Abstract
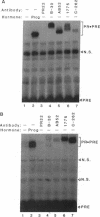
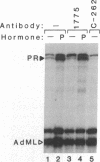
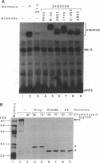
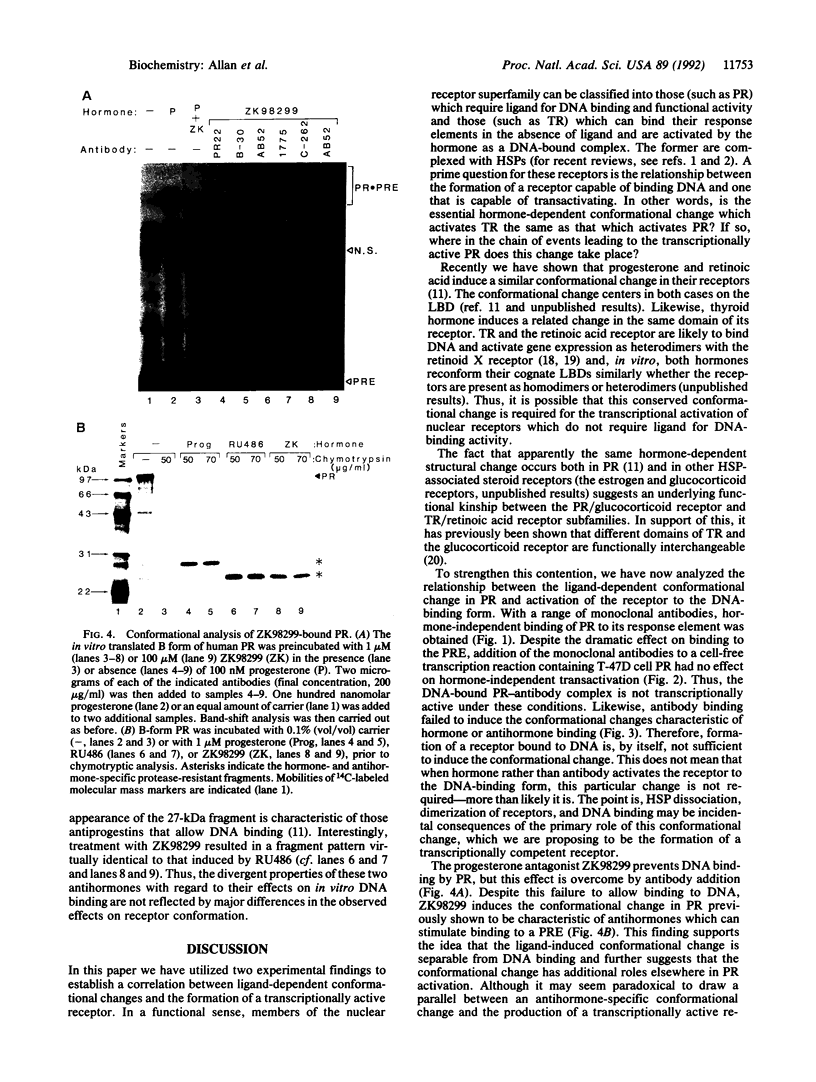
Hormones and antihormones induce related, but distinct, conformational changes in the progesterone receptor [Allan, G. F., Leng, X., Tsai, S. Y., Weigel, N. L., Edwards, D. P., Tsai, M.-J. & O'Malley, B. W. (1992) J. Biol. Chem. 267, 19513-19520]. In both cases the conformational change precedes the dissociation of heat shock proteins and binding to DNA. We have now investigated the steps in hormone action which are dependent upon this conformational change. We show that in the absence of ligand, monoclonal antibodies directed against different regions of the progesterone receptor can induce high-affinity binding to its response element in vitro. This antibody-induced DNA binding is presumably facilitated by enhanced dimerization of receptor monomers. However, antibodies do not induce the hormone-specific conformational change in the progesterone receptor and do not induce in vitro transcription by the receptor. In contrast, the antiprogestin ZK98299, which inhibits receptor binding to DNA, fully induces the antihormone-specific conformational change. Thus, our data imply that steroids induce a conformational change in their receptors which is necessary for events subsequent to DNA binding, most likely for transactivation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan G. F., Leng X., Tsai S. Y., Weigel N. L., Edwards D. P., Tsai M. J., O'Malley B. W. Hormone and antihormone induce distinct conformational changes which are central to steroid receptor activation. J Biol Chem. 1992 Sep 25;267(27):19513–19520. [PubMed] [Google Scholar]
- Bagchi M. K., Tsai M. J., O'Malley B. W., Tsai S. Y. Analysis of the mechanism of steroid hormone receptor-dependent gene activation in cell-free systems. Endocr Rev. 1992 Aug;13(3):525–535. doi: 10.1210/edrv-13-3-525. [DOI] [PubMed] [Google Scholar]
- Bagchi M. K., Tsai S. Y., Tsai M. J., O'Malley B. W. Identification of a functional intermediate in receptor activation in progesterone-dependent cell-free transcription. Nature. 1990 Jun 7;345(6275):547–550. doi: 10.1038/345547a0. [DOI] [PubMed] [Google Scholar]
- Bagchi M. K., Tsai S. Y., Tsai M. J., O'Malley B. W. Progesterone enhances target gene transcription by receptor free of heat shock proteins hsp90, hsp56, and hsp70. Mol Cell Biol. 1991 Oct;11(10):4998–5004. doi: 10.1128/mcb.11.10.4998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baniahmad A., Köhne A. C., Renkawitz R. A transferable silencing domain is present in the thyroid hormone receptor, in the v-erbA oncogene product and in the retinoic acid receptor. EMBO J. 1992 Mar;11(3):1015–1023. doi: 10.1002/j.1460-2075.1992.tb05140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baulieu E. E. Steroid hormone antagonists at the receptor level: a role for the heat-shock protein MW 90,000 (hsp 90). J Cell Biochem. 1987 Oct;35(2):161–174. doi: 10.1002/jcb.240350209. [DOI] [PubMed] [Google Scholar]
- Benhamou B., Garcia T., Lerouge T., Vergezac A., Gofflo D., Bigogne C., Chambon P., Gronemeyer H. A single amino acid that determines the sensitivity of progesterone receptors to RU486. Science. 1992 Jan 10;255(5041):206–209. doi: 10.1126/science.1372753. [DOI] [PubMed] [Google Scholar]
- Dalman F. C., Koenig R. J., Perdew G. H., Massa E., Pratt W. B. In contrast to the glucocorticoid receptor, the thyroid hormone receptor is translated in the DNA binding state and is not associated with hsp90. J Biol Chem. 1990 Mar 5;265(7):3615–3618. [PubMed] [Google Scholar]
