Abstract
Fossil fuel combustion and fertilizer use has increased the amount of biologically available N entering terrestrial ecosystems. Nonetheless, our understanding of how anthropogenic N may alter the physiological mechanisms by which soil microorganisms cycle N in soil is still developing. Here, we applied shotgun metagenomics to a replicated long-term field experiment to determine how two decades of experimental N deposition, at a rate expected by mid-century, has affected the genetic potential of the soil microbial community to cycle N in soils. Experimental N deposition lead to a significant and persistent increase in functional assemblages mediating N cycle transformations associated with ecosystem N loss (i.e., denitrification and nitrification), whereas functional assemblages associated with N input and retention (i.e., N fixation and microbial N assimilation) were less positively affected. Furthermore, the abundance and composition of microbial taxa, as well as functional assemblages involved in housekeeping functions (i.e., DNA replication) were unaffected by experimental N deposition. Taken together, our results suggest that functional genes and gene pathways associated with ecosystem N loss have been favored by experimental N deposition, which may represent a genetic mechanism fostering increased N loss as anthropogenic N deposition increases in the future.
Introduction
Anthropogenic deposition of biologically-available nitrogen (N) is projected to continue throughout this century [1, 2] and understanding the fate of anthropogenic N has a myriad of implications for the function of terrestrial ecosystems. For example, the potential health and environmental damages of anthropogenic N are estimated to be $210 billion USD yr−1 in the United States alone [3]. In N-limited temperate forests [4], increased N availability may lead to a phenomenon called N saturation, which predicts ecosystem responses as N limitation to plants is alleviated, resulting in greater N loss via leaching and denitrification [5, 6]. Sugar maple (Acer saccharum Marsh.) dominated forests in the upper Great Lakes region, U. S. A., are particularly prone to N saturation, due to their high rates of net N mineralization (80–120 kg N ha-1 y-1) and moderate rates of atmospheric N deposition (7–12 kg N ha-1 y-1 [7–9]). Anthropogenic N deposition can decrease microbial respiration and biomass [10–12], and alter bacterial and fungal community composition [13–16]. However, we have a limited understanding of whether anthropogenic N deposition can alter the abundance and composition of soil microbial communities mediating N cycling processes and whether any changes will exacerbate ecosystem N loss [17, 18].
Whole-community surveys (e.g., shotgun metagenomes) are essential to understand of microbial responses to anthropogenic N deposition, especially regarding the functional assemblages mediating soil N cycling processes. For twenty years, we have experimentally increased N deposition in replicate northern hardwood forest stands to investigate the ecosystem-level consequences of chronic anthropogenic N deposition (Table 1 and Fig 1 [1]). To date, experimental N deposition has increased NO3- leaching and soil C storage [8, 19, 20]. It has also altered the composition of bacteria [21, 22] and fungi [23, 24] in forest floor. Furthermore, experimental N deposition altered the bacterial and fungal potential to degrade lignocellulose [16, 25, 26].
Table 1. Site, climatic, overstory, and ambient nitrogen deposition rates of four study sites receiving experimental N additions.
| Characteristic | Site A | Site B | Site C | Site D | |
|---|---|---|---|---|---|
| Location | |||||
| Latitude (N) | 46°52” | 45°33” | 44°23” | 43°40” | |
| Longitude (W) | 88°53” | 84°52” | 85°50” | 86°9” | |
| Climate | |||||
| Mean annual temperature (°C) | 4.8 | 6.1 | 6.5 | 7.7 | |
| Mean annual precipitation (cm) | 91.9 | 93.3 | 92.8 | 86.6 | |
| Wet + Dry Ambient N Deposition (Kg N ha−1 yr−1) | 6.8 | 9.1 | 11.7 | 11.8 | |
| Vegetation | |||||
| Overstory biomass (Mg ha-1) | 261 | 261 | 274 | 234 | |
| Acer saccharum biomass (Mg ha-1) | 237 | 224 | 216 | 201 | |
| Environment | |||||
| Leaf Litter (Oe/Oa horizons) | |||||
| Litter C:N | 63.7 | 57.1 | 52.9 | 43.4 | |
| Litter mass (g) | 412 | 396 | 591 | 550 | |
| Soil (0–10 cm) | |||||
| Sand (%) | 85 | 89 | 89 | 87 | |
| pH (1:1 soil/H2O) | 4.8 | 5.0 | 4.5 | 4.7 | |
| Base saturation, % | 71 | 96 | 73 | 80 | |
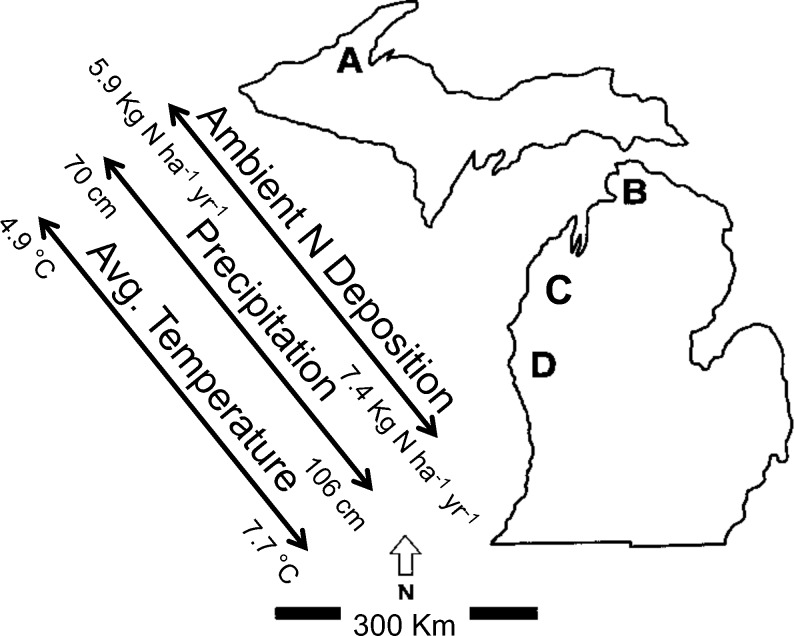
Fig 1. The geographic distribution of the study sites in Lower and Upper Michigan.
In each stand beginning in 1994, three plots received ambient atmospheric N deposition and three plots received ambient plus 30 kg NO3−–N ha−1 yr−1.
In this study, we applied shotgun metagenomics to decipher whether nearly two decades of experimental N deposition has altered the functional capacity of the soil microbial community to cycle N in our long-term field experiment. Nitrogen saturation theory predicts that northern forest ecosystems will exhibit greater rates of N cycling processes (i.e., denitrification and NO3- leaching) with reduced N limitation to plants. We hypothesized that forest stands exposed to future rates of N deposition will harbor functional genes associated with N cycle processes in a greater abundance than forest stands exposed to ambient N deposition, especially those mediating denitrification. We analyzed the shotgun metagenomes with commonly used functional gene databases (i.e., FunGene and Subsystems) to investigate the effect of experimental N deposition on the genetic capacity of soil bacteria to mediate soil N cycling processes. Using these approaches, we obtained insight to the taxonomic composition as well as the genetic capacity of the soil microbial community, which has implications to understanding the fate of anthropogenic N in soil.
Materials and Methods
Site description and sample collection
The influence of experimental N deposition on the N-cycling capacity of the forest floor (Oe/a horizon) microbial community was investigated in four stands of northern hardwood forest in Lower and Upper Michigan, USA (Table 1, Fig 1). The forest stands span the north-south range of the sugar maple (Acer saccharum Marsh) dominated northern hardwood forests in the Great Lakes region of North America [27]. All sites lie along a climatic gradient and are floristically and edaphically similar [28]. The Oi horizon is mainly comprised of sugar maple leaf litter, and the Oe/Oa horizon (i.e., forest floor) is interpenetrated by a dense mat of fine roots (~0.5 mm dia.). Soils are sandy (85–90%), well-drained, isotic, frigid Typic Haplorthods of the Kalkaska series. In 1994, six plots (30-m by 30-m) were established at each stand; three receive ambient N deposition and three receive experimental N deposition. The experimental N deposition treatment is applied during the growing season and consists of six uniform applications of NaNO3 pellets broadcast over the forest floor (30 kg N ha-1y-1). In our study sites, NO3- comprises ~60% of atmospheric N deposition [20, 29]. Sampling was allowed under a special use permit issued by the USDA Forest Service and the Michigan Department of Natural Resources.
