Abstract
Objectives
We present a novel, patient-centric, longitudinal summary of patient progress through the HIV care continuum. Using this new approach, we compare person-time spent alive, in care, on antiretroviral therapy (ART) and virally suppressed among persons who inject drugs (PWID) and those who do not (non-IDU).
Design
Prospective clinical observational cohort study.
Methods
We followed ART-naïve patients with detectable HIV viral loads who enrolled in the Johns Hopkins HIV Clinical Cohort from enrollment until the occurrence of several care continuum-related milestones, including ART initiation and viral suppression, and until several care continuum-related failures, including loss-to-clinic (LTC) and death. We added and subtracted cumulative incidence curves to estimate the proportion of the cohort in each of seven continuum stages across the 10 years following enrollment in clinical care.
Results
PWID composed 32% of the study sample (n=1,443). Over ten years following enrollment in care, PWID and non-IDU spent only 23% and 37% of person-time in care, on ART and virally suppressed. PWID lost 8.9 more months of life compared to non-IDU and spent an additional 5.0 months on ART but not virally suppressed, and an additional 5.5 months in care but not on ART. There were not meaningful improvements in the 5-year restricted mean person-time differences comparing PWID to non-IDU across enrollment cohorts (2000-03, 2004-07, 2008-14).
Conclusions
Efforts to increase viral suppression among PWID should focus on increasing ART initiation and improving adherence to therapy.
Keywords: Competing risks, HIV care continuum, Injection drug use, Survival analysis
Introduction
Viral suppression is the ultimate goal of HIV care, given the associated reduced morbidity, mortality and infectiousness [1, 2]. The HIV care continuum is a convenient framework for visualizing the path to viral suppression, and is typically presented as the proportion of HIV-infected persons diagnosed, linked to medical care, retained in care, prescribed antiretroviral therapy (ART) and virally suppressed in a given population in a given year [3, 4]. The cross-sectional care continuum does not address how individuals move through the continuum over time [5].
Injection drug use (IDU) is associated with poorer HIV-related outcomes [6, 7]. Particularly, people who inject drugs (PWID) experience delays linking to HIV medical care, and are less likely to be retained in care, prescribed ART and virally suppressed [8-10]. PWID also have a higher mortality risk than non-IDU [11, 12]. “Longitudinal” analyses of the care continuum are typically serial snapshots, with people who die removed from the denominator for continuum estimates in subsequent years [13], artificially inflating the reported proportion of PWID who are virally suppressed [14, 15]. Furthermore, excluding deceased persons precludes consideration of death as a relevant end-point for the care continuum.
We present a novel method for visualizing the care continuum for PWID and non-IDU, subsequent to engagement in care which accounts for differences in survival and shifts consideration of the care continuum from a cross-sectional to a longitudinal perspective.
METHODS
Study population
The Johns Hopkins HIV Clinic provides HIV continuity care to HIV-infected persons principally residing in the Baltimore, Maryland area, but also from surrounding states. HIV-infected patients both self-refer and are referred to the clinic by other health care providers across the region. Patients are enrolled into care without regard to demographic, socioeconomic status or medical insurance and the clinic population reflects the HIV epidemic in Baltimore and the surrounding region. The clinic is staffed by pharmacists, nurses and caseworkers that supports antiretroviral adherence through methods tailored to the individual patient. The Johns Hopkins HIV Clinical Cohort (JHHCC) consists of all HIV-infected persons age ≥18 years who enroll in care at Johns Hopkins HIV clinic and consent to share their data (>90% of persons enrolled into continuity care). For this study, we included persons enrolled in the JHHCC from January 2000 to August 2015 who were ART naïve (prior exposure to mono- or dual- therapy only) and not virally suppressed (≤400 copies/mL) at enrollment. Collection of data on patients in the JHHCC, and this analysis, were approved by the Johns Hopkins Hospital Institutional Review Board.
