ABSTRACT
Objective:
Conventional bronchoscopy has a low diagnostic yield for peripheral pulmonary lesions. Radial-probe EBUS employs a rotating ultrasound transducer at the end of a probe that is passed through the working channel of the bronchoscope. Radial-probe EBUS facilitates the localization of peripheral pulmonary nodules, thus increasing the diagnostic yield. The objective of this study was to present our initial experience using radial-probe EBUS in the diagnosis of peripheral pulmonary lesions at a tertiary hospital.
Methods:
We conducted a retrospective analysis of 54 patients who underwent radial-probe EBUS-guided bronchoscopy for the investigation of pulmonary nodules or masses between February of 2012 and September of 2013. Radial-probe EBUS was performed with a flexible 20-MHz probe, which was passed through the working channel of the bronchoscope and advanced through the bronchus to the target lesion. For localization of the lesion and for collection procedures (bronchial brushing, transbronchial needle aspiration, and transbronchial biopsy), we used fluoroscopy.
Results:
Radial-probe EBUS identified 39 nodules (mean diameter, 1.9 ∓ 0.7 cm) and 19 masses (mean diameter, 4.1 ∓ 0.9 cm). The overall sensitivity of the method was 66.7% (79.5% and 25.0%, respectively, for lesions that were visible and not visible by radial-probe EBUS). Among the lesions that were visible by radial-probe EBUS, the sensitivity was 91.7% for masses and 74.1% for nodules. The complications were pneumothorax (in 3.7%) and bronchial bleeding, which was controlled bronchoscopically (in 9.3%).
Conclusions:
Radial-probe EBUS shows a good safety profile, a low complication rate, and high sensitivity for the diagnosis of peripheral pulmonary lesions.
Keywords: Diagnostic techniques, respiratory system; Lung/ultrasonography; Bronchoscopy/methods; Bronchoscopy/instrumentation.
RESUMO
Objetivo:
A broncoscopia convencional possui baixo rendimento diagnóstico para lesões pulmonares periféricas. A ecobroncoscopia radial (EBUS radial) emprega um transdutor ultrassonográfico rotatório na extremidade de uma sonda que é inserida no canal de trabalho do broncoscópio. O EBUS radial facilita a localização de nódulos pulmonares periféricos, aumentando assim o rendimento diagnóstico. O objetivo deste estudo foi apresentar nossa experiência inicial com o uso de EBUS radial para o diagnóstico de lesões pulmonares periféricas em um hospital terciário.
Métodos:
Foi realizada uma análise retrospectiva de 54 pacientes submetidos à broncoscopia guiada por EBUS radial para a investigação de nódulos ou massas pulmonares entre fevereiro de 2012 e setembro de 2013. O EBUS radial foi realizado com uma sonda flexível de 20 MHz, que foi inserida no canal de trabalho do broncoscópio até chegar à lesão-alvo. A fluoroscopia foi usada para localizar a lesão e realizar procedimentos de coleta (escovado brônquico, aspiração transbrônquica com agulha e biópsia transbrônquica).
Resultados:
O EBUS radial identificou 39 nódulos (média de diâmetro: 1,9 ± 0,7 cm) e 19 massas (média de diâmetro: 4,1 ± 0,9 cm). A sensibilidade global do EBUS radial foi de 66,7% (79,5% para as lesões visíveis pelo método e 25% para as lesões não visíveis pelo método). Nas lesões visíveis pelo método, a sensibilidade foi de 91,7% para massas e de 74,1% para nódulos. As complicações foram pneumotórax (3,7%) e sangramento brônquico controlado broncoscopicamente (9,3%).
Conclusões:
O EBUS radial apresenta bom perfil de segurança, baixo índice de complicações e alta sensibilidade para o diagnóstico de lesões pulmonares periféricas.
