ABSTRACT
Objective:
To determine whether COPD severity correlates with sputum cell counts, atopy, and asthma.
Methods:
This was a cross-sectional study involving 37 patients with COPD and 22 healthy subjects with normal lung function (controls). Sputum cell counts were determined by microscopy after centrifugation of samples. Skin prick tests were performed, and serum cytokines were determined by ELISA.
Results:
Patients were stratified by bronchodilator response: a non-reversible airflow limitation (nonRAL) group comprised 24 patients showing no significant post-bronchodilator change in FEV1; and a partially reversible airflow limitation (partialRAL) group comprised 13 patients showing FEV1 reversibility (post-bronchodilator FEV1 increase ≥ 12%). The proportion of eosinophils in sputum was higher in the partialRAL group than in the nonRAL group (p < 0.01), and there was an inverse correlation between the proportion of eosinophils and FEV1 (p < 0.05). However, none of the patients had a history of asthma and skin prick test results did not differ between the two groups. In the patient sputum samples, neutrophils predominated. Serum levels of TNF, IL-6, IL-8, and RANTES (CCL5) were higher in patients than in controls (p < 0.001) but did not differ between the two patient groups.
Conclusions:
COPD patients with partial FEV1 reversibility appear to have higher sputum eosinophil counts and greater airway hyperresponsiveness than do those with no FEV1 reversibility. However, we found that COPD severity did not correlate with atopy or with the cytokine profile.
Keywords: Pulmonary disease, chronic obstructive; Cytokines; Chemokines; Eosinophils; Sputum/cytology; Forced expiratory volume
RESUMO
Objetivo:
Determinar se a gravidade da DPOC se correlaciona com a contagem de células no escarro, atopia e asma.
Métodos:
Estudo transversal com 37 pacientes com DPOC e 22 indivíduos saudáveis com função pulmonar normal (controles). As contagens de células no escarro foram determinadas por microscopia após a centrifugação das amostras. Foram realizados testes cutâneos de puntura, e as citocinas séricas foram determinadas por ELISA.
Resultados:
Os pacientes foram estratificados pela resposta ao broncodilatador: o grupo de limitação ao fluxo aéreo não reversível (LFAnr) envolveu 24 pacientes sem alteração significativa do VEF1 pós-broncodilatador, e o grupo de limitação ao fluxo aéreo parcialmente reversível (LFApr) envolveu 13 pacientes com reversibilidade do VEF1 (aumento do VEF1 pós-broncodilatador ≥ 12%). A proporção de eosinófilos no escarro foi maior no grupo LFApr do que no LFAnr (p < 0,01), e houve uma correlação inversa entre a proporção de eosinófilos e VEF1 (p < 0,05). Entretanto, nenhum dos pacientes apresentou histórico de asma e os resultados dos testes cutâneos não diferiram entre os dois grupos. Nas amostras de escarro dos pacientes, os neutrófilos predominaram. Os níveis séricos de TNF, IL-6, IL-8 e RANTES (CCL5) foram maiores nos pacientes que nos controles (p < 0,001), mas não diferiram entre os dois grupos de pacientes.
Conclusões:
Pacientes com DPOC e reversibilidade parcial do VEF1 parecem apresentar maiores contagens de eosinófilos no escarro e maior hiper-responsividade das vias aéreas que aqueles sem reversibilidade do VEF1. Entretanto, a gravidade da DPOC não se correlacionou com atopia ou perfil das citocinas.
INTRODUCTION
COPD is an inflammatory disorder that affects the airways, pulmonary parenchyma, and pulmonary vessels, progressing slowly to irreversible airway obstruction. Although studies have shown that neutrophil and eosinophil counts are both elevated during COPD exacerbations, neutrophilic inflammation is the norm in COPD. 1 However, even in the stable phase of the disease, eosinophils are found in up to 40% of patients. 2 It is thought that this feature is related to a COPD subtype-COPD with asthma, also known as the asthma-COPD overlap syndrome. 2 Patients with that syndrome show FEV1 reversibility after bronchodilator use.
