Abstract
Background
The purpose of this study was to determine the effect of mild hypothermia therapy on gastric mucosa after cardiopulmonary resuscitation (CPR) and the underlying mechanism.
Material/Methods
Ventricular fibrillation was induced in pigs. After CPR, the surviving pigs were divided into mild hypothermia-treated and control groups. The changes in vital signs and hemodynamic parameters were monitored before cardiac arrest and at intervals of 0.5, 1, 2, 4, 6, 12, and 24 h after restoration of spontaneous circulation. Serum IL-6 was determined at the same time, and gastroscopy was performed. The pathologic changes were noted, and the expression of IL-6 was determined by hematoxylin and eosin (HE) staining and immunohistochemistry under light.
Results
The heart rate, mean arterial blood pressure, and cardiac output in both groups did not differ significantly. The gastric mucosa ulcer index evaluated by gastroscopy 2 h and 24 h after restoration of spontaneous circulation (ROSC) in the mild hypothermic group was lower than that the control group (P<0.05). The inflammatory pathologic score of gastric mucosa in the mild hypothermic group 6–24 h after ROSC was lower than that in the control group (P<0.05). Serum and gastric mucosa IL-6 expression 0.5–4 h and 6, 12, and 24 h after ROSC was lower in the mild hypothermic group than in the control group (P<0.05).
Conclusions
Mild hypothermia treatment protects gastric mucosa after ROSC via inhibiting IL-6 production and relieving the inflammatory reaction.
MeSH Keywords: Cardiopulmonary Resuscitation, Reperfusion Injury, Stomach Diseases
Background
With the development and popularization of cardiopulmonary resuscitation (CPR), the success rate of recovery has been significantly improved; however, the rate of survival to hospital discharge has only improved 15~20% [1,2]. The main reason for high mortality can be attributed to the complicated systemic ischemia-reperfusion reaction after restoration of spontaneous circulation (ROSC), which includes hypoxic brain injury, myocardial dysfunction, and multiple organ dysfunction syndrome (MODS) [3]. Moreover, MODS reduces the long-term survival rate. Clinical studies have investigated different methods by which to improve the long-term survival rate following CPR, with a particular focus on the protection of cerebral and cardiac function. Indeed, it has shown that mild hypothermia treatment ameliorates cerebral and cardiac injuries [4]. Few studies, however, have investigated the influence of mild hypothermia treatment on gastrointestinal injury after CPR [5]. Thus, we designed the current study to investigate gastrointestinal injury after CPR and determined whether or not hypothermia treatment attenuates injury induced by CPR.
Material and Methods
Animal preparation
Thirty-three male Wuzhishan miniature pigs purchased from The Animal Husbandry Institute of the Chinese Academy of Agricultural Sciences were used for the experiments (8–10 weeks old, 28±2 kg). The pigs were isolated from water for 12 h before the experiments.
Experimental protocols
Anesthesia was induced by the intramuscular injection of ketamine, which was maintained with continuous marginal infusion of 3% pentobarbital sodium. A physiologic monitor was used to document the vital signs. All pigs were intubated and given respiratory support via mechanical ventilation (VC+SIMV+PSV mode; tidal volume, 15 mL/kg; respiratory frequency, 12 per minute with room air) to maintain an end-tidal carbon dioxide pressure of 35–40 mm Hg. A blood pressure monitor catheter was inserted into the right femoral artery to monitor blood pressure. A Swan-Ganz catheter was inserted into the left femoral artery and advanced to the pulmonary artery to monitor hemodynamics. A temporary pacemaker was inserted into the right external jugular vein and advanced to the right ventricle to induce ventricular fibrillation. A urethral catheter with a temperature monitor was inserted into the bladder to monitor the body core temperature and urine volume.
With stable vital signs, a computer-controlled programmable stimulator connected to the external end of a temporary pacemaker was used in the S1S2 mode (300/200 ms) at ratio of 8: 1 with a –10 ms pace length until ventricular fibrillation (VF) was established, and respiratory support was paused. VF was demonstrated by electrocardiograph and arterial blood pressure depression. After 8 min of VF [6], standard CPR [4] was initiated immediately for 1 cycle with continuing respiratory support using pure oxygen for 2 min at a frequency of 100 compressions per min. If VF existed after 1 cycle of CPR, defibrillation with 150 J of diphase wave was applied and another cycle of CRP followed. Defibrillation and CPR were repeated unless ROSC was demonstrated or this procedure was performed for 15 min and the pig was declared to be dead. ROSC was defined as a systolic blood pressure >50 mm Hg, lasting for >5 min.
