano L. At the right distance: ER-mitochondria juxtaposition in cell life and death. Biochim. Biophys. Acta. 2014;1843:2184–2194. doi: 10.1016/j.bbamcr.2014.05.011. [DOI] [PubMed] [Google Scholar]
- 20.Suzuki J, Kanemaru K, Ishii K, Ohkura M, Okubo Y, Iino M. Imaging intraorganellar Ca2+ at subcellular resolution using CEPIA. Nat. Commun. 2014;5:4153. doi: 10.1038/ncomms5153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stewart A, Ghosh M, Spencer DM, Leslie CC. Enzymatic properties of human cytosolic phospholipase A2γ. J. Biol. Chem. 2002;277:29526–29536. doi: 10.1074/jbc.M204856200. [DOI] [PubMed] [Google Scholar]
- 22.Ghosh M, Stewart A, Tucker DE, Bonventre JV, Murphy RC, Leslie CC. Role of cytosolic phospholipase A2 in prostaglandin E2 production by lung fibroblasts. Amer. J. Respir. Cell Mol. Biol. 2004;30:91–100. doi: 10.1165/rcmb.2003-0005OC. [DOI] [PubMed] [Google Scholar]
- 23.Thastrup O, Cullen PJ, Drobak BK, Hanley MR, Dawson AP. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2+-ATPase. Proc. Natl. Acad. Sci. U.S.A. 1990;87:2466–2470. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Drummond IA, Lee AS, Resendez E, Jr, Steinhardt RA. Depletion of intracellular calcium stores by calcium ionophore A23187 induces the genes for glucose-regulated proteins in hamster fibroblasts. J. Biol. Chem. 1987;262:12801–12805. [PubMed] [Google Scholar]
- 25.Evans JH, Spencer DM, Zweifach A, Leslie CC. Intracellular calcium signals regulating cytosolic phospholipase A2 translocation to internal membranes. J. Biol. Chem. 2001;276:30150–30160. doi: 10.1074/jbc.M100943200. [DOI] [PubMed] [Google Scholar]
- 26.Evans JH, Leslie CC. The cytosolic phospholipase A2 catalytic domain modulates association and residence time at Golgi membranes. J. Biol. Chem. 2004;279:6005–6016. doi: 10.1074/jbc.M311246200. [DOI] [PubMed] [Google Scholar]
- 27.Gelb MH, Svaren JP, Abeles RH. Fluoro ketone inhibitors of hydrolytic enzymes. Biochemistry. 1985;24:1813–1817. doi: 10.1021/bi00329a001. [DOI] [PubMed] [Google Scholar]
- 28.Street IP, Lin HK, Laliberté F, Ghomashchi F, Wang Z, Perrier H, Tremblay NM, Huang Z, Weech PK, Gelb MH. Slow- and tight-binding inhibitors of the 85-kDa human phospholipase A2 . Biochemistry. 1993;32:5935–5940. doi: 10.1021/bi00074a003. [DOI] [PubMed] [Google Scholar]
- 29.Barbayianni E, Stephens D, Grkovich A, Magrioti V, Hsu YH, Dolatzas P, Kalogiannidis D, Dennis EA, Kokotos G. 2-Oxoamide inhibitors of phospholipase A2 activity and cellular arachidonate release based on dipeptides and pseudodipeptides. Bioorg. Medicinal Chem. 2009;17:4833–4843. doi: 10.1016/j.bmc.2009.03.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berridge MJ. Inositol trisphosphate and calcium signalling. Nature. 1993;361:315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- 31.Rizzuto R, Pinton P, Carrington W, Fay FS, Fogarty KE, Lifshitz LM, Tuft RA, Pozzan T. Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science. 1998;280:1763–1766. doi: 10.1126/science.280.5370.1763. [DOI] [PubMed] [Google Scholar]
- 32.Vance JE. MAM (mitochondria-associated membranes) in mammalian cells: Lipids and beyond. Biochim. Biophys. Acta. 2013;1841:595–609. doi: 10.1016/j.bbalip.2013.11.014. [DOI] [PubMed] [Google Scholar]
- 33.Pinton P, Giorgi C, Siviero R, Zecchini E, Rizzuto R. Calcium and apoptosis: ER-mitochondria Ca2+ transfer in the control of apoptosis. Oncogene. 2008;27:6407–6418. doi: 10.1038/onc.2008.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lo TN, Saul W, Beaven MA. The actions of Ca2+ ionophores on rat basophilic (2H3) cells are dependent on cellular ATP and hydrolysis of inositol phospholipids. A comparison with antigen stimulation. J. Biol. Chem. 1987;262:4141–4145. [PubMed] [Google Scholar]
- 35.Knepper SM, Rutledge CO. Effects of calcium depletion on norepinephrine- and A23187-induced stimulation of inositol phosphate formation. Biochem. Pharmacol. 1987;36:3043–3050. doi: 10.1016/0006-2952(87)90222-x. [DOI] [PubMed] [Google Scholar]
- 36.Wigginton SA, Minneman KP. Comparison of calcium ionophore and receptor-activated inositol phosphate formation in primary glial cell cultures. Eur. J. Pharmacol. 1991;208:239–247. doi: 10.1016/0922-4106(91)90101-m. [DOI] [PubMed] [Google Scholar]
- 37.Jackson TR, Patterson SI, Thastrup O, Hanley MR. A novel tumour promoter, thapsigargin, transiently increases cytoplasmic free Ca2+ without generation of inositol phosphates in NG115-401L neuronal cells. Biochem. J. 1988;253:81–86. doi: 10.1042/bj2530081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Collins TJ, Lipp P, Berridge MJ, Bootman MD. Mitochondrial Ca2+ uptake depends on the spatial and temporal profile of cytosolic Ca2+ signals. J. Biol. Chem. 2001;276:26411–26420. doi: 10.1074/jbc.M101101200. [DOI] [PubMed] [Google Scholar]
- 39.Deak AT, Blass S, Khan MJ, Groschner LN, Waldeck-Weiermair M, Hallstrom S, Graier WF, Malli R. IP3-mediated STIM1 oligomerization requires intact mitochondrial Ca2+ uptake. J. Cell Sci. 2014;127:2944–2955. doi: 10.1242/jcs.149807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.La Rovere RM, Roest G, Bultynck G, Parys JB. Intracellular Ca2+ signaling and Ca2+ microdomains in the control of cell survival, apoptosis and autophagy. Cell Calcium. 2016 doi: 10.1016/j.ceca.2016.04.005. in press. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.