The inflammatory response of the airways has received special attention in recent years. 3 , 4 Levels of inflammatory mediators such as C-reactive protein, IL-8, IL-6, TNF, and RANTES (CCL5) have been found to be elevated in COPD. 5 - 7 In addition, neutrophil counts are higher in smokers with COPD, as are levels of IL-8 and eosinophil cationic protein. 4 According to Lapperre et al., 8 it is possible that inflammation associated with smoking occurs in two stages: an initial phase, during which neutrophils and macrophages are present in the epithelium and submucosa, and a late stage, with the additional participation of lymphocytes and eosinophils. Nevertheless, the association between COPD and asthma has been controversial, and the influence that eosinophils have on airway inflammation and on the severity of COPD is not completely understood.
The severity of airflow obstruction can be determined by quantifying the magnitude of the decrease in FEV1, and the stages of COPD are based on the post-bronchodilator FEV1. 9 Once the diagnosis of COPD has been made, pulmonary function tests are useful for quantitative monitoring of the course of the disease. To determine the severity of COPD, FEV1 and its reversibility are reconciled with the Global Initiative for Chronic Obstructive Lung Disease (GOLD) classification. 10 The aim of the present study was to determine whether COPD severity correlates with FEV1 reversibility, asthma, and atopy. In addition, we evaluated the relationship between serum cytokine levels and COPD subtypes based on FEV1 reversibility.
METHODS
This was a cross-sectional study involving 37 patients diagnosed with COPD on the basis of the GOLD criteria. 11 All patients were under treatment in the Pulmonology Department of Professor Edgard Santos University Hospital, in the city of Salvador, Brazil. The study was approved by the Research Ethics Committee of the Hospital (Protocol no. 113/2012), and all participating patients gave written informed consent. All of the patients completed questionnaires designed to evaluate the history of asthma in childhood, smoking, passive smoking, and allergic rhinitis. All patients also underwent physical examinations and pulmonary function tests with an emphasis on the functional parameters FEV1, FVC, and FEV1/FVC ratio. According to the GOLD criteria, 11 an FEV1/FVC ratio ≤ 70% of the predicted value is diagnostic of COPD. Spontaneous or induced sputum samples were obtained, skin prick tests were performed in order to assess sensitivity to allergens, and 10-mL blood samples were collected for determination of serum cytokine levels. A group of 22 healthy subjects without COPD (with normal lung function) were used as controls.
On the basis of the pulmonary function test parameters established in the 2010 GOLD guidelines and the response to bronchodilator, the COPD patients were divided into two groups: non-reversible airflow limitation (nonRAL), comprising the patients who showed no significant post-bronchodilator change in FEV1 (n = 24); and partially reversible airflow limitation (partialRAL), comprising the patients who showed FEV1 reversibility (n = 13). Post-bronchodilator FEV1 reversibility was defined as a ≥ 12% increase in FEV1, as proposed in the joint American Thoracic Society (ATS)/European Respiratory Society (ERS)/GOLD guidelines. 12 The control group was composed of (N = 22) healthy subjects without COPD and with normal lung function.
All patients underwent spirometry in accordance with the ATS/ERS/GOLD joint guidelines. 12 Bronchodilator testing was performed with 100 µg/mL of albuterol sulfate (Aerolin(r) [Ventolin(r)]; GlaxoSmithKline Brasil Ltda., Rio de Janeiro, Brazil). In brief, four puffs (400 µg/mL) were administered with the aid of a spacer (Fumax(r); GlaxoSmithKline Brasil Ltda.). At 15 min after administration of the bronchodilator, the pulmonary function tests were repeated.
Patients were submitted to the immediate hypersensitivity skin prick tests, as described by Pepys et al. 13 and modified by Osterbalee and Weeke. 14 The allergens tested included dog dander, cat dander, airborne fungi (Aspergillus fumigatus ), cockroach allergens (from Blattella germanica and Periplaneta americana ), and dust mite allergens (from Dermatophagoides pteronyssinus and Blomia tropicalis ). Reagents were obtained from Immunotech ([a division of] FDA Allergenic Ltda., Rio de Janeiro, Brazil).
Sputum induction was performed in accordance with the modified protocol described by Pavord et al., 15 with inhalation of hypertonic saline solution (3%, 4%, and 5%) in an ultrasonic nebulizer (Fisoneb(r); Canadian Medical Products, Ltd, Markham, Ontario, Canada) at a low flow rate (0.87 L/min). Peripheral blood samples (10 mL each) were centrifuged at 2,000 rpm for 10 min. Serum was collected and stored at −20°C for subsequent cytokine measurements. Cytokines and chemokines were quantified by sandwich ELISA according to the manufacturer's protocol (R&D Systems, Minneapolis, MN, USA). To quantify TNF and IL-6, we used high sensitivity kits (Quantikine HS ELISA; R&D Systems). To quantify RANTES (CCL5) and IL-8, we used DuoSet ELISA kits (R&D Systems).