Pigs that achieved ROSC were randomly divided into two groups (mild hypothermia-treated and control groups). A heat exchange catheter was immediately inserted into pigs after ROSC in the mild hypothermia-treated group; they then received endovascular treatment with mild hypothermia. The cooling technology, involving a heat exchange catheter connected to a COOL GARD 3000 system, was inserted into the inferior vena cava and regulated the core body temperature using recyclable brine. The core body temperature was lowered to 33°C as soon as possible and maintained for 12 h, then slowly rewarmed to 36.5°C at a rewarming rate of 0.25°C/h. Both groups received saline and other treatment to maintain life. All of the pigs were sacrificed 24 h post-resuscitation with an overdose of pentobarbital.
Measurements
(1) Hemodynamic parameters, including core body temperature (T), heart rate (HR), mean arterial pressure (MAP), and cardiac output (CO), were observed and recorded at baseline (before cardiac arrest) and then 0.5, 1, 2, 4, 6, 12, and 24 h post-ROSC. (2) Changes in serum IL-6 were measured by ELISA. (3) The degree of gastric mucosa injury was observed by gastroscopy, and the gastric mucosa damage index was evaluated using the Guth method [7]. The biopsy specimens from the gastric antrum were stained with hematoxylin and eosin (HE), and then the pathologic integrity of the gastric mucosa was calculated based on neutrophil submersion [8]. (4) The biopsy specimens were also subjected to immunohistochemical analysis of IL-6 expression; positive expression of IL-6 was indicated by brown-yellow particles in the cytoplasm or cytoblasts. Five non-overlapping views were randomly selected to analyze the mean optical density using Image-Pro Plus V6.0 software (Media Cybernetics, Inc,). The mean optical density of the same-size views represented the expression of IL-6 [9].
Statistical analysis
Statistical analysis was performed with SPSS 19.0 software (SPSS, Inc., Chicago, Illinois, USA). Measurement data are shown as the mean ± standard deviation (SD). Data between groups were analyzed using Student’s t-test, and data within groups were analyzed using repeated-measure analysis of variance (ANOVA). Values of P<0.05 were considered statistically significant.
Results
Of the 33 pigs, 30 were resuscitated successfully and randomly divided into two groups (mild hypothermia-treated and control groups). Eleven pigs died within 24 h after ROSC in the 2 groups; 19 pigs survived (11 pigs in the mild hypothermia-treated group and 8 pigs in the control group). There was no significant difference in the weights of the pigs between the two groups (P>0.05); therefore, the data between the two groups were comparable.
Hemodynamic parameters
At baseline before VF was induced, the HR, MAP, CO, and T did not differ significantly between the two groups (P>0.05). Pigs in the mild hypothermia-treated group had a lower body temperature from use of an intravascular cryogenic technique and were kept at 33°C. Pigs receiving mild hypothermia treatment had no significant differences in HR (P=0.124), MAP (P=0.079), and CO (P=0.390) compared to the control group after ROSC (P>0.05).
Serum IL-6
Before VF was induced, the serum IL-6 level was not different between the two groups. After ROSC, the serum IL-6 level in both groups increased and reached a peak at 4 h, then gradually decreased to the baseline level. Between 0.5 and 4 h, the serum IL-6 level in the mild hypothermia-treated group was significantly lower than that in the control group (P<0.05; Table 1).
Table 1.
Serum IL-6 level in the mild hypothermia-treated and control groups (χ̄±SD).
| Group | Mild hypothermia (pg/ml) | Control (pg/ml) | P value |
|---|---|---|---|
| Baseline | 9.064±0.724 | 9.995±1.263 | 0.090 |
| 0.5 h | 10.589±0.936 | 12.031±1.383 | 0.015* |
| 1 h | 13.008±2.401 | 15.459±2.453 | 0.044* |
| 2 h | 16.545±1.875 | 20.309±1.089 | 0.000** |
| 4 h | 23.771±1.405 | 26.002±1.229 | 0.002** |
| 6 h | 18.641±3.040 | 21.012±4.106 | 0.165 |
| 12 h | 12.466±0.995 | 12.609±1.015 | 0.763 |
| 24 h | 10.012±1.117 | 10.159±0.671 | 0.726 |
Compared with the control group,
P<0.05;
P<0.01.
