Abstract
Total arterial revascularization is the leading trend in coronary artery bypass grafting (CABG) for the treatment of coronary artery disease (CAD). Adding to its superiority to vein conduits, arteries allow for a high degree of versatility and long-term patency, while minimizing the need for reintervention. This is especially important for patients with multi-vessel coronary artery disease, as well as young patients. However, arterial revascularization has come a long way before being widely appreciated, with some yet unresolved debates, and advances that never cease to impress. In this review, we discuss the evolution of this surgical technique and its clinical success, as well as its most conspicuous limitations in light of accumulated published date from decades of experience.
MeSH Keywords: Arteries, Coronary Artery Bypass, Myocardial Revascularization
Background
Coronary artery disease (CAD), typically caused by atherosclerosis, is a leading cause of cardiovascular events (e.g., myocardial infarction, stroke, and heart failure), which claim the lives of millions worldwide [1]. Narrowing (stenosis) of 1 or more of the coronary arteries leads to myocardial deprivation of adequate blood supply. The severity of the disease is governed by the type and number of coronary arteries involved, and the treatment option is determined accordingly. In less severe conditions, percutaneous coronary intervention (PCI), or coronary angioplasty, is a widely accepted less invasive treatment [2]. However, in case of severe multi-vessel CAD (MCAD), coronary artery bypass grafting (CABG) represents a superior, yet aggressive, surgical intervention for myocardial revascularization [2–4].
CABG History
The world’s first clinically successful coronary artery bypass operation was reported by Robert Goetz in 1961, using a titanium ring non-suture method to create an internal mammary artery (IMA)-coronary artery anastomosis [5]. Shortly after, Vasilii Kolesov revolutionized the art of CABG surgery by introducing a suture method and performed his first clinically successful CABG operation in 1964 [6]. Despite the novelty of the surgery and the successes made by Kolesov, CABG was received disappointing responses from the surgical community, which doubted its safety and effectiveness as a treatment for CAD.
In 1967, a team at the Cleveland Clinic successfully reported the use of the saphenous vein (SV) as a graft for CABG. The trend started to expand favoring the use of SV [7]. However, not long after it was adopted as the graft of choice for CABG, its disadvantages started to surface. SV grafts (SVG) were associated with short- and long-term complications severely affecting its patency.
Problems with saphenous vein grafts (SVG):
– Short-term complications: postoperative graft thrombosis is a major concern with vein grafts, with up to 12% occlusion rates within the first 6 months after CABG, an issue which necessitates vigilant antiplatelet and antithrombotic therapy [8].
– Delayed complications: such a high thrombosis rate predisposes the vein graft to intimal hyperplasia, usually within the first year of grafting in nearly all graft [9].
– Late complications: graft atherosclerosis is the inevitable ramification for thrombosis and intimal thickening [8]. Indeed, pharmacological interventions, such as lipid-lowering drugs, were shown to improve graft patency [10]. For more detailed information on pharmacological protocols to improve SVG patency please, refer to the review by Kim et al. [8]. Nevertheless, atherosclerosis remains the primary cause of graft failure and poor patency, necessitating repeated revascularization in almost 10% of patients within 10 years of SVG implementation [11].
Back to the 60s
Due to the aforementioned drawbacks of SVG, surgeons were encouraged to reconsider the arterial conduits pioneered by Goetz and Kolesov [6]. Though their early work using arterial conduits dates back to the 60s, it was not, however, until the 80s that the superiority of arterial grafts over venous ones was appreciated. Being the first arterial grafts, internal mammary arteries (IMAs) have earned international conspicuity and are currently considered the conduits of first choice for arterial revascularization [6].
Left vs. Right IMA
The accumulated experience with left internal mammary artery (LIMA) has proved its excellent aptness as a conduit for CABG. LIMA grafts offer 98% patency at 5 years and 95% at 10 years [12]. A recent study by Tatoulis et al. has shown that at 10 years, LIMA has a patency of over 96% to the left anterior descending coronary artery (LAD) and 89% to the circumflex (Cx) [13]. As a result, LIMA has been extensively used and LIMA-LAD has been a staple in CABG over the past 3 decades [14].
However, in the case of MCAD, the need for extensive CABG to other coronary arteries has prompted the use of more than just 1 graft (total arterial revascularization). The right internal mammary artery (RIMA) is the second-choice conduit, which, though biologically identical to LIMA, has been much less frequently used for CABG than LIMA. However, recent studies have shown that RIMA has excellent patency, equivalent to that of LIMA. The overall patency of RIMA is 90%. At 10 years, RIMA patency to LAD was reported to be 95% and 90% at 15 years [13]. Moreover, RIMA patency to the right coronary artery was reported to be 84%, 91% to the Cx, and 86% to the posterior descending artery at 10 years [13].