In the statistical analysis, we used measures of central tendency, including means and medians, for demographic and clinical variables. Data were analyzed using the Statistical Package for the Social Sciences, version 17.0 for Windows (SPSS Inc., Chicago, IL, USA). The values obtained for the variables body mass index, SpO2, and lung function, which typically have a normal distribution, were analyzed with Student's t-tests. For the comparison between sputum cell counts and the stages of COPD severity, we used the Mann-Whitney test. The correlation between eosinophils in sputum and FEV1 (before and after bronchodilator use) was analyzed by Spearman's correlation coefficient. The comparisons among the nonRAL, partialRAL, and control groups, in terms of cytokine production, sputum cell counts, and COPD severity, were made with the Kruskal-Wallis test, followed by Dunn's post-test for multiple comparisons.
RESULTS
Characteristics of the sample
The demographic features, smoking status, and pulmonary function test results of the COPD patients, by group (with or without post-bronchodilator FEV1 reversibility), are shown in Table 1. There were no differences between the two patient groups regarding age, gender, or smoking status. There were also no differences between the two groups in terms of the body mass index, SpO2, or age at the onset of symptoms. All patients in the sample had an FEV1/FVC ratio ≤ 70% of the predicted value. The median values for pre- and post-bronchodilator FEV1 were 48.2% (range, 30-66%) and 51% (range, 35-71%), respectively, in the nonRAL group, compared with 35% (range, 28-44%) and 47% (range, 36-52%), respectively, in the partialRAL group (p < 0.04), whereas they were 79% (range, 65-82%) and 84% (range, 69-89%), respectively, in the control group. None of the control subjects were smokers or former smokers.
Table 1. Demographic, clinical, and pulmonary function characteristics of patients with COPD.a .
| Characteristic | Group | p* | |
|---|---|---|---|
| nonRAL | partialRAL | ||
| (n = 24) | (n = 13) | ||
| Gender, n (%) | |||
| Male | 11 (45.8) | 6 (46.2) | 0.72 |
| Female | 13 (54.2) | 7 (53.8) | |
| Age, years | 66 (57-73) | 69 (59-76) | 0.88 |
| Smoking, n (%) | 8 (27.3) | 4 (30.8) | 0.67 |
| Former smokers, n (%) | 16 (72.7) | 9 (69.2) | 0.43 |
| Body mass index, kg/m2 | 22 (19-25) | 18 (18-21) | 0.001 |
| SpO2, % | 96 (94-97) | 95 (93-97) | 0.93 |
| Age at symptom onset, years | 54 (42-58) | 55 (43-56) | 0.97 |
| FEV1/FVC ratio, % of predicted | |||
| Pre-BD | 64 (53-70) | 59 (53-69) | 0.44 |
| Post-BD | 66 (51-62) | 62 (57-68) | |
| FEV1, % of predicted | |||
| Pre-BD | 48 (30-66) | 35 (28-42) | 0.04 |
| Post-BD | 51 (35-71) | 40 (35-50) | |
| FVC, % of predicted | |||
| Pre-BD | 69 (57-84) | 58 (53-66) | 0.06 |
| Post-BD | 76 (64-89) | 69 (59-77) | |
nonRAL: non-reversible airflow limitation; partialRAL: partially reversible airflow limitation; and BD: bronchodilator. aValues expressed as median (interquartile range), except where otherwise indicated. *Student's t-test (two-tailed); level of statistical significance set at p < 0.05.
COPD severity, sputum cell counts, and atopy
As can be seen in Table 2, the severity of COPD was assessed in accordance with the 2010 GOLD guidelines. The 24 patients in the nonRAL group were fairly equally distributed among the COPD stages II, III, and IV, whereas 12 (92.3%) of the 13 patients in the partialRAL group were categorized as having stage III COPD. Although neutrophil counts increased in proportion with the severity of COPD, eosinophils were not detected in patients with stage IV COPD (data not shown).