Patients who reported IDU as the likely source of their HIV infection (i.e., who had a history of injection prior to their HIV diagnosis) were classified as PWID. PWID may not have been actively injecting throughout the study period. Furthermore, non-IDU may have been using illicit drugs through routes other than injection (it is possible but unlikely that some patients started injecting drugs after enrollment). Heroin and cocaine are the most commonly injected drugs in this cohort; injection of amphetamine and other drugs is rare. Baseline laboratory values were defined as those measured closest to enrollment, within a window 6 months prior to and 1 month after enrollment. We excluded 14 patients without baseline CD4 cell count.
Outcomes measurement
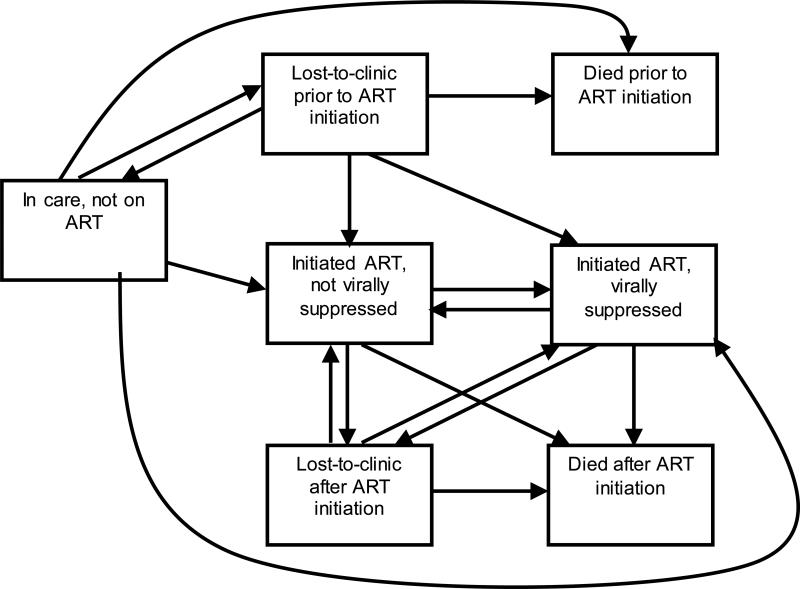
Because our study sample was, by definition, linked to care, we focused on care continuum outcomes subsequent to linkage to care: loss-to-care, ART initiation, viral suppression, and death. We stratified death and loss-to-care by whether they occurred before or after ART initiation. Thus there were seven care continuum stages in our framework (listed below; also in Appendix A, Figure 1). Loss-to-care was approximated as loss-to-clinic (LTC), with the understanding that patients lost to the Johns Hopkins HIV clinic may reengage in care elsewhere. Patients were classified as LTC after 12 months with no HIV laboratory measurements or clinical visits in the HIV outpatient center. Patients re-entered care with any new CD4 count, viral load or clinical visit. ART initiation was defined as the initiation of ≥3 antiretroviral medications on the same day. Viral suppression was defined as most recent viral load ≤400 copies/mL. Dates of death were obtained from clinic sources and regular matches against the Social Security Death Index.
Analysis
We estimated the proportion of the cohort in each stage of the care continuum over time, stratified by history of IDU. Complete details of our approach are available in appendix A. Briefly, we estimated the cumulative incidence from enrollment to the following events, nonparametrically [14]. Events in bold correspond to transition into a continuum state (regardless of prior state); events in italics correspond to transition out of a continuum state (regardless of future state).
Death before ART initiation;
LTC before ART initiation;
No longer LTC before ART initiation (composite outcome of return-to-clinic and death prior to return-to-clinic);
ART initiation;
Viral suppression after ART initiation;
No longer virally suppressed after ART initiation (composite outcome including viral load measurement >400 copies/mL, death, or LTC);
LTC after ART initiation;
No longer LTC after ART initiation; and
Death after ART initiation.
Note that ART initiation corresponds to transition into the continuum state “on ART and not suppressed” only if ART initiation is not also accompanied by viral suppression or death on the same day. Events other than ART initiation and death could occur more than once in the analysis. The maximum numbers of occurrences of each event in our data are available in Appendix A Table 1. By estimating cumulative incidence functions, we have appropriately accounted for competing events (i.e., events that preclude occurrence of the event of interest). For example, death prior to ART initiation precludes a patient from ever initiating ART; the cumulative incidence function for ART initiation can only ever go up to (100 – D)% where D is the cumulative incidence of death before ART initiation. Competing event(s) for each continuum-related event are listed in Appendix A Table 1.