INTRODUCTION
Bronchoscopy has been used worldwide for the diagnosis of pulmonary nodules and centrally located masses. However, for the diagnosis of smaller lesions, the reported sensitivity of routine bronchoscopy remains low (34%; range, 5-76%), albeit higher for larger lesions (63%; range, 31-82%). 1 The use of fluoroscopic guidance increases the diagnostic accuracy of conventional bronchoscopy from 14% to 71%, depending on factors such as location of the nodule, lesion size, presence of the bronchus sign, and other technical aspects of the procedure. 2 However, fluoroscopy has some limitations, because it is not a tridimensional method and there is therefore no guarantee that the lesion is adequately sampled, as well as because it exposes patients to radiation. Although transthoracic needle aspiration (TTNA) has been shown to have excellent diagnostic sensitivity (approximately 90% in most studies), it can provoke pneumothorax or bleeding, requiring interventions such as chest tube drainage and transfusion (7% and 18%, respectively), which are a serious concern in clinical practice. 3 - 5
Radial-probe EBUS has emerged as a widely accepted procedure that can increase sensitivity and accuracy for the diagnosis of peripheral pulmonary nodules. 6 - 8 Radial-probe EBUS can precisely locate pulmonary nodules or masses based on differences in echogenicity between normal lung parenchyma and the lesion itself. Studies have shown that radial-probe EBUS improves diagnostic rates for peripheral pulmonary nodules, particularly for lesions smaller than 2 cm in diameter. Although it is not mandatory, the routine use of fluoroscopy plus radial-probe EBUS has been shown to produce better results than does either technique alone. 9 - 11
The aim of this study was to evaluate our initial experience in using radial-probe EBUS for the diagnosis of peripheral pulmonary lesions in a tertiary hospital setting.
METHODS
This was a retrospective, cross-sectional analysis of radial-probe EBUS procedures performed in patients with peripheral pulmonary nodules or masses who were seen at the Heart Institute of the University of São Paulo School of Medicine Hospital das Clínicas , in the city of São Paulo, Brazil, between February of 2012 and September of 2013. The data were obtained from the Heart Institute database. The study was approved by the Hospital das Clínicas Research Ethics Committee.
To measure the lesion and locate the corresponding bronchial segment, CT scans of the chest were evaluated. The inclusion criterion was the referral for the diagnosis of an indeterminate pulmonary nodule or mass. Patients were excluded if an endobronchial lesion was observed during conventional bronchoscopy or if they were lost to follow-up. Patients with pulmonary masses (defined as lesions with a diameter greater than 3 cm) were referred for radial-probe EBUS if a previous bronchoscopy was nondiagnostic.
All radial-probe EBUS procedures were preceded by conventional bronchoscopy with a flexible bronchoscope (BF-1T180; Olympus Medical Systems Corp., Tokyo, Japan), in order to access the airways and identify any endobronchial lesions. All patients were under conscious sedation (with midazolam and fentanyl) and topical anesthesia (with 1% lidocaine). Upon completion of the conventional bronchoscopy, a 20-MHz radial probe (UM-3R; Olympus Medical Systems Corp.) was inserted through the 2.8-mm working channel of the bronchoscope toward the lesion in the lung parenchyma (Figure 1). In most cases, fluoroscopy was used in order to check the position of the probe after its correct positioning (within or adjacent to the lesion) had been ascertained by radial-probe EBUS (Figures 2 and 3). Thereafter, collection procedures were performed, such procedures including bronchial brushing, for cytology; transbronchial needle aspiration (TBNA), using a 21-gauge needle, for cytological and cell-block analysis; and transbronchial biopsy (TBB), for histological analysis. When infectious disease (especially granulomatous disease) was suspected, BAL fluid was collected for microbiological analysis.
Figure 1. Radial-probe EBUS: (a) probe drive unit; (b) distal end of the radial probe outside the bronchoscope; c) radial probe being inserted into the working channel of the bronchoscope; and d) bronchoscopic image of the probe within the segmental bronchus.
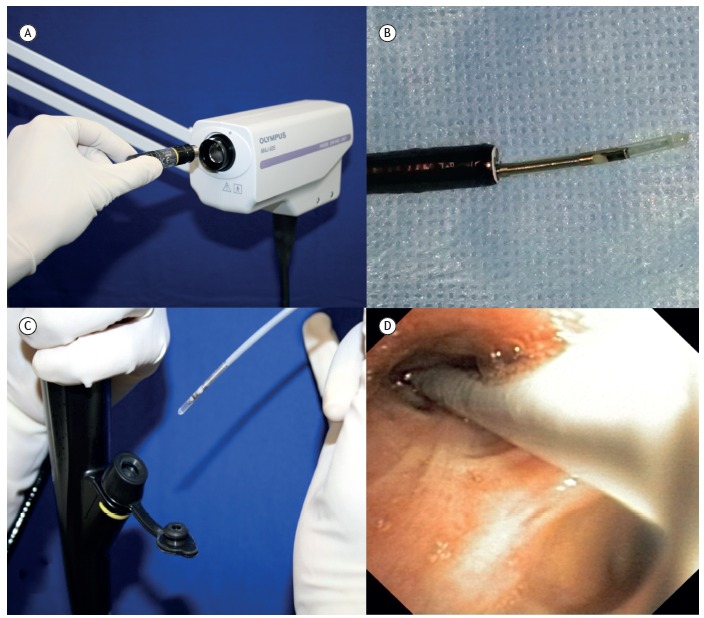
Figure 2. Comparison between CT and radial-probe EBUS: a) CT of the chest, showing a 2.4 cm nodule in the left upper lobe; and (b) radial-probe EBUS image with well-defined, echogenic borders (probe positioned within the lesion). The final diagnosis in this case was non-small cell lung cancer (squamous cell lung carcinoma).