Samples from all four sites were taken during a period that was phenologically similar across the expanse of northern hardwood forest (i.e., late May to early June 2013), a time at which high rates of microbial activity are supported by ample soil moisture. Within each 30-m-by-30-m plot, 10 random 0.1-m-by-0.1-m samples of the forest floor (Oe/Oa horizons) were collected after removing the Oi horizon, composited and hand-homogenized by plot, and immediately flash frozen with liquid N2 for nucleic acid extraction. The forest floor was the focus of this study, as prior work has shown the microbial community inhabiting the Oe/Oa horizons to be both a sink for anthropogenic N as well as sensitive to chronic N deposition [16, 25, 30]. Samples were transported to the University of Michigan within 48 hours, where they were stored at -80°C prior to nucleic acid extraction.
DNA extraction and metagenome generation
DNA was extracted from 0.60 g (fresh weight; six replicate extractions) of forest floor sample using a PowerLyzer PowerSoil DNA isolation kit and a PowerLyzer 24 homogenizer (MoBio Laboratories, Carlsbad, CA). The manufacturer’s protocol was followed and was further optimized by the initial addition of 250 μL phenol:chloroform:isoamyl alcohol (25:24:1; pH 6.7); bead beating for 45 seconds (4,000 rpm); all centrifugation steps at 4°C; and an overnight ethanol precipitation at -20°C. A PowerClean DNA Cleanup kit (MoBio) was used to purify the extracted DNA. Purified DNA quality was determined using a Nanodrop ND8000 (Thermo Scientific, Waltham, MA, USA) and then quantified by PicoGreen (Invitrogen, Carlsbad, CA, USA) on a Synergy HT fluorometer.
Shotgun sequencing of purified environmental DNA was performed at the University of Michigan DNA sequencing core. The sample DNA was barcoded by plot (n = 24) and sequenced across six lanes of an Illumina Hiseq 2500 (IIllumina, San Diego, CA, USA), with 150 base single-end reads. Cutadapt was used to remove sequences containing adapter contamination (version 1.7.1 [31]). Sequences were processed for quality control in MG-RAST [32] using default parameters and a minimum acceptable phred score of 20 in DynamicTrim [33]. Metagenome sequence data are available for public use in MG-RAST under accession numbers 4614815.3–4614838.3.
Taxonomic identification and diversity of the forest floor metagenome
Taxonomic information was obtained from the shotgun metagenomes within MG-RAST using the “best-hit classification” against the RDP database [34]. Metagenome sequences were taxonomically assigned using a maximum e-value of 1 × 10−5, minimum percent identity cutoff of 80%, and a minimum alignment length cutoff of 75. Metagenomic diversity was evaluated using the alpha diversity estimator in MG-RAST.
Identification of Functional genes
To determine the impact of experimental N deposition on the genetic potential of the forest floor microbial community to cycle N, we assessed the metagenomes as i) bacterial genes associated with N-cycle function using the FunGene database [35], and ii) functional pathways (i.e., including promoters, transport proteins, etc.) using a Subsystems-based approach in MG-RAST [36]. Inasmuch, we obtained complimentary assessments of how chronic N deposition impacted the genetic capacity of the forest floor microbial community to mediate N cycling processes.
Sequences from shotgun metagenomes were assigned to a putative function by homology to reference databases for genes associated with N cycle transformations. Reference databases were downloaded from FunGene, sequences were included that achieved a minimum score of 100 and a greater than 50% coverage to the Hidden Markov Model; the minimum score was increased for some genes to remove non-specific reference sequences from the database. (S1 Table; sensu [37]). FunGene databases for typical and atypical nosZ were combined as a single nosZ database to reduce the possibility of redundant annotations. A total of twelve FunGene databases for genes associated with N cycle function were used in further analyses (S1 Table). The abundance of genes associated with N cycle transformations was determined by the assignment of metagenome sequences to each functional gene database using the ‘blastx’ function in DIAMOND (v. 0.7.9.58 [38]). FunGene databases were retrieved on 6/16/2016 and are publicly available on GitHub (https://github.com/zacf/UM-gradient-metagenome-databases).
For Subsystems analysis, metagenome sequences were annotated to functional pathways using a maximum e-value of 1 × 10−5, a minimum percent identity cutoff of 60%, and a minimum alignment length cutoff of 25 amino acids. To gain insight in to how microbial N cycling is affected by experimental N deposition, we examined the functional pathways (i.e., Subsystem level three) within the “N cycle” level-one classification.
Housekeeping gene identification
We assessed genes involved in cellular “housekeeping” functions (e.g., DNA repair and replication) to assess whether experimental N deposition increased the relative abundance of bacterial genes mediating soil N cycling processes. It is plausible that experimental N deposition could increase the occurrence of both housekeeping genes and those involved in mediating soil N cycling processes; or, the abundance of function genes could increase, while the abundance of housekeeping genes remained constant. We evaluated the effect of N deposition on housekeeping genes by querying the metagenomes against i) housekeeping gene databases downloaded from FunGene, including DNA gyrase (gyrB), DNA recombinase (recA), and RNA polymerase (β-subunit; rpoB) using DIAMOND (S1 Table), and ii) 36 gene pathways within the “DNA metabolism” Subsystems level 3 classification in MG-RAST. We used this information to assess whether experimental N deposition increased the abundance of functional genes that mediate ecosystem processes, relative to those involved with basal metabolic processes. By doing so, we could determine if experimental N deposition increased the overall prevalence of particular N cycling genes in the forest floor bacterial community.
Statistical Analyses
For each metagenome, functional gene and gene pathway assignments were normalized to the number of reads with predicted functions (n = 24; sensu [39]). In the circumstance in which sequences were assigned to multiple Subsystem or gene categories, all categories were counted as additional hits (sensu [40]). All univariate analyses were performed in R (Version 3.01 [41]). The effect of experimental N deposition on the relative abundance and diversity of hits attributed to functional genes and pathways were evaluated by two-way ANOVA with site, treatment, and their interaction as factors; means were compared with a Tukey’s Honestly Significant Difference test (HSD [42]). Significance was accepted at α = 0.05; when applicable, P-values were corrected for multiple comparisons using the Benjamini & Hochberg false discovery rate correction [43].
Calculation of beta-diversity and associated statistics were performed in R and Primer (version 6, Primer-E Ltd., Plymouth, UK). The normalized metagenome abundance table was used to generated a Euclidian distance matrix, from which, the compositional differences between microbial N-cycling and housekeeping assemblages exposed to ambient and experimental N deposition were evaluated by permutational multivariate analysis of variance using the “adonis” function in R (PerMANOVA [44]). Site, treatment, and their interaction were included as factors in the two-way PerMANOVA model. Contributions of each gene or gene pathway to the metagenomic dissimilarity between the ambient and experimental N deposition condition were assessed using Similarity Percentage analysis (SIMPER [45]) in Primer.
Results
Taxonomic assessment of the soil microbial community
We analyzed high-quality single-end reads to determine if experimental N deposition altered the genetic capacity of the soil microbial community to cycle N in soil (sensu [39, 46, 47]). Across the 24 metagenomes (i.e., 12 plots receiving ambient N deposition and 12 plots receiving experimental N deposition), sequencing and quality control generated 717,933,077 high-quality reads totaling 108.4 Gbases. 26–33% of reads could be assigned to a putative function, percentages that are similar to other metagenomic surveys of soil [39, 48].
We determined the taxonomic classification of metagenome sequences using the RDP database within MG-RAST. Experimental N deposition did not affect the taxonomic composition of the soil microbial community at the phylum (PerMANOVA; P = 0.27), class (P = 0.30), or species (P = 0.21) level. Reads associated with Bacteria dominated the metagenomes (~98% of annotated reads); the bacterial phyla Actinobacteria (S1 Fig; ~46%;), Proteobacteria (~ 26%), and Bacteroidetes (~ 11%) were the major taxa across the metagenomes. Fungi (1.0 ± 0.6%) and Archaea (0.03 ± 0.01%) were less well represented across the metagenomes. The estimated alpha diversity of the metagenomes was not affected by experimental N deposition (S2 Fig; P = 0.80).
