Abstract
Mitomycin C (MC), a commonly used anticancer drug, induces DNA damage via DNA alkylation. Decarbamoyl mitomycin C (DMC), another mitomycin lacking the carbamate at C10, generates similar lesions as MC. Interstrand cross-links (ICLs) are believed to be the lesions primarily responsible for the cytotoxicity of MC and DMC. The major ICL generated by MC (α-ICL) has a trans stereochemistry at the guanine-drug linkage whereas the major ICL from DMC (β-ICL) has the opposite, cis, stereochemistry. In addition, DMC can provoke strong p53-independent cell death. Our hypothesis is that the stereochemistry of the major unique β-ICL generated by DMC is responsible for this p53-independent cell death signaling. p53 gene is inactively mutated in more than half of human cancers. p21WAF1/CIP1 known as a major effector of p53 is involved in p53-dependent and -independent control of cell proliferation and death. This study revealed the role of p21WAF1/CIP1 on MC and DMC triggered cell damage. MCF-7 (p53-proficient) and K562 (p53-deficient) cells were used. Cell cycle distributions were shifted to the G1/S phase in MCF-7 treated with MC and DMC, but were shifted to the S phase in K562. p21WAF1/CIP1 activation was observed in both cells treated with MC and DMC, and DMC triggered more significant activation. Knocking down p53 in MCF-7 did not attenuate MC and DMC induced p21WAF1/CIP1 activation. The α-ICL itself was enough to cause p21WAF1/CIP1 activation.
Keywords: mitomycin C, decarbamoyl mitomycin C, p21, p53
Introduction
Mitomycin C (MC) is a bioreductive anticancer drug produced by Streptomyces casespitosus (1,2) and commonly used to treat many cancers, such as stomach, anal, and lung cancers (3,4). However, the response rates are only ~15–20% (4). Although its mode of action has been extensively examined (5), it is still the center of numerous research endeavors (6–10). In particular, this drug is used intensively to investigate the mechanisms involved in DNA repair (8–10). Studies showed that mitomycin C induces DNA damage via DNA alkylation to produce DNA mono-adducts, intrastrand cross-links and interstrand cross-links (ICLs) (10,11). The main toxicity of MC is due to these interstrand MC-DNA crosslinks (12).
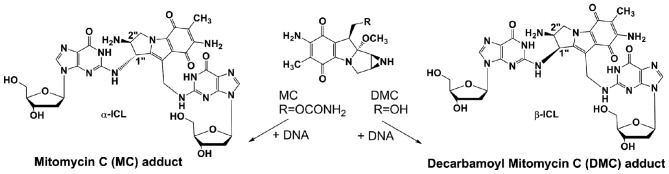
The MC analog, 10-decarbamoyl mitomycin C (DMC), has not been fully investigated. It has recently been found that the major ICLs produced by MC and DMC have opposite stereochemistry. MC generates an ICL with trans stereochemistry (α-ICL) and DMC generates an ICL with cis stereochemistry (β isomer, β-ICL) (Fig. 1). Experimental evidence points to crosslinks α-ICL and β-ICL as the lesions primarily responsible for the cytotoxicity of the mitomycins (13). When the cellular and molecular response of human cancer cells treated with MC and DMC were compared, it was found that DMC was more toxic in human cancer cells with or without a functioning p53 (14) and that DMC provokes a strong p53-independent cell death (7). Xiao et al (15) further suggested that DMC could enhance Chk1 checkpoint activation and Rad51 chromatin recruitment via a p53-independent disassociation of ATR chromatin eviction.
Figure 1.
Mitomycin C (MC) and decarbamoyl mitomycin C (DMC) interstrand crosslinks (ICLs).
The study of anticancer drug induced DNA damage mechanisms has mainly focused on cell cycle and checkpoint control proteins. However, there are other mechanisms which can regulate cell cycle progression, such as p21WAF1/CIP1. Esposito et al (16) demonstrated that nucleolar stressors, 5-fluouracil (5-FU) and oxaliplatin (L-OHP), trigger cell cycle arrest and apoptosis by altering rpL3-regulated p21 expression. Moreover, this alteration of p21 expression by rpL3 could occur in a p53-independent manner (17). In many human cancers, abnormal expressions of cyclin D1 and cyclin E (which can promote the transition of G1/S phase) have been observed (18). This accelerated G1/S transition effect triggered by cyclin D1 and cyclin E can be inhibited by p21WAF1/CIP1 when anticancer drugs induce DNA damage (19,20). Choi et al (20) observed that MC inhibited the G1/S transition by p53-dependent p21WAF1/CIP1, but at sublethal MC concentrations, the accumulation of cyclin E with a delayed increase of p21WAF1/CIP1 promoted G1/S transition. It suggests that the cell cycle G1/S transition is controlled by cyclin E and p21WAF1/CIP1 in a MC dose-dependent manner.
The p53 tumor suppressor protein is one of the key players for keeping genetic stability following DNA damage and is the target of many chemotherapeutic drugs (21,22). However, p53 gene is inactively mutated in more than half of human cancers (22–24). p21WAF1/CIP1, known as a protein cyclin-dependent kinase inhibitor, is a major effector of p53 and is involved in p53-dependent and -independent control of cell proliferation and death (25). p21WAF1/CIP1 expression has been linked with irreversible cell cycle arrest in both G1 and G2/M. In this study, MCF-7 (p53-proficient cell line) and K562 (p53-deficient cell line) were used to elucidate the role of p21WAF1/CIP1 in the signaling mechanism of MC and DMC and their effects on cell cycle. An oligonucleotide (18 mer) bearing the major MC-ICL (α-ICL) was also synthesized and transfected into cells to unveil the effect of the α-ICL on the regulation of p21WAF1/CIP1.
Materials and methods
Cell culture and reagents
Human breast cancer cells (MCF-7) and leukemia cancer cells (K562) were obtained from the American Type Tissue Culture (Manassas, VA, USA). Both cell lines have been used for mitomycin C studies (7,26). MCF-7 cell line is a p53-proficient cell line. K562 cell line is a p53-deficient cell line with an inactivation mutation in exon 5 (27). Dulbecco's modified Eagle's medium (DMEM), RPMI-1640, fetal bovine serum (FBS), heat-inactivated horse serum, gentamicin (50 mg/ml) were obtained from Invitrogen (Gaithersburg, MD, USA). MCF-7 cells were cultured within DMEM supplemented with 10% FBS and 50 μg/ml gentamicin. K562 cells were cultured within RPMI-1640 supplemented with 10% FBS, 2 mM glutamine and 50 μg/ml gentamicin. Both cells were maintained in a humidified atmosphere at 37°C and 5% CO2. For chemical treatment, cells were cultured in 60-mm Petri dishes one day prior to the experiment. Cells were grown until medium density (~80% confluence) before chemical treatments.
Mitomycin C was a generous gift from Dr Maria Tomasz (Hunter College, City University of New York, New York, USA). Decarbamoyl mitomycin C was synthesized based on the protocol of Kinoshita et al (28).
