Abstract
Background
Intracerebral hemorrhage (ICH) is one severe subtype of stroke, with a very complex pathology. Stem cell-based therapy holds promising potential in the treatment of neurological disorders. Human umbilical cord-derived mesenchymal stem cells (UC-MSCs) have a therapeutic effect in recovery from brain damage following ICH. The aim of this study was to identify an effective and convenient way of using UC-MSCs in the ICH rat model.
Material/Methods
CM-DiI-labeled human UC-MSCs were transplanted intracerebrally or intravenously into collagenase VII-induced ICH rat models. Neurological function was evaluated before ICH and at 0, 7, 14, 21, and 28 days after treatment. ICH rats were sacrificed to evaluate the injury volume. Neurogenesis and angiogenesis and vascular areas were investigated using microtubule-associated protein 2 (MAP2), glial fibrillary acidic protein (GFAP), and 4′,6-diamidino-2-phenylindole (DAPI) immunohistochemistry at two weeks after transplantation.
Results
The intracerebral and intravenous administration of UC-MSCs both resulted in significant improvement in neurological function and decrease in injury volume of ICH rats. Transplanted UC-MSCs were chemotactic in vivo and showed a predominant distribution around the ICH region. In addition, UC-MSCs could integrate into the cerebral vasculature in both groups.
Conclusions
Both intracerebral and intravenous administration of UC-MSCs could have a favorable effect on recovery of neurological function in ICH rats, although the fundamental mechanisms may be different between the two groups. Our data suggest that intravenous implantation of UC-MSCs could serve as a favorable approach for cell-based therapy in central nervous system (CNS) diseases according to clinical needs.
MeSH Keywords: Angiogenesis Inducing Agents; Human Umbilical Vein Endothelial Cells; Intracranial Hemorrhage, Hypertensive; Mesenchymal Stromal Cells
Background
Mesenchymal stromal cells (MSCs, also known as mesenchymal stem cells) are very promising candidates for cell-based therapy, based on their multiple differentiation capacity [1], low immunogenicity [2,3], and the potential to produce various bioactive factors that are beneficial in the repair of tissue injury [4], while other therapies such as autologous chondrocyte implantation have those limitations, more or less. MSC transplantation has been widely reported to be effective in improving functional outcome in a variety of diseases including central nervous system (CNS) disease [5] such as cerebral ischemia [6], spinal cord injury [7], and experimental autoimmune encephalomyelitis (EAE) [8]. These data suggest that MSCs provide potential utility in the treatment of CNS diseases [9,10].
MSCs have been successfully isolated from multiple tissues since they were originally obtained from adult bone marrow [11]. Studies revealed that MSCs reside in almost all organs and tissues [12], suggesting the extensive distribution of these cells. Human umbilical cord tissue, which is traditionally regarded as medical waste, is gaining large attention as it seems to be a rich source of MSCs and has advantages in many ways over MSCs derived from bone marrow, cord blood, and other tissues [13–15]. In this regard, we suggest that human umbilical cord tissue-derived MSCs (UC-MSCs) could serve as an ideal cell candidate in regard to MSC-based therapy.
Intracerebral hemorrhage (ICH) is a subtype of stroke known as sudden death of brain cells in a localized area due to inadequate blood flow, which frequently leads to severe and persistent neurological defects because of the loss of parenchymal neural cells [16]. It has been reported that bone marrow mesenchymal stem cells can ameliorate neurological deficits and blood-brain barrier dysfunction after ICH in spontaneously hypertensive rats [9]. As a consequence, MSC-based therapy is as a good candidate for ICH treatment. However, until today, there have been no effective strategies available to repair the functional defects after ICH [17]. Since stem cell–based therapy is a possible treatment strategy for neurological diseases, we hypothesized that UC-MSC transplantation could be beneficial to neurological function recovery after ICH. Using intracerebral or intravenous delivery as a measure of functional recovery in a IHC rat model, we showed that UC-MSCs may be an effective and convenient way to improve functional recovery after IHC.
Material and Methods
Isolation and identification of UC-MSCs
From full-term deliveries with the consent of the mother after cesarian section, human umbilical cords were collected and then stored in phosphate-buffered saline (PBS) with penicillin-streptomycin at 4°C aseptically. The process was approved by the institutional review board of the Chinese Academy of Medical Science and Peking Union Medical College. The isolation of MSCs was as described previously [14]. The cords were promptly cut into small pieces (about 1 mm3) with scissors, followed with digestion for 30 minutes with 0.075% collagenase II (Sigma, St Louis, Missouri, USA) and gentle agitation for another 30 minutes with 0.125% trypsin (Gibco, Grand Island, New York, USA) at 37°C in a water bath. The mixture was diluted with PBS and filtered through strainer mesh, and the supernatant was centrifuged at the speed of 250 g for 15 minutes. After rinsing with PBS, the cells were suspended in fresh growth media for plating onto non-coated plastic flasks. Through changing the media after 3 days, nonadherent cells were removed. The growth media was DMEM/F12 (1:1) (Gibco) supplemented with 10% fetal bovine serum (FBS; Hyclone, Logan, Utah, USA), penicillin-streptomycin 100 U/mL (Sigma), 1% glutamine (Sigma), and epidermal growth factor 10 ng/mL (Sigma) used as the maintenance and expansion system. Cells were cultured at 37°C in humidified 5% CO2 and formed colonies of spindle-shaped cells. At 60%–80% confluence, cells were harvested after treatment with 0.25% trypsin-EDTA (Gibco) and were reseeded for expansion. Passage 3 to passage 6 cells were used in this study. Flow cytometry to analyze phenotype and osteogenic and adipogenic differentiation were used to characterize UC-MSCs by previously described protocols [14,18].