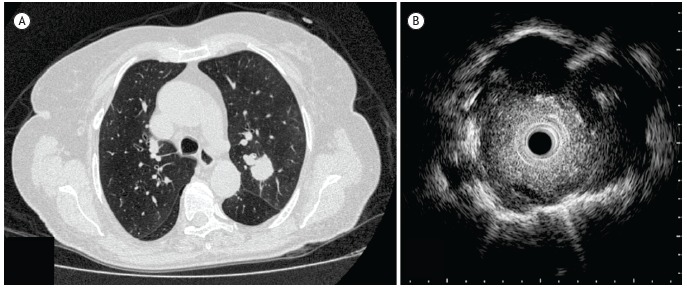
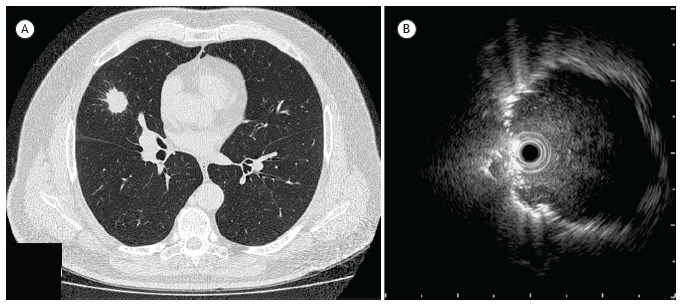
Figure 3. Comparison between CT and radial-probe EBUS: a) CT of the chest, showing a 2.5 cm nodule in the middle lobe; and (b) radial-probe EBUS image with the probe positioned adjacent to the lesion. The final diagnosis in this case was non-small cell lung cancer (adenocarcinoma).
Paulo (SP) Brasil.
Sample collection followed standardized routine protocols. 6 Biopsy fragments were transported in 10% formaldehyde; TBNA aspirates were handled carefully to ensure that an adequate amount of material was sent for analysis (mounting on glass slides for cytology, fixation in formaldehyde for cell-block analysis, and, when necessary, storage in a sterile device for microbiology); and samples obtained by bronchial brushing were mounted on glass slides for direct examination. 12 Rapid on-site evaluation (ROSE) was used in order to determine the quality of the cytology specimens obtained from some patients.
The radial-probe EBUS procedure was considered successful if it resulted in the specific diagnosis of malignancy or inflammatory processes. The procedure was also classified as a success if a lesion determined to present nonspecific benign disease by radial-probe EBUS was subsequently proven to be benign by further investigation, or if the lesion remained stable for six months on CT scans.
Statistical analysis
Sensitivity was calculated as the number of successful diagnoses made by radial-probe EBUS-guided bronchoscopy, divided by the total number of procedures. We performed descriptive analysis of absolute and relative frequencies. Patients with pulmonary nodules and patients with pulmonary masses were compared by Fisher's exact test. The IBM SPSS Statistics software package, version 19.0 (IBM Corporation, Armonk, NY, USA) was used for all analysis.
RESULTS
A total of 54 patients underwent flexible bronchoscopy with radial-probe EBUS. There was predominance of females, who accounted for 57.4% of the sample, and the mean patient age was 64.8 ∓ 11.1 years (range, 43-87 years). Three patients (5.5%) were excluded because they were lost to follow-up, and a final diagnosis was therefore neither obtained nor confirmed for those patients. Consequently, we analyzed 51 patients, of whom 37 (72.5%) were referred for the investigation of pulmonary nodules and 14 (27.5%) were referred for the investigation of pulmonary masses. The overall sensitivity of radial-probe EBUS was 66.7% for the diagnosis of pulmonary nodules or masses (Table 1).