β-actin antibody was obtained from Sigma-Aldrich (St. Louis, MO, USA). p145 p21WAF1/CIP1 and p146 p21WAF1/CIP1, and p21WAF1/CIP1 antibodies were from Santa Cruz. Bio-Rad DC (detergent compatible) protein assay reagents were purchased from Bio-Rad (Hercules, CA, USA). The Super Signal West Pico chemiluminescent kit, secondary antibodies containing anti-rabbit IgG or anti-mouse IgG conjugated to horseradish peroxidase, mammalian protein extraction reagent (M-PER) lysis buffer, Halt protease inhibitor cocktail, and stripping buffer were obtained from Pierce (Rockford, IL, USA). SuperFect™ transfection reagent was purchased from Qiagen (Valencia, CA, USA). All other reagents were purchased from Sigma-Aldrich.
α-interstrand cross-link (α-ICL) synthesis
The synthesis protocol was adapted from Borowy-Borowski et al (5) with minor modifications. Briefly, a 1:1 molar mixture of the 18 mer and its complementary strand (0.415 μmol of oligo 1: ACTGATCTCGTTAGTCAT and 0.415 μmol of oligo 2 -complementary-: ATGACTAACGAGATCAGT) were mixed with 50 μmol of MC (from a 20-μmol/ml solution in 70% water and 30% methanol). The mixture was lyophilized and redisolved in 4.5 ml of Tris buffer (0.1 M, pH 7.4). The mixture was heated to 55°C for 10 min and then cooled down to 4°C over the course of 5 h. The mixture was then deaerated for 30 min by bubbling argon. An equal amount of a freshly prepared sodium dithionite solution (100 μl of the same Tris buffer and 2.78 mg of sodium dithionite i.e., 16 μmol of sodium dithionite) was added to the solution every 10 min. The addition was repeated 4 times. The last addition was made as a titration i.e., sodium dithionite was added until the purple color disappeared. The solution was then stirred open to air for 20 min to reoxidize the hydroquinones. The cross-link was pre-purified by sep-pak chromatography and ethanol precipitation. Final purification was achieved by 20% urea polyacrylamide gel (29).
Evidence of crosslink formation was obtained from enzymatic digestion of the cross-linked oligonucleotide and HPLC analysis of the digest (Buffer A, 20 mM ammonium acetate; Buffer B, 30% acetonitrile and 70% Buffer A; 0.8 ml/min flow rate. Gradient, 20% B to 60% B in 60 min. Elution times: dC, 12 min; dG, 17 min; dT, 21 min; dA, 24 min; dG-MC-dG, 27 min) (data not shown). Thermal denaturation analysis was conducted to confirm cross-link formation and showed Tm of the unmodified duplex of 46.72°C and Tm of MC-α-ICL of 61.29°C.
Chemical treatments
MC and DMC were dissolved in 30% methanol and freshly diluted with cell culture media to obtain tested concentrations. Dosages were chosen based on previous studies (6). Cells were treated with chemicals and incubated at 37°C for different periods of incubation time as indicated. The final methanol concentration in the treatment was <0.15%, which is not toxic to the cells.
Cell cycle analysis
The cells were washed twice with ice cold phosphate-buffered saline (PBS) and fixed in 70% ethanol at 4°C overnight. Then the fixed cells were stained with a 50 μg/ml propidium iodide solution containing 100 μg/ml RNase A and glucose buffer at room temperature for 30 min, and the cell cycle distribution was analyzed by Attune® Acoustic Focusing Cytometer using Attune® Cytometric Software.
Western blot analysis
After 24 h of chemical exposure, cells were lysed with a mixture of M-PER, Halt protease inhibitor, and a phosphatase inhibitor cocktail. The concentration of protein samples was determined by using Bio-Rad DC (detergent compatible) protein assay reagents (Bio-Rad).
Protein samples (40 μg) from each treatment condition were resolved via 8% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to PVDF membranes. Membranes were blocked in PBS/0.05% Tween-20, containing 5% BSA, and were then probed overnight with primary antibody. The antibody was detected with corresponding horseradish peroxidase-linked secondary antibody. Blots were developed using Super Signal West Pico Chemiluminescent Substrate detection reagents. Membranes were then stripped with stripping buffer for 15 min at room temperature and re-probed with β-actin (1 μg/μl) or p21WAF1/CIP1 antibody as the loading control. Chemiluminescent signals were captured using the Geliance 600 imaging system (Perkin-Elmer, Shelton, CT, USA) and analyzed by GeneTools software (Syngene, Frederick, MD, USA).
Transfection
p53 shRNA plasmid from Santa Cruz was transiently transfected into MCF-7 cells to knock down the expression of p53 by using SuperFect™ transfection reagent 24 h before chemical treatments. Briefly, p53 shRNA plasmid was mixed with SuperFect in a ratio of 1 μg DNA to 2 μl transfection reagent according to the manufacturer's instructions. The mixtures were added in 30 μl of serum-free DMEM for 10 min at room temperature. The mixtures were then mixed with 0.6 ml of complete cell culture media and transferred to the cells for a 3-h incubation at 37°C in humidified air containing 5% CO2. The transfection mixtures were then removed and replaced with fresh media after washing the cells twice with PBS. The cells were then incubated for an additional 24 h at 37°C in humidified air containing 5% CO2 prior to chemical treatments.
The isolated α-interstrand cross-link was also transfected into cells by SuperFect transfection reagent using the same protocol as p53 shRNA plasmid transfection.
Statistical analyses
All experiments were performed at least in triplicate, and results are reported as means ± SEM. Statistical significance was determined using one-way analysis of variance (ANOVA) followed by Dunnett's post hoc test (p<0.05, with control at 100%) by using GraphPad PRISM® 6 software.