Effects of experimental N deposition on functional genes associated with N Cycle transformations
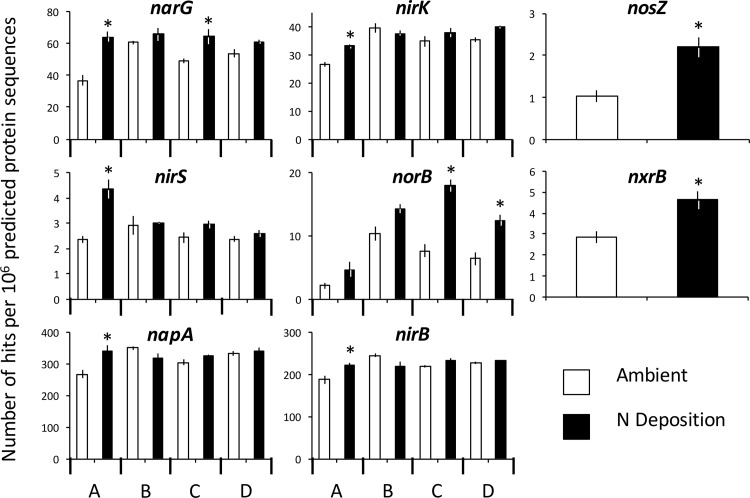
Experimental N deposition increased the abundance of functional genes in the FunGene database associated with N cycle processes, but this response mainly occurred in a site-specific manner (site × treatment; adjusted P < 0.05; Fig 2, S2 Table). For example, genes associated with assimilatory NO3- reduction (i.e., napA, nirB) increased in abundance under experimental N deposition; however, this response was driven by increased gene abundances in site A, which is the northern-most site with the least amount of ambient N deposition (napA, +28% increase; nirB, +19% increase; Tukey’s HSD; P < 0.05). These gene assemblages were unaffected by N deposition in the southern-most three sites. Further, genes associated with denitrification increased in abundance under experimental N deposition, and again this response was driven by increased gene abundances in site A (e.g., narG (+74% change from ambient), nirK (+25%), and nirS (+85% change), with no change in gene abundances in the southern-most three sites. The norB gene, which also is associated with denitrification, increased in abundance under experimental N deposition in the southern-most two sites C (+135% increase from ambient) and D (+74% increase) and not in the northern two sites. However, the nxrB gene (+63% increase from ambient), which is associated with nitrification, as well as the nosZ gene (+111% increase), which is associated with denitrification, both increased in abundance under experimental N deposition consistently across sites (site × treatment; adjusted P > 0.05).
Fig 2. The effect of experimental N deposition on the relative abundance of genes associated with N cycle transformations.
All genes presented were significantly different in abundance between the ambient (open bars) and experimental N deposition treatment (closed bars; adjusted P < 0.05). Data are shown by treatment unless the site × treatment P < 0.05, in which case the data are presented by site. Abundance data can be found in S2 and S3 Tables. * P < 0.05; result of pairwise tests can be found in S3 Table.
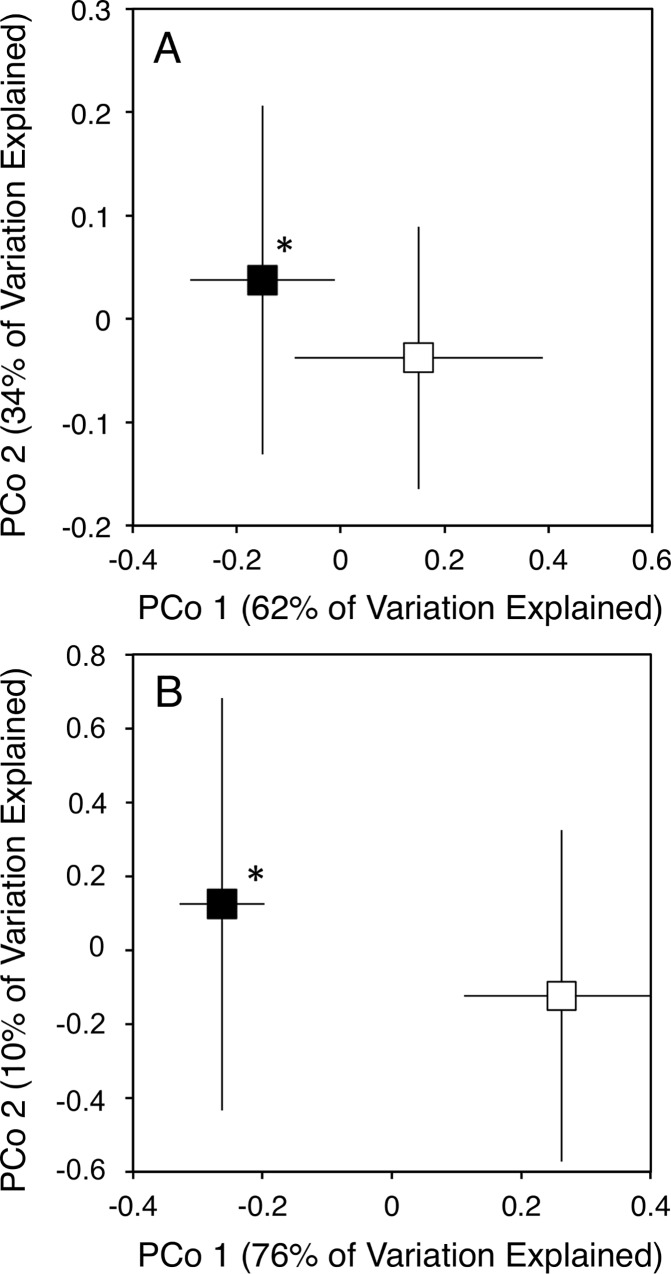
The overall composition of functional genes mediating N cycle processes was altered in a consistent manner by experimental N deposition, as there was no significant site × treatment interaction (Fig 3A; treatment effect, P = 0.003; site × treatment, P = 0.25). As indicated by SIMPER, genes associated with denitrification (Table 2; i.e., napA (25%), narG (19%)), contributed the greatest proportion of multivariate dissimilarity between the ambient and experimental N deposition treatment, followed by genes associated with assimilatory NO3 reduction (i.e., nirB, (12%) and nirA (10%)). Together, functional genes mediating denitrification and assimilatory NO3 reduction contributed 89% of the total dissimilarity between the microbial community under ambient and experimental N deposition.
Fig 3.
The effect of experimental N deposition on the composition of functional genes (A) and Subsystems gene pathways (B) associated with the N cycle. Ordinations were obtained from Principle Coordinates Analysis on based Euclidian distances of normalized data. Open and closed boxes indicate the composition of functional gene pathways in the ambient and experimental N deposition treatment, respectively. * P < 0.05 by PerMANOVA.