Table 2. Cell counts in induced sputum samples, by COPD stage, and results of allergy testing in patients with COPD, by group.a .
| Variable | Group | p | |
| nonRAL | partialRAL | ||
| (n = 24) | (n = 13) | ||
| GOLD stage | |||
| II | 6 (27.3) | 0 (0.0) | 0.03 |
| III | 12 (45.5) | 12 (92.3) | 0.77 |
| IV | 6 (27.3) | 1 (7.7) | 0.43 |
| Skin prick test | |||
| Positive | 5 (20.8) | 4 (30.8) | 0.58 |
| Negative | 17 (70.8) | 9 (69.2) | 0.09 |
| Proportion of neutrophilsb | 38 (24-52) | 37 (26-47) | 0.67 |
| Proportion of eosinophilsb | 0 (0-7) | 9 (2-15) | 0.01 |
nonRAL: non-reversible airflow limitation; partialRAL: partially reversible airflow limitation; and GOLD: Global Initiative for Chronic Obstructive Lung Disease. aValues expressed as n (%). bValues expressed as median (interquartile range). *Mann-Whitney test (two-tailed); level of statistical significance set at p < 0.05.
In both patient groups, we performed differential counts of neutrophils and eosinophils in the sputum samples (Table 2). The neutrophil counts did not differ between the two groups (p > 0.05). However, the median eosinophil count was significantly higher in the partialRAL group than in the nonRAL group (p < 0.01).
In our evaluation of atopy, with the skin prick test, we found no difference between the two patient groups in terms of positivity for any of the antigens tested (Table 2). Of the 24 patients in the nonRAL group, 5 (21%) had at last one positive test, compared with 4 (31%) of the 13 patients in the partialRAL group (p > 0.05). Moreover none of the patients in either patient group had a history of asthma. In 2 of the patients in the nonRAL group, the response to histamine was negative.
Relationship between the proportion of eosinophils and FEV1
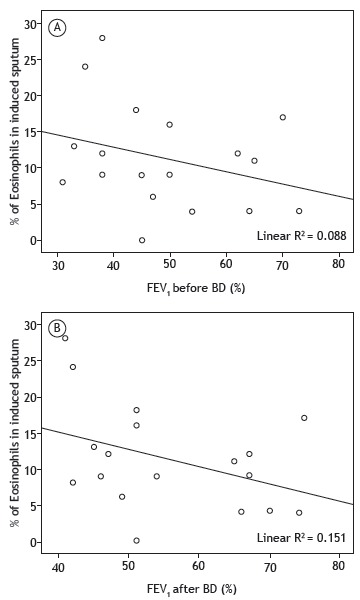
The relationship between the proportion of eosinophils in the sputum and FEV1 (before and after bronchodilator use) is shown in Figure 1. There was an inverse relationship between the proportion of eosinophils in the sputum and FEV1 before and after bronchodilator use (p < 0.01).
Figure 1. Scatter plots showing the proportion of eosinophils in induced sputum and the FEV1 of COPD patients with non-reversible airflow limitation or partially reversible airflow limitation, before and after bronchodilator (BD) use (A and B, respectively). Statistics derived by Spearman's correlation coefficient; level of statistical significance set at p < 0.05.