Results
Cell cycle distribution patterns of MCF-7 and K562 cells treated with MC and DMC are shifted to G1/S phase and to S phase, respectively
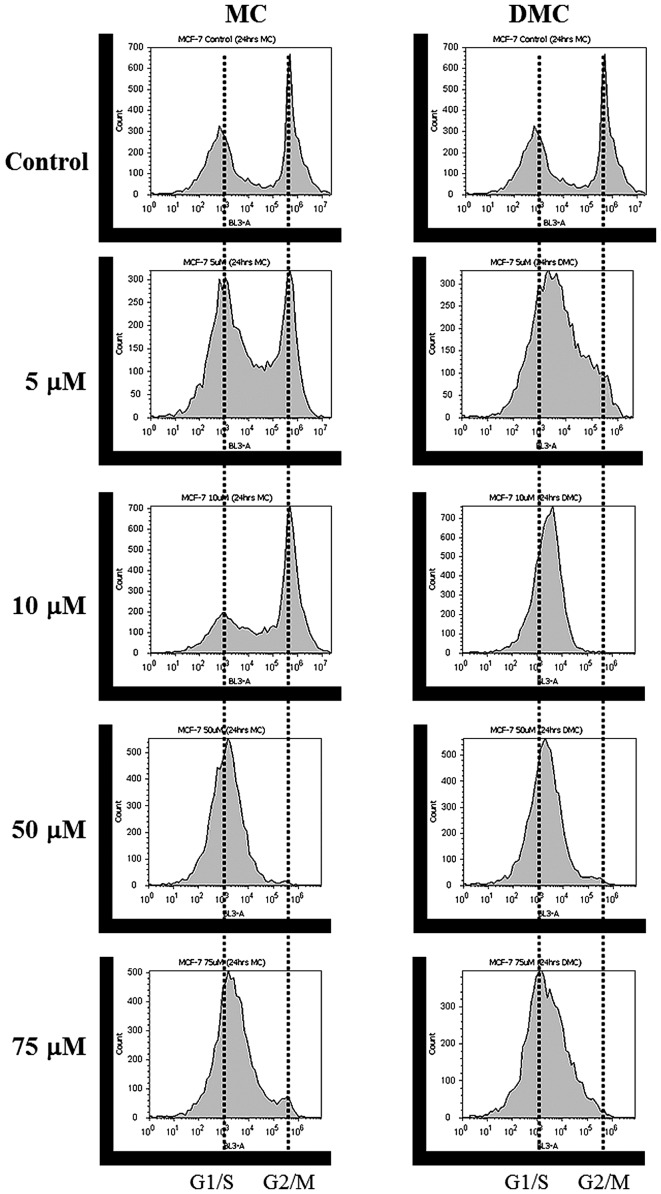
DNA content distribution profiles of MCF-7 treated with various concentrations of MC and DMC for 24 h are shown in Fig. 2 and Tables I and II. The cell cycle pattern of MCF-7 control cells appeared normal, with most cells halted at the G1/G0 phase (49.4% cell population at G1/G0 phase, 10.1% at S phase and 32.6% at G2/M phase). MC exposure induced a shift of MCF-7 cell cycle distribution pattern from G2/M to G1/S phase as MC concentration increased. When MCF-7 cells were treated with 50 μM and 75 μM of MC, >90% of cells halted at the G1/S phase (Fig. 2, left panel and Table I). These results concur with previous studies which suggest that MC shifts cell cycles to G1/S or S phase (30,31). DMC exposure also induced a shift of MCF-7 cell cycle distribution pattern from G2/M to G1/S as DMC concentration increased (Fig. 2, right panel and Table II). Furthermore, DMC induced a shift to G1/S (43.5±39.5% = 83%) at the concentration as low as 5 μM and at lower concentration range as compared to MC.
Figure 2.
Effects of MC and DMC on cell cycle of MCF-7 cells. MCF-7 cells (1.0×106 per well) were plated and treated with vehicle (cell culture media), MC and DMC for 24 h. After chemical treatments, cells were stained with 50 μg/ml propidium iodide and analyzed for cell cycle distribution patterns by flow cytometer. Cell cycle distribution patterns of MCF-7 are shifted to G1/S phase after chemical exposures.
Table I.
Cell cycle profiles of MCF-7 treated with various concentrations of mitomycin C (MC) for 24 h.
| Cell cycle phase | |||
|---|---|---|---|
|
|
|||
| Concentration of MC | G1/G0 (%) | S (%) | G2/M (%) |
| Control | 49.4 | 10.1 | 32.6 |
| 5 μM | 55.8 | 14.8 | 24.6 |
| 10 μM | 37.7 | 5.9 | 50.3 |
| 50 μM | 94.0 | 3.5 | 0 |
| 75 μM | 90.3 | 4.4 | 0 |
Table II.
Cell cycle profiles of MCF-7 treated with various concentrations of 10-decarbamoyl mitomycin C (DMC) for 24 h.
| Cell cycle phase | |||
|---|---|---|---|
|
|
|||
| Concentration of DMC | G1/G0 (%) | S (%) | G2/M (%) |
| Control | 49.4 | 10.1 | 32.6 |
| 5 μM | 43.5 | 39.5 | 8.7 |
| 10 μM | 95.7 | 1.1 | 0 |
| 50 μM | >90.0 | <10 | |
| 75 μM | >90.0 | <10 | |
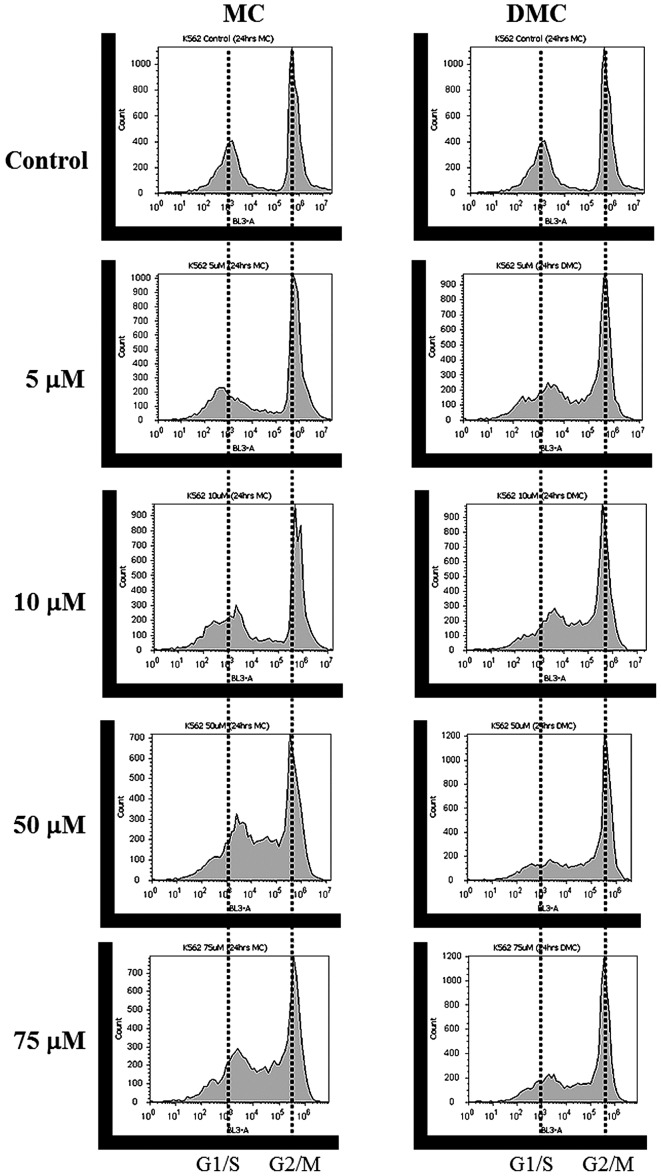
For K562 cells, DNA content distribution profiles of cells treated with various concentrations of MC and DMC for 24 h are shown in Fig. 3 and Table III and IV. Most of K562 control cells were halted at the G2/M phase (41.4% of the cell population at G1/G0 phase, 4.2% at S phase and 52.5% at G2/M phase). MC exposure increased cells halted at the S phase for cells treated with 50 μM and 100 μM MC (Fig. 3, left panel and Table III). The cell population of K562 at G2/M was also reduced at 50 and 100 μM. DMC exposure not only increased cells halted in the S phase, but also decreased cells arrested in the G1/G0 phase as DMC concentration increased (Fig. 3, right panel and Table IV). DMC exposure increased cells halted at the S phase at as low as 5 μM and at lower concentration range as compared to MC.
Figure 3.
Effects of MC and DMC on cell cycle of K562 cells. K562 cells (1.0×106 per well) were plated and treated with vehicle (cell culture media), MC and DMC for 24 h. After chemical treatments, cells were stained with 50 μg/ml propidium iodide and analyzed for cell cycle distribution patterns by flow cytomer. Cell cycle distribution patterns of K562 cells are shifted to S phase after chemical exposures.