The cumulative incidence for events above represent the proportion entering and exiting each of the continuum stages. To estimate the proportion present in each continuum stage, we added and subtracted cumulative incidence curves [16, 17] as follows:
Dead before ART initiation = cumulative incidence of death prior to ART initiation.
LTC before ART initiation = sum of cumulative incidences for LTC before ART initiation, less the sum of the cumulative incidences for no longer LTC before ART initiation.
Dead after ART initiation = cumulative incidence of death after ART initiation.
LTC after ART initiation = sum of the cumulative incidences for LTC after ART initiation, less the sum of the cumulative incidences for no longer LTC after ART initiation.
On ART, virally suppressed = sum of the cumulative incidences of viral suppression on ART, less the sum of the cumulative incidences of no longer being virally suppressed.
On ART and not suppressed = cumulative incidence of ART initiation, less the cumulative incidences of death after ART initiation, LTC after ART initiation, and virally suppressed on ART.
In care and not on ART = 1 minus the cumulative incidence of death before ART initiation, minus the proportion LTC before ART initiation (#2 in this list), minus the cumulative incidence of ART initiation.
By design, the proportions above sum to 1 at each time point. Thus we can present the distribution of the cohort over time since enrollment as a set of stacked curves. Integrating the area between adjacent curves (or equivalently, the area under each individual curve graphed separately) gives the (restricted) mean time spent in each continuum stage (or the mean months of life lost) over 10 years of follow-up. Here, the term “restricted” is used to clarify that the total follow-up time is capped at 10 years, and thus the average time in each state is smaller than it would be had we been able to follow everyone from enrollment until death. We estimated the restricted mean time in state empirically, and then took the difference in estimates for PWID and non-IDU.
We compare our metric to the difference in the proportion of PWID and non-IDU in each stage of the care continuum at 10 years. This latter quantity is similar to traditional care continuum estimates in that it provides a snapshot of the population at a specific time, however it is anchored to time since clinic enrollment, rather than to calendar time.
We used inverse probability weights [18, 19] to adjust for sex, black race, MSM transmission risk, and baseline age, CD4 cell count, HIV1 viral load, AIDS diagnosis and antiretroviral therapy exposure. Adjustment served to balance the distribution of these covariates among PWID and non-IDU at clinic enrollment, such that adjusted results are not due to differences in demographics or initial care-seeking behavior of PWID and non-IDU.
To examine changes in the continuum over calendar time we stratified the cohort according to year of enrollment (2000-2003, 2004-2007 and 2008-2014) and compared the difference in 5-year restricted mean time spent in each stage of the care continuum associated with IDU for each enrollment cohort.
We formed 95% Wald confidence intervals using standard error estimated by the standard deviation of estimates based on 200 non-parametric bootstrap resamples of the data [20]. We conducted all analyses in SAS 9.4 (SAS Institute Inc., Cary, NC).
RESULTS
Of 1,443 ART-naïve, virally unsuppressed, HIV-infected persons enrolled in the JHHCC between January 2000 and August 2015, the majority were male (65%), black (77%) and heterosexual (57%). Median age at enrollment was 40 years [interquartile range (IQR): 34, 46], median CD4 count was 284 cells/μL (IQR: 119, 477) and median log10 HIV viral load was 4.4 copies/mL (IQR: 3.8, 5.0). One third of persons (33%) had prior exposure to antiretroviral medications and 21% had a prior AIDS diagnosis. PWID composed 32% of the study sample. PWID were slightly older than non-IDU and fewer reported MSM or heterosexual sex as another possible risk factor for HIV infection (Table 1).
Table 1.