Two Are Better Than One
For extensive arterial revascularization, such as in case of MCAD, RIMA has been used along with LIMA for a bilateral bypass (BIMA); this technique was reported as early as the mid-70s [15]. However, as with LIMA alone (Figure 1), BIMA bypass had its share of skepticism until it started to gain acceptance [16]. This is because early studies reporting on the use of BIMA for CABG lacked randomization and long-term follow-up, as the technique was mainly confined to younger and lower-risk patients, who are naturally expected to benefit more from it [17]. More recently, however, studies have reported excellent results with BIMA with regard to freedom from postoperative complications, and needlessness for reintervention, but the most striking results were in terms of long-term survival benefit [16–18]. One of the largest systemic studies conducted on the comparison between BIMA and single IMA (SIMA), comprising 16 000 patients, has demonstrated a significant survival benefit in favor of BIMA at a mean of 10 years of follow-up [19]. In another study, by Lytle et al., the benefits of BIMA were reported to be more apparent in the second postoperative decade, compared to SIMA [20]. In this study, survival in the BIMA-receiving group was 50% at 20 years, compared to 37% in the SIMA-receiving group, in this case LIMA. Another recent study, by Kurlansky et al.., reported on their 30-year follow-up results comparing long-term survival benefits of SIMA vs. BIMA [21]. At 15 years, survival was 39% for SIMA and 53.5% for BIMA, while at 25 years survival was 16.5%±2.1% for SIMA and 28.5%±2.2% for BIMA (p=0.001). When they compared cohorts of optimally matched groups, patients who received a second IMA displayed a clear survival benefit of 34% prolongation of median survival. Furthermore, long-term survival benefits of BIMA over SIMA were more recently reported to hold true even among diabetic patients, wherein, according to a recent study, the 8-year survival for diabetic patients receiving BIMA was 87.4% compared to 60.6% for those receiving SIMA [22].
Figure 1.
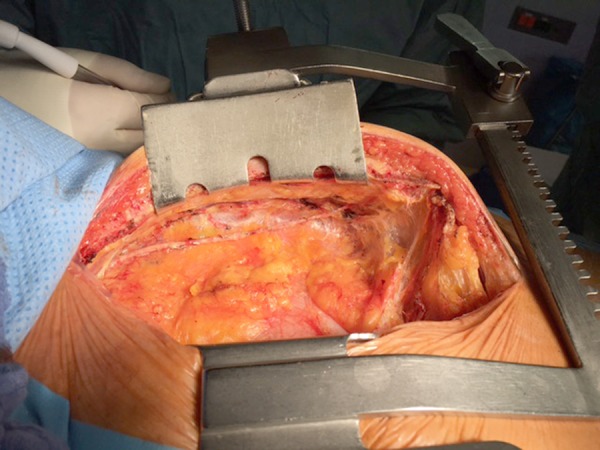
Intraoperative image demonstrating the skeletonized left internal mammary artery. The pleural cavity was not opened during preparation of the graft.
Graft Configuration
The traditional method of total arterial revascularization using BIMA adopts in situ use to bypass the left side of the heart (i.e., LAD). In the early 90s, Tector and others revolutionized the art by utilizing BIMA in novel ways of composite grafting. He developed the so-called “T-grafts” (Figure 2), as well as sequential grafts for multi-vessel CAD [23]. Typically, the attached LIMA bypasses the narrowed or stenotic coronaries in the anterior and/or anterolateral heart, while the free RIMA is utilized to create a so-called “Y-anastomosis” to LIMA and bypass in the inferior, inferolateral, or posterior region of the heart.
Figure 2.
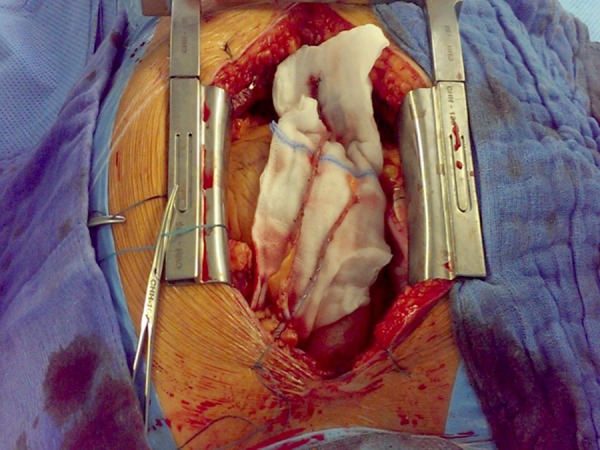
Intraoperative image showing a completed T-graft with both skeletonized internal mammary arteries (LIMA+RIMA) sutured together with 8-0 Prolene.
Earlier studies, however, attributed the aforementioned benefits of BIMA to their in situ use and to grafting the left side of the heart, undermining the anastomosis of free grafts from the aorta or placement to the right coronary artery [24,25]. Nevertheless, more recent studies have reported high short- and long-term patency rates (exceeding 96%) for all IMA anastomoses, regardless of the coronary artery grafted, highlighting the utility of Y-grafts and sequential grafting [26,27].
Nowadays, contemporary methods of total arterial revascularization make extensive use of the free RIMA. For example, a short length of RIMA can be freely used for the Y-anastomosis with LIMA to bypass the diagonal branch of LAD, while the remaining attached RIMA bypasses the Cx or the right coronary artery [14].