Table 2. Contribution of functional genes implicated in the N cycle to compositional dissimilarity as determined by SIMPER.
| Process | Gene | Average Dissimilarity | Percent Contribution | Cumulative Percent Contribution |
|---|---|---|---|---|
| Denitrification | 67.2 | 67.2 | ||
| napA | 1.8 | 24.9 | ||
| narG | 1.4 | 19.1 | ||
| norB | 1.1 | 15.3 | ||
| nosZ | 0.3 | 3.8 | ||
| nirK | 0.2 | 2.7 | ||
| nirS | 0.1 | 1.4 | ||
| Assimilatory NO3 reduction | 21.5 | 88.7 | ||
| nirB | 0.9 | 12.0 | ||
| nirA | 0.7 | 9.5 | ||
| Nitrification | 6.6 | 95.3 | ||
| nxrB | 0.3 | 4.4 | ||
| ureA | 0.2 | 2.2 | ||
| N Fixation | 4.8 | 100 | ||
| nifD | 0.2 | 2.8 | ||
| nifH | 0.2 | 2.0 |
Effects of N deposition on functional pathways of the N cycle
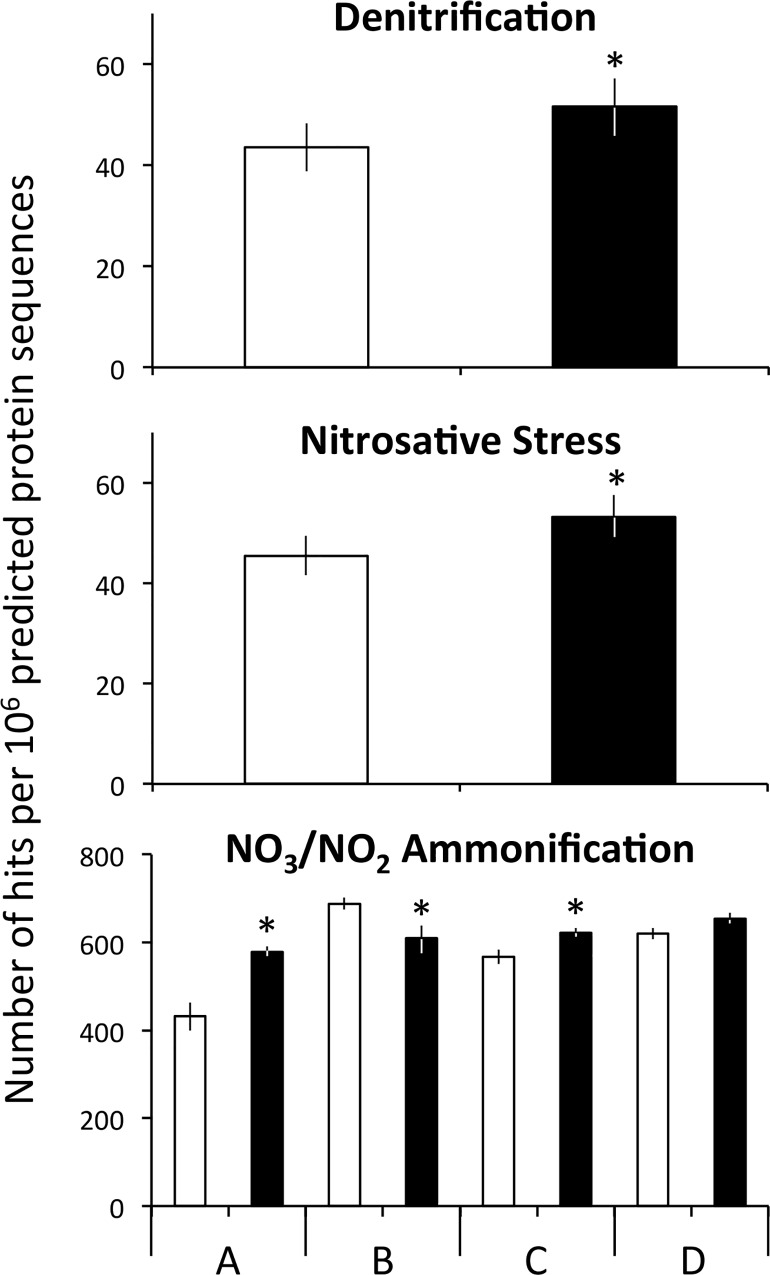
Experimental N deposition altered the abundance of 3 of 11 total Subsystems level 3 functional pathways within the “N Cycle” level 1 classification (Fig 4 and S4 Table; adjusted P < 0.05). Further, all 3 affected gene pathways increased in abundance in the experimental N deposition treatment, relative to the ambient treatment. For example, gene pathways associated with denitrification (+18% change from ambient) and nitrosative stress protection (+19%) were most positively affected by experimental N deposition, followed by gene pathways associated with NO3/NO2 ammonification (i.e., assimilatory and respiratory pathways; +9%). Gene pathways mediating nitrosative stress protection and denitrification were affected similarly by experimental N deposition (site × treatment; adjusted P > 0.05), however, gene pathways associated with NO3/NO2 ammonification were affected in a site-specific manner (S5 Table; site × treatment; adjusted P < 0.05). Pairwise tests indicate that gene pathways associated with NO3/NO2 ammonification were significantly more abundant in sites A (+34%) and C (+10%), were less abundant in site B (-11%), and were unaffected by experimental N deposition in site D.
Fig 4. The effect of experimental N deposition on the relative abundance of Subsystems level 3 functional pathways.
All pathways presented were significantly different in abundance between the ambient (open bars) and experimental N deposition treatment (closed bars; adjusted P < 0.05). Data are shown by treatment unless the site × treatment P < 0.05, in which case the data are presented by site. The full datasets can be found in S3 Table. × Site × Treatment; adjusted P < 0.05.
The composition of Subsystems level 3 functional pathways was altered by experimental N deposition in a consistent fashion across all sites (Fig 3B; treatment effect, P = 0.04; site × treatment, P = 0.19). SIMPER determined that functional pathways associated with nitrilase (Table 3; 31%), denitrification (23%), and NO synthase (19%) contributed greatest to the dissimilarity in the functional pathway composition under ambient and experimental N deposition.
Table 3. Contribution of Subsystems pathways to compositional dissimilarity as determined by SIMPER.
| Subsystems Pathway | Average Dissimilarity | Percent Contribution | Cumulative Percent Contribution Explained |
|---|---|---|---|
| Nitrilase | 2.2 | 30.7 | 30.7 |
| Denitrification | 1.6 | 22.9 | 53.6 |
| Nitric oxide synthase | 1.4 | 18.8 | 72.4 |
| Nitrosative stress protection | 0.7 | 9.5 | 81.9 |
| Nitrogen fixation | 0.4 | 5.7 | 87.6 |
* Only those Subsystems level 3 pathways that contributed greater than 5% to the total multivariate dissimilarity was included.
Effect of N deposition on housekeeping genes
Experimental N deposition did not affect any of the 36 Subsystems level 3 functional pathways within the “DNA metabolism” level 1 classification (S5 Table; adjusted P > 0.05). Experimental N deposition also did not affect the relative abundance of three functional genes associated with housekeeping functions, namely gyrB, recA, and rpoB (Table 4; adjusted P > 0.05). Similarly, experimental N deposition neither affected the composition of DNA metabolism functional pathways nor housekeeping genes (data not shown; adjusted P > 0.05). Thus, chronic experimental N deposition selects for functional genes and gene pathways that mediate N cycle transformations, as it did not affect genes or gene pathways involved with basal metabolic processes.
Table 4. The relative abundance of metagenomic hits to genes associated with “housekeeping” functions in DIAMOND.
| Gene | Ambient | Experimental N Deposition |
|---|---|---|
| gyrB | 486.7 ± 11.5 | 482.5 ± 12.1 |
| recA | 177.5 ± 4.3 | 178.3 ± 5.3 |
| rpoB | 1043.3 ± 15.7 | 1040.8 ± 25.0 |
Data are presented as number of hits per 1,000,000 predicted protein sequences. None were significantly different between the ambient and experimental N deposition treatment.
Discussion
Selection of genes associated with the N cycle under future rates of N deposition
Two decades of experimental N deposition significantly increased the abundance of functional genes and gene pathways associated with certain N cycle processes. Further, this effect was most pronounced in the northernmost sites in our long-term field experiment that have historically experienced the lowest amounts of ambient N deposition. In contrast, experimental N deposition did not affect the relative abundance, composition, or diversity of microbial taxa, as well as genes associated with housekeeping functions (e.g., DNA and protein synthesis). Consistent with N saturation theory, our findings suggest that chronic N deposition has favored a microbial community with the genetic capacity for N cycle transformations, i.e., denitrification and NO3/NO2 ammonification, which may lead to ecosystem N loss [5, 17]. However, this result differs from others who found ecological coherence in the response of microbial taxa and functional genes to N enrichment [39, 49]. Importantly, experimental N deposition in our study occurs at a rate ~10-fold lower than in other studies (30 vs. ~300 kg N ha-1y-1). It is possible that chronic N deposition, at a rate expected by mid-century across parts of eastern North America and Europe (i.e., as in our study), is sufficient to select for the presence of functional genes associated with the cycling of N, but not to cause concomitant shift in taxonomic composition. Though obtaining taxonomic data from metagenomes is not without limitations [50], a concurrent analysis of bacterial and fungal rRNA gene sequences revealed that neither were affected by experimental N deposition [24]. Taken together, these observations suggest a selection of certain genes and gene pathways mediating N cycle processes, rather than a shift in taxonomic composition that elicits a concomitant change in functional capacity. In our case, the analysis of functional genes and gene pathways, in addition to both ribosomal genes and genes associated with housekeeping functions, supports the notion that genes associated with soil N transformations are favored by experimental N deposition.