Table III.
Cell cycle profiles of K562 treated with various concentrations of mitomycin C (MC) for 24 h.
| Cell cycle phase | |||
|---|---|---|---|
|
|
|||
| Concentration of MC | G1/G0 (%) | S (%) | G2/M (%) |
| Control | 41.4 | 4.2 | 52.5 |
| 5 μM | 33.9 | 6.0 | 53.1 |
| 10 μM | 47.2 | 3.8 | 46.1 |
| 50 μM | 40.5 | 13.1 | 43.5 |
| 75 μM | 42.8 | 18.8 | 35.2 |
Table IV.
Cell cycle profiles of K562 treated with various concentrations of 10-decarbamoyl mitomycin C (DMC) for 24 h.
| Cell cycle phase | |||
|---|---|---|---|
|
|
|||
| Concentration of DMC | G1/G0 (%) | S (%) | G2/M (%) |
| Control | 41.4 | 4.2 | 52.5 |
| 5 μM | 40.8 | 9.7 | 47.0 |
| 10 μM | 34.8 | 12.5 | 50.2 |
| 50 μM | 30.6 | 10.0 | 57.8 |
| 75 μM | 33.9 | 17.5 | 48.1 |
p21WAF1/CIP1 in MCF-7 and K562 cells is activated by MC and DMC
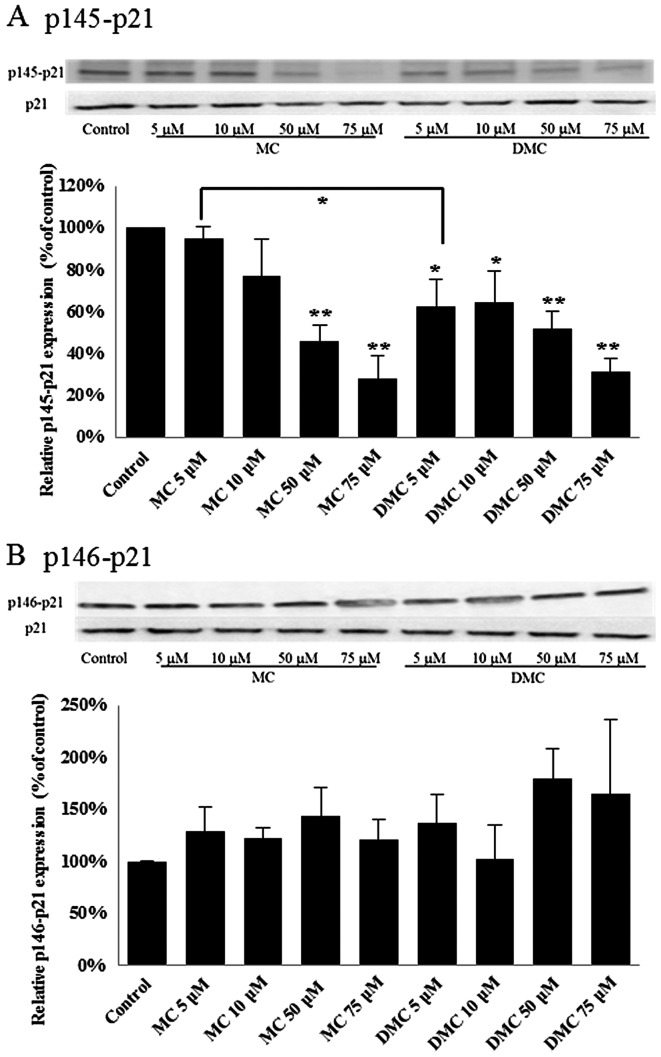
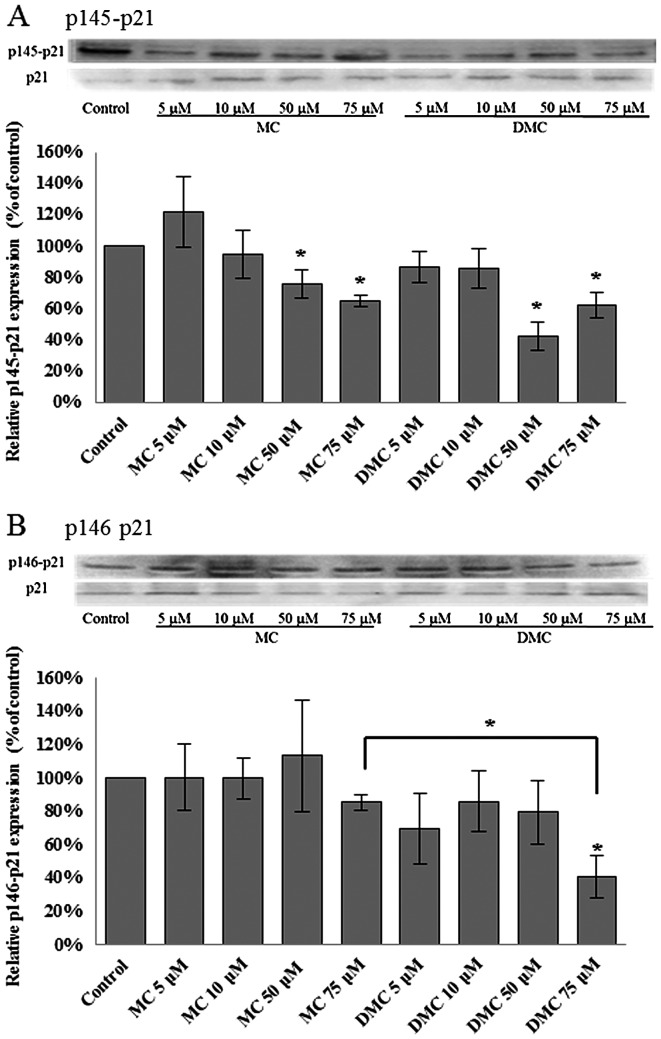
p21WAF1/CIP1 has been linked with irreversible cell cycle arrest in both G1 and G2/M. Phosphorylation of p21WAF1/CIP1 at Thr145 (p145-p21) induces its cytoplasmic accumulation to promote cell proliferation (32). In addition, phosphorylation p21WAF1/CIP1 at Ser146 (p146-p21) leads to the stability of p21WAF1/CIP1 and cell survival (33). Dephosphorylation of p21WAF1/CIP1 will increase the translocation of active p21WAF1/CIP1 to nuclei and subsequently trigger gene expression for cell proliferation suppression. In order to study the p21WAF1/CIP1 activation, the levels of phosphorylated p21WAF1/CIP1 at Thr145 and Ser146 were detected for cells treated with MC and DMC by western blot analysis.
After 24 h of chemical exposure, MC significantly dephosphorylated the p21WAF1/CIP1 at Thr145 (p145-p21) in 50 μM and 75 μM treated MCF-7 cells (Fig. 4A). DMC significantly dephosphorylated p21WAF1/CIP1 at Thr145 (p145-p21) in MCF-7 cells exposed with the dosage as low as 5 μM (Fig. 4A). DMC at 5 μM reduced more p145-p21 in MCF-7 cells as compared with cells treated with 5 μM MC. However, there was no significant statistical change on the level of phosphorylation of p21WAF1/CIP1 at Ser146 (p146-p21) in MCF-7 cells treated with MC and DMC for 24 h (Fig. 4B).