Table 1. Visibility of lesions on radial-probe EBUS, lesion size, and diagnostic sensitivity.
| Visibility | (N = 51) | Pulmonary lesions | |
|---|---|---|---|
| Nodules | Masses | ||
| All lesions | |||
| n (%) | 37 (72.5) | 14 (27.5) | |
| Size (cm), mean ∓ SD | 2.5 ∓ 1.3 | 1.9 ∓ 0.7 | 4.1 ∓ 0.9 |
| Identified by radial-probe EBUS, n (sensitivity) | 34 (66.7%) | 23 (62.2%) | 11 (78.6%) |
| Lesions visible by radial-probe EBUS | |||
| n (%) | 39 (76.5) | 27 (69.2) | 12 (30.8) |
| Size (cm), mean ∓ SD | 2.6 ∓ 1.2 | 1.9 ∓ 0.7 | 3.9 ∓ 0.9 |
| Identified by radial-probe EBUS, n (sensitivity) | 31 (79.5%) | 20 (74.1%) | 11 (91.7%) |
| Lesions not visible by radial-probe EBUS | |||
| n (%) | 12 (23.5) | 10 (83.3) | 2 (16.7) |
| Size (cm), mean ∓ SD | 1.6 ∓ 1.1 | 1.3 ∓ 0.6 | 3.7 ∓ 0.7 |
| Identified by radial-probe EBUS, n (sensitivity) | 3 (25.0%) | 3 (30.0%) | 0 (0.0) |
The pulmonary lesions were visible and not visible by radial-probe EBUS in 39 patients (76.5%) and 12 patients (23.5%), respectively, the latter category including 10 nodules (1.3 ∓ 0.6 cm) and 2 masses (3.7 ∓ 0.7 cm). The sensitivity of the procedure was 79.5% and 25.0% for the diagnosis of lesions that were visible and not visible by radial-probe EBUS, respectively (p = 0.005).
Nodules
Among the 37 patients referred for the investigation of pulmonary nodules, the nodules were visible by radial-probe EBUS in 27 (73.0%). The probe was positioned adjacent to the lesion in 17 (63.0%) of those 27 cases. Comparing the cases in which the probe was positioned adjacent to the lesion and those in which it was positioned within the lesion, we found that the mean size of the nodules was significantly smaller in the former group (1.7 ∓ 0.3 cm vs. 2.3 ∓ 0.3 cm, p = 0.033). Among those same 27 cases, the diagnosis was obtained by radial-probe EBUS and confirmed surgically in 20 (74.1%), compared with only 3 (30.0%) of the 10 cases in which the nodule was not visible by radial-probe EBUS (Table 1). Malignant nodules were found in 14 (51.8%) of the 27 cases, with a predominance of non-small cell lung cancer. The radial-probe EBUS results were positive in 10 (71.4%) of those 14 malignant nodules. The bronchus sign was present on CT scans in 16 (59.3%) of the 27 cases. Neither bronchus sign nor probe location were found to correlate with the final diagnosis (p = 0.895). In 15 patients (56.0%), cytology specimens were submitted to ROSE, the results of which were positive in 8 (54.0%) of those 15. Fluoroscopic guidance was possible in 16 (59.3%) of the 27 patients with nodules that were visible by radial-probe EBUS.
Masses
Lesions that were visible by radial-probe EBUS were identified in 12 (85.7%) of the 14 patients referred for the investigation of pulmonary masses. Among those 12 patients, a definitive diagnosis was obtained in 11 (91.7%), 10 (83.3%) being diagnosed with tumors and 1 (8.3%) being diagnosed with bronchiolitis obliterans organizing pneumonia. In 9 (75.0%) of those same 12 cases, CT showed the bronchus sign, and the radial probe was positioned within the lesion in all 12 cases. Fluoroscopic guidance was used in 7 (58.3%). In 10 (83.3%) of the 12 cases, cytology specimens were submitted to ROSE, the results of which were positive in 8 (80.0%) of the 10. A definitive diagnosis was obtained by TTNA in only 1 patient (8.3%). The final diagnoses and sensitivity of radial-probe EBUS are summarized in Table 2.