Immunological profile
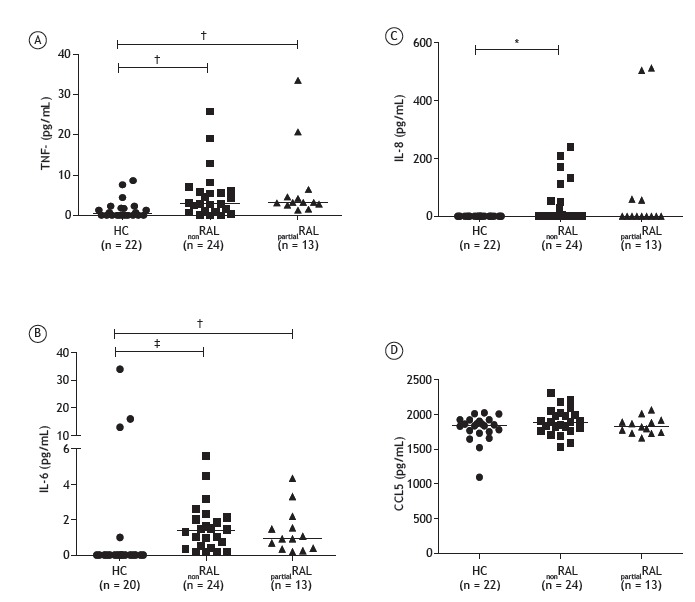
The cytokine and chemokine levels in the nonRAL group, partialRAL group, and control group are shown in Figure 2. The median TNF levels were 2.9 pg/mL (range, 0.95-6.03 pg/mL) in the nonRAL group and 3.2 pg/mL (range, 2.65-5.50 pg/mL) in the partialRAL group, both of which were significantly higher than the 0.35 pg/mL (range, 0-1.9 pg/mL) observed for the controls (p < 0.01). The median IL-6 levels were also significantly higher in the nonRAL group, and partialRAL group than in the control group-1.4 pg/mL (range, 0.42-2.10 pg/mL) and 0.92 pg/mL (range, 0.37-1.89 pg/mL), respectively, versus 0 pg/mL (p < 0.01). In addition, IL-8 levels were significantly higher in the nonRAL group than in the control group (p < 0.05) and RANTES (CCL5) levels did not differ significantly between the two patient groups or between either patient group and the control group (p > 0.05 for all). As depicted in Figure 2, the median IL-8 values for the nonRAL, partialRAL, and control groups were 0 (range, 0-57.50), 0 (range, 0-51.75), and 0 (range, 0-0), respectively, the difference between the nonRAL group and the control group being statistically significant (p < 0.05). There were no statistical differences among the three groups in terms of the serum levels of RANTES (CCL5; Figure 2). The severity of COPD did not correlate significantly with the serum levels of TNF, IL-6, IL-8, or RANTES (CCL5; data not shown).
Figure 2. Dot plots of serum levels of the cytokines TNF (A), IL-6 (B), and IL-8 (C), as well as those of RANTES (CCL5; D), in healthy controls (HC), COPD patients with non-reversible airflow limitation (nonRAL), and COPD patients with partially reversible airflow limitation (partialRAL). *p < 0.05, †p < 0.01, and ‡p < 0.001; Kruskal-Wallis test followed by Dunn's post-test for multiple comparisons.
DISCUSSION
COPD is a severe progressive inflammatory disease and is the fourth leading cause of death in the United States. 16 The prevalence of and mortality associated with COPD continue to rise. In addition, COPD has become a major cause of death and disability worldwide. 17 Two COPD subtypes, based on FEV1 reversibility, have recently been identified. Here, we attempted to determine whether those subtypes are associated with sputum cell counts, cytokine levels, and symptom severity. We found that COPD patients with reversibility of FEV1 had higher sputum eosinophil counts and greater airway hyperresponsiveness than did those without such reversibility, and that there was an inverse correlation between the proportion of eosinophils in the sputum and the FEV1 before and after bronchodilator use. Furthermore, we showed that COPD with FEV1 reversibility was not associated with atopy or asthma. In fact, our findings corroborate those of previous studies showing that, although IL-6, IL-8 and TNF levels are higher in COPD patients than in healthy subjects, the production of those cytokines is comparable between the two types of COPD.
Historically, the incidence of COPD has been highest among males and among smokers. However, the proportional representation of women has been increasing. 18 In addition, a marked female predominance has been reported among patients with an early onset of severe COPD. 19 In the present study, the proportional distribution of males and females was similar between the two patient groups, as was the proportion of smokers.