Gastroscopy and HE staining
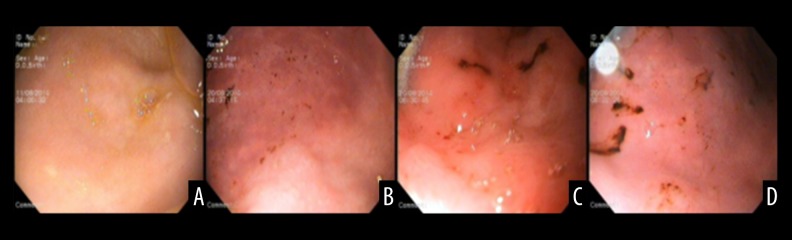
At baseline before VF, the degree of gastric mucosa injury observed by gastroscopy was similar in the two groups (P>0.05). Various degrees of gastric mucosa damage after ROSC occurred. After ROSC, the gastric mucosa gradually appeared as a large pale ischemic region with strip hyperemia, local erosions, necrosis with superficial ulceration, and white patches. The degree of gastric mucosa injury was quantified using the Guth method. The damage index of the gastric mucosa increased in both groups, but was lower in the mild hypothermia-treated group within 24 h after ROSC (P<0.05; Figure 1).
Figure 1.
Gastric mucosa damage observed by gastroscopy. (A) Normal gastric mucosa before VF. (B) Petechial hemorrhages. (C) Long strips of ulcer hemorrhages. (D) Patchy hemorrhage.
Histopathology
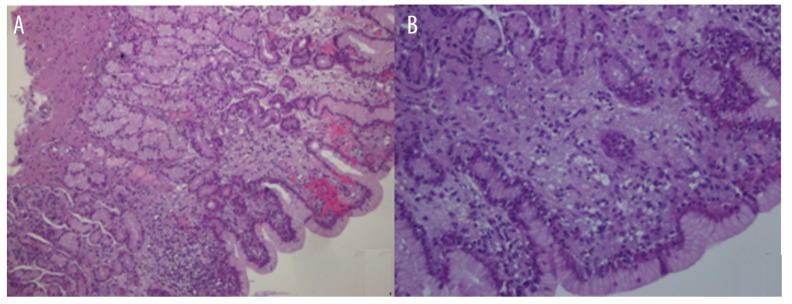
Slices of gastric tissue samples were observed under light microscopy. There was no significant difference in the pathologic integrals of the gastric mucosa prior to induction of VF. After ROSC, gastric mucosal injury was noted, including dropsical glands, capillary hyperemia, inflammatory exudate, mucosal hemorrhage, necrosis, and a significant inflammatory cellular infiltration, which was mainly composed of neutrophils. The pathologic integrals of the gastric mucosa in both groups increased continuously within 24 h after ROSC, but increased more slowly in the mild hypothermia-treated group between 6 and 24 h (all P<0.05). Therefore, mild hypothermia treatment alleviated the gastric mucosa inflammation (Table 2, Figure 2).
Table 2.
Gastric mucosa damage in the mild hypothermia-treated and control groups (χ̄±SD).
| Group | Damage index of gastric mucosa | Pathologic integrals of gastric mucosa | ||||
|---|---|---|---|---|---|---|
| Mild hypothermia | Control | P value | Mild hypothermia | Control | P value | |
| Baseline | 0.0±0.0 | 0.0±0.0 | – | 0.4±0.5 | 0.4±0.5 | 0.962 |
| 2 h | 4.5±0.8 | 6.6±2.1 | 0.024* | 5.7±0.8 | 6.1±0.8 | 0.303 |
| 6 h | 12.2±4.2 | 16.0±2.3 | 0.034* | 7.6±1.0 | 9.0±0.8 | 0.006** |
| 12 h | 20.9±4.4 | 28.4±6.1 | 0.006* | 10.5±0.8 | 12.6±1.3 | 0.001** |
| 24 h | 28.5±5.1 | 35.0±5.3 | 0.014* | 13.0±1.2 | 14.3±0.9 | 0.022* |
Compared with the control group,
P<0.05;
P<0.01.