Table 2. Final diagnoses of the lesions that were visible by radial-probe EBUS and sensitivity of the procedure.
| Diagnosis | Pulmonary lesions | |||
|---|---|---|---|---|
| Nodules | Masses | |||
| Cases | Sensitivity | Cases | Sensitivity | |
| N (%) | N diagnosed (%) | N (%) | N diagnosed (%) | |
| Malignancy | 14 (51.8) | 10 (71.4) | 10 (83.3) | 9 (90.0) |
| Non-small cell lung cancer | 10 (37.0) | 7 (70.0) | 8 (66.7) | 7 (87.5) |
| Small cell lung cancer | 2 (7.4) | 2 (100.0) | 1 (8.3) | 1 (100.0) |
| Adenoid cystic carcinoma | 1 (3.7) | 1 (100.0) | ||
| Hamartoma | 1 (3.7) | 0 (0.0) | ||
| Metastatic breast cancer | 1 (8.3) | 1 (100.0) | ||
| Tuberculosis or fungal infection | 4 (14.8) | 2 (50.0) | ||
| Inflammatory disease | 3 (11.1) | 3 (100.0) | 2 (16.7) | 2 (100.0) |
| Nonspecific benign disease | 6 (22.2) | 6 (100.0) | ||
| Total | 27 (100.0) | 20 (74.1) | 12 (100.0) | 11 (91.7) |
Complications
Procedure related complications occurred in 7 (13.0%) of the 54 patients. Pneumothorax requiring chest tube drainage occurred in 2 (3.7%) and moderate bleeding occurred in 5 (9.3%) and were managed with topical application of cold saline solution with epinephrine. All complications occurred in patients with pulmonary nodules.
DISCUSSION
This is a report of our initial experience with radial-probe EBUS for the diagnosis of peripheral pulmonary lesions in patients treated in Brazil. Since 2001, when it was introduced into clinical practice, radial-probe EBUS has been used as an adjunct to TBB and to other bronchoscopic procedures for the evaluation of peripheral pulmonary lesions. The equipment consists of a thin, flexible catheter with a small probe at the end that can capture 360° ultrasound images of the lung parenchyma and the target lesion. Although it is easy to perform, radial-probe EBUS requires training because the operator must visually differentiate among normal lung parenchyma, vessels, and specific intrapulmonary lesions (e.g., nodules and masses). Pulmonary lesions are hypoechoic and usually have sharply defined borders, due to the strong reflective interface between the aerated lung and the lesion itself.
Radial-probe EBUS can be a valuable tool for localizing pulmonary lesions and guiding tissue sampling, particularly for small nodules. 8 , 13 , 14 In a prospective randomized trial, 13 the diagnostic accuracy of radial-probe EBUS-guided TBB was found to be similar to that of CT-guided TTNA (87.5% and 93.3%, respectively), although the complication rate was significantly higher in the latter (27% vs. 3%). Paone et al. demonstrated that radial-probe EBUS-guided TBB has a sensitivity of 75% and 71% for detecting lesions that are < 2 cm and < 3 cm in diameter, respectively, compared with 31% and 23%, respectively, for conventional TBB. 15 The authors also found that, although radial-probe EBUS-guided TBB and fluoroscopy-guided bronchoscopic biopsy provide comparable results, the radiation exposure associated with the latter constitutes a major disadvantage.
Our preliminary experience with radial-probe EBUS indicates that the procedure has high sensitivity for nodules and masses (74.1% and 92%, respectively), which is in agreement with the findings of other studies. 16 Various studies have focused on factors affecting the diagnostic yield of radial-probe EBUS, such factors including nodule size, the capacity to visualize the lesion, and whether the probe is positioned within the lesion. Huang et al. 17 found that lesion size and ultrasound visualization were important factors for the diagnostic yield. Steinfort et al. 13 reported higher diagnostic accuracy when the probe was positioned within the lesion. In our study, nodules were visible by radial-probe EBUS in 27 (73%) of the 37 cases and the probe was positioned within the nodule in 10 (37%). The sensitivity of the procedure tripled for lesions that were visible by radial-probe EBUS compared to those that were not visible (73% vs. 25%). The diagnostic sensitivity was also better for masses than for nodules (92% vs. 74%).
The presence of the bronchus sign can also influence the results of radial-probe EBUS. In the present study, this finding correlated to a better diagnostic sensitivity as reported by other authors. 18 The bronchus sign was seen in 9 (75.0%) of the 12 patients with pulmonary masses that were visible by radial-probe EBUS, and the position of the lesion was determined by the radial probe. Among the pulmonary nodules that were visible by radial-probe EBUS, the bronchus sign was seen in 16 (59.3%), although no correlation was found between the presence of the bronchus sign, the position of the probe or the diagnosis.