Characteristics of 1,443 ART-naïve, virally unsuppressed, HIV-infected persons who enrolled in the Johns Hopkins HIV Clinical Cohort from 2000-2014, stratified by self-report of injection drug use as their likely route of HIV acquisition
| PWID | Non-IDU | Total | |
|---|---|---|---|
| N | 456 | 987 | 1443 |
| Male sex† | 291 (64%) | 641 (65%) | 932 (65%) |
| Age‡ | 42 (37, 48) | 38 (31, 47) | 40 (34, 46) |
| Race | |||
| Black | 357 (78%) | 751 (76%) | 1108 (77%) |
| White | 89 (20%) | 187 (19%) | 276 (19%) |
| Other | 10 (2%) | 49 (5%) | 59 (4%) |
| Transmission risk | |||
| MSM | 34 (7%) | 329 (33%) | 363 (25%) |
| Heterosexual | 222 (49%) | 597 (60%) | 819 (57%) |
| History of any antiretroviral use | 151 (33%) | 320 (32%) | 471 (33%) |
| AIDS | 108 (24%) | 198 (20%) | 306 (21%) |
| CD4 cell count (cells/μl)‡ | 273 (104, 451) | 296 (126, 482) | 284 (119, 477) |
| <50 | 78 (17%) | 167 (17%) | 245 (17%) |
| 50-199 cells/μL | 101 (23%) | 195 (20%) | 296 (21%) |
| 200-349 | 103 (23%) | 218 (22%) | 321 (23%) |
| ≥350 cells/μl | 165 (37%) | 390 (40%) | 555 (39%) |
| Missing | 9 | 17 | 26 |
| Viral load (HIV RNA log10 copies/mL)‡ | 4.5 (3.8, 5.0) | 4.4 (3.7, 5.0) | 4.4 (3.8, 5.0) |
* Abbreviations: HIV, human immunodeficiency virus; PWID, persons who inject drugs; IDU, injection drug use; MSM, men who have sex with men; BMI, body mass index; ART, antiretroviral therapy (defined as initiating 3+ drugs on the same day); AIDS, acquired immune deficiency syndrome
N(%) unless otherwise specified
Median (IQR)
§ As measured by any positive laboratory test for antibody, antigen, or DNA/RNA
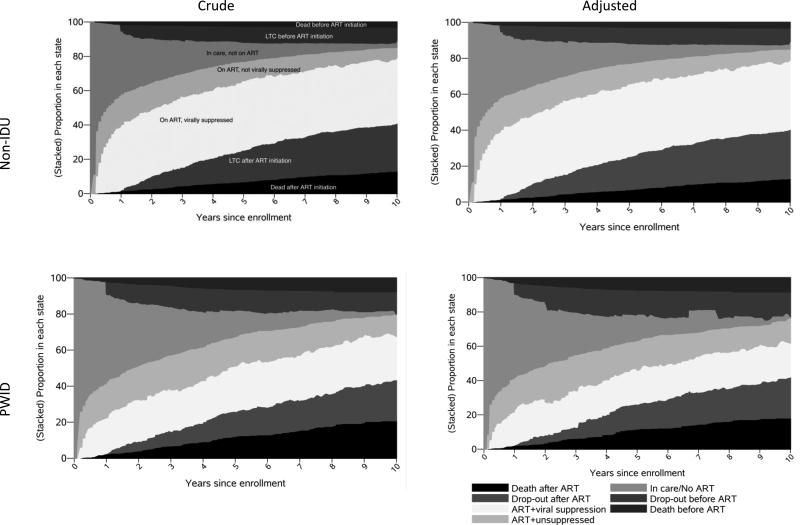
During the ten years following enrollment in the JHHCC, both non-IDU and PWID spent the most time in any one continuum stage, on ART and virally suppressed (44.9 and 27.9 months, respectively) (figure 1, table 2). However, this represented only 37% and 23% of total person-time for non-IDU and PWID. On average, PWID spent 17.0 fewer months (95% CI: −21.2, −12.8) on ART and virally suppressed compared to non-IDU. In contrast, PWID lost 8.9 more months of life, spent 5.0 more months on ART but not virally suppressed (95% CI: 2.4, 7.6) and spent 5.5 more months in care but not on ART (95% CI: 3.0, 7.9) (table 3). PWID spent 4.9 fewer months lost-to-clinic after ART initiation, but that may not be attributable to better retention; PWID had lower incidence of ART initiation and higher incidence of death after ART initiation. Results from the weighted analysis were similar to unweighted results.