ICH model construction
In this study, male Sprague-Dawley rats [19] (230 to 260 g) were chosen as experimental animals. All experimental procedures were approved by the Care of Experimental Animals Committee of Alliancells Key Institute of Stem Cells and Translational Regenerative Medicine and by the institutional review board for the use of human cells. Intrastriatal administration of bacterial collagenase used to induce ICH was as described in previous reports with minor modification [19]. Briefly, the anesthetized rats were injected intraperitoneally with 10% chloral hydrate (30 mg/kg) and placed in a stereotaxic frame. Collagenase type VII solution (0.5 U in 2 μL of sterilized PBS, Sigma) was injected into the striatum over a period of 5 minutes (0.4 μL/min) through a drilled burr hole based on the coordinates: 0.5 mm posterior, 6.0 mm ventral, and 3.0 mm lateral to the bregma. After injection, the syringe was retained in place for 5 minutes. Bone wax was used to seal the hole, and then the wound was sutured. In the meantime, 2 μL of PBS was injected at the same site in the sham group. The body temperature was maintained at 37±0.5°C, monitored with a thermistor-controlled heat blanket.
UC-MSC labeling and transplantation procedure
We used CM-DiI 3 μg/mL (Molecular Probes, Eugene, Oregon, USA) to label UC-MSCs at 37°C for 10 minutes and then at 4°C for another 15 minutes. After washing with PBS three times, UC-MSCs diluted at the certain concentration were ready for following procedures. Animals were divided into four groups. (1) In the MSC-IC group (n=20), 2×105 cells (10 μL) were transplanted into rats intracerebrally at a speed of 1 μL/minute and placed for another 5 minutes (coordinates: 0.5 mm posterior, 3.5 mm ventral, and 3.0 mm lateral to the bregma). (2) In the MSC-IV group (n=16), rats received 2×106 cells (1 mL) intravenously through the tail vein in one minute. (3) In the control group (n=16), rats received no treatment after ICH. (4) Group 4 was a sham group (n=10). In all groups, rats had no immunosuppressive medications.
Neurological evaluation
To assess the neurological function, the modified neurological severity score (mNSS) scale was adopted for use before ICH and at 0, 7, 14, 21, and 28 days after treatment by 2 individuals blinded to the experimental groups. The mNSS scale [20] is a composite of motor, sensory, reflex, and balance tests. A scale of 0 to 18 (normal score, 0; maximal deficit score, 18) was used to grade for neurological function, wherein 1 score point was awarded for the inability to perform the test or for the lack of a tested reflex; the higher the score, the more severe the injury.
Injury volume assay
Fourteen days after treatment was selected as the time point for quantitative analysis of injury volume. Rat brains were equally cut into consecutive 2-mm thick sections with brain matrix, and total injury volume was calculated with Image-Pro Plus software (Media Cybernetics, Carlsbad, California, USA) by summation of the lesioned areas of all slices. Two observers blinded to the experimental treatment carried out volume quantitation.
Immunohistochemical staining
Transcardial perfusion with saline was used to fix anesthetized animals and rat brains, followed by perfusion and immersion in 4% paraformaldehyde, and dehydration with 20% sucrose in 0.1 M PBS overnight. Corresponding to the coronal coordinates bregma 0.5 mm to −1.5 mm, the brain tissue was cut into a series of adjacent 10 μm thick coronal cryostat sections. Immunofluorescence of sections was done for visualization of the CM-DiI-labeled UC-MSC expression of neuron/glia-specific markers, which employed primary antibodies against microtubule-associated protein 2 (MAP2, 1:200; Chemicon), glial fibrillary acidic protein (GFAP, 1:1000; Dako, Carpinteria, California, USA), and von Willebrand factor (vWF, 1:400; Dako). Immunofluorescence identification was conducted with fluorescein isothiocyanate conjugated antibody (FITC; Chemicon). Negative control sections from each animal received identical preparations for immunohistochemical staining, except that primary antibodies were omitted. 4′,6-Diamidino-2-phenylindole (DAPI; Invitrogen) was for nucleus identification.
Vascular density quantitation
At 14 days post-transplantation, visualization of vascular regions was accomplished with vWF immunostaining. Five equidistant slices (from 0.5 mm anterior to 1.5 mm posterior to the bregma) from three animals per group were prepared for analysis. At a magnification of 200×, five random areas of the peri-ICH region per slice were photographed for measurement. The Image-Pro Plus software package was employed to calculate vascular areas, and vascular density was expressed by the percentage of vascular areas.
Statistical analysis
All data are presented as mean ±SD. Continuous variables between the two groups were compared by two-way analysis of variance (ANOVA). Results were considered statistically significant if P was less than 0.05. The statistical tests were conducted with the GraphPad Prism 5 software (GraphPad Prism, San Diego, California, USA).