Functional assemblages mediating ecosystem N loss are favored by experimental N deposition
In our study, complimentary metagenomic analyses (i.e., homology of metagenomic reads to FunGene databases and Subsystems functional pathways) determined that the genetic potential of the soil microbial community to cycle N has been altered by experimental N deposition, which may be one mechanism mediating ecosystem N-loss under future rates of N deposition. For example, anthropogenic N enrichment can favor ecosystem N loss in forests through increased rates of denitrification and nitrification [51, 52]. Thus, it would be expected that the abundance of functional genes and gene pathways associated with denitrification and nitrification would be greater in soils exposed to experimental N deposition as compared to the ambient N treatment. Indeed, functional gene pathways associated with denitrification increased under experimental N deposition (19% increase from the ambient treatment; Fig 4), although functional gene associated with denitrification from the FunGene database were impacted in a site-specific fashion (Fig 4; see Site-specific responses of N cycle functional assemblages to experimental N deposition). Moreover, functional assemblages associated with denitrification accounted for a significant portion of the dissimilarity between bacterial functional assemblages associated with N-cycling processes, accounting for 67% and 23% of the dissimilarity of the gene and gene pathway assemblage composition, respectively (Tables 2 and 3). The genetic potential for nitrification was also favored by experimental N deposition, as indicated by an increased abundance of nxrB (+63% increase from ambient). However, the rate-limiting step in nitrification is considered to be the oxidation of ammonia into nitrite, which is performed by ammonia oxidizing archaea and bacteria (AOA and AOB, respectively). The metagenomes were interrogated for the presence of ammonia-oxidizing archaea and bacteria in our experiment; however, the abundance of these functional genes was below the limit of detection of our analyses. These metagenomic changes may have ecosystem-level consequences, as a recent meta-analysis determined the abundance of genes associated with denitrification and nitrification were positively correlated with their respective process rates [17]. In our field experiment, experimental N deposition increased annual net nitrification (+27% [8]) as well as NO3- leaching (+680% [53]), a finding consistent with other studies as well as N saturation theory [54, 55]; unfortunately process rates for denitrification in our experiment were unavailable at this time. Previous estimations indicated denitrification rates increased 5-fold with NO3- fertilization, however, the potential for denitrification in our long-term field experiment is low since the sandy soils are well drained and highly aerobic [56]. While these findings represent a plausible genetic mechanism mediating ecosystem N loss in our field experiment, a robust analysis of microbial N cycling in the forest floor is needed to more clearly determine if the observed compositional shifts may produce a concomitant functional change in response to experimental N deposition.
Functional gene pathways associated with nitrosative stress protection increased in abundance under chronic anthropogenic N deposition (+18% change from ambient; Fig 2), which suggests an increased impact of reactive nitrogen species (e.g., NO-derived compounds; NOx) on an N enriched Earth. NOx can arise from fossil fuel combustion as well as incomplete denitrification and nitrification (e.g., the “hole-in-the-pipe” model [57]) and has been linked to acid rain, biodiversity loss, and ozone depletion, among other environmental consequences [58–60]. In deciduous forests, NOx emission increases with N enrichment and is expected to increase in the Anthropocene [61–63], although in situ measurements of NOx emissions in our experiment were unavailable. One of the most understood mechanisms for bacterial NO detoxification involves flavohemoprotein, which enzymatically converts NO to NO3- [64]. Genes encoding flavohemoprotein accounted for 46% of metagenome hits to the nitrosative stress protection Subsystem (data not shown), thus the increased microbial response to nitrosative stress may be one factor mediating NO3- leaching in our long-term experiment. Microbial nitrosative stress protection may be a favored trait in the Anthropocene, and a conduit of ecosystem N loss in an N-enriched world.
Response of functional genes mediating ecosystem N input and retention
The response of functional genes and gene pathways associated with N cycle transformations leading to ecosystem N input and retention (i.e., N fixation) were not affected by experimental N deposition in a robust fashion, which may exacerbate ecosystem N loss under future rates of N deposition. For example, functional genes and gene pathways associated with N2 fixation did not change in relative abundance due to experimental N deposition (Figs 2 and 4), but did account for 26% and 5% of compositional dissimilarity between functional assemblages exposed to ambient and experimental N deposition, respectively. The link between gene abundance and function for functional genes associated with N2 fixation remains elusive [17]. However, free-living N2-fixing bacteria are typically facultative in nature and can reduce N2 fixation as N limitation is alleviated, which can foster slowed or reduced N2 fixation under N enrichment [65–67].
The incorporation of N to microbial biomass and subsequently into soil organic matter is a potential a mechanism fostering N-retention as northern hardwood forests acclimate to future rates of N deposition. For example, microbial assimilation of 15NO3- occurred within hours of addition in experimental N deposition plots in our long-term experiment [30]; similar findings have also been reported in lab incubations [8] and agricultural soils [68]. From this, it would be expected that functional assemblages associated with N assimilation would increase under experimental N deposition. Here, the abundance of two functional genes and a gene pathway mediating microbial N assimilation (i.e., napA, nirB and the NO3/NO2 ammonification pathway) increased under experimental N deposition, albeit in a site-dependent manner. Specifically, napA and nirB both increased in abundance in the northern-most site A (28% and 19% increase from ambient, respectively), but not in the southern-most three sites, B, C, and D. Whereas, gene pathways associated with NO3/NO2 ammonification, which includes assimilatory pathways (e.g., NAS and Nar genes), increased in abundance in sites A (+34%) and C (+10%), but decreased in abundance in site B (11% decrease from ambient) and was unaffected by N deposition in site D. However, gene pathways associated with ammonia assimilation (e.g., via glutamate dehydrogenase) were unaffected by N deposition. Together, this suggests an increased potential for nitrate and nitrite assimilation, but not ammonia assimilation under future rates of atmospheric N deposition. Nitrilase genes are associated with N assimilation in some bacteria [69, 70]. Here, gene pathways associated with nitrilase contributed most to compositional dissimilarity between functional assemblages exposed to ambient and experimental N deposition (31%; Table 3), but the abundance of this gene pathway was not affected by experimental N deposition in a significant fashion. Counter to our predictions, the genetic potential of the soil microbial community to assimilate N was not affected by experimental N deposition in a robust fashion.
Site-specific responses of N cycle functional assemblages to experimental N deposition
The significant site by treatment interactions that were observed for functional assemblages associated with denitrification and NO3/NO2 ammonification suggests a site-specific response to experimental N deposition. The four experimental forest stands were selected to encompass the north-south range of the northern hardwood forests in the Great Lakes region of North America, which is an expansive ecosystem. Over the last decade, ambient N deposition has ranged from ~4 to ~12 kg N ha-1 y-1 across our study sites (sites A to D, respectively; Fig 1 [71, 72]). Because the N deposition treatment (30 kg N ha-1 y-1) is constant across sites, the increase in N deposition ranges from ~10-fold at site A to ~3-fold at the southern-most site D. It is possible that functional assemblages associated with denitrification and N ammonification previously exposed to relatively high ambient N deposition (i.e., the southern-most sites) may not have been impacted by our treatment in a way we would be able to detect. It is also plausible that functional assemblages associated with denitrification and N ammonification in our southernmost sites have acclimated to the experimental N deposition treatments, whereas those in the northernmost sites have not.