Figure 1.
Proportion of N=1443 ART-naïve, virally unsuppressed, HIV-infected PWID and non-IDU in each compartment of the HIV care continuum following enrollment in the Johns Hopkins HIV Clinical Cohort to 10 years follow-up
Table 2.
Crude and adjusted* average number of months over 10 years of follow-up spent in each of the HIV care continuum stages by ART-naïve, virally unsuppressed persons who inject drugs (PWID) and those who do not (non-IDU) who enrolled in the Johns Hopkins HIV Clinical Cohort, 2000-2014
| PWID | Non-IDU | Difference | |
|---|---|---|---|
| Crude | |||
| Dead before ART initiation | 6.5 (5.4, 7.5) | 2.7 (2.2, 3.1) | 3.8 (2.6, 5.0) |
| Lost-to-care before ART initiation | 12.1 (9.7, 14.5) | 9.6 (8.1, 11.0) | 2.5 (−0.4, 5.4) |
| In care, not ART initiated | 25.9 (23.9, 28.0) | 20.5 (19.1, 21.9) | 5.5 (3.0, 7.9) |
| On ART, not virally suppressed | 19.5 (17.3, 21.7) | 14.5 (12.9, 16.0) | 5.0 (2.4, 7.6) |
| On ART, virally suppressed | 27.9 (24.7, 31.1) | 44.9 (42.2, 47.5) | −17.0 (−21.2, −12.8) |
| Lost-to-care after ART initiation | 14.7 (12.0, 17.5) | 19.6 (17.5, 21.8) | −4.9 (−8.4, −1.3) |
| Dead after ART initiation | 13.4 (11.9, 14.8) | 8.3 (7.6, 9.1) | 5.1 (3.4, 6.7) |
| Adjusted* | |||
| Dead before ART initiation | 5.9 (4.5, 7.4) | 2.9 (2.4, 3.4) | 3.0 (1.4, 4.6) |
| Lost-to-care before ART initiation | 14.4 (7.4, 21.4) | 9.3 (7.9, 10.8) | 5.1 (−2.1, 12.2) |
| In care, not ART initiated | 25.9 (22.8, 28.9) | 20.4 (18.9, 21.8) | 5.5 (2.3, 8.7) |
| On ART, not virally suppressed | 20.1 (15.6, 24.6) | 14.8 (13.2, 16.4) | 5.3 (0.5, 10.0) |
| On ART, virally suppressed | 24.5 (19.9, 29.1) | 44.8 (42.1, 47.6) | −20.3 (−25.5, −15.1) |
| Lost-to-care after ART initiation | 15.8 (10.1, 21.4) | 19.4 (17.1, 21.6) | −3.6 (−9.8, 2.5) |
| Dead after ART initiation | 13.4 (11.9, 14.8) | 8.3 (7.6, 9.1) | 5.1 (3.4, 6.7) |
Abbreviations: ART, antiretroviral therapy defined as 3+ drugs initiated on the same day; HIV, human immunodeficiency virus; IDU, injection drug use; PWID, persons who inject drugs; MSM, men who have sex with men; AIDS, acquired immunodeficiency syndrome
Adjusted for sex, age, black race, MSM transmission risk, CD4 cell count, HIV viral load, prior AIDS diagnosis and prior exposure to mono- or dual-antiretroviral therapy
Table 3.