Results
Characterization of UC-MSCs
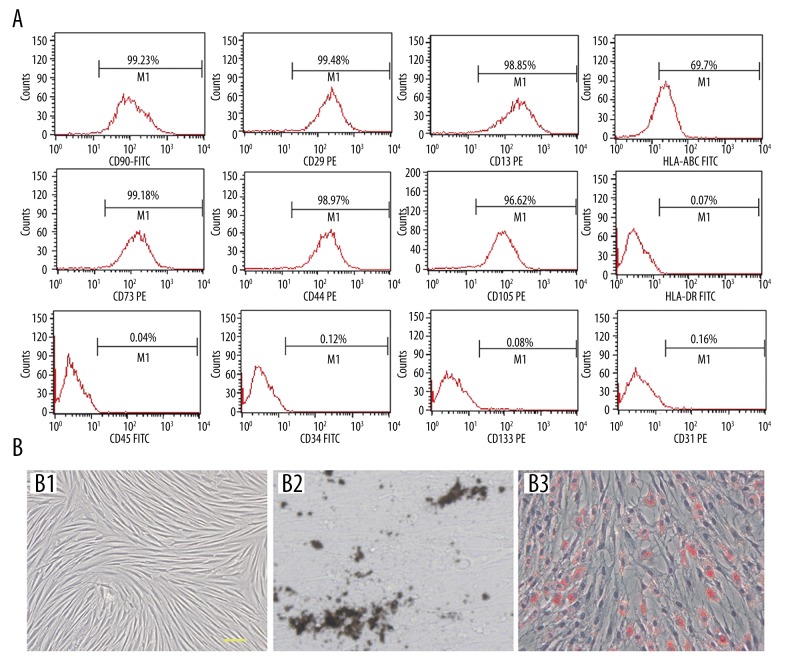
MSCs were isolated from human umbilical cord with a success rate of 100%, and they could be culture-expanded up to 30 passages without morphological change. Flow cytometry analysis showed that they were positive for CD90, CD29, CD13, CD73, CD44, and CD105; and negative for hematopoietic markers CD34 and CD45 and endothelial cell markers CD31 and CD133. In addition, UC-MSCs can express moderate HLA-ABC but no HLA-DR (Figure 1A). As shown by Van Kossa and Oil red staining, under specific culture condition, UC-MSCs were able to differentiate into both osteocytes and adipocytes, respectively, in vitro (Figure 1B).
Figure 1.
Characterization of UC-MSCs. (A) Flow cytometry analysis shows that UC-MSCs are positive for CD90, CD29, CD13, CD73, CD44, CD105, and HLA-ABC, while they are negative for CD34, CD45, CD31, and CD133. (B) UC-MSCs display fibroblastic morphology in plastic culture (B1). Von kossa (B2) and Oil red (B3) stainings reveal osteogenic and adipogenic potential of UC-MSCs in vitro. Scale bar=50 μm.
In vivo distribution of UC-MSCs after transplantation
At 28 days after transplantation, UC-MSCs, as labeled by red fluorescent CM-DiI, could be found in both the MSC-IC and MSC-IV groups. In the MSC-IC group, we found a cluster of cells that stayed where they were injected, with the majority of them distributed around the ICH region in the ipsilateral hemisphere; we also found that a small amount of donor cells had migrated to the contralateral hemisphere (data not shown). Interestingly, UC-MSCs injected via the tail vein also could be found in the ICH rat brain at 28 days after treatment, though in a limited amount. The preferred location of these cells also was around the ICH zone (data not shown).
Neurological improvement after UC-MSC treatment
One day after ICH, rats displayed severe neurological deficits as indicated by the high scores on the mNSS scale (Figure 2). The rats in the control group showed a gradual reduction of the score throughout the evaluation period, suggesting that they could to some extent recover from the ICH-induced injury, which may be attributed to endogenous restorative mechanisms. In rats that received UC-MSC treatment, both intracerebrally (t=6.462, P=0.000; t=4.393, P=0.000; in 7 and 21 days) and intravenously (t=6.343, P=0.000; t=2.746, P=0.013; t=4.501, P=0.000; t=2.317, P=0.028; in 7, 14, 21, and 28 days), we found accelerated improvement of neurological function as compared to the control group. There was no difference between the MSC-IC and MSC-IV groups (t=1.352, P=0.185) as to the improvement (Figure 2).
Figure 2.
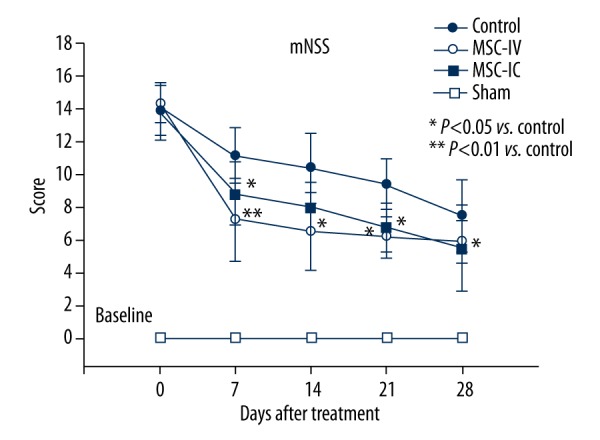
Neurological function improvement after UC-MSC treatment. Neurological evaluation with the mNSS scale shows that both intracerebral (MSC-IC) and intravenous (MSC-IV) injection of UC-MSCs significantly reduced neurological defects as compared with the control group; the sham group was used as the baseline (n=6 in each group).
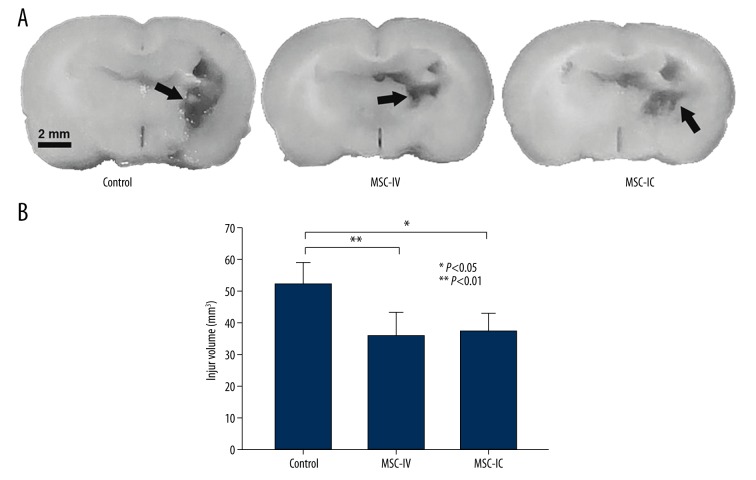
Hematoma, the primary consequence of ICH, can contribute to secondary brain injury, which leads to severe neurological deficits and sometimes delayed fatality [21]. At 14 days after UC-MSC treatment, the injury volume was found significantly reduced in both the MSC-IC (37.23±5.45 mm3, t=2.814, P=0.012) and MSC-IV (35.87±7.26 mm3, t=2.314, P=0.030) groups as compared with the control group (52.12±6.78 mm3). There also was no difference between the two cell-treated groups (t=1.293, P=0.142) (Figure 3). These data suggest the neuroprotective effect of UC-MSCs in ICH could be obtained with an alternative pathway of injection.