Radial Artery (RA) as a Conduit
The search for more arterial conduits for CABG did not stop at internal thoracic arteries (ITAs). In the early 70s, Carpentier et al. reported the use of RA as a conduit for CABG [28]. The earlier experience with such a graft was highly discouraging due to reported intimal hyperplasia and occlusion, which led to high rates of graft failure, and it was abandoned shortly thereafter [29,30]. More than a decade later, the use of RA was revived by several studies, and modifications were introduced, including the harvesting technique (Figure 3), as well as antispasmodic drug treatments, and excellent outcomes were reported with regard to short- and mid-term patency [31,32]. Nowadays, RA is seen to have value as a graft for CABG, as recent studies have demonstrated high rates of short- and long-term patency, as well as lower rates of graft failure, compared to SV. In a comprehensive meta-analysis study of 5 randomized controlled trials, Cao et al. reported RA to be significantly less likely to failure than SV (6% for RA vs. 17.5% for SV), and significantly more likely to be completely patent than SV (89.9% for RA vs. 63.1% for SV) beyond 4 years of follow-up [33]. Compared to IMAs, RA was shown to be as valuable. A recent single institution’s retrospective cohort study has evaluated the overall patency of RA in 1851 patients, and reported excellent short- and long-term patency, which was similar to those of LIMA, regardless of which coronary artery it was grafted to [34]. As a second arterial conduit in combination with ITA, a study by Schwann et al. has addressed late survival benefits of RA compared to SV, and reported a significantly better survival advantage for ITA/RA compared to ITA/SV grafts [35].
Figure 3.
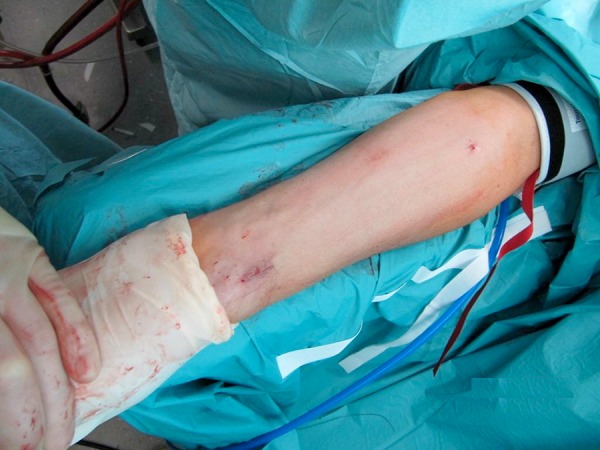
Cosmetic result after endoscopic harvest of the left radial artery.
RA is used along with IMAs in a traditional configuration (BIMA/RA), where RA bypasses the right coronary artery, while both attached RIMA and LIMA bypass LAD and Cx, respectively [36]. A more contemporary example for the use of RA in total arterial revascularization is as an extension to RIMA to bypass the distal ends of the posterolateral coronary as a single anastomosis, or alternatively, as a sequential graft to the posterior descending artery (side-to-side) and the posterolateral branch (end-to-end) [14].
Gastroepiploic Artery (GEA) as a Conduit
The first attempt at using GEA as a bypass graft on the right coronary artery was introduced in the early 1970s by Mills [37]. GEA was used also in the Vineberg procedure, in which arterial grafts are directly implanted in the myocardium. The right GEA has emerged as an alternative arterial conduit for CABG when bypass surgeries were first introduced [38]. The branch of the gastroduodenal artery originates from common hepatic artery, which gives rise to the GEA among the 4 arteries supplying the stomach. In rare occasions, the GEA can also arise from the superior mesenteric artery [39]. The surgeon must be aware of this anatomic variety. Histologically, no differences were observed between structure of the wall of GEA and IMAs. However, the GEA has more smooth muscle cells in media than IMAs, resulting in a more spastic response upon simple surgical manipulation [40]. According to recent studies, the initial patency of GEA (97.1% at 1 month, 92.3% 1 year, 80.9% at 7 years, and 66.5% after 10 years) was improved after using skeletonized grafts: 97.8% immediately, 97.8% after 5 years, and 90.2% at 8 years after surgery [41]. Nevertheless, there is no exact guideline for decision-making among the users and non-users of this conduit.
Limitations and Contraindications
Despite the unprecedented success achieved with arterial grafts in patients with CAD, total arterial revascularization faces some limitations and contraindications that must be carefully considered.
Diabetes
Diabetes has long been a concern and, to some extent, a contraindication when it came to revascularization with BIMA, as it was demonstrated by several early studies to be associated with worse outcomes [22]. Diabetes was considered an independent risk factor for late cardiac death and lower survival in patients receiving BIMA, as well as a 10-fold increase in deep sternal wound infection (DSWI), the latter being especially dependent on the harvesting technique [42–45]. In 2003, Endo and colleagues reported a significant improvement in the long-term survival for diabetic patients receiving skeletonized BIMA grafts compared to those receiving SIMA [46]. According to their results, preserved ejection fraction (higher than 0.4) was imperative to reap the benefits of BIMA in diabetic patients, for whom the 10-year survival rate using BIMA was significantly higher than with SIMA (87.8±3.5% vs. 75.2±3.4%, P=0.04). More recent studies have also confirmed the long-term survival benefits to diabetic patients receiving BIMA compared to SIMA [22].