Figure 4.
Effects of MC and DMC on p21WAF1/CIP1 activation in MCF-7 cells. The relative levels of phosphorylated p21WAF1/CIP1 at Thr145 (p145-p21) and at Ser146 (p146-p21) in MCF-7 cells treated with MC and DMC for 24 h were determined by western blot analysis. p21WAF1/CIP1 expression was also measured as the loading control. The integrated density values (IDV) of western blot images were normalized with the IDV of the corresponding p21WAF1/CIP1 images. The final data for all chemically treated groups were expressed as a percentage of the control data. The data of control samples were taken as 100%. (A) p145-p21, *p<0.05. **p<0.01; (B) p146-p21, there was no statistical difference between the groups.
For K562 cells, MC and DMC significantly reduced the level of phosphorylated p21WAF1/CIP1 at Thr145 (p145-p21) in K562 cells treated with 50 μM and 75 μM of chemicals for 24 h (Fig. 5A). The level of phosphorylated p21WAF1/CIP1 at Ser146 (p146-p21) was only significantly decreased when K562 cells were treated with 75 μM of DMC for 24 h (Fig. 5B). DMC at 75 μM reduced more p146-p21 in K562 cells as compared with cells treated with 75 μM MC.
Figure 5.
Effects of MC and DMC on p21WAF1/CIP1 activation in K562 cells. The relative levels of phosphorylated p21WAF1/CIP1 at Thr145 (p145-p21) and at Ser146 (p146-p21) in K562 cells treated with MC and DMC for 24 h were determined by western blot analysis. p21WAF1/CIP1 expression was also measured as the loading control. The integrated density values (IDV) of western blot images were normalized with the IDV of the corresponding p21WAF1/CIP1 images. The final data for all chemically treated groups were expressed as a percentage of the control data. The data of control samples were taken as 100%. (A) p145-p21; (B) p146-p21. *p<0.05.
Activation of p21WAF1/CIP1 by MC and DMC in MCF-7 is p53-independent
p21WAF1/CIP1 expression can be regulated p53-dependently and -independently. In order to investigate the role of p53 in MC and DMC induced p21WAF1/CIP1 activation, p53 shRNA plasmid was transfected into MCF-7 cells to knock down p53 expression. p53 expression was reduced by 73.6% after 24 h of p53 shRNA knockdown (Fig. 6A). After 24 h of p53 shRNA knockdown, cells were treated with 75 μM of MC and DMC for 24 h. The level of phosphorylated p21WAF1/CIP1 at Thr145 (p145-p21) in p53 knockdown cells (no chemical exposure) was the same as one in normal MCF-7 cells (no chemical exposure). The levels of p145-p21 in p53 knockdown cells treated with MC and DMC for 24 h were the same as the one in normal MCF-7 cells treated under the same condition, with ~70% reduction as compared with control cells (Fig. 6B).
Figure 6.
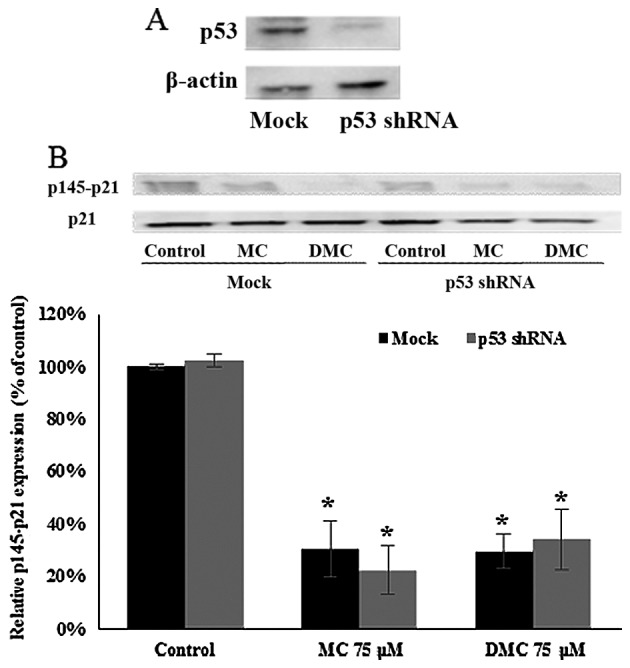
Activation of p21WAF1/CIP1 by MC and DMC in MCF-7 cells was p53-independent. p53 shRNA plasmid was transfected into MCF-7 cells 24 h prior MC and DMC exposures. (A) Expression level of p53 in MCF-7 cells and p53 knockdown cells were detected by western blot analysis. Representive blot is shown. (B) p53 knock-down MCF-7 cells were treated with MC and DMC for 24 h. The relative levels of phosphorylated p21WAF1/CIP1 at Thr145 (p145-p21) were determined by western blot analysis. p21WAF1/CIP1 expression was also measured as the loading control. The integrated density values (IDV) of western blot images were normalized with the IDV of the corresponding p21WAF1/CIP1 images. The final data for all chemically treated groups were expressed as a percentage of the control data. The data of control samples were taken as 100%. *p<0.01.