Figure 3.
Reduced injury volume in ICH brain after UC-MSC treatment. (A) Representative images show that injury regions (arrows) in both the MSC-IV and MSC-IC groups were obviously smaller compared to that in the control group at 14 days after treatment. (B) Injury volume quantitation. MSC-IC versus control, P<0.05; MSC-IV versus control, P<0.01. The difference in injury volume between the MSC-IC and MSC-IV groups was not significant (P>0.05); n=5 per group.
In vivo neural-specific protein expression of UC-MSCs
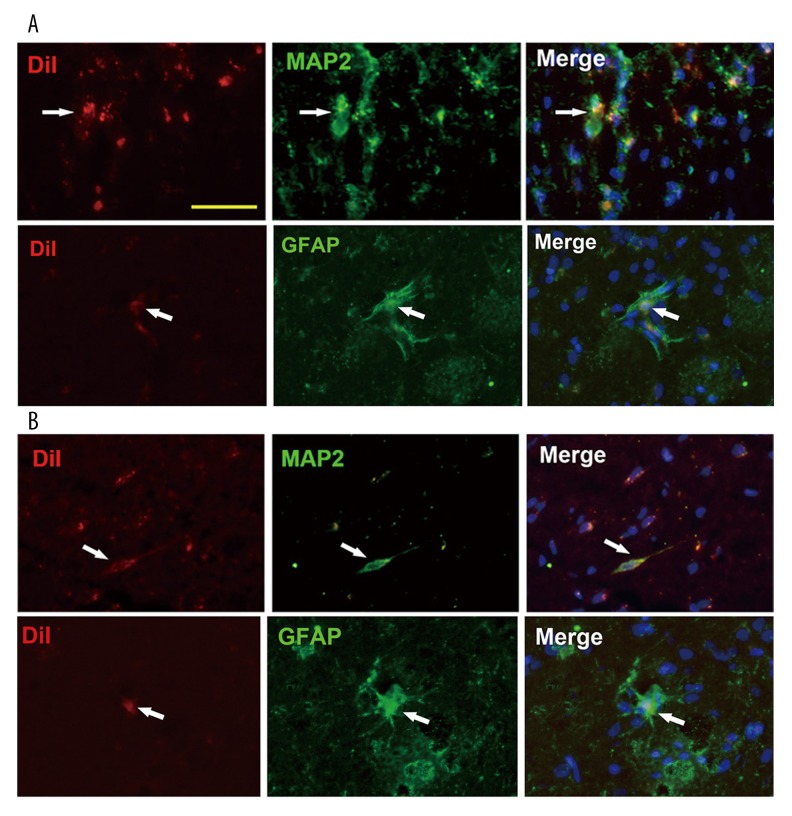
UC-MSCs have been reported to be able to differentiate into neural cells by several studies including our previous in vitro induction [14]. UC-MSCs could be found in rat brain in both the MSC-IC and MSC-IV groups at 4 weeks after injection. We detected whether these cells could express neural cell marker MAP2 or astrocyte marker GFAP in rat brain. By immunostaining, we observed that a subset of UC-MSCs colocalized with neural or glial markers in vivo in both the MSC-IC and MSC-IV groups (Figure 4). However, the frequency of the colocalization was very slow (<5%).
Figure 4.
Expression of neural-specific proteins of UC-MSCs in vivo. At 4 weeks post- transplantation, CM-DiI (red) labeled UC-MSCs could be found in rat brain in both the MSC-IC (A) and MSC-IV (B) groups. Immunostaining assay shows that a few donor cells colocalized with neural cell marker MAP2 or astrocyte marker GFAP in two groups (arrows). DAPI (blue) was used to identify the nucleus. Scale bar=50 μm.