Deep Sternal Wound Infection (DSWI)
The traditional pedicled manner of harvesting of IMAs for grafting additionally involves harvest of surrounding parietal pleura, venae comitantes, muscle, and fascia, leaving the chest wall completely devascularized, especially upon BIMA harvesting [17]. This leads to a significant decrease in sternal blood flow, which impairs wound healing, and subsequently leads to sternal infection [45,47]. Hence, DSWI has long been the most commonly reported complication for BIMA-CABG in earlier studies [45]. Patients with initially compromised wound healing abilities (e.g., diabetic patients) are at greater risk for DSWI, especially upon BIMA grafting [22,48]. According to a large retrospective study by Loop et al. in 1990, diabetic patients receiving BIMA were 5 times more likely to suffer from DWSI [49]. Another study, by Borger et al., incorporating over 12 000 patients, reported that the risk of DSWI in diabetic patients increased from 1.3% to 14.3% upon BIMA grafting [49]. Other risk factors for DSWI include female sex, peripheral vascular disease, chronic obstructive pulmonary disease, chronic renal insufficiency, and body mass index higher than 35 kg/m2 [48,50]. Harvesting IMA by “skeletonization”, on the other hand, avoids this complication. This is done by harvesting only the IMA from the endothoracic fascia, while preserving the surrounding venous, lymphatic, and collateral blood supply, which in turn facilitates wound healing [17,51]. Several studies have reported excellent outcomes with skeletonized BIMA grafts. Calafiore et al. reported a lower incidence of DSWI in diabetic patients receiving skeletonized BIMA grafts compared to pedicled grafts (2.2% vs. 10%, p<0.05) [52]. In a more recent study comparing matching diabetic patients receiving BIMA grafts, DSWI was significantly lower in the skeletonized vs. the pedicled group (1.3 vs. 11.1, p=0.03) [53]. In a recent meta-analysis study, Saso et al. reviewed 12 studies and reported a reduction in the odds of sternal wound infection in all patients receiving skeletonized BIMA grafts (odds ratio, 0.41; 95%CI, 0.26 to 0.64) [54]. The incidence of DSWI in all patients receiving skeletonized BIMA grafts is estimated to be 1.1% to 1.7%, which can increase up to 2.2% in diabetic patients [51].
Competitive Flow
The benefits of CABG with IMAs are quiet tempting for use in treating CAD, but not all narrow coronary arteries should be grafted. Evidence from linear regression analyses from earlier studies underscored the risk of arterial graft failure by occlusion or non-functionality due to competitive flow, which was strongly correlated with degree of native coronary artery stenosis [55,56]. The more well-preserved the flow in the native coronary artery being grafted, the higher was the risk for graft failure. A postoperative angiographic study by Shimizu et al. has beautifully demonstrated this correlation [57]. They divided patients undergoing CABG in 3 groups according to the degree of stenosis in their native coronary arteries; high (H): 80% stenosis or greater; moderate (M): 60% to 79% stenosis; and low (L): 40% to 59%. Using intravascular Doppler velocimetry, they measured the graft phasic flow, as well as graft flow volume. Their results were as follows: Average peak velocity (group H, 27.1±8.6 cm/s; group M, 16.9±3.9 cm/s; group L, 7.2±3.7 cm/s), distal graft diameter (group H, 2.27±0.23 mm; group M, 2. 00±0.28 mm; group L, 1.07±0.27 mm), and graft flow volume (group H, 33.1±12.0 mL/min; group M, 16.2±5.8 mL/min; group L, 2.3±2.0 mL/min). The risk of graft occlusion due to competitive flow becomes more prominent in grafts to non-LAD, women, RIMA grafts, and smokers [58]. It is advised that coronary arteries with less than 70% stenosis in the left heart side and less than 90% in the dominant right system should be left untouched [14]. However, there remain intense debate on whether to graft moderately stenotic coronary arteries. More recently, the concept of “prophylactic” grafting has emerged as an option for patients with even mildly stenotic lesions but with concomitant severe medical illness [59]. In those patients, their minimally diseased vessels are expected deteriorate with time, and the risk from reoperation increases. Interestingly, this principle was suggested more than 2 decades ago by Lust and colleagues, but it was far from conceivable back then [60].
RA Limitations
The following discussion highlights the most important considerations for the use of RA for CABG. See Gaudino et al., 2014 for more details [61].
Adequacy of ulnar flow: usually evaluated by a modified Allen’s test (a hyperemic response of later than 10 s in the ischemic hand indicates a poor collateral ulnar circulation and excludes the RA from use, whereas a good response is usually seen within 5 s), but could also be complemented with pulse oximetry and echo-Doppler; the latter is beneficial for morphometric evaluation of the RA.
RA morphology: RAs with inner diameter less than 2 mm or calcifications should be excluded.
Sensory abnormalities and weakness in the forearm after RA removal.
Use of vasodilators: RA are notorious for their marked spastic responses to vasoconstrictors and hypothermia, necessitating pharmacologic interventions during harvesting, postoperatively, and in the early follow-up period.
Pedicled vs. skeletonized RA: although skeletonization is reported to yield a longer graft with larger diameters, less spasms, and higher patency rates, it significantly increases the harvest time and the risk of severe graft injury.
GEA Limitations
Earlier studies have shown that GEA is associated with a high risk of early graft failure [62]. This has been confirmed by the most recent research and meta-analyses revealing that GEA bears the highest risk of functional and complete graft occlusion. GEA is the least preferable choice for arterial CABG, as it demonstrates no angiographic superiority compared with saphenous vein graft [63].