Previous efforts to interrogate the impact of anthropogenic N enrichment on functional genes and gene pathways associated with soil N cycling processes in our long-term field experiment determined that experimental N deposition led to a less abundant and less rich functional assemblage associated with N cycle transformations [73], which differs from results presented here. In this study, samples were taken for during late spring, whereas samples for the previous study were taken in early fall. It is plausible that differences in phenology between the spring and fall sampling times may explain the observed differences in microbial responses to chronic N deposition. For example, the microbial decomposition of fine roots is a major C source for the soil microbiome [74], and fine roots are the major source of recalcitrant plant litter in our field experiment [75]. In northern hardwood forests, rates of fine root mortality are evenly spread throughout the year, although a pulse of fine root growth occurs during spring whereas fall is a time of increased fine root mortality and loss [76]. Nitrogen cycling functional assemblages are sensitive to substrate addition (i.e., exogenous C substrates [77]), thus, seasonal differences in substrate availability from fine roots may constrain N cycling functional assemblages in our field experiment. A seasonal assessment of N cycling transformations is needed to more accurately assess the temporal robustness of the microbial response to anthropogenic N enrichment.
Conclusion
The results presented here support the hypothesis that chronic N deposition selects for bacteria harboring functional genes and gene pathways mediating N cycling processes that are associated with ecosystem N loss (i.e., denitrification, nitrification), whereas functional assemblages associated with ecosystem N retention (i.e., N assimilation) were less positively affected by this agent of global change. The increased abundance of certain N-cycling functional assemblages occurred as part of a broad microbial response to anthropogenic N deposition which results in increased NO3 leaching and soil C storage, as well as reduced litter decay. Taken together, results presented here suggest that anthropogenic N deposition may fundamentally alter the potential of soil microbial communities to cycle N in soils, which is a plausible mechanism mediating increased N loss in our long-term field experiment.
Supporting Information
Data presented represent the mean ± SE (n = 12). “Other” includes phyla with less than 1% relative abundance, including the bacterial phyla Chlamydiae, Cyanobacteria, Chloroflexi, Spirochaetes, Tenericutes, Gemmatimonadetes, Chlorobi, Thermotogae, Deferribacteres, and Deinococcus-Thermus, and Eukaryotes Ascomycota and Basidiomycota, Bacillariophyta and Archaea Thaumarchaeota.
(TIFF)
Data presented represent the mean ± SE (n = 12).
(TIFF)
(DOCX)
Data are presented as mean number ± SE (n = 12) of hits per 1,000,000 predicted protein sequences.
(DOCX)
Data are presented as mean number ± SE (n = 3) of hits per 1,000,000 predicted protein sequences.
(DOCX)
Data are presented as mean number ± SE (n = 12) of hits per 1,000,000 predicted protein sequences.
(DOCX)
Data are presented as mean number ± SE (n = 12) of hits per 1,000,000 predicted protein sequences.
(DOCX)
Acknowledgments
We thank Karl Romanowicz for his work in sample preparation and Will Argiroff and Lauren Cline for their assistance in shotgun metagenome analyses. We also wish to thank the two Reviewers as well as other members of the Zak lab for their thoughtful feedback on this manuscript.
Data Availability
Metagenome sequence data are publically available in MG-RAST (Meyer et al., 2008) under accession numbers 4614815.3-4614838.3. All FunGene databases were accessed on 6/6/2016 and are publically available on GitHub (https://github.com/zacf/UM-gradient-metagenome-databases).
Funding Statement
Grants from the United States Department of Energy’s Biological and Environmental Research program and the National Science Foundation's Long Term Research in Environmental Biology program provided support for our work.
References
- 1.Galloway JN, Dentener FJ, Capone DG, Boyer EW, Howarth RW, Seitzinger SP, et al. Nitrogen cycles: past, present, and future. Biogeochemistry. 2004;70(2):153–226. 10.1007/s10533-004-0370-0 [DOI] [Google Scholar]
- 2.Torseth K, Aas W, Breivik K, Fjaeraa AM, Fiebig M, Hjellbrekke AG, et al. Introduction to the European Monitoring and Evaluation Programme (EMEP) and observed atmospheric composition change during 1972–2009. Atmos Chem Phys. 2012;12(12):5447–81. 10.5194/Acp-12-5447-2012 . [DOI] [Google Scholar]
- 3.Sobota DJ, Compton JE, McCrackin ML, Singh S. Cost of reactive nitrogen release from human activities to the environment in the United States. Environmental Research Letters. 2015;10(2):025006. [Google Scholar]
- 4.Vitousek PM, Hattenschwiler S, Olander L, Allison S. Nitrogen and nature. Ambio. 2002;31(2):97–101. . [DOI] [PubMed] [Google Scholar]
- 5.Aber J, McDowell W, Nadelhoffer K, Magill A, Berntson G, Kamakea M, et al. Nitrogen saturation in temperate forest ecosystems—hypotheses revisited. Bioscience. 1998;48(11):921–34. . [Google Scholar]
- 6.Aber JD, Nadelhoffer KJ, Steudler P, Melillo JM. Nitrogen saturation in northern forest ecosystems. Bioscience. 1989;39(6):378–86. . [Google Scholar]
- 7.MacDonald NW, Burton AJ, Jurgensen MF, Mclaughlin JW, Mroz GD. Variation in forest soil properties along a great-lakes air-pollution gradient. Soil Sci Soc Am J. 1991;55(6):1709–15. . [Google Scholar]
- 8.Zak DR, Holmes WE, Tomlinson MJ, Pregitzer KS, Burton AJ. Microbial cycling of C and N in northern hardwood forests receiving chronic atmospheric NO3- deposition. Ecosystems. 2006;9(2):242–53. 10.1007/S10021-005-0085-7 . [DOI] [Google Scholar]
- 9.Zak DR, Pregitzer KS. Spatial and temporal variability of nitrogen cycling in northern lower Michigan. Forest Sci. 1990;36(2):367–80. . [Google Scholar]
- 10.Janssens IA, Dieleman W, Luyssaert S, Subke JA, Reichstein M, Ceulemans R, et al. Reduction of forest soil respiration in response to nitrogen deposition. Nature Geoscience. 2010;3(5):315–22. 10.1038/Ngeo844 . [DOI] [Google Scholar]
- 11.Burton AJ, Pregitzer KS, Crawford JN, Zogg GP, Zak DR. Simulated chronic NO3- deposition reduces soil respiration in northern hardwood forests. Glob Chang Biol. 2004;10(7):1080–91. 10.1111/J.1365-2486.2004.00737.X . [DOI] [Google Scholar]
- 12.Wallenstein MD, McNulty S, Fernandez IJ, Boggs J, Schlesinger WH. Nitrogen fertilization decreases forest soil fungal and bacterial biomass in three long-term experiments. Forest Ecol Manag. 2006;222(1–3):459–68. 10.1016/J.Foreco.2005.11.002 . [DOI] [Google Scholar]
- 13.Fierer N, Lauber CL, Ramirez KS, Zaneveld J, Bradford MA, Knight R. Comparative metagenomic, phylogenetic and physiological analyses of soil microbial communities across nitrogen gradients. Isme J. 2011. Epub 2011/12/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nemergut DR, Townsend AR, Sattin SR, Freeman KR, Fierer N, Neff JC, et al. The effects of chronic nitrogen fertilization on alpine tundra soil microbial communities: implications for carbon and nitrogen cycling. Environ Microbiol. 2008;10(11):3093–105. Epub 2008/09/04. 10.1111/j.1462-2920.2008.01735.x . [DOI] [PubMed] [Google Scholar]
- 15.Allison SD, Hanson CA, Treseder KK. Nitrogen fertilization reduces diversity and alters community structure of active fungi in boreal ecosystems. Soil Biol Biochem. 2007;39(8):1878–87. 10.1016/J.Soilbio.2007.02.001 . [DOI] [Google Scholar]