The analysis of sputum is an useful tool in the evaluation of inflammation of the airways. 15 Induced sputum was initially used for the diagnosis of lung cancer and later for infectious diseases. In the early 1990s, this method was employed in the investigation of bronchial inflammation associated with asthma. 20 More recently, because of its safety, reproducibility and low cost, it has been used for investigation of the pathogenesis of asthma and COPD. Local neutrophil recruitment in inflammation is a hallmark of COPD, as is an increase in the levels of inflammatory mediators in the airways and in circulating blood. 21 The release of neutrophil elastase, acid phosphatase, and myeloperoxidase that occurs during neutrophilic inflammation is characteristic of COPD. 22 - 24 Neutrophils are the predominant cells in the sputum of COPD patients, and high proportions of neutrophils were found in the sputum of the patients in both of the COPD groups evaluated in the present study. 3 However, it is also possible that the hypertonic saline solution used for the induced sputum techniques contributes to increasing the number of neutrophils. 25
The role that eosinophils in the sputum play in COPD is not clear. It was once thought that the presence of eosinophils was related to a subgroup of COPD patients with features of asthma 2 , 5 and that it was associated with a better response to corticosteroid therapy. 26 In fact, an association between asthma and COPD would further the development of strategies for the therapeutic management of COPD. 27 , 28 However, our data argue against the occurrence of asthma in patients who show post-bronchodilator FEV1 reversibility. None of our patients had a personal or family history of asthma, and the prevalence of atopy, as determined with the skin prick test, was comparable between the two patient groups. In addition, the immunological profile (cytokine and chemokine production) was similar in the two groups, and there was no increase in RANTES (CCL5), which is a typical Th2 cytokine in COPD patients with post-bronchodilator FEV1 reversibility. In a previous study, we found an association between nasal eosinophils and atopy in patients with COPD. 29 However, in the present study, the presence of eosinophils in the sputum was not found to be associated with atopy or asthma. Although the role that eosinophils play in COPD is not completely understood, we observed that eosinophil counts were increased in COPD patients with post-bronchodilator FEV1 reversibility. We also identified an inverse correlation between the proportion of eosinophils in the sputum and the decrease in FEV1. Although our findings might suggest that eosinophils are related to the severity of COPD, no eosinophils were found in the sputum of the patients with stage IV COPD. It is possible that, as in the later stages of the disease, the numbers of inflammatory cells are decreased in that phase of the disease.
Increased levels of IL-6, IL-1β, TNF, and IL-8 have been observed in the induced sputum of patients with stable COPD. 5 There is also evidence of a relationship between elevated cytokine levels in COPD and cigarette smoking. 6 , 30 However, the relationship that chemokines have with eosinophils in the sputum or with FEV1 reversibility has not been evaluated. Serum IL-6 is considered the best biomarker of COPD severity when associated with the degree of airway obstruction and has been associated with mortality. 7 We found that the severity of COPD did not correlate with the serum levels of IL-6, IL-8, TNF, or RANTES (CCL5). In the present study, it was possible to evaluate the levels of cytokines (IL-6 and TNF) and chemokines-IL-8 and RANTES (CCL5)-in COPD patients with and without FEV1 reversibility after bronchodilator use. However, the cytokine and chemokine levels were similar between the two patient groups.
Eosinophilic airway inflammation has been associated with COPD exacerbations. 31 A reduction in sputum eosinophil counts has been associated with a reduction in COPD exacerbations. 31 Because this was a cross-sectional study, we did not assess the relationship between the inflammatory response and exacerbation. However, we found an association between eosinophil inflammation and airway obstruction. That supports the relationship between eosinophilic airway inflammation and COPD exacerbation, 31 as well as the association between the peripheral blood eosinophil count and death from exacerbations of COPD. 32
One limitation of the present study is the small number of participants. However, it is clear that patients in the partialRAL group had greater airway hyperresponsiveness. In addition, the observation that COPD with FEV1 reversibility was not related to asthma but was associated with increased numbers of eosinophils in the sputum, together with the inverse correlation observed between the proportion of eosinophils in the sputum and FEV1, suggests that eosinophils play an important role in the inflammatory response in COPD patients with post-bronchodilator FEV1 reversibility.
Our data do not support the occurrence of asthma-COPD overlap syndrome in patients who show airway responsiveness to bronchodilator use. Although we cannot rule out the possibility that eosinophilic inflammation is a subtype of COPD, our data indicate that it is a phase of the disease that is associated with greater airway obstruction.
Footnotes
Financial support: None.
Study carried out in the Serviço de Pneumologia, Ambulatório Magalhães Neto, Complexo Hospitalar Universitário Professor Edgard Santos, Universidade Federal da Bahia, Salvador (BA) Brasil.