Future Prospects and Conclusions
Total arterial revascularization is the current surgical trend. However, compared to conventional bypass surgery, it requires higher levels of surgical dexterity. Despite its early inauguration, it has remained an esoteric procedure that is regarded skeptically by many. This is probably the reason why use of SVG was so widespread, although the use of IMA as a conduit for CABG was reported years earlier. Nevertheless, the advances in the art kept incessantly growing, especially as SVG was no longer as appealing. But the call for 2 IMAs instead of 1 was still too much for the surgical community to digest, let alone the use of free grafts, or even other non-IMA conduits. It is hard to dispute the innate superiority of arterial conduits to veins for CABG. It appears to be common sense that if you must bypass an artery, you should use another artery, and if you need more bypasses, you need more arteries. But surgeons speak the “Kaplan-Meier” language, and there has got to be time-bound clinical evidence favoring the utility of arterial conduits, as well as more arterial conduits, but how can this be done for such a nongeneric procedure? The predicament is the lack of properly structured studies with matching cohorts. The result was many single-institution studies, from which a solid statement is sometimes difficult to make without reservations. More recently, however, and as discussed above, the growing experience with arterial conduits has clearly shown the benefits of total arterial revascularization, even in high-risk patients. In fact, not only are arterial grafts superior in terms of patency and survival, but they also protect native coronary arteries against further progression of atherosclerotic disease [64].
The advances in harvesting techniques, although adding to the complexity and time of the procedure, have greatly augmented the safety of BIMA grafts, especially for diabetic patients, in whom BIMA has been contraindicated due to the high risk of postoperative morbidity and DSWI. In fact, modern surgical technologies such as robotic totally endoscopic coronary artery bypass (TECAB) and minimally invasive coronary artery bypass (MIDCAB) grafting eliminate these risks, as they allow access for IMAs and the heart without sternal division [51]. Furthermore, the advances in off-pump CABG (OPCAB) are likely to expand the selection criteria to include elderly patients, whereas CABG [65,66] using cardiopulmonary bypass entails higher risks. However, given the high level of surgical adroitness involved, it will most probably remain a recondite procedure.
It is also noteworthy to highlight to importance of early preclinical research and experimental work in large animals, which inspired many surgical refinements we have witnessed, if not the whole art [6]. With the current expanding technologies in tissue engineering, one cannot exclude the possibility of not having to harvest any artery in the near future, but rather use engineered conduits. Such engineered conduits can come in the desired length and branching, with genetically modified endothelium to eliminate vasospasms.
We are yet to be impressed about what the future has to bring. However, we have learned from the evolution of CABG was that no patient with moderate or advanced CAD should be denied the benefits of total arterial revascularization, provided that an experienced surgeon is available.
Footnotes
Conflicts of interest
The authors have no conflicts of interest to declare.
Source of support: Departmental sources
References
- 1.Lavoie L, Khoury H, Welner S, et al. Burden and prevention of adverse cardiac events in patients with concomitant chronic heart failure and coronary artery disease: A literature review. Cardiovasc Ther. 2016;34:152–60. doi: 10.1111/1755-5922.12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mohr FW, Morice MC, Kappetein AP, et al. Coronary artery bypass graft surgery versus percutaneous coronary intervention in patients with three-vessel disease and left main coronary disease: 5-year follow-up of the randomised, clinical SYNTAX trial. Lancet. 2013;381:629–38. doi: 10.1016/S0140-6736(13)60141-5. [DOI] [PubMed] [Google Scholar]
- 3.Cao C, Manganas C, Bannon P, et al. Drug-eluting stents versus coronary artery bypass graft surgery in left main coronary artery disease: A meta-analysis of early outcomes from randomized and nonrandomized studies. J Thorac Cardiovasc Surg. 2013;145:738–47. doi: 10.1016/j.jtcvs.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 4.Zhu P, Zhou P, Sun Y, et al. Hybrid coronary revascularization versus coronary artery bypass grafting for multivessel coronary artery disease: Systematic review and meta-analysis. J Cardiothorac Surg. 2015;10:63. doi: 10.1186/s13019-015-0262-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goetz RH, Rohman M, Haller JD, et al. Internal mammary-coronary artery anastomosis. A nonsuture method employing tantalum rings. J Thorac Cardiovasc Surg. 1961;41:378–86. [PubMed] [Google Scholar]