Increased vascular density after intracerebral administration of UC-MSCs
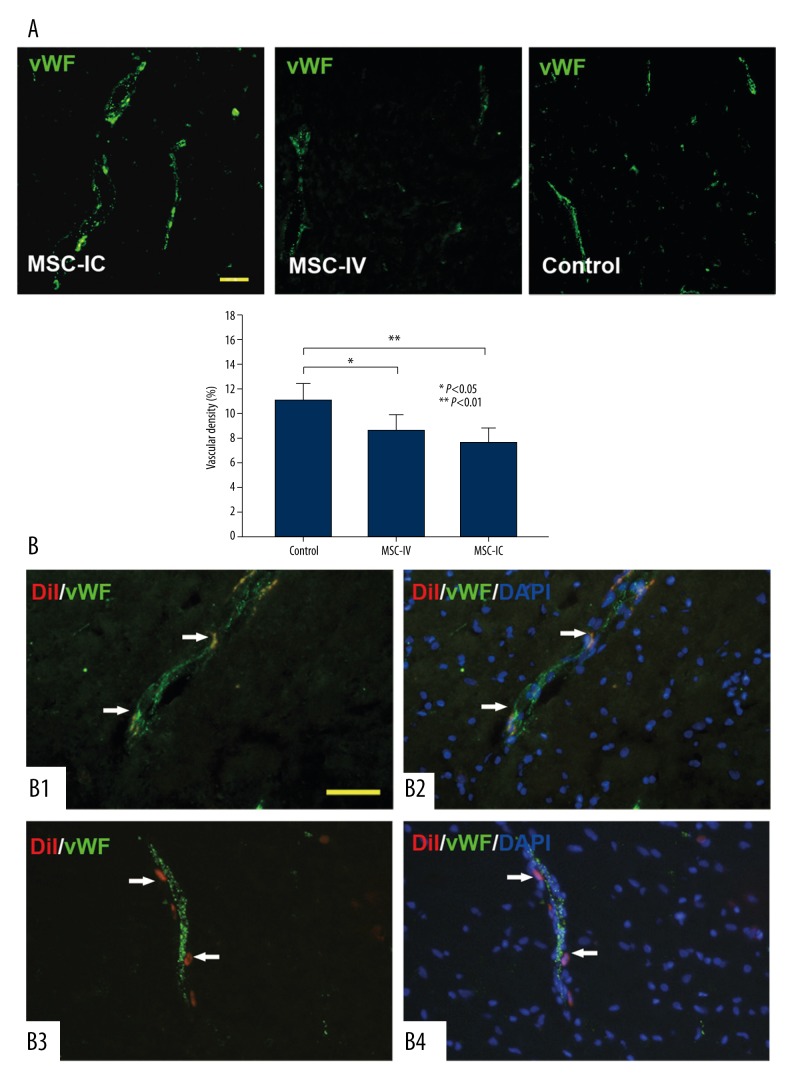
We have demonstrated that UC-MSCs could be beneficial in several ischemic animal models and that the mechanism may be associated with the ability to promote angiogenesis under injured condition [18,22]. Here, we also found that UC-MSC treatment was able to increase the vascular density in peri-ICH regions of the ipsilateral hemisphere. Of note is that this observation was only made in the MSC-IC group (11.02±1.33%) as compared to the control group (7.56±1.31%, t=3.721, P=0.002); we did not find a significant difference in vascular density between the MSC-IV group (8.58±1.27%, t=1.453, P=0.102) and the control group (Figure 5A).
Figure 5.
Pro-angiogenic effect of UC-MSCs. (A) Blood vessels are recognized by vWF immunostaining (green); representative images show that the vascular density in the MSC-IC group is significantly higher than that in the MSC-IV and control groups. The vascular density is not different between the MSC-IV group and the control group. n=3 per group. (B) At 14 days after treatment, in the MSC-IV group, a population of CM-DiI-labeled UC-MSC cells (red) either located in the endothelium and expressed endothelial-specific marker vWF (B1, B2) or located in blood vessel walls (B3, B4). DAPI (blue) was used to identify the nucleus. Scale bars=50 μm.
In addition, in the MSC-IV group, we observed that donor cells labeled by CM-DiI showed a proximity to blood vessels. They either incorporated into the cerebral endothelium (Figure 5B–B1,B2) or located in blood vessel walls (Figure 5B–B3,B4), and a portion of the incorporated cells colocalized with endothelial cell–specific protein vWF (Figure 5B–B1,B2). This proximity of UC-MSCs to cerebral vasculature was also observed in the MSC-IV group (data not shown).
Discussion
There are several stem cell candidates for cell-based therapy in CNS diseases, including embryonic stem cells (ESCs), neural stem cells (NSCs)/olfactory ensheathing cells (OECs), and MSCs. ESCs are pluripotent cells that can give rise to all cell and tissue types, while obstacles such as ethical problems and immune rejection hinder their utilization. The newly reported induced pluripotent stem (iPS) cells are an intriguing substitute for ESCs in regard to the direct reprogramming from somatic cells without destruction of the embryo [23]. There are, however, still many concerns including low efficiency of reprogramming and risk of tumorigenesis. As to NSCs or OECs, drawbacks like limited cell sources and expansion capacity also handicap their application in neural diseases. MSCs can self-renew and differentiate into multiple tissue cells such as osteocytes, cardiocytes, and neural cells [1,6]. In addition, MSCs are capable of releasing a variety of cytokines and growth factors that have both paracrine and autocrine activities [4,24]. In addition, MSCs are immune tolerant, which enables them to cross HLA-mismatched barriers; also, they have immunosuppressive functions to limit the inflammation after tissue injury, which may be good for the tissue injury repair [3,25,26]. These data imply that MSCs might be promising candidates for cell-based therapy.
In this study, we demonstrated that UC-MSCs could bring about neurological benefits in ICH rats via intracerebral or intravenous delivery. The transplanted UC-MSCs could survive up to 4 weeks in rat brain, which on one hand may be attributed to the relatively immunoprivileged environment of the brain [27] and on another may correlate with the immune-tolerant feature of MSCs, due to their low or no expression of HLA-DR (Figure 1) and costimulatory factors [2]. Therefore, CNS may be regarded as a promising tissue target for cell therapy, especially using MSCs. Accordingly, others have demonstrated that porcine or human UC-MSCs were immunotolerant in rat brain or spinal cord [28–30].
It is important to optimize the delivery pathway by which stem cells are injected with respect to feasibility and efficiency in future clinical applications. In terms of cell-based therapy in CNS diseases, the potential pathways include intracerebral (focal site or around), intravenous, intracarotid, intracerebroventricular, and subarachnoid injection. Here, we showed that both intracerebral and intravenous administration of UC-MSCs was effective to promote neurological recovery of ICH rats, suggesting that UC-MSCs may be delivered via alternative methods with therapeutic effects. Consistently, previous reports also demonstrated that focal or systemic delivery of MSCs equally improved the neurological functions of animals after cerebral ischemia [20,31]. Interestingly, we observed that UC-MSCs had a tendency to migrate from the injection site to the injury site in both the intracerebral and intravenous groups, suggesting that injured tissues may provide chemotactic cues for UC-MSCs’ migration, even crossing the blood-brain barrier. Indeed, MSCs could express a broad array of chemokines receptors [32], and a couple of studies have reported the injured tissue-oriented migration of MSCs in cerebral ischemia; the underlying mechanism may be associated with the effect of the SDF-1/CXCR4 axis [33–35]. With respect to convenience, the intravenous implantation of UC-MSCs could serve as a favorable approach for cell-based therapy in CNS diseases according to clinical needs.