Figure 2.
Pathologic changes of the gastric mucosa observed by light microscopy. (A) Original magnification ×100. (B) Original magnification ×400. Gastric mucosal glands with edema, hemorrhage, necrosis, and numerous infiltrating inflammatory cells were present.
Gastric mucosal IL-6
Before VF was induced, the gastric mucosal IL-6 level did not differ between the two groups, but increased after ROSC and peaked at 12 h in both groups. Between 6 and 24 h, the gastric mucosal IL-6 level in the mild hypothermia-treated group was significantly lower than that in the control group (all P<0.05; Table 3, Figure 3).
Table 3.
Gastric mucosal IL-6 in the mild hypothermia-treated and control groups (χ̄±SD).
| Group | Mild hypothermia | Control | P value |
|---|---|---|---|
| Baseline | 0.0715±0.0093 | 0.0780±0.0088 | 0.142 |
| 2 h | 0.1079±0.0129 | 0.1175±0.0095 | 0.094 |
| 6 h | 0.1449±0.0173 | 0.1697±0.0138 | 0.004* |
| 12 h | 0.2204±0.0218 | 0.2652±0.0178 | 0.000** |
| 24 h | 0.1853±0.0155 | 0.2005±0.0146 | 0.046* |
Compared with the control group,
P<0.05;
P<0.01.
Figure 3.
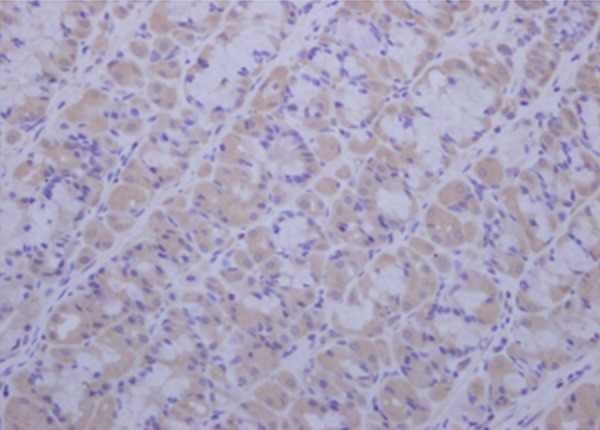
The expression of gastric mucosa IL-6 detected by immunohistochemistry, observed by light microscopy. Original magnification ×400.
Discussion
Many animal experiments and clinical trials have shown that the high mortality rate after ROSC is associated with an ischemia-reperfusion reaction [3]. There are a number of methods for the detection of cardiac and brain function in animals; however, there is a lack of methods for determining gastrointestinal function. Many physiologic characteristics of pigs are similar to humans with respect to the circulatory system, gastrointestinal system, and metabolism [10]. Thus, pigs were chosen as the experimental animal to approximate the physiologic and pathologic findings in humans after ROSC. VF is the most common consequence of cardiac arrest and accurately reflects the time of the cardiac arrest [11]. Eight minutes without intervention after a cardiac arrest is the length of time patients can endure without CPR [6].
We observed the same experimental animal at different time points by gastroscopy, and the dynamic changes in the gastric mucosa were assessed by biopsy.
Our results demonstrated that the survival rate was higher in the mild hypothermia-treated group than in the control group. Indeed, mild hypothermia treatment reduces the mortality following cardiac arrest, as reported in animal and clinical studies [12,13]. Thus, mild hypothermia treatment is recommended by the American Heart Association guidelines [4].
In the current experiments, with the exception of temperature as an intervention factor, HR, MAP, and CO did not differ significantly between the two groups. Thus, lowering the body temperature has no influence on vital signs and hemodynamic parameters, as reported by Yang et al. [14] and Zhao et al. [15] in similar experiments.