- 6.Konstantinov IE, Vasilii I. Kolesov: A surgeon to remember. Tex Heart Inst J. 2004;31:349–58. [PMC free article] [PubMed] [Google Scholar]
- 7.Favaloro RG. Saphenous vein autograft replacement of severe segmental coronary artery occlusion: Operative technique. Ann Thorac Surg. 1968;5:334–39. doi: 10.1016/s0003-4975(10)66351-5. [DOI] [PubMed] [Google Scholar]
- 8.Kim FY, Marhefka G, Ruggiero NJ, et al. Saphenous vein graft disease: Review of pathophysiology, prevention, and treatment. Cardiol Rev. 2013;21:101–9. doi: 10.1097/CRD.0b013e3182736190. [DOI] [PubMed] [Google Scholar]
- 9.Motwani JG, Topol EJ. Aortocoronary saphenous vein graft disease: Pathogenesis, predisposition, and prevention. Circulation. 1998;97:916–31. doi: 10.1161/01.cir.97.9.916. [DOI] [PubMed] [Google Scholar]
- 10.Knatterud GL, Rosenberg Y, Campeau L, et al. Long-term effects on clinical outcomes of aggressive lowering of low-density lipoprotein cholesterol levels and low-dose anticoagulation in the post coronary artery bypass graft trial. Post CABG Investigators. Circulation. 2000;102:157–65. doi: 10.1161/01.cir.102.2.157. [DOI] [PubMed] [Google Scholar]
- 11.Cohn LH. Cardiac surgery in the adult. New York; London: McGraw-Hill; Medical: 2008. [Google Scholar]
- 12.Tatoulis J, Buxton BF, Fuller JA. Patencies of 2127 arterial to coronary conduits over 15 years. Ann Thorac Surg. 2004;77:93–101. doi: 10.1016/s0003-4975(03)01331-6. [DOI] [PubMed] [Google Scholar]
- 13.Tatoulis J, Buxton BF, Fuller JA. The right internal thoracic artery: the forgotten conduite5,766 patients and 991 angiograms. Ann Thorac Surg. 2011;92:9–15. doi: 10.1016/j.athoracsur.2011.03.099. discussion 15–17. [DOI] [PubMed] [Google Scholar]
- 14.Buxton BF, Hayward PA. The art of arterial revascularization – total arterial revascularization in patients with triple vessel coronary artery disease. Ann Cardiothorac Surg. 2013;2:543–51. doi: 10.3978/j.issn.2225-319X.2013.07.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barner HB. Double internal mammary-coronary artery bypass. Arch Surg. 1974;109:627–30. doi: 10.1001/archsurg.1974.01360050025007. [DOI] [PubMed] [Google Scholar]
- 16.Lytle BW, Blackstone EH, Loop FD, et al. Two internal thoracic arteries are better than one. J Thorac Cardiovasc Surg. 1999;117:855–72. doi: 10.1016/S0022-5223(99)70365-X. [DOI] [PubMed] [Google Scholar]
- 17.Taggart DP. Bilateral internal mammary artery grafting: Are BIMA better? Heart. 2002;88(1):7–9. doi: 10.1136/heart.88.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fiore A, Naunheim K, Dean P, et al. Results of internal thoracic artery grafting over 15 years: Single versus double grafts. Ann Thorac Surg. 1990;49:202–8. doi: 10.1016/0003-4975(90)90139-w. discussion 208–9. [DOI] [PubMed] [Google Scholar]
- 19.Taggart DP, D’Amico R, Altman DG. Effect of arterial revascularisation on survival: A systematic review of studies comparing bilateral and single internal mammary arteries. Lancet. 2001;358:870–75. doi: 10.1016/S0140-6736(01)06069-X. [DOI] [PubMed] [Google Scholar]
- 20.Lytle BW, Blackstone EH, Sabik JF, et al. The effect of bilateral internal thoracic artery grafting on survival during 20 postoperative years. Ann Thorac Surg. 2004;78:2005–14. doi: 10.1016/j.athoracsur.2004.05.070. [DOI] [PubMed] [Google Scholar]
- 21.Kurlansky PA, Traad EA, Dorman MJ, et al. Thirty-year follow-up defines survival benefit for second internal mammary artery in propensity-matched groups. Ann Thorac Surg. 2010;90:101–8. doi: 10.1016/j.athoracsur.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 22.Puskas JD, Sadiq A, Vassiliades TA, et al. Bilateral internal thoracic artery grafting is associated with significantly improved long-term survival, even among diabetic patients. Ann Thorac Surg. 2012;94:710–15. doi: 10.1016/j.athoracsur.2012.03.082. [DOI] [PubMed] [Google Scholar]
- 23.Tector AJ, Kress DC, Schmahl TM, et al. T-graft: A new method of coronary arterial revascularization. J Cardiovasc Surg (Torino) 1994;35:19–23. [PubMed] [Google Scholar]
- 24.Verhelst R, Etienne PY, El Khoury G, et al. Free internal mammary artery graft in myocardial revascularization. Cardiovasc Surg. 1996;4:212–16. doi: 10.1016/0967-2109(96)82318-0. [DOI] [PubMed] [Google Scholar]
- 25.Buxton BF, Ruengsakulrach P, Fuller J, et al. The right internal thoracic artery graft – benefits of grafting the left coronary system and native vessels with a high grade stenosis. Eur J Cardiothorac Surg. 2000;18:255–61. doi: 10.1016/s1010-7940(00)00527-3. [DOI] [PubMed] [Google Scholar]
- 26.Calafiore AM, Contini M, Vitolla G, et al. Bilateral internal thoracic artery grafting: Long-term clinical and angiographic results of in situ versus Y grafts. J Thorac Cardiovasc Surg. 2000;120:990–96. doi: 10.1067/mtc.2000.110249. [DOI] [PubMed] [Google Scholar]