References
- 1.Saetta M, Di Stefano A, Maestrelli P, Turato G, Ruggieri MP, Roggeri A. Airway eosinophilia in chronic bronchitis during exacerbations. Pt 1Am J Respir Crit Care Med. 1995;152(6):1926–1931. doi: 10.1164/ajrccm.150.6.7952628. [DOI] [PubMed] [Google Scholar]
- 2.Brightling CE, Monteiro W, Ward R, Parker D, Morgan MD, Wardlaw AJ. Sputum eosinophilia and short-term response to prednisolone in chronic obstructive pulmonary disease a randomized controlled trial. Lancet. 2000;356(9240):1480–1485. doi: 10.1016/S0140-6736(00)02872-5. [DOI] [PubMed] [Google Scholar]
- 3.Rufino R, Costa CH, Souza HS, Madi K, Silva JR. Induced sputum and peripheral blood cell profile in chronic obstructive pulmonary disease. J Bras Pneumol. 2007;33(5):510–518. doi: 10.1590/s1806-37132007000500005. [DOI] [PubMed] [Google Scholar]
- 4.Willemse BW, ten Hacken NH, Rutgers B, Lesman-Leegte IG, Postma DS, Timens W. Effect of 1-year smoking cessation on airway inflammation in COPD and asymptomatic smokers. Eur Respir J. 2005;26(5):835–845. doi: 10.1183/09031936.05.00108904. [DOI] [PubMed] [Google Scholar]
- 5.Keatings VM, Collins PD, Scott DM, Barnes PJ. Differences in interleukin-8 and tumor necrosis factor-alpha in induced sputum from patients with chronic obstructive pulmonary disease or asthma. Am J Respir Crit Care Med. 1996;153(2):530–534. doi: 10.1164/ajrccm.153.2.8564092. [DOI] [PubMed] [Google Scholar]
- 6.Lim S, Roche N, Oliver BG, Mattos W, Barnes PJ, Chung KF. Balance of matrix metalloprotease-9 and tissue inhibitor of metalloprotease-1 from alveolar macrophages in cigarette smokers Regulation by interleukin-10. Pt 1Am J Respir Crit Care Med. 2000;162(4):1355–1360. doi: 10.1164/ajrccm.162.4.9910097. [DOI] [PubMed] [Google Scholar]
- 7.Celli BR, Locantore N, Yates J, Tal-Singer R, Miller BE, Bakke P. Inflammatory biomarkers improve clinical prediction of mortality in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;185(10):1065–1072. doi: 10.1164/rccm.201110-1792OC. [DOI] [PubMed] [Google Scholar]
- 8.Lapperre TS, Postma DS, Gosman MM, Snoeck-Stroband JB, ten Hacken NH, Hiemstra PS. Relation between duration of smoking cessation and bronchial inflammation in COPD. Thorax. 2006;61(2):115–121. doi: 10.1136/thx.2005.040519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hurd S. The impact of COPD on lung health worldwide epidemiology and incidence. Chest. 2000;117(2 Suppl):1S–4S. doi: 10.1378/chest.117.2_suppl.1S. [DOI] [PubMed] [Google Scholar]
- 10.Senior RM, Silverman EK. Chronic obstructive pulmonary disease. Hamilton, Canada: Decker; 2011. http://www.medicinanet.com.br/m/conteudos/acp-medicine/6413/doenca_pulmonar_obstrutiva_cronica.htm [Google Scholar]
- 11.Global initiative for chronic obstructive lung disease. Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary Disease. Bethesda: GOLD; 2011. http://www.goldcopd.org/guidelines-global-strategy-for-diagnosis-management.html [Google Scholar]
- 12.Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease American Thoracic Society. Pt 2Am J Respir Crit Care Med. 1995;152(5):S77–121. [PubMed] [Google Scholar]
- 13.Pepys J, Roth A, Carrol KB. RAST, skin and nasal tests and the history in grass pollen allergy. Clin Allergy. 1975;5(4):431–442. doi: 10.1111/j.1365-2222.1975.tb01882.x. [DOI] [PubMed] [Google Scholar]
- 14.Osterbalee O, Weeke B. A new lancet for skin prick testing. Allergy. 1979;34(4):209–212. doi: 10.1111/j.1398-9995.1979.tb01700.x. [DOI] [PubMed] [Google Scholar]
- 15.Pavord ID, Pizzichini MM, Pizzichini E, Hargreave FE. The use of induced sputum to investigate airway inflammation. Thorax. 1997;52(6):498–501. doi: 10.1136/thx.52.6.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoyert DL, Kung HC, Smith BL. Deaths preliminary data for 2003. Natl Vital Stat Rep. 2005;53(15):1–48. [PubMed] [Google Scholar]