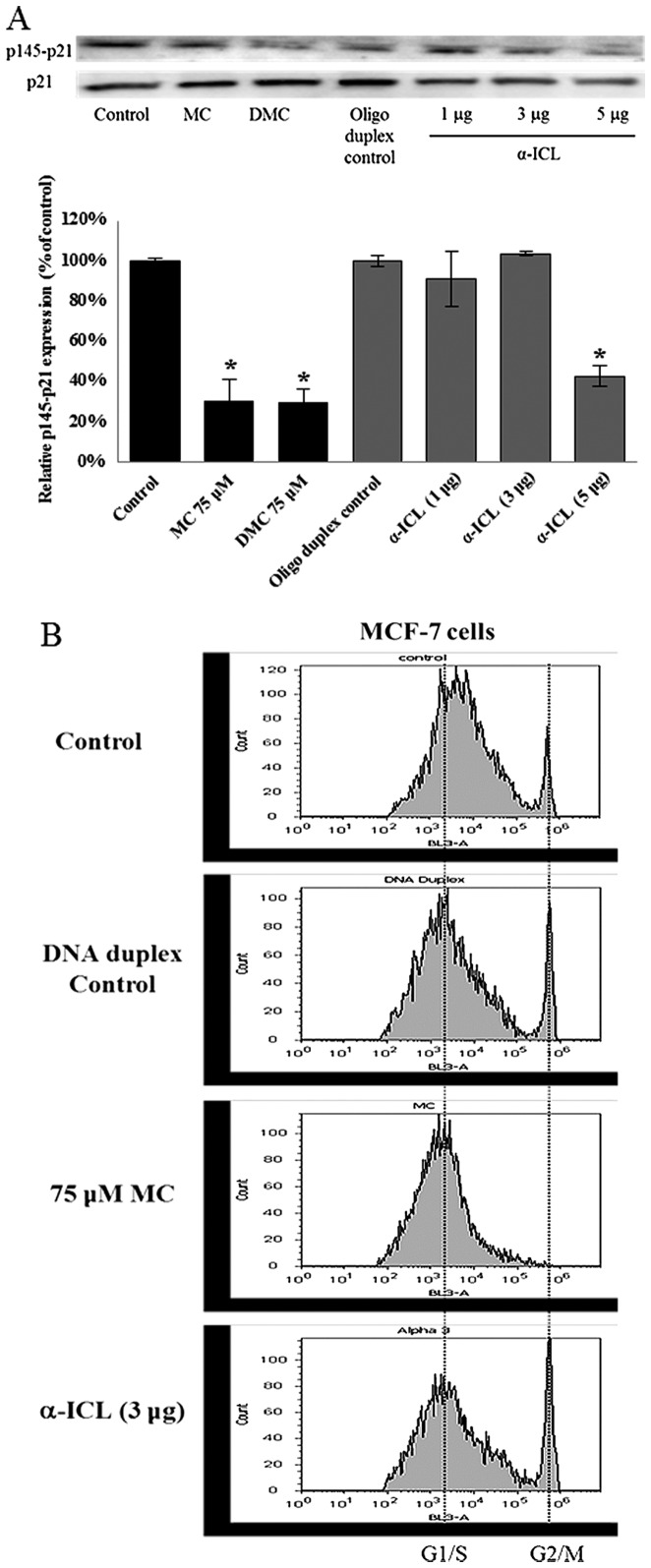
α-ICL itself can activate p21WAF1/CIP1, but not halt the cell cycle at G1/S phase in MCF-7
The α-ICL produced by MC plays a major role in MC induced cytotoxicity. In order to study whether the α-ICL itself can induce p21WAF1/CIP1 activation as MC did, MCF-7 cells were transfected with the α-ICL (1, 3 or 5 μg) and with DNA duplex (5 μg) as transfection control. Concomitantly some MCF-7 cells (no transfection) were treated with 75 μM MC or DMC. After 24-h exposure to the α-ICL, MC, and DMC, the levels of phosphorylated p21WAF1/CIP1 at Thr145 (p145-p21) were decreased by ~60% for the 5 μg α-ICL transfected group (Fig. 7A) as compared to DNA duplex control. However, no significant change in the cell cycle distribution pattern was observed between the α-ICL transfected group and the DNA duplex control group (Fig. 7B).
Figure 7.
Effects of α-ICL on activation of p21WAF1/CIP1 and cell cycle in MCF-7 cells. (A) α-interstrand cross-link (α-ICL) induced p21WAF1/CIP1 activation in MCF-7 cells after 24-h exposure. α-ICL was transfected into MCF-7 cells when cells were treated with MC and DMC for 24 h. The relative levels of phosphorylated p21WAF1/CIP1 at Thr145 (p145-p21) were determined by western blot analysis. p21WAF1/CIP1 expression was also measured as the loading control. *p<0.05. **p<0.01. (B) α-interstrand cross-link (α-ICL) did not trigger significant cell cycle distribution pattern change in MCF-7 cells after 24-h exposure. α-ICL was transfected into MCF-7 cells when cells were treated with MC and DMC for 24 h. Then cells were stained with 50 μg/ml propidium iodide and analyzed for cell cycle distribution patterns by flow cytometer.
Discussion
Mitomycin C (MC) is a commonly used chemotherapy agent. Both MC and its analog, DMC (decarbamoyl mitomycin C), cause various DNA lesions, such as α DNA interstrand cross-links (β-ICL) for MC and β DNA interstrand cross-links (β-ICL) for DMC. These ICLs are among the most toxic DNA lesions (13). However, only ~20% of cancers respond to MC treatment (4). DMC is more toxic in human cancer cells with or without functioning p53 (14,34) as compared to MC. Both MC and DMC also regulate cell cycle by different mechanisms to cause cell cycle arrest at different phases (20,34). MC at 5 μM has been known to trigger cell cycle arrest at S phase in human hepatocellular carcinoma cells, HepG2 (p53 wild-type cells) and Hep3B (p53 null cells) (20). Boamah et al (34) also demonstrated that 1 μM DMC halted cell proliferation at the S and G2/M phases and MC at 10 μM arrested cells at S phase in p53-null DLD1 colon cancer cells. The present cell cycle analysis is in agreement with those studies and showed that both MC and DMC increased the cell population at the G1/S phase in MCF-7 (p53 wild-type) cells and at the S phase in K562 (p53-deficient) cells. p53, also known as cell cycle check protein, can mediate a G1 arrest to preserve the genetic stability under DNA damage (35). p53 deficiency in K562 could possibly lead cells to lose the regulation of G1 progression. Thus, there was no significant change on G1 phase cell population observed in this study.
Abbas et al (7) and Boamah et al (34) indicated that DMC triggers stronger p53-independent cell damage. This suggests that another signaling pathway besides p53 could be responsible for DMC induced cell death. p21WAF1/CIP1 is a p53 downstream effector and inhibits the G1-S transition. However, p21WAF1/CIP1 expression can also be regulated by a p53-independent mechanism (36). p21WAF1/CIP1 has been shown to be upregulated in ML-1 p53 wild-type cells by MC and DMC (7) and in HepG2 p53 wild-type cells by MC (20). In this study, the status of p21WAF1/CIP1 activation induced by MC and DMC in MCF-7 and K562 cells was measured by the level of phosphorylated p21WAF1/CIP1. Dephosphorylation of p21WAF1/CIP1 will activate p21WAF1/CIP1 and translocate p21WAF1/CIP1 to nuclei. Our results showed that p21WAF1/CIP1 was dephosphorylated by MC and DMC in both MCF-7 and K562 cells. DMC showed stronger p21WAF1/CIP1 activation as compared to MC.
Since p21WAF1/CIP1 can be regulated by p53-dependent and -independent mechanisms (36) and p21WAF1/CIP1 was also activated in K562 (p53 deficient) cells after MC and DMC exposures, it is possible that p21WAF1/CIP1 activation by MC and DMC in MCF-7 could be regulated by a p53-independent pathway. Russo et al (17) and Esposito et al (16) suggested p21WAF1/CIP1 regulates cell cycle arrest and apoptosis in p53-independent mechanism in response to ribosome protein rpL3. In this study, p53 shRNA knockdown did lower the expression of p53 in MCF-7, but did not attenuate MC and DMC induced p21WAF1/CIP1 dephosphorylation. This indicates that activation of p21WAF1/CIP1 in MCF-7 cells by MC and DMC did not require p53. DNA lesions formed by MC and DMC have been linked to their cytotoxic mechanisms. In this study, the major DNA lesion of MC (α-ICL) was introduced into MCF-7 cells. The activation of p21WAF1/CIP1 was observed to a similar extent when compared to cells treated with MC and DMC. However, there was no significant change in cell cycle distribution pattern after the α-ICL was introduced into MCF-7 cells when compared with DNA duplex control. This indicates that the α-ICL itself is sufficient to trigger p21WAF1/CIP1 activation, but not to set the cell cycle at rest. Several reasons may explain this phenomenon. ICLs are extremely toxic to cells (37). MC generates a total of 6 different covalent DNA lesions (38,39) and other DNA adducts that are not examined in this study may contribute to MC-induced cell cycle arrest. Secondly, the transfected oligonucleotide bearing the ICL may not set the cell cycle at rest because it is not part of a replicating machinery i.e., DNA itself or part of a replicating plasmid. Moreover, another CIP/KIP family member, p27, could play a role in this regulation. p27 is also a cyclin-dependent kinase inhibitor as p21WAF1/CIP1 and has similar cellular function as p21WAF1/CIP1. Rao et al (40) demonstrated that p21WAF1/CIP1 and p27 are involved in lovastatin triggered G1 cell cycle arrest in a p53-independent manner. Therefore, it would be of interest to compare p27 expression in response to MC, DMC, and ICLs in cells with or without functioning p53.