We found that a subset of UC-MSCs could express neural-specific protein in rat brain in both the intracerebral and intravenous injection groups; it is, however, hard to ascribe the benefit to the cell replacement since the phenomenon was uncommon, which is parallel to the observation of others [20,36]. Also, it should be cautioned that it may have resulted from cell fusion under in vivo condition [37], though several studies have shown that MSCs could differentiate into neural cells in vitro [38,39]. What’s more, whether they communicated with host tissue functionally remains unknown even if those cells really differentiated into neural cells.
The mechanism underlying the benefits of UC-MSC injection in ICH may relate to UC-MSCs’ paracrine activities, which are neuroprotective to injured brain tissue. It is well documented that MSCs are able to secrete a variety of factors such as NGF, BDNF, HGF, G-CSF, and VEGF [13,17,40,41], upregulation of which seems to be involved in the repair of neural injury by MSC treatment [42,43]. Therefore, in the present study the enhanced angiogenesis after UC-MSC treatment may have been associated with the pro-angiogenic factors’ upregulation. Supporting evidence could also be derived from the fact that angiogenesis plays a crucial role in brain development and repair [44], and endogenous cerebral angiogenesis is induced and seems to be a restorative pathway after ICH [19]. In addition to the ability to produce angiogenic factors, MSCs can differentiate into endothelial cells [45] or function as vascular supporting cells as observed in our previous and present studies [18,22]. This may implicate another mechanism of MSCs through remodeling or stabilizing vasculature to promote the angiogenic process after ICH. Vedat et al. also reported that “Sodium nitrite provides angiogenic and proliferative effects in vivo and in vitro” [46]. In line with this, recent advances reveal that the perivascular region very likely represents the in vivo niche of MSCs [47], suggesting the close relationship between MSCs and the vascular system.
Of note is that the enhanced vascular density was only found in rats that received UC-MSCs intracerebrally but not intravenously, although we observed that donor cells migrated into rat brain and integrated into blood vessels in the peri-ICH zone through systemic injection. This may be attributed to the limited numbers of cells that migrated. Given the comparability of the functional benefit in the two groups, the underlying mechanisms that mediate the benefits may be different between them. Therefore, it is speculated that a combination of these two means of delivering MSCs may have synergetic effects and result in more significant neurological improvement. Further studies are required to test this hypothesis and clarify the detailed mechanisms.
Conclusions
We demonstrated that UC-MSC treatment, via the intracerebral or the intravenous pathway, was able to promote neurological recovery in rats after ICH, and these effects may be associated with augmentation of angiogenesis in the intracerebral injection group. Apart from angiogenesis [31], previous experience from MSC transplantation in cerebral ischemia has implied that MSCs also had effects in reducing scar formation, inhibiting apoptosis, remodeling astrocyte axons, and stimulating endogenous NSC activity [48,49]. Whether these effects occur in ICH after MSC transplantation warrants further investigation. Nevertheless, our results suggest that UC-MSC transplantation provides a promising approach in the treatment of CNS diseases in future clinics and that intravenous implantation of UC-MSCs could serve as a favorable approach for cell-based therapy in CNS diseases according to clinical needs.
Acknowledgement
The authors acknowledge Dr. Hanyu Wang for critical reading of the manuscript.
Footnotes
Source of support: Departmental sources
References
- 1.Jiang Y, Jahagirdar BN, Reinhardt RL, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–49. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- 2.Weiss ML, Anderson C, Medicetty S, et al. Immune properties of human umbilical cord Wharton’s jelly-derived cells. Stem Cells. 2008;26:2865–74. doi: 10.1634/stemcells.2007-1028. [DOI] [PubMed] [Google Scholar]
- 3.Suzdaltseva YG, Burunova VV, Vakhrushev IV, et al. In vitro comparison of immunological properties of cultured human mesenchymal cells from various sources. Bull Exp Biol Med. 2008;145:228–31. doi: 10.1007/s10517-008-0057-y. [DOI] [PubMed] [Google Scholar]
- 4.Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98:1076–84. doi: 10.1002/jcb.20886. [DOI] [PubMed] [Google Scholar]
- 5.Zhu J, Liu Q, Jiang Y, et al. Enhanced angiogenesis promoted by human umbilical mesenchymal stem cell transplantation in stroked mouse is Notch1 signaling associated. Neuroscience. 2015;290:288–99. doi: 10.1016/j.neuroscience.2015.01.038. [DOI] [PubMed] [Google Scholar]
- 6.Koh SH, Kim KS, Choi MR, et al. Implantation of human umbilical cord-derived mesenchymal stem cells as a neuroprotective therapy for ischemic stroke in rats. Brain Res. 2008;1229:233–48. doi: 10.1016/j.brainres.2008.06.087. [DOI] [PubMed] [Google Scholar]