- 27.Dion R, Glineur D, Derouck D, et al. Long-term clinical and angiographic follow-up of sequential internal thoracic artery grafting. Eur J Cardiothorac Surg. 2000;17:407–14. doi: 10.1016/s1010-7940(00)00370-5. [DOI] [PubMed] [Google Scholar]
- 28.Carpentier A, Guermonprez JL, Deloche A, et al. The aorta-to-coronary radial bypass graft: a technique avoiding pathological changes in grafts. Ann Thorac Surg. 1973;16:111–21. doi: 10.1016/s0003-4975(10)65825-0. [DOI] [PubMed] [Google Scholar]
- 29.Curtis JJ, Stoney WS, Alford WC, et al. Intimal hyperplasia: A cause of radial artery aortocoronary bypass graft failure. Ann Thorac Surg. 1975;20:628–35. doi: 10.1016/s0003-4975(10)65754-2. [DOI] [PubMed] [Google Scholar]
- 30.Fisk RL, Brooks CH, Callaghan JC, et al. Experience with the radial artery graft for coronary artery bypass. Ann Thorac Surg. 1976;21:513–18. doi: 10.1016/s0003-4975(10)63919-7. [DOI] [PubMed] [Google Scholar]
- 31.Acar C, Ramsheyi A, Pagny JY, et al. The radial artery for coronary artery bypass grafting: Clinical and angiographic results at five years. J Thorac Cardiovasc Surg. 1998;116:981–89. doi: 10.1016/S0022-5223(98)70050-9. [DOI] [PubMed] [Google Scholar]
- 32.Acar C, Jebara VA, Portoghese M, et al. Revival of the radial artery for coronary artery bypass grafting. Ann Thorac Surg. 1992;54:652–60. doi: 10.1016/0003-4975(92)91007-v. [DOI] [PubMed] [Google Scholar]
- 33.Cao C, Manganas C, Horton M, et al. Angiographic outcomes of radial artery versus saphenous vein in coronary artery bypass graft surgery: A meta-analysis of randomized controlled trials. J Thorac Cardiovasc Surg. 2013;146:255–61. doi: 10.1016/j.jtcvs.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 34.Tranbaugh RF, Dimitrova KR, Friedmann P, et al. Coronary artery bypass grafting using the radial artery: Clinical outcomes, patency, and need for reintervention. Circulation. 2012;126:S170–75. doi: 10.1161/CIRCULATIONAHA.111.083048. [DOI] [PubMed] [Google Scholar]
- 35.Schwann TA, Engoren M, Bonnell M, et al. Comparison of late coronary artery bypass graft survival effects of radial artery versus saphenous vein grafting in male and female patients. Ann Thorac Surg. 2012;94:1485–91. doi: 10.1016/j.athoracsur.2012.05.029. [DOI] [PubMed] [Google Scholar]
- 36.Locker C, Schaff HV, Dearani JA, Daly RC. Improved late survival with arterial revascularization. Ann Cardiothorac Surg. 2013;2(4):467–74. doi: 10.3978/j.issn.2225-319X.2013.06.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mills NL, Everson CT. Right gastroepiploic artery: A third arterial conduit for coronary artery bypass. Ann Thorac Surg. 1989;47:706–11. doi: 10.1016/0003-4975(89)90122-7. [DOI] [PubMed] [Google Scholar]
- 38.Bailey CP, Hirose T, Brancato R, et al. Revascularization of the posterior (diaphragmatic) portion of the heart. Ann Thorac Surg. 1966;2:791–805. [Google Scholar]
- 39.Suma H, Sato H. The in situ right gastroepiploic artery graft via the superior mesenteric artery. J Thorac Cardiovasc Surg. 1989;98:1150. [PubMed] [Google Scholar]
- 40.Van Son JA, Smedts F, Vincent JG, et al. Comparative anatomic studies of various arterial conduits for myocardial revascularization. J Thorac Cardiovasc Surg. 1990;99:703–7. [PubMed] [Google Scholar]
- 41.Suzuki T, Asai T, Nota H, et al. Early and long-term patency of in situ skeletonized gastroepiploic artery after off-pump coronary artery bypass graft surgery. Ann Thorac Surg. 2013;96:90–95. doi: 10.1016/j.athoracsur.2013.04.018. [DOI] [PubMed] [Google Scholar]
- 42.Pick AW, Orszulak TA, Anderson BJ, Schaff HV. Single versus bilateral internal mammary artery grafts: 10-year outcome analysis. Ann Thorac Surg. 1997;64:599–605. doi: 10.1016/s0003-4975(97)00620-6. [DOI] [PubMed] [Google Scholar]
- 43.Accola KD, Jones EL, Craver JM, et al. Bilateral mammary artery grafting: Avoidance of complications with extended use. Ann Thorac Surg. 1993;56:872–79. doi: 10.1016/0003-4975(93)90347-k. [DOI] [PubMed] [Google Scholar]
- 44.Matsa M, Paz Y, Gurevitch J, et al. Bilateral skeletonized internal thoracic artery grafts in patients with diabetes mellitus. J Thorac Cardiovasc Surg. 2001;121:668–74. doi: 10.1067/mtc.2001.112824. [DOI] [PubMed] [Google Scholar]
- 45.De Paulis R, deNotaris S, Scaffa R, et al. The effect of bilateral internal thoracic artery harvesting on superficial and deep sternal infection: The role of skeletonization. J Thorac Cardiovasc Surg. 2005;129:536–43. doi: 10.1016/j.jtcvs.2004.07.059. [DOI] [PubMed] [Google Scholar]
- 46.Endo M, Tomizawa Y, Nishida H. Bilateral versus unilateral internalthoracic revascularization in patients with diabetes. Circulation. 2003;108:1343–49. doi: 10.1161/01.CIR.0000085995.87982.6E. [DOI] [PubMed] [Google Scholar]
- 47.Seyfer AE, Shriver CD, Miller TR, Graeber GM. Sternal blood flow after median sternotomy and mobilization of the internal mammary arteries. Surgery. 1988;104:899–904. [PubMed] [Google Scholar]