In conclusion, mitomycin C and its derivative 10-decar-bamoyl mitomycin C triggered cell cycle arrest at G1/S phase in MCF-7 cells and at S phase in K562 cells. DMC induced the arrest at lower concentrations as compared with MC. MC and DMC both decreased the phosphorylated p21WAF1/CIP1 at Thr145 in MCF-7 and K562 cells. DMC showed stronger effect on dephosphorylation of p21WAF1/CIP1 at Thr145 in MCF-7 cells. Knocking down p53 did not change the effect of MC and DMC on p21WAF1/CIP1 activation. The major DNA lesion produced by MC, α-ICL, did activate p21WAF1/CIP1, but did not halt the cell cycle. This study demonstrated that MC and DMC activated p21WAF1/CIP1 in p53-independent manner and α-ICL can trigger the activation of p21WAF1/CIP1. In the future, p21WAF1/CIP1 and other p53-independent signaling pathways in response to the β-ICL will require further investigation.
Acknowledgements
This study was supported by NIH grant 5SC3GM105460 and the PRISM program at John Jay. Special thanks to John Jay College of Criminal Justice Start-up Fund, PRISM fund, and PSC CUNY grant. PRISM is the Program for Research Initiatives for Science Majors at John Jay College and funded by the Title V, HSI-STEM and MSEIP programs within the US Department of Education; the PAESMEM program through the National Science Foundation; and New York State's Graduate Research and Teaching Initiative.
Abbreviations
- MC
mitomycin C
- DMC
10-decarbamoyl mitomycin C
- α-ICL
α interstrand cross-link
References
- 1.Hata T, Hoshi T, Kanamori K, Matsumae A, Sano Y, Shima T, Sugawara R. Mitomycin, a new antibiotic from Streptomyces. I. J Antibiot (Tokyo) 1956;9:141–146. [PubMed] [Google Scholar]
- 2.Sartorelli AC, Hodnick WF, Belcourt MF, Tomasz M, Haffty B, Fischer JJ, Rockwell S. Mitomycin C: A prototype bioreductive agent. Oncol Res. 1994;6:501–508. [PubMed] [Google Scholar]
- 3.Verweij J, Pinedo H. Cancer Chemotherapy and Biological Response Modifiers. In: Pinedo HM, Chabner BA, Longo DL, editors. Annual 11. Vol. 67. Elsevier Science Publishers B.V; Amsterdam: 1990. [Google Scholar]
- 4.Bradner WT. Mitomycin C: A clinical update. Cancer Treat Rev. 2001;27:35–50. doi: 10.1053/ctrv.2000.0202. [DOI] [PubMed] [Google Scholar]
- 5.Borowy-Borowski H, Lipman R, Tomasz M. Recognition between mitomycin C and specific DNA sequences for cross-link formation. Biochemistry. 1990;29:2999–3006. doi: 10.1021/bi00464a016. [DOI] [PubMed] [Google Scholar]
- 6.Boamah EK, White DE, Talbott KE, Arva NC, Berman D, Tomasz M, Bargonetti J. Mitomycin-DNA adducts induce p53-dependent and p53-independent cell death pathways. ACS Chem Biol. 2007;2:399–407. doi: 10.1021/cb700060t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abbas T, Olivier M, Lopez J, Houser S, Xiao G, Kumar GS, Tomasz M, Bargonetti J. Differential activation of p53 by the various adducts of mitomycin C. J Biol Chem. 2002;277:40513–40519. doi: 10.1074/jbc.M205495200. [DOI] [PubMed] [Google Scholar]
- 8.Ben-Yehoyada M, Wang LC, Kozekov ID, Rizzo CJ, Gottesman ME, Gautier J. Checkpoint signaling from a single DNA interstrand crosslink. Mol Cell. 2009;35:704–715. doi: 10.1016/j.molcel.2009.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Räschle M, Knipscheer P, Enoiu M, Angelov T, Sun J, Griffith JD, Ellenberger TE, Schärer OD, Walter JC. Mechanism of replication-coupled DNA interstrand crosslink repair. Cell. 2008;134:969–980. doi: 10.1016/j.cell.2008.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weng MW, Zheng Y, Jasti VP, Champeil E, Tomasz M, Wang Y, Basu AK, Tang MS. Repair of mitomycin C mono- and inter-strand cross-linked DNA adducts by UvrABC: A new model. Nucleic Acids Res. 2010;38:6976–6984. doi: 10.1093/nar/gkq576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shinohara K, Bando T, Sasaki S, Sakakibara Y, Minoshima M, Sugiyama H. Antitumor activity of sequence-specific alkylating agents: Pyrolle-imidazole CBI conjugates with indole linker. Cancer Sci. 2006;97:219–225. doi: 10.1111/j.1349-7006.2006.00158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Palom Y, Suresh Kumar G, Tang LQ, Paz MM, Musser SM, Rockwell S, Tomasz M. Relative toxicities of DNA cross-links and monoadducts: New insights from studies of decarbamoyl mitomycin C and mitomycin C. Chem Res Toxicol. 2002;15:1398–1406. doi: 10.1021/tx020044g. [DOI] [PubMed] [Google Scholar]
- 13.Kaspárková J, Brabec V. Recognition of DNA interstrand cross-links of cis-diamminedichloroplatinum(II) and its trans isomer by DNA-binding proteins. Biochemistry. 1995;34:12379–12387. doi: 10.1021/bi00038a035. [DOI] [PubMed] [Google Scholar]
- 14.Patrick SM, Tillison K, Horn JM. Recognition of cisplatin-DNA interstrand cross-links by replication protein A. Biochemistry. 2008;47:10188–10196. doi: 10.1021/bi800460d. [DOI] [PubMed] [Google Scholar]
- 15.Xiao G, Kue P, Bhosle R, Bargonetti J. Decarbamoyl mitomycin C (DMC) activates p53-independent ataxia telangiectasia and rad3 related protein (ATR) chromatin eviction. Cell Cycle. 2015;14:744–754. doi: 10.1080/15384101.2014.997517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Esposito D, Crescenzi E, Sagar V, Loreni F, Russo A, Russo G. Human rpL3 plays a crucial role in cell response to nucleolar stress induced by 5-FU and L-OHP. Oncotarget. 2014;5:11737–11751. doi: 10.18632/oncotarget.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Russo A, Esposito D, Catillo M, Pietropaolo C, Crescenzi E, Russo G. Human rpL3 induces G1/S arrest or apoptosis by modulating p21 (waf1/cip1) levels in a p53-independent manner. Cell Cycle. 2013;12:76–87. doi: 10.4161/cc.22963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Resnitzky D, Gossen M, Bujard H, Reed SI. Acceleration of the G1/S phase transition by expression of cyclins D1 and E with an inducible system. Mol Cell Biol. 1994;14:1669–1679. doi: 10.1128/MCB.14.3.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He G, Kuang J, Huang Z, Koomen J, Kobayashi R, Khokhar AR, Siddik ZH. Upregulation of p27 and its inhibition of CDK2/cyclin E activity following DNA damage by a novel platinum agent are dependent on the expression of p21. Br J Cancer. 2006;95:1514–1524. doi: 10.1038/sj.bjc.6603448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choi SY, Shen YN, Woo SR, Yun M, Park JE, Ju YJ, Jeong J, Shin HJ, Joo HY, Park ER, et al. Mitomycin C and doxorubicin elicit conflicting signals by causing accumulation of cyclin E prior to p21WAF1/CIP1 elevation in human hepatocellular carcinoma cells. Int J Oncol. 2012;40:277–286. doi: 10.3892/ijo.2011.1184. [DOI] [PubMed] [Google Scholar]