- 16.Edwards IP, Zak DR, Kellner H, Eisenlord SD, Pregitzer KS. Simulated atmospheric N deposition alters fungal community composition and suppresses ligninolytic gene expression in a northern hardwood forest. PLoS One. 2011;6(6):e20421 Epub 2011/06/28. 10.1371/journal.pone.0020421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rocca JD, Hall EK, Lennon JT, Evans SE, Waldrop MP, Cotner JB, et al. Relationships between protein-encoding gene abundance and corresponding process are commonly assumed yet rarely observed. Isme J. 2015;9(8):1693–9. 10.1038/ismej.2014.252 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Graham EB, Knelman JE, Schindlbacher A, Siciliano S, Breulmann M, Yannarell A, et al. Microbes as engines of ecosystem function: when does community structure enhance predictions of ecosystem processes? Frontiers in Microbiology. 2016;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pregitzer KS, Burton AJ, Zak DR, Talhelm AF. Simulated chronic nitrogen deposition increases carbon storage in Northern Temperate forests. Glob Chang Biol. 2008;14(1):142–53. 10.1111/j.1365-2486.2007.01465.x [DOI] [Google Scholar]
- 20.Zak DR, Holmes WE, Burton AJ, Pregitzer KS, Talhelm AF. Simulated atmospheric NO3- deposition increases soil organic matter by slowing decomposition. Ecol Appl. 2008;18(8):2016–27. Epub 2009/03/07. . [DOI] [PubMed] [Google Scholar]
- 21.Eisenlord SD, Zak DR. Simulated atmospheric nitrogen deposition alters actinobacterial community composition in forest soils. Soil Sci Soc Am J. 2010;74(4):1157–66. [Google Scholar]
- 22.Freedman ZB, Zak DR. Atmospheric N deposition alters connectance, but not functional potential among saprotrophic bacterial communities. Mol Ecol. 2015;24(12):3170–80. Epub 5/5/2015. 10.1111/mec.13224 . [DOI] [PubMed] [Google Scholar]
- 23.Entwistle EM, Zak DR, Edwards IP. Long-Term Experimental Nitrogen Deposition Alters the Composition of the Active Fungal Community in the Forest Floor. Soil Sci Soc Am J. 2013;77(5):1648–58. 10.2136/sssaj2013.05.0179 [DOI] [Google Scholar]
- 24.Freedman ZB, Romanowicz KJ, Upchurch RA, Zak DR. Differential responses of total and active soil microbial communities to long-term experimental N deposition. Soil Biol Biochem. 2015;90:275–82. 10.1016/j.soilbio.2015.08.014 [DOI] [Google Scholar]
- 25.Freedman Z, Zak DR. Atmospheric N deposition increases bacterial laccase-like multicopper oxidases: implications for organic matter decay. Appl Environ Microbiol. 2014;80(14):4460–8. 10.1128/AEM.01224-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Freedman ZB, Upchurch RA, Zak DR, Cline LC. Anthropogenic N deposition slows decay by favoring bacterial metabolism: Insights from metagenomic analyses. Frontiers in Microbiology. 2016;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Braun EL. Deciduous forests of eastern North America. New York, NY: Macmillan Publishing Co, Inc.; 1950. 596 p. [Google Scholar]
- 28.Burton AJ, Ramm CW, Pregitzer KS, Reed DD. Use of Multivariate Methods in Forest Research Site Selection. Can J Forest Res. 1991;21(11):1573–80. 10.1139/x91-219 . [DOI] [Google Scholar]
- 29.Barnard RL, Osborne CA, Firestone MK. Responses of soil bacterial and fungal communities to extreme desiccation and rewetting. ISME J. 2013;7(11):2229–41. 10.1038/ismej.2013.104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zogg GP, Zak DR, Pregitzer KS, Burton AJ. Microbial immobilization and the retention of anthropogenic nitrate in a northern hardwood forest. Ecology. 2000;81(7):1858–66. . [Google Scholar]
- 31.Martin M. Cutadapt removes adapter sequences from high-throughput sequencing reads. 2011. 2011;17(1). 10.14806/ej.17.1.200 pp. 10–12. [DOI] [Google Scholar]
- 32.Meyer F, Paarmann D, D'Souza M, Olson R, Glass EM, Kubal M, et al. The metagenomics RAST server—a public resource for the automatic phylogenetic and functional analysis of metagenomes. BMC Bioinformatics. 2008;9:386–93. Artn 386 10.1186/1471-2105-9-386 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cox MP, Peterson DA, Biggs PJ. SolexaQA: At-a-glance quality assessment of Illumina second-generation sequencing data. BMC Bioinformatics. 2010;11:485 10.1186/1471-2105-11-485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cole JR, Wang Q, Fish JA, Chai B, McGarrell DM, Sun Y, et al. Ribosomal Database Project: data and tools for high throughput rRNA analysis. Nucleic Acids Res. 2014;42(Database issue):D633–42. 10.1093/nar/gkt1244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fish JA, Chai BL, Wang Q, Sun YN, Brown CT, Tiedje JM, et al. FunGene: the functional gene pipeline and repository. Frontiers in Microbiology. 2013;4 ARTN 291 10.3389/fmicb.2013.00291 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Overbeek R, Olson R, Pusch GD, Olsen GJ, Davis JJ, Disz T, et al. The SEED and the Rapid Annotation of microbial genomes using Subsystems Technology (RAST). Nucleic Acids Res. 2014;42(D1):D206–D14. 10.1093/nar/gkt1226 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Penton CR, St Louis D, Cole JR, Luo YQ, Wu LY, Schuur EAG, et al. Fungal Diversity in Permafrost and Tallgrass Prairie Soils under Experimental Warming Conditions. Appl Environ Microbiol. 2013;79(22):7063–72. 10.1128/Aem.01702-13 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Buchfink B, Xie C, Huson DH. Fast and sensitive protein alignment using DIAMOND. Nat Methods. 2015;12(1):59–60. 10.1038/nmeth.3176 [DOI] [PubMed] [Google Scholar]
- 39.Fierer N, Lauber CL, Ramirez KS, Zaneveld J, Bradford MA, Knight R. Comparative metagenomic, phylogenetic and physiological analyses of soil microbial communities across nitrogen gradients. ISME J. 2012;6(5):1007–17. Epub 2011/12/03. 10.1038/ismej.2011.159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zifcakova L, Vetrovsky T, Howe A, Baldrian P. Microbial activity in forest soil reflects the changes in ecosystem properties between summer and winter. Environ Microbiol. 2016;18(1):288–301. 10.1111/1462-2920.13026 . [DOI] [PubMed] [Google Scholar]
- 41.R Code Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2013. [Google Scholar]
- 42.Tukey JW. Comparing individual means in the analysis of variance. Biometrics. 1949;5(2):99–114. . [PubMed] [Google Scholar]
- 43.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate—a Practical and Powerful Approach to Multiple Testing. J Roy Stat Soc B Met. 1995;57(1):289–300. . [Google Scholar]
- 44.Anderson MJ. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 2001;26(1):32–46. . [Google Scholar]
- 45.Clarke KR. Non-parametric multivariate analyses of changes in community structure. Australian Journal of Ecology. 1993;18(1):117–43. 10.1111/j.1442-9993.1993.tb00438.x [DOI] [Google Scholar]
- 46.Cline LC, Zak DR. Soil microbial communities are shaped by plant-driven changes in resource availability during secondary succession. Ecology. 2015. 10.1890/15-0184.1 [DOI] [PubMed] [Google Scholar]
- 47.Cardenas E, Kranabetter JM, Hope G, Maas KR, Hallam S, Mohn WW. Forest harvesting reduces the soil metagenomic potential for biomass decomposition. ISME J. 2015. 10.1038/ismej.2015.57 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Uroz S, Ioannidis P, Lengelle J, Cebron A, Morin E, Buee M, et al. Functional Assays and Metagenomic Analyses Reveals Differences between the Microbial Communities Inhabiting the Soil Horizons of a Norway Spruce Plantation. Plos One. 2013;8(2). ARTN e55929 10.1371/journal.pone.0055929 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ramirez KS, Craine JM, Fierer N. Consistent effects of nitrogen amendments on soil microbial communities and processes across biomes. Glob Chang Biol. 2012;18(6):1918–27. 10.1111/j.1365-2486.2012.02639.x [DOI] [Google Scholar]