After ROSC, the gastric mucosa was damaged in both groups and the damage index of gastric mucosa increased, but the damage index in the mild hypothermia-treated group was lower than that in the control group. Therefore, mild hypothermia treatment could alleviate the extent of damage of the gastric mucosa. Gastric mucosal glands were injured, including hemorrhage, necrosis, and an inflammatory cellular infiltration. The pathologic integrals of the gastric mucosa increased in both groups, but were lower in the mild hypothermia-treated group. The inflammatory reaction of the gastric mucosa in the mild hypothermia-treated group was superior to that in the control group. Based on the morphologic and histopathologic changes of the gastric mucosa, mild hypothermia treatment protects the gastric mucosa after ROSC.
IL-6 is a multi-functional cytokine secreted by various cells, including activated T cells, B cells, monocytes, macrophages, and vascular endothelial cells. IL-6 participates in the inflammatory reaction and immune response, and plays a key role in the process of the inflammatory reaction. When organs undergo ischemia-reperfusion, vascular endothelial cells promote monocytes and macrophages to synthesize and secrete IL-6, which plays an important role in inducing the immunologic cascade [16,17]. With the increased concentration of IL-6, injury of the blood vessel endothelium is aggravated, which increases vascular permeability and induces thrombosis. Furthermore, IL-6 accelerates injury by activating injured vascular endothelial and inflammatory cells to release tumor necrosis factor-α, resulting in a cessation of perfusion in capillary vessels with an inflammatory cell attachment [18]. Therefore, IL-6 can be used as an indicator of the degree of the inflammatory reaction.
In this experiment, we detected the change in IL-6 in serum and gastric mucosa; both levels increased dramatically in a short time. The peak level of gastric mucosa IL-6 at 6 h was later than that of the serum IL-6 at 4 h, likely reflecting gastric mucosal persistent ischemia after ROSC.
In stressor shock conditions, the systemic blood circulation will adjust to be redistributed to ensure the perfusion of vital organs, such as the brain and heart; however, perfusion of the gastrointestinal tract decreases significantly [19]. The result of a lower level of IL-6 in serum and gastric mucosa in the mild hypothermia-treated group demonstrates that mild hypothermia treatment may decrease the level of IL-6 and diminish the inflammatory reaction.
Conclusions
Mild hypothermia could improve survival rate due to reducing the damage to the heart, brain, and other vital organs, as well as to gastric mucosa. After ROSC, gastrointestinal tract perfusion decreases significantly and gastric mucosa injury is severe. With mild hypothermia treatment, gastric mucosal injury becomes better, with fewer gastrointestinal complications. We believe that an underlying mechanism is inhibiting IL-6 production and relieving the inflammatory reaction. With the popularization of mild hypothermia in critically ill patients, we consider that it is also beneficial to gastric mucosa.
Footnotes
Conflict of interest
There are no conflict of interests associated with this study.
Source of support: Departmental sources
References
- 1.Gupta P, Rettiganti M, Jeffries HE, et al. Risk factors and outcomes of in-hospital cardiac arrest following pediatric heart operations of varying complexity. Resuscitation. 2016;105:1–7. doi: 10.1016/j.resuscitation.2016.04.022. [DOI] [PubMed] [Google Scholar]
- 2.Miranzadeh S, Adib-Hajbaghery M, Hosseinpour N. a prospective study of survival after in-hospital cardiopulmonary resuscitation and its related factors. Trauma Mon. 2016;21(1):e31796. doi: 10.5812/traumamon.31796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stub D, Bernard S, Duffy SJ, Kaye DM. Post cardiac arrest syndrome: a review of therapeutic strategies. Circulation. 2011;123:1428–35. doi: 10.1161/CIRCULATIONAHA.110.988725. [DOI] [PubMed] [Google Scholar]