- 7.Lee KH, Suh-Kim H, Choi JS, et al. Human mesenchymal stem cell transplantation promotes functional recovery following acute spinal cord injury in rats. Acta Neurobiol Exp (Wars) 2007;67:13–22. doi: 10.55782/ane-2007-1628. [DOI] [PubMed] [Google Scholar]
- 8.Gerdoni E, Gallo B, Casazza S, et al. Mesenchymal stem cells effectively modulate pathogenic immune response in experimental autoimmune encephalomyelitis. Ann Neurol. 2007;61:219–27. doi: 10.1002/ana.21076. [DOI] [PubMed] [Google Scholar]
- 9.Wang C, Fei Y, Xu C, et al. Bone marrow mesenchymal stem cells ameliorate neurological deficits and blood-brain barrier dysfunction after intracerebral hemorrhage in spontaneously hypertensive rats. Int J Clin Exp Pathol. 2015;8:4715–24. [PMC free article] [PubMed] [Google Scholar]
- 10.Yamauchi T, Kuroda Y, Morita T, et al. Therapeutic effects of human multilineage-differentiating stress enduring (MUSE) cell transplantation into infarct brain of mice. PLoS One. 2015;10:e0116009. doi: 10.1371/journal.pone.0116009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Friedenstein AJ, Gorskaja JF, Kulagina NN. Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Exp Hematol. 1976;4:267–74. [PubMed] [Google Scholar]
- 12.da Silva Meirelles L, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci. 2006;119:2204–13. doi: 10.1242/jcs.02932. [DOI] [PubMed] [Google Scholar]
- 13.Friedman R, Betancur M, Boissel L, et al. Umbilical cord mesenchymal stem cells: Adjuvants for human cell transplantation. Biol Blood Marrow Transplant. 2007;13:1477–86. doi: 10.1016/j.bbmt.2007.08.048. [DOI] [PubMed] [Google Scholar]
- 14.Lu LL, Liu YJ, Yang SG, et al. Isolation and characterization of human umbilical cord mesenchymal stem cells with hematopoiesis-supportive function and other potentials. Haematologica. 2006;91:1017–26. [PubMed] [Google Scholar]
- 15.Hsieh JY, Wang HW, Chang SJ, et al. Mesenchymal stem cells from human umbilical cord express preferentially secreted factors related to neuroprotection, neurogenesis, and angiogenesis. PLoS One. 2013;8:e72604. doi: 10.1371/journal.pone.0072604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aronowski J, Hall CE. New horizons for primary intracerebral hemorrhage treatment: experience from preclinical studies. Neurol Res. 2005;27:268–79. doi: 10.1179/016164105X25225. [DOI] [PubMed] [Google Scholar]
- 17.Quittet MS, Touzani O, Sindji L, et al. Effects of mesenchymal stem cell therapy, in association with pharmacologically active microcarriers releasing VEGF, in an ischaemic stroke model in the rat. Acta Biomater. 2015;15:77–88. doi: 10.1016/j.actbio.2014.12.017. [DOI] [PubMed] [Google Scholar]
- 18.Wu KH, Zhou B, Lu SH, et al. In vitro and in vivo differentiation of human umbilical cord derived stem cells into endothelial cells. J Cell Biochem. 2007;100:608–16. doi: 10.1002/jcb.21078. [DOI] [PubMed] [Google Scholar]
- 19.Tang T, Liu XJ, Zhang ZQ, et al. Cerebral angiogenesis after collagenase-induced intracerebral hemorrhage in rats. Brain Res. 2007;1175:134–42. doi: 10.1016/j.brainres.2007.08.028. [DOI] [PubMed] [Google Scholar]
- 20.Chen J, Li Y, Wang L, et al. Therapeutic benefit of intracerebral transplantation of bone marrow stromal cells after cerebral ischemia in rats. J Neurol Sci. 2001;189:49–57. doi: 10.1016/s0022-510x(01)00557-3. [DOI] [PubMed] [Google Scholar]
- 21.Zazulia AR, Diringer MN, Derdeyn CP, Powers WJ. Progression of mass effect after intracerebral hemorrhage. Stroke. 1999;30:1167–73. doi: 10.1161/01.str.30.6.1167. [DOI] [PubMed] [Google Scholar]
- 22.Wu KH, Zhou B, Yu CT, et al. Therapeutic potential of human umbilical cord derived stem cells in a rat myocardial infarction model. Ann Thorac Surg. 2007;83:1491–98. doi: 10.1016/j.athoracsur.2006.10.066. [DOI] [PubMed] [Google Scholar]
- 23.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–76. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 24.Mayer H, Bertram H, Lindenmaier W, et al. Vascular endothelial growth factor (VEGF-A) expression in human mesenchymal stem cells: Autocrine and paracrine role on osteoblastic and endothelial differentiation. J Cell Biochem. 2005;95:827–39. doi: 10.1002/jcb.20462. [DOI] [PubMed] [Google Scholar]
- 25.Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007;110:3499–506. doi: 10.1182/blood-2007-02-069716. [DOI] [PubMed] [Google Scholar]
- 26.Chen M, Li X, Zhang X, et al. The inhibitory effect of mesenchymal stem cell on blood-brain barrier disruption following intracerebral hemorrhage in rats: Contribution of TSG-6. J Neuroinflammation. 2015;12:61. doi: 10.1186/s12974-015-0284-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matyszak MK. Inflammation in the CNS: Balance between immunological privilege and immune responses. Prog Neurobiol. 1998;56:19–35. doi: 10.1016/s0301-0082(98)00014-8. [DOI] [PubMed] [Google Scholar]
- 28.Weiss ML, Mitchell KE, Hix JE, et al. Transplantation of porcine umbilical cord matrix cells into the rat brain. Exp Neurol. 2003;182:288–99. doi: 10.1016/s0014-4886(03)00128-6. [DOI] [PubMed] [Google Scholar]