- 48.Savage EB, Grab JD, O’Brien SM, et al. Use of bilateral internal thoracic arteries in diabetic patients increases deep sternal wound infection. Ann Thorac Surg. 2007;83:1002–6. doi: 10.1016/j.athoracsur.2006.09.094. [DOI] [PubMed] [Google Scholar]
- 49.Borger MA, Rao V, Weisel RD, et al. Deep sternal wound infection: risk factors and outcomes. Ann Thorac Surg. 1998;65(4):1050–56. doi: 10.1016/s0003-4975(98)00063-0. [DOI] [PubMed] [Google Scholar]
- 50.Loop FD, Lytle BW, Cosgrove DM, et al. Sternal wound complication after isolated coronary artery bypass grafting: Early and late mortality, morbidity and cost of care. Ann Thorac Surg. 1990;49:179–87. doi: 10.1016/0003-4975(90)90136-t. [DOI] [PubMed] [Google Scholar]
- 51.Wehman B, Taylor B. Coronary revascularization using bilateral internal thoracic arteries: Safe with skeletonization? J Clin Exp Cardiol. 2013;(Suppl 7):007. doi: 10.4172/2155-9880.S7-007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Calafiore AM, Vitolla G, Iaco AL, et al. Bilateral internal mammary artery grafting: midterm results of pedicled versus skeletonized conduits. Ann Thorac Surg. 1999;67:1637–42. doi: 10.1016/s0003-4975(99)00282-9. [DOI] [PubMed] [Google Scholar]
- 53.Peterson MD, Borger MA, Rao V, et al. Skeletonization of bilateral internal thoracic artery grafts lowers the risk of sternal infection in patients with diabetes. J Thorac Cardiovasc Surg. 2003;126:1314–19. doi: 10.1016/s0022-5223(03)00808-0. [DOI] [PubMed] [Google Scholar]
- 54.Saso S, James D, Vecht JA, et al. Effect of skeletonization of the internal thoracic artery for coronary revascularization on the incidence of sternal wound infection. Ann Thorac Surg. 2010;89:661–70. doi: 10.1016/j.athoracsur.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 55.Seki T, Kitamura S, Kawachi K, et al. A quantitative study of postoperative luminal narrowing of the internal thoracic artery graft in coronary artery bypass surgery. J Thorac Cardiovasc Surg. 1992;104:1532–38. [PubMed] [Google Scholar]
- 56.Hashimoto H, Isshiki T, Ikari Y, et al. Effects of competitive blood flow on arterial graft patency and diameter. Medium-term postoperative follow-up. J Thorac Cardiovasc Surg. 1996;111:399–407. doi: 10.1016/s0022-5223(96)70449-x. [DOI] [PubMed] [Google Scholar]
- 57.Shimizu T, Hirayama T, Suesada H, et al. Effect of flow competition on internal thoracic artery graft: Postoperative velocimetric and angiographic study. J Thorac Cardiovasc Surg. 2000;120:459–65. doi: 10.1067/mtc.2000.108166. [DOI] [PubMed] [Google Scholar]
- 58.Sabik JF, 3rd, Lytle BW, Blackstone EH, et al. Does competitive flow reduce internal thoracic artery graft patency? Ann Thorac Surg. 2003;76(5):1490–96. doi: 10.1016/s0003-4975(03)01022-1. [DOI] [PubMed] [Google Scholar]
- 59.Evora PRB, Arcêncio L, Schmidt A, et al. Prophylactic left internal mammary artery graft In mildly-stenosed coronary lesions. Still an open discussion. Arq Bras Cardiol. 2016;106(3):168–70. doi: 10.5935/abc.20160032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lust RM, Zeri RS, Spence PA, et al. Effect of chronic native flow competition on internal thoracic artery grafts. Ann Thorac Surg. 1994;57(1):45–50. doi: 10.1016/0003-4975(94)90363-8. [DOI] [PubMed] [Google Scholar]
- 61.Gaudino M, Crea F, Cammertoni F, et al. Technical issues in the use of the radial artery as a coronary artery bypass conduit. Ann Thorac Surg. 2014;98(6):2247–25. doi: 10.1016/j.athoracsur.2014.07.039. [DOI] [PubMed] [Google Scholar]
- 62.Santos GG, Stolf NA, Moreira LF, et al. Randomized comparative study of radial artery and right gastroepiploic artery in composite arterial graft for CABG. Eur J Cardiothorac Surg. 2002;21:1009–14. doi: 10.1016/s1010-7940(02)00180-x. [DOI] [PubMed] [Google Scholar]
- 63.Benedetto U, Raja SG, Albanese A, et al. Searching for the second best graft for coronary artery bypass surgery: A network meta-analysis of randomized controlled trials. Eur J Cardiothorac Surg. 2015;47:59–65. doi: 10.1093/ejcts/ezu111. [DOI] [PubMed] [Google Scholar]
- 64.Dimitrova KR, Hoffman DM, Geller CM, et al. Arterial grafts protect the native coronary vessels from atherosclerotic disease progression. Ann Thorac Surg. 2012;94:475–78. doi: 10.1016/j.athoracsur.2012.04.035. [DOI] [PubMed] [Google Scholar]
- 65.Płotek W, Pielok J, Cybulski M, Samborska R. Emotional processes in patients undergoing coronary artery bypass graft surgeries with extracorporeal circulation in view of selected indicators of the inflammatory condition. Med Sci Monit. 2015;21:105–17. doi: 10.12659/MSM.892372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wysocka A, Cybulski M, Berbeć H, et al. Prognostic value of paraoxonase 1 in patients undergoing coronary artery bypass grafting surgery. Med Sci Monit. 2014;20:594–600. doi: 10.12659/MSM.890025. [DOI] [PMC free article] [PubMed] [Google Scholar]