- 4.Neumar RW, Shuster M, Callaway CW, et al. Part 1: Executive summary: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2015;132(18 Suppl 2):S315–67. doi: 10.1161/CIR.0000000000000252. [DOI] [PubMed] [Google Scholar]
- 5.Kulstad EB, Courtney DM, Waller D. Induction of therapeutic hypothermia via the esophagus: a proof of concept study. World J Emerg Med. 2012;3:118–22. doi: 10.5847/wjem.j.issn.1920-8642.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beesems SG, Wijmans L, Tijssen JG, Koster RW. Duration of ventilations during cardiopulmonary resuscitation by lay rescuers and first responders: Relationship between delivering chest compressions and outcomes. Circulation. 2013;127:1585–90. doi: 10.1161/CIRCULATIONAHA.112.000841. [DOI] [PubMed] [Google Scholar]
- 7.Chandra P, Kishore K, Ghosh AK. Assessment of antisecretory, gastroprotective, and in-vitro antacid potential of Daucus carota in experimental rats. Osong Public Health Res Perspect. 2015;6:329–35. doi: 10.1016/j.phrp.2015.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rogers AB. Classification and grading of gastritis. Histologic scoring of gastritis and gastric cancer in mouse models. Methods Mol Biol. 2012;921:189–203. doi: 10.1007/978-1-62703-005-2_22. [DOI] [PubMed] [Google Scholar]
- 9.Prasad K, Prabhu GK. Image analysis tools for evaluation of microscopic views of immunohistochemically stained specimen in medical research – a review. J Med Syst. 2012;36:2621–31. doi: 10.1007/s10916-011-9737-7. [DOI] [PubMed] [Google Scholar]
- 10.Frantz TL, Steenburg SD, Gaski GE, et al. Tissue damage volume predicts organ dysfunction and inflammation after injury. J Surg Res. 2016;202:188–95. doi: 10.1016/j.jss.2015.12.043. [DOI] [PubMed] [Google Scholar]
- 11.Mozaffarian D, Benjamin EJ, Go AS, et al. Executive summary: Heart Disease and Stroke Statistics – 2016 Update: A Report From the American Heart Association. Circulation. 2016;133:447–54. doi: 10.1161/CIR.0000000000000366. [DOI] [PubMed] [Google Scholar]
- 12.Arrich J, Holzer M, Havel C, et al. Hypothermia for neuroprotection in adults after cardiopulmonary resuscitation. Cochrane Database Syst Rev. 2012;9:CD004128. doi: 10.1002/14651858.CD004128.pub3. 2012. [DOI] [PubMed] [Google Scholar]
- 13.Jin Y, Lin Y, Feng JF, et al. Moderate hypothermia significantly decreases hippocampal cell death involving autophagy pathway after moderate traumatic brain injury. J J Neurotrauma. 2015;32:1090–100. doi: 10.1089/neu.2014.3649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang L, Li C, Gao C, et al. Investigation of myocardial stunning after cardiopulmonary resuscitation in pigs. Biomed Environ Sci. 2011;24:155–62. doi: 10.3967/0895-3988.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 15.Zhao H, Li CS, Gong P, et al. Molecular mechanisms of therapeutic hypothermia on neurological function in a swine model of cardiopulmonary resuscitation. Resuscitation. 2012;83:913–20. doi: 10.1016/j.resuscitation.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 16.Liang H, Yu F, Tong Z, et al. Effect of ischemia post-conditioning on skeletal muscle oxidative injury, mTOR, Bax, Bcl-2 proteins expression, and HIF-1alpha/beta-actin mRNA, IL-6/beta-actin mRNA and caveolin-3/beta-actin mRNA expression in ischemia-reperfusion rabbits. Mol Biol Rep. 2013;40:507–14. doi: 10.1007/s11033-012-2087-9. [DOI] [PubMed] [Google Scholar]
- 17.Teke Z, Bostanci EB, Yenisey C, et al. Caffeic acid phenethyl ester alleviates mesenteric ischemia/reperfusion injury. Invest Surg. 2012;25:354–65. doi: 10.3109/08941939.2012.677968. [DOI] [PubMed] [Google Scholar]
- 18.Karshovska E, Zhao Z, Blanchet X, et al. Hyperreactivity of junctional adhesion molecule A-deficient platelets accelerates atherosclerosis in hyperlipidemic mice. Circ Res. 2015;116:587–99. doi: 10.1161/CIRCRESAHA.116.304035. [DOI] [PubMed] [Google Scholar]
- 19.Ng JW, Cairns SA, O’Boyle CP. Management of the lower gastrointestinal system in burn: A comprehensive review. Burns. 2016;42(4):728–37. doi: 10.1016/j.burns.2015.08.007. [DOI] [PubMed] [Google Scholar]