In 2004, Kurimoto et al. 8 reported the use of a guide sheath, which is a flexible guide catheter that acts like an extension to the working channel of the bronchoscope. The guide sheath is left in place at the target site after the radial probe has been retracted. It is radiopaque and allows biopsy, brushing, or needle aspiration in the regions of interest defined by the radial-probe EBUS. It also enables repeat collection procedures to be performed at those same sites and minimizes the associated risk of bleeding. Some studies have shown that using a guide sheath during radial-probe EBUS-guided TBB provides higher diagnostic yields for pulmonary masses and nodules, 12 - 15 especially for smaller lesions. In our study, we did not use a guide sheath, because the device is still awaiting regulatory approval for use in Brazil.
The differential diagnosis between malignancy and infectious disease is important in Brazil. In the present study, we identified non-neoplastic disease in 13 (48.1%) of the 27 pulmonary nodules that were visible by radial-probe EBUS and in 2 (16.7%) of the 12 pulmonary masses that were visible by radial-probe EBUS, the final diagnoses including fungal infections and tuberculosis. Those diagnoses were subsequently confirmed by surgical methods, and the patients were treated accordingly. It is important to include infectious diseases, especially tuberculosis, in the differential diagnosis of pulmonary nodules and masses.
Our study has limitations. Due to the small sample size, we analyzed the various bronchoscopic methods (BAL, TBB, Brush, and needle aspiration) collectively, rather than separately. It is important to standardize the procedure; to choose the best method to use in each case; to collect as much material as possible; and to send the material for cytological, cell-block, histological, and microbiological analysis, as needed, although such tests are not universally available. In our patients, the cell-block analysis of material obtained from needle aspiration was important for the diagnosis, particularly in pulmonary nodules. Three of our patients were lost to follow-up reducing the sample size. In addition, fluoroscopy and ROSE were not employed in all cases.
In general, EBUS is a safe procedure with a low complication rate. Steinfort et al. 13 studied the effectiveness and complications of radial-probe EBUS-guided TBB, in comparison with those of CT-guided TTNA. They found that the incidence of pneumothorax was much higher in CT-guided TTNA, and that the diagnostic accuracy of radial-probe EBUS-guided TBB was comparable to CT-guided TTNA. In our study, we found that pneumothorax requiring chest tube drainage occurred in only approximately 4% of the patients, and that bleeding (mild to moderate), which was controlled with local hemostatic measures, occurred in less than 10% of the patients. All such complications occurred in patients with pulmonary nodules.
In conclusion, radial-probe EBUS showed a good safety profile and a high diagnostic yield for peripheral pulmonary masses and nodules.
Footnotes
Financial support: None.
Study performed at the Serviço de Endoscopia Respiratória, Instituto do Coração-InCor-Hospital das Clínicas, Faculdade de Medicina, Universidade de São Paulo, São Paulo (SP) Brasil
References
- 1.Rivera MP, Mehta AC. American College of Chest Physicians Initial diagnosis of lung cancer ACCP evidence-based clinical practice guidelines (2nd edition) Chest. 2007;132(3 Suppl):131S–148S. doi: 10.1378/chest.07-1357. [DOI] [PubMed] [Google Scholar]
- 2.Baaklini WA, Reinoso MA, Gorin AB, Sharafkaneh A, Manian P. Diagnostic yield of fiberoptic bronchoscopy in evaluating solitary pulmonary nodules. Chest. 2000;117(4):1049–1054. doi: 10.1378/chest.117.4.1049. [DOI] [PubMed] [Google Scholar]
- 3.Rivera MP, Mehta AC, Wahidi MM. Establishing the diagnosis of lung cancer Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5 Suppl):142S–165S. doi: 10.1378/chest.12-2353. [DOI] [PubMed] [Google Scholar]