- 29.Yang CC, Shih YH, Ko MH, et al. Transplantation of human umbilical mesenchymal stem cells from Wharton’s jelly after complete transection of the rat spinal cord. PLoS One. 2008;3:e3336. doi: 10.1371/journal.pone.0003336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Divya MS, Roshin GE, Divya TS, et al. Umbilical cord blood-derived mesenchymal stem cells consist of a unique population of progenitors co-expressing mesenchymal stem cell and neuronal markers capable of instantaneous neuronal differentiation. Stem Cell Res Ther. 2012;3:57. doi: 10.1186/scrt148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen J, Zhang ZG, Li Y, et al. Intravenous administration of human bone marrow stromal cells induces angiogenesis in the ischemic boundary zone after stroke in rats. Circ Res. 2003;92:692–99. doi: 10.1161/01.RES.0000063425.51108.8D. [DOI] [PubMed] [Google Scholar]
- 32.Ringe J, Strassburg S, Neumann K, et al. Towards in situ tissue repair: human mesenchymal stem cells express chemokine receptors CXCR1, CXCR2 and CCR2, and migrate upon stimulation with CXCL8 but not CCL2. J Cell Biochem. 2007;101:135–46. doi: 10.1002/jcb.21172. [DOI] [PubMed] [Google Scholar]
- 33.Cui X, Chen J, Zacharek A, et al. Nitric oxide donor upregulation of stromal cell-derived factor-1/chemokine (CXC motif) receptor 4 enhances bone marrow stromal cell migration into ischemic brain after stroke. Stem Cells. 2007;25:2777–85. doi: 10.1634/stemcells.2007-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shichinohe H, Kuroda S, Yano S, et al. Role of SDF-1/CXCR4 system in survival and migration of bone marrow stromal cells after transplantation into mice cerebral infarct. Brain Res. 2007;1183:138–47. doi: 10.1016/j.brainres.2007.08.091. [DOI] [PubMed] [Google Scholar]
- 35.Wang Y, Deng Y, Zhou GQ. SDF-1alpha/CXCR4-mediated migration of systemically transplanted bone marrow stromal cells towards ischemic brain lesion in a rat model. Brain Res. 2008;1195:104–12. doi: 10.1016/j.brainres.2007.11.068. [DOI] [PubMed] [Google Scholar]
- 36.Lund RD, Wang S, Lu B, et al. Cells isolated from umbilical cord tissue rescue photoreceptors and visual functions in a rodent model of retinal disease. Stem Cells. 2007;25:602–11. doi: 10.1634/stemcells.2006-0308. [DOI] [PubMed] [Google Scholar]
- 37.Alvarez-Dolado M, Pardal R, Garcia-Verdugo JM, et al. Fusion of bone-marrow-derived cells with Purkinje neurons, cardiomyocytes and hepatocytes. Nature. 2003;425:968–73. doi: 10.1038/nature02069. [DOI] [PubMed] [Google Scholar]
- 38.Fu YS, Shih YT, Cheng YC, Min MY. Transformation of human umbilical mesenchymal cells into neurons in vitro. J Biomed Sci. 2004;11:652–60. doi: 10.1007/BF02256131. [DOI] [PubMed] [Google Scholar]
- 39.Woodbury D, Schwarz EJ, Prockop DJ, Black IB. Adult rat and human bone marrow stromal cells differentiate into neurons. J Neurosci Res. 2000;61:364–70. doi: 10.1002/1097-4547(20000815)61:4<364::AID-JNR2>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 40.Kim DH, Yoo KH, Choi KS, et al. Gene expression profile of cytokine and growth factor during differentiation of bone marrow-derived mesenchymal stem cell. Cytokine. 2005;31:119–26. doi: 10.1016/j.cyto.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 41.Chen X, Li Y, Wang L, et al. Ischemic rat brain extracts induce human marrow stromal cell growth factor production. Neuropathology. 2002;22:275–79. doi: 10.1046/j.1440-1789.2002.00450.x. [DOI] [PubMed] [Google Scholar]
- 42.Zacharek A, Chen J, Cui X, et al. Angiopoietin1/Tie2 and VEGF/Flk1 induced by MSC treatment amplifies angiogenesis and vascular stabilization after stroke. J Cereb Blood Flow Metab. 2007;27:1684–91. doi: 10.1038/sj.jcbfm.9600475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mahmood A, Lu D, Chopp M. Intravenous administration of marrow stromal cells (MSCs) increases the expression of growth factors in rat brain after traumatic brain injury. J Neurotrauma. 2004;21:33–39. doi: 10.1089/089771504772695922. [DOI] [PubMed] [Google Scholar]
- 44.Zadeh G, Guha A. Angiogenesis in nervous system disorders. Neurosurgery. 2003;53:1362–74. doi: 10.1227/01.neu.0000093425.98136.31. discussion 1374–76. [DOI] [PubMed] [Google Scholar]
- 45.Oswald J, Boxberger S, Jorgensen B, et al. Mesenchymal stem cells can be differentiated into endothelial cells in vitro. Stem Cells. 2004;22:377–84. doi: 10.1634/stemcells.22-3-377. [DOI] [PubMed] [Google Scholar]
- 46.Vedat Y, Suat D, Fatih Y, et al. Sodium nitrite provides angiogenic and proliferative effects in vivo and in vitro. Med Sci Monit Basic Res. 2015;21:41–46. doi: 10.12659/MSMBR.893727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Crisan M, Yap S, Casteilla L, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–13. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 48.Li Y, McIntosh K, Chen J, et al. Allogeneic bone marrow stromal cells promote glial-axonal remodeling without immunologic sensitization after stroke in rats. Exp Neurol. 2006;198:313–25. doi: 10.1016/j.expneurol.2005.11.029. [DOI] [PubMed] [Google Scholar]
- 49.Chen J, Li Y, Katakowski M, et al. Intravenous bone marrow stromal cell therapy reduces apoptosis and promotes endogenous cell proliferation after stroke in female rat. J Neurosci Res. 2003;73:778–86. doi: 10.1002/jnr.10691. [DOI] [PubMed] [Google Scholar]