- 17.Mannino DM. COPD: epidemiology, prevalence, morbidity and mortality, and disease heterogeneity. Chest. 2002;121(5):121S–126S. doi: 10.1378/chest.121.5_suppl.121S. [DOI] [PubMed] [Google Scholar]
- 18.Mannino DM, Homa DM, Akinbami LJ, Ford ES, Redd SC. Chronic obstructive pulmonary disease surveillance--United States, 1971-2000. MMWR Surveill Summ. 2002;51(6):1–16. [PubMed] [Google Scholar]
- 19.Silverman EK, Weiss ST, Drazen JM, Chapman HA, Carey V, Campbell EJ. Gender-related differences in severe, early-onset chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000;162(6):2152–2158. doi: 10.1164/ajrccm.162.6.2003112. [DOI] [PubMed] [Google Scholar]
- 20.Pin I, Gibson PG, Kolendowicz R, Girgis-Gabardo A, Denburg JA, Hargreave FE. Use of induced sputum cell counts to investigate airway inflammation in asthma. Thorax. 1992;47(1):25–29. doi: 10.1136/thx.47.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moermans C, Heinen V, Nguyen M, Henket M, Sele J, Manise M. Local and systemic cellular inflammation and cytokine release in chronic obstructive pulmonary disease. Cytokine. 2011;56(2):298–304. doi: 10.1016/j.cyto.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 22.Saetta M. Airway inflammation in chronic obstructive pulmonary disease. Pt 2Am J Respir Crit Care Med. 1999;160(5):S17–S20. doi: 10.1164/ajrccm.160.supplement_1.6. [DOI] [PubMed] [Google Scholar]
- 23.Cosio MG, Majo J, Cosio MG. Inflammation of the airways and lung parenchyma in COPD role of T cells. Chest. 2002;121(5 Suppl):160S–165S. doi: 10.1378/chest.121.5_suppl.160S. [DOI] [PubMed] [Google Scholar]
- 24.Barnes PJ, Chowdhury B, Kharitonov SA, Magnussen H, Page CP, Postma D. Pulmonary biomarkers in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2006;174(1):6–14. doi: 10.1164/rccm.200510-1659PP. [DOI] [PubMed] [Google Scholar]
- 25.Kips JC, Fahy JV, Hargreave FE, Ind PW, in't Veen JC. Methods for sputum induction and analysis of induced sputum a method for assessing airway inflammation in asthma. Eur Respir J Suppl. 1998;26:9S–12S. [PubMed] [Google Scholar]
- 26.Fujimoto K, Kubo K, Yamamoto H, Yamaguchi S, Matzuzawa Y. Eosinophilic inflammation in the airway is related to glucocorticoid reversibility in patients with pulmonary emphysema. Chest. 1999;115(3):697–702. doi: 10.1378/chest.115.3.697. [DOI] [PubMed] [Google Scholar]
- 27.Kanazawa M. Diseases to differentiate from COPD, with emphasis on bronchial asthma [Article in Japanese] Nihon Rinsho. 2007;65(4):675–681. [PubMed] [Google Scholar]
- 28.Miravitlles M, Morera J. It's time for an aetiology-based definition of chronic obstructive pulmonary disease. Respirology. 2007;12(3):317–319. doi: 10.1111/j.1440-1843.2007.01082.x. [DOI] [PubMed] [Google Scholar]
- 29.Neves MC, Neves YC, Mendes CM, Bastos MN, Camelier AA, Queiroz CF. Evaluation of atopy in patients with COPD. J Bras Pneumol. 2013;39(3):296–305. doi: 10.1590/S1806-37132013000300006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuschner WG, D'Alessandro A, Wong H, Blanc PD. Dose-dependent cigarette smoking-related inflammatory responses in healthy adults. Eur Respir J. 1996;9(10):1989–1994. doi: 10.1183/09031936.96.09101989. [DOI] [PubMed] [Google Scholar]
- 31.Siva R, Green RH, Brightling CE, Shelley M, Hargadon B, McKenna S. Eosinophilic airway inflammation and exacerbations of COPD a randomized controlled trial. Eur Respir J. 2007;29(5):906–913. doi: 10.1183/09031936.00146306. [DOI] [PubMed] [Google Scholar]
- 32.Hospers JJ, Schouten JP, Weiss ST, Rijcken B, Postma DS. Asthma attacks with eosinophilia predict mortality from chronic obstructive pulmonary disease in a general population sample. Am J Respir Crit Care Med. 1999;160(6):1869–1874. doi: 10.1164/ajrccm.160.6.9811041. [DOI] [PubMed] [Google Scholar]