- Dalman F. C., Sturzenbecker L. J., Levin A. A., Lucas D. A., Perdew G. H., Petkovitch M., Chambon P., Grippo J. F., Pratt W. B. Retinoic acid receptor belongs to a subclass of nuclear receptors that do not form "docking" complexes with hsp90. Biochemistry. 1991 Jun 4;30(22):5605–5608. doi: 10.1021/bi00236a038. [DOI] [PubMed] [Google Scholar]
- Damm K., Thompson C. C., Evans R. M. Protein encoded by v-erbA functions as a thyroid-hormone receptor antagonist. Nature. 1989 Jun 22;339(6226):593–597. doi: 10.1038/339593a0. [DOI] [PubMed] [Google Scholar]
- DeMarzo A. M., Beck C. A., Onate S. A., Edwards D. P. Dimerization of mammalian progesterone receptors occurs in the absence of DNA and is related to the release of the 90-kDa heat shock protein. Proc Natl Acad Sci U S A. 1991 Jan 1;88(1):72–76. doi: 10.1073/pnas.88.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawell S. E., White R., Hoare S., Sydenham M., Page M., Parker M. G. Inhibition of estrogen receptor-DNA binding by the "pure" antiestrogen ICI 164,384 appears to be mediated by impaired receptor dimerization. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6883–6887. doi: 10.1073/pnas.87.17.6883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronemeyer H. Transcription activation by estrogen and progesterone receptors. Annu Rev Genet. 1991;25:89–123. doi: 10.1146/annurev.ge.25.120191.000513. [DOI] [PubMed] [Google Scholar]
- Klein-Hitpass L., Cato A. C., Henderson D., Ryffel G. U. Two types of antiprogestins identified by their differential action in transcriptionally active extracts from T47D cells. Nucleic Acids Res. 1991 Mar 25;19(6):1227–1234. doi: 10.1093/nar/19.6.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks M. S., Hallenbeck P. L., Nagata T., Segars J. H., Appella E., Nikodem V. M., Ozato K. H-2RIIBP (RXR beta) heterodimerization provides a mechanism for combinatorial diversity in the regulation of retinoic acid and thyroid hormone responsive genes. EMBO J. 1992 Apr;11(4):1419–1435. doi: 10.1002/j.1460-2075.1992.tb05187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard D., Salser S. J., Yamamoto K. R. A movable and regulable inactivation function within the steroid binding domain of the glucocorticoid receptor. Cell. 1988 Sep 23;54(7):1073–1080. doi: 10.1016/0092-8674(88)90122-5. [DOI] [PubMed] [Google Scholar]
- Thompson C. C., Evans R. M. Trans-activation by thyroid hormone receptors: functional parallels with steroid hormone receptors. Proc Natl Acad Sci U S A. 1989 May;86(10):3494–3498. doi: 10.1073/pnas.86.10.3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai S. Y., Carlstedt-Duke J., Weigel N. L., Dahlman K., Gustafsson J. A., Tsai M. J., O'Malley B. W. Molecular interactions of steroid hormone receptor with its enhancer element: evidence for receptor dimer formation. Cell. 1988 Oct 21;55(2):361–369. doi: 10.1016/0092-8674(88)90059-1. [DOI] [PubMed] [Google Scholar]
- Vegeto E., Allan G. F., Schrader W. T., Tsai M. J., McDonnell D. P., O'Malley B. W. The mechanism of RU486 antagonism is dependent on the conformation of the carboxy-terminal tail of the human progesterone receptor. Cell. 1992 May 15;69(4):703–713. doi: 10.1016/0092-8674(92)90234-4. [DOI] [PubMed] [Google Scholar]
- Yu V. C., Delsert C., Andersen B., Holloway J. M., Devary O. V., När A. M., Kim S. Y., Boutin J. M., Glass C. K., Rosenfeld M. G. RXR beta: a coregulator that enhances binding of retinoic acid, thyroid hormone, and vitamin D receptors to their cognate response elements. Cell. 1991 Dec 20;67(6):1251–1266. doi: 10.1016/0092-8674(91)90301-e. [DOI] [PubMed] [Google Scholar]
- el-Ashry D., Oñate S. A., Nordeen S. K., Edwards D. P. Human progesterone receptor complexed with the antagonist RU 486 binds to hormone response elements in a structurally altered form. Mol Endocrinol. 1989 Oct;3(10):1545–1558. doi: 10.1210/mend-3-10-1545. [DOI] [PubMed] [Google Scholar]