- 50.Sharpton TJ. An introduction to the analysis of shotgun metagenomic data. Front Plant Sci. 2014;5 ARTN 209 10.3389/fpls.2014.00209 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Niu S, Classen AT, Dukes JS, Kardol P, Liu L, Luo Y, et al. Global patterns and substrate-based mechanisms of the terrestrial nitrogen cycle. Ecol Lett. 2016:n/a-n/a. 10.1111/ele.12591 [DOI] [PubMed] [Google Scholar]
- 52.Lu M, Yang Y, Luo Y, Fang C, Zhou X, Chen J, et al. Responses of ecosystem nitrogen cycle to nitrogen addition: a meta-analysis. New Phytol. 2011;189(4):1040–50. Epub 2010/12/09. 10.1111/j.1469-8137.2010.03563.x . [DOI] [PubMed] [Google Scholar]
- 53.Pregitzer KS, Zak DR, Burton AJ, Ashby JA, MacDonald NW. Chronic nitrate additions dramatically increase the export of carbon and nitrogen from northern hardwood ecosystems. Biogeochemistry. 2004;68(2):179–97. . [Google Scholar]
- 54.Jefts S, Fernandez IJ, Rustad LE, Dail DB. Decadal responses in soil N dynamics at the Bear Brook Watershed in Maine, USA. Forest Ecol Manag. 2004;189(1–3):189–205. 10.1016/J.Foreco.2003.08.011 . [DOI] [Google Scholar]
- 55.Lamontagne S, Schiff SL. Response of soil microorganisms to an elevated nitrate input in an open Pinus banksiana—Cladina forest. Forest Ecol Manag. 2000;137(1–3):13–22. . [Google Scholar]
- 56.Merrill AG, Zak DR. Factors Controlling Denitrification Rates in Upland and Swamp Forests. Can J Forest Res. 1992;22(11):1597–604. 10.1139/x92-212 . [DOI] [Google Scholar]
- 57.Firestone M, Davidson E, Andreae M, Schimel D. Microbiological basis of NO and N2O production and consumption in soil. Exchange of trace gases between terrestrial ecosystems and the atmosphere: John Wiley & Sons; 1989. p. 7–21. [Google Scholar]
- 58.Johnston H. Reduction of Stratospheric Ozone by Nitrogen Oxide Catalysts from Supersonic Transport Exhaust. Science. 1971;173(3996):517-&. 10.1126/science.173.3996.517 . [DOI] [PubMed] [Google Scholar]
- 59.Erisman JW, Galloway JN, Seitzinger S, Bleeker A, Dise NB, Petrescu AMR, et al. Consequences of human modification of the global nitrogen cycle. Philos T R Soc B. 2013;368(1621). 10.1098/rstb.2013.0116 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fields S. Global nitrogen—Cycling out of control. Environ Health Persp. 2004;112(10):A556–A63. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huang Y, Li DJ. Soil nitric oxide emissions from terrestrial ecosystems in China: a synthesis of modeling and measurements. Sci Rep-Uk. 2014;4 ARTN 7406 10.1038/srep07406 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Buhlmann T, Hiltbrunner E, Korner C, Rihm B, Achermann B. Induction of indirect N2O and NO emissions by atmospheric nitrogen deposition in (semi-)natural ecosystems in Switzerland. Atmos Environ. 2015;103:94–101. 10.1016/j.atmosenv.2014.12.037 . [DOI] [Google Scholar]
- 63.Pinder RW, Davidson EA, Goodale CL, Greaver TL, Herrick JD, Liu LL. Climate change impacts of US reactive nitrogen. P Natl Acad Sci USA. 2012;109(20):7671–5. 10.1073/pnas.1114243109 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Poole RK. Nitric oxide and nitrosative stress tolerance in bacteria. Biochemical Society Transactions. 2005;33:176–80. 10.1042/BST0330176 [DOI] [PubMed] [Google Scholar]
- 65.Berthrong ST, Yeager CM, Gallegos-Graves L, Steven B, Eichorst SA, Jackson RB, et al. Nitrogen Fertilization Has a Stronger Effect on Soil Nitrogen-Fixing Bacterial Communities than Elevated Atmospheric CO2. Appl Environ Microbiol. 2014;80(10):3103–12. 10.1128/Aem.04034-13 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Reed SC, Cleveland CC, Townsend AR. Functional Ecology of Free-Living Nitrogen Fixation: A Contemporary Perspective. Annu Rev Ecol Evol Syst. 2011;42(1):489–512. 10.1146/annurev-ecolsys-102710-145034 [DOI] [Google Scholar]
- 67.Ochoa-Hueso R, Maestre FT, de Los Rios A, Valea S, Theobald MR, Vivanco MG, et al. Nitrogen deposition alters nitrogen cycling and reduces soil carbon content in low-productivity semiarid Mediterranean ecosystems. Environ Pollut. 2013;179:185–93. 10.1016/j.envpol.2013.03.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Burger M, Jackson LE. Microbial immobilization of ammonium and nitrate in relation to ammonification and nitrification rates in organic and conventional cropping systems. Soil Biol Biochem. 2003;35(1):29–36. [Google Scholar]
- 69.Luque I, Herrero A, Flores E, Madueno F. Clustering of genes involved in nitrate assimilation in the Cyanobacterium Synechococcus. Mol Gen Genet. 1992;232(1):7–11. 10.1007/Bf00299130 . [DOI] [PubMed] [Google Scholar]
- 70.Martiny AC, Kathuria S, Berube PM. Widespread metabolic potential for nitrite and nitrate assimilation among Prochlorococcus ecotypes. P Natl Acad Sci USA. 2009;106(26):10787–92. 10.1073/pnas.0902532106 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Burton AJ, Pregitzer KS, Macdonald NW. Foliar Nutrients in Sugar Maple Forests Along a Regional Pollution-Climate Gradient. Soil Sci Soc Am J. 1993;57(6):1619–28. . [Google Scholar]
- 72.Program NAD. National Atmospheric Deposition Program 2004 Annual Summary. Champaign, IL: Illinois State Water Survey, 2005. [Google Scholar]
- 73.Freedman Z, Eisenlord SD, Zak DR, Xue K, He Z, Zhou J. Towards a molecular understanding of N cycling in northern hardwood forests under future rates of N deposition. Soil Biol Biochem. 2013;66:130–8. 10.1016/j.soilbio.2013.07.010 [DOI] [Google Scholar]
- 74.Zhang XY, Wang W. The decomposition of fine and coarse roots: their global patterns and controlling factors. Sci Rep-Uk. 2015;5 ARTN 09940 10.1038/srep09940 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xia MX, Talhelm AF, Pregitzer KS. Fine roots are the dominant source of recalcitrant plant litter in sugar maple-dominated northern hardwood forests. New Phytologist. 2015;208(3):715–26. 10.1111/nph.13494 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hendrick RL, Pregitzer KS. The Demography of Fine Roots in a Northern Hardwood Forest. Ecology. 1992;73(3):1094–104. 10.2307/1940183 . [DOI] [Google Scholar]
- 77.Bottomley PJ, Taylor AE, Myrold DD. A consideration of the relative contributions of different microbial subpopulations to the soil N cycle. Front Microbiol. 2012;3:373 10.3389/fmicb.2012.00373 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data presented represent the mean ± SE (n = 12). “Other” includes phyla with less than 1% relative abundance, including the bacterial phyla Chlamydiae, Cyanobacteria, Chloroflexi, Spirochaetes, Tenericutes, Gemmatimonadetes, Chlorobi, Thermotogae, Deferribacteres, and Deinococcus-Thermus, and Eukaryotes Ascomycota and Basidiomycota, Bacillariophyta and Archaea Thaumarchaeota.
(TIFF)
Data presented represent the mean ± SE (n = 12).
(TIFF)
(DOCX)
Data are presented as mean number ± SE (n = 12) of hits per 1,000,000 predicted protein sequences.
(DOCX)
Data are presented as mean number ± SE (n = 3) of hits per 1,000,000 predicted protein sequences.
(DOCX)
Data are presented as mean number ± SE (n = 12) of hits per 1,000,000 predicted protein sequences.
(DOCX)
Data are presented as mean number ± SE (n = 12) of hits per 1,000,000 predicted protein sequences.
(DOCX)
Data Availability Statement
Metagenome sequence data are publically available in MG-RAST (Meyer et al., 2008) under accession numbers 4614815.3-4614838.3. All FunGene databases were accessed on 6/6/2016 and are publically available on GitHub (https://github.com/zacf/UM-gradient-metagenome-databases).