- 21.Willers H, Dahm-Daphi J, Powell SN. Repair of radiation damage to DNA. Br J Cancer. 2004;90:1297–1301. doi: 10.1038/sj.bjc.6601729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harris SL, Levine AJ. The p53 pathway: Positive and negative feedback loops. Oncogene. 2005;24:2899–2908. doi: 10.1038/sj.onc.1208615. [DOI] [PubMed] [Google Scholar]
- 23.Petitjean A, Achatz MI, Borresen-Dale AL, Hainaut P, Olivier M. TP53 mutations in human cancers: Functional selection and impact on cancer prognosis and outcomes. Oncogene. 2007;26:2157–2165. doi: 10.1038/sj.onc.1210302. [DOI] [PubMed] [Google Scholar]
- 24.Faulhaber O, Bristow RG. Basis of cell kill following clinical radiotherapy. In: Sluyser M, editor. Application of Apoptosis to Cancer Treatment. Springer; Amsterdam: 2005. pp. 293–320. [DOI] [Google Scholar]
- 25.Fang L, Igarashi M, Leung J, Sugrue MM, Lee SW, Aaronson SA. p21Waf1/Cip1/Sdi1 induces permanent growth arrest with markers of replicative senescence in human tumor cells lacking functional p53. Oncogene. 1999;18:2789–2797. doi: 10.1038/sj.onc.1202615. [DOI] [PubMed] [Google Scholar]
- 26.Sugiyama K, Shimizu M, Akiyama T, Tamaoki T, Yamaguchi K, Takahashi R, Eastman A, Akinaga S. UCN-01 selectively enhances mitomycin C cytotoxicity in p53 defective cells which is mediated through S and/or G(2) checkpoint abrogation. Int J Cancer. 2000;85:703–709. doi: 10.1002/(SICI)1097-0215(20000301)85:5<703::AID-IJC17>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 27.Law JC, Ritke MK, Yalowich JC, Leder GH, Ferrell RE. Mutational inactivation of the p53 gene in the human erythroid leukemic K562 cell line. Leuk Res. 1993;17:1045–1050. doi: 10.1016/0145-2126(93)90161-D. [DOI] [PubMed] [Google Scholar]
- 28.Kinoshita S, Uzu K, Nakano K, Takahashi T. Mitomycin derivatives. 2. Derivatives of decarbamoylmitosane and decar-bamoylmitosene. J Med Chem. 1971;14:109–112. doi: 10.1021/jm00284a006. [DOI] [PubMed] [Google Scholar]
- 29.Summer H, Grämer R, Dröge P. Denaturing urea polyacrylamide gel electrophoresis (Urea PAGE) J Vis Exp. 2009;32:e1485. doi: 10.3791/1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou QM, Wang XF, Liu XJ, Zhang H, Lu YY, Su SB. Curcumin enhanced antiproliferative effect of mitomycin C in human breast cancer MCF-7 cells in vitro and in vivo. Acta Pharmacol Sin. 2011;32:1402–1410. doi: 10.1038/aps.2011.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu J, Zhao L, Li Y, Li N, He M, Bai X, Yu Z, Zheng Z, Mi X, Wang E, et al. Silencing of Fanconi anemia complementation group F exhibits potent chemosensitization of mitomycin C activity in breast cancer cells. J Breast Cancer. 2013;16:291–299. doi: 10.4048/jbc.2013.16.3.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rössig L, Jadidi AS, Urbich C, Badorff C, Zeiher AM, Dimmeler S. Akt-dependent phosphorylation of p21(Cip1) regulates PCNA binding and proliferation of endothelial cells. Mol Cell Biol. 2001;21:5644–5657. doi: 10.1128/MCB.21.16.5644-5657.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li Y, Dowbenko D, Lasky LA. AKT/PKB phosphorylation of p21Cip/WAF1 enhances protein stability of p21Cip/WAF1 and promotes cell survival. J Biol Chem. 2002;277:11352–11361. doi: 10.1074/jbc.M109062200. [DOI] [PubMed] [Google Scholar]
- 34.Boamah EK, Brekman A, Tomasz M, Myeku N, Figueiredo-Pereira M, Hunter S, Meyer J, Bhosle RC, Bargonetti J. DNA adducts of decarbamoyl mitomycin C efficiently kill cells without wild-type p53 resulting from proteasome-mediated degradation of checkpoint protein 1. Chem Res Toxicol. 2010;23:1151–1162. doi: 10.1021/tx900420k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Di Leonardo A, Linke SP, Clarkin K, Wahl GM. DNA damage triggers a prolonged p53-dependent G1 arrest and long-term induction of Cip1 in normal human fibroblasts. Genes Dev. 1994;8:2540–2551. doi: 10.1101/gad.8.21.2540. [DOI] [PubMed] [Google Scholar]
- 36.Macleod KF, Sherry N, Hannon G, Beach D, Tokino T, Kinzler K, Vogelstein B, Jacks T. p53-dependent and independent expression of p21 during cell growth, differentiation, and DNA damage. Genes Dev. 1995;9:935–944. doi: 10.1101/gad.9.8.935. [DOI] [PubMed] [Google Scholar]
- 37.Lawley PD, Phillips DH. DNA adducts from chemotherapeutic agents. Mutat Res. 1996;355:13–40. doi: 10.1016/0027-5107(96)00020-6. [DOI] [PubMed] [Google Scholar]
- 38.Bargonetti J, Champeil E, Tomasz M. Differential toxicity of DNA adducts of mitomycin C. J Nucleic Acids. 2010;2010:698960. doi: 10.4061/2010/698960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tomasz M. Mitomycin C: Small, fast and deadly (but very selective) Chem Biol. 1995;2:575–579. doi: 10.1016/1074-5521(95)90120-5. [DOI] [PubMed] [Google Scholar]
- 40.Rao S, Lowe M, Herliczek TW, Keyomarsi K. Lovastatin mediated G1 arrest in normal and tumor breast cells is through inhibition of CDK2 activity and redistribution of p21 and p27, independent of p53. Oncogene. 1998;17:2393–2402. doi: 10.1038/sj.onc.1202322. [DOI] [PubMed] [Google Scholar]