- 4.Wahidi MM, Govert JA, Goudar RK, Gould MK, McCrory DC. Evidence for the treatment of patients with pulmonary nodules when is it lung cancer?: ACCP evidence-based clinical practice guidelines (2nd edition) Chest. 2007;132(3 Suppl):94S–107S. doi: 10.1378/chest.07-1352. [DOI] [PubMed] [Google Scholar]
- 5.Lee SM, Park CM, Lee KH, Bahn YE, Kim JI, Goo JM. C-arm cone-beam CT-guided percutaneous transthoracic needle biopsy of lung nodules clinical experience in 1108 patients. Radiology. 2014;271(1):291–300. doi: 10.1148/radiol.13131265. [DOI] [PubMed] [Google Scholar]
- 6.Sheski FD, Mathur PN. Endobronchial ultrasound. Chest. 2008;133(1):264–270. doi: 10.1378/chest.06-1735. [DOI] [PubMed] [Google Scholar]
- 7.Steinfort DP, Khor YH, Manser RL, Irving LB. Radial probe endobronchial ultrasound for the diagnosis of peripheral lung cancer systematic review and meta-analysis. Eur Respir J. 2011;37(4):902–910. doi: 10.1183/09031936.00075310. [DOI] [PubMed] [Google Scholar]
- 8.Kurimoto N, Miyazawa T, Okimasa S, Maeda A, Oiwa H, Miyazu Y. Endobronchial ultrasonography using a guide sheath increases the ability to diagnose peripheral pulmonary lesions endoscopically. Chest. 2004;126(3):959–965. doi: 10.1378/chest.126.3.959. [DOI] [PubMed] [Google Scholar]
- 9.Kurimoto N, Murayama M, Yoshioka S, Nishisaka T. Analysis of the internal structure of peripheral pulmonary lesions using endobronchial ultrasonography. Chest. 2002;122(6):1887–1894. doi: 10.1378/chest.122.6.1887. [DOI] [PubMed] [Google Scholar]
- 10.Hergott CA, Tremblay A. Role of bronchoscopy in the evaluation of solitary pulmonary nodules. Clin Chest Med. 2010;31(1):49–63. doi: 10.1016/j.ccm.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 11.Sanchez-Font A, Giralt L, Vollmer I, Pijuan L, Gea J, Curull V. Endobronchial ultrasound for the diagnosis of peripheral pulmonary lesions A controlled study with fluoroscopy. Arch Bronconeumol. 2014;50(5):166–171. doi: 10.1016/j.arbr.2014.03.014. [DOI] [PubMed] [Google Scholar]
- 12.Figueiredo VR, Jacomelli M, Rodrigues AJ, Canzian M, Cardoso PF, Jatene FB. Current status and clinical applicability of endobronchial ultrasound-guided transbronchial needle aspiration. J Bras Pneumol. 2013;39(2):226–237. doi: 10.1590/S1806-37132013000200015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Steinfort DP, Vincent J, Heinze S, Antippa P, Irving LB. Comparative effectiveness of radial probe endobronchial ultrasound versus CT-guided needle biopsy for evaluation of peripheral pulmonary lesions a randomized pragmatic trial. Respir Med. 2011;105(11):1704–1711. doi: 10.1016/j.rmed.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 14.Herth FJ, Ernst A, Becker HD. Endobronchial ultrasound-guided transbronchial lung biopsy in solitary pulmonary nodules and peripheral lesions. Eur Respir J. 2002;20(4):972–974. doi: 10.1183/09031936.02.00032001. [DOI] [PubMed] [Google Scholar]
- 15.Paone G, Nicastri E, Lucantoni G, Dello Iacono R, Battistoni P, D'Angeli AL. Endobronchial ultrasound-driven biopsy in the diagnosis of peripheral lung lesions. Chest. 2005;128(5):3551–3557. doi: 10.1378/chest.128.5.3551. [DOI] [PubMed] [Google Scholar]
- 16.Steinfort DP, Khor YH, Manser RL, Irving LB. Radial probe endobronchial ultrasound for the diagnosis of peripheral lung cancer systematic review and meta-analysis. Eur Respir J. 2011;37(4):902–910. doi: 10.1183/09031936.00075310. [DOI] [PubMed] [Google Scholar]
- 17.Huang CT, Ho CC, Tsai YJ, Yu CJ, Yang PC. Factors influencing visibility and diagnostic yield of transbronchial biopsy using endobronchial ultrasound in peripheral pulmonary lesions. Respirology. 2009;14(6):859–864. doi: 10.1111/j.1440-1843.2009.01585.x. [DOI] [PubMed] [Google Scholar]
- 18.Herth FJ, Eberhardt R, Becker HD, Ernst A. Endobronchial ultrasound-guided transbronchial lung biopsy in fluoroscopically invisible solitary pulmonary nodules a prospective trial. Chest. 2006;129(1):147–150. doi: 10.1378/chest.129.1.147. [DOI] [PubMed] [Google Scholar]