Crude average number of months and difference comparing PWID and non-IDU over 5 years of follow-up spent in each of the HIV care continuum stages, for ART-naïve, virally unsuppressed, HIV-infected persons who enrolled in the Johns Hopkins HIV Clinical Cohort, stratified by calendar period of enrollment
| PWID | Non-IDU | Difference | |
|---|---|---|---|
| 2000-2003 (N=630) | |||
| Dead before ART initiation | 2.6 (2.0, 3.2) | 2.0 (1.5, 2.4) | 0.6 (−0.2, 1.4) |
| Lost-to-care before ART initiation | 6.0 (4.6, 7.5) | 4.2 (3.1, 5.2) | 1.9 (0.1, 3.7) |
| In care, not ART initiated | 22.6 (20.6, 24.7) | 19.2 (17.6, 20.8) | 3.4 (0.8, 6.0) |
| On ART, not virally suppressed | 11.9 (10.2, 13.6) | 11.1 (9.6, 12.6) | 0.8 (−1.5, 3.1) |
| On ART, virally suppressed | 9.8 (7.9, 11.6) | 17.8 (15.8, 19.8) | −8.0 (−10.9, −5.2) |
| Lost-to-care after ART initiation | 3.5 (2.4, 4.7) | 3.3 (2.4, 4.2) | 0.2 (−1.2, 1.7) |
| Dead after ART initiation | 3.6 (2.9, 4.2) | 2.5 (2.0, 3.0) | 1.1 (0.3, 1.9) |
| 2004-2007 (N=326) | |||
| Dead before ART initiation | 3.2 (2.0, 4.4) | 0.9 (0.5, 1.3) | 2.4 (1.1, 3.6) |
| Lost-to-care before ART initiation | 5.9 (3.5, 8.3) | 3.7 (2.4, 5.0) | 2.2 (−0.4, 4.9) |
| In care, not ART initiated | 17.5 (14.9, 20.0) | 17.1 (15.3, 18.9) | 0.3 (−2.5, 3.2) |
| On ART, not virally suppressed | 9.8 (7.4, 12.2) | 7.7 (6.2, 9.2) | 2.1 (−0.7, 4.9) |
| On ART, virally suppressed | 17.7 (13.8, 21.5) | 23.4 (20.6, 26.2) | −5.8 (−10.4, −1.1) |
| Lost-to-care after ART initiation | 2.6 (1.1, 4.1) | 4.2 (2.7, 5.7) | −1.6 (−3.7, 0.4) |
| Dead after ART initiation | 3.3 (2.2, 4.4) | 2.9 (2.3, 3.6) | 0.4 (−0.9, 1.6) |
| 2008-2014 (N=461) | |||
| Dead before ART initiation | 1.0 (0.4, 1.6) | 0.2 (0.1, 0.3) | 0.8 (0.2, 1.4) |
| Lost-to-care before ART initiation | 4.2 (2.0, 6.3) | 4.0 (2.7, 5.2) | 0.2 (−2.4, 2.8) |
| In care, not ART initiated | 15.4 (12.9, 17.9) | 10.4 (9.3, 11.6) | 5.0 (2.2, 7.7) |
| On ART, not virally suppressed | 9.7 (7.5, 11.9) | 7.2 (6.1, 8.4) | 2.5 (0.0, 5.0) |
| On ART, virally suppressed | 17.6 (13.9, 21.4) | 28.0 (25.6, 30.3) | −10.3 (−14.5, −6.1) |
| Lost-to-care after ART initiation | 7.1 (4.7, 9.5) | 8.8 (7.2, 10.3) | −1.7 (−4.6, 1.2) |
| Dead after ART initiation | 4.0 (2.8, 5.2) | 1.5 (1.1, 1.9) | 2.5 (1.3, 3.8) |
Abbreviations: ART, antiretroviral therapy defined as 3+ drugs initiated on the same day; HIV, human immunodeficiency virus; IDU, injection drug use; PWID, persons who inject drugs
Comparing the proportion of PWID and non-IDU in each stage of the care continuum at 10 years after enrollment resulted in similar substantive conclusions: PWID were less likely to be on ART and virally suppressed. However, the relative magnitude of differences between PWID and non-IDU differed. For example, while PWID spent 5% more person-time over 10 years of follow-up in care but not yet on ART (Table 2), at the end of 10 years, 4% fewer PWID were in care but not yet on ART (Supplemental Table 1). In contrast, while PWID spent 4% more person-time over 10 years on ART but not virally suppressed, at the end of 10 years, the proportion of PWID on ART but not virally suppressed was 7% higher than among non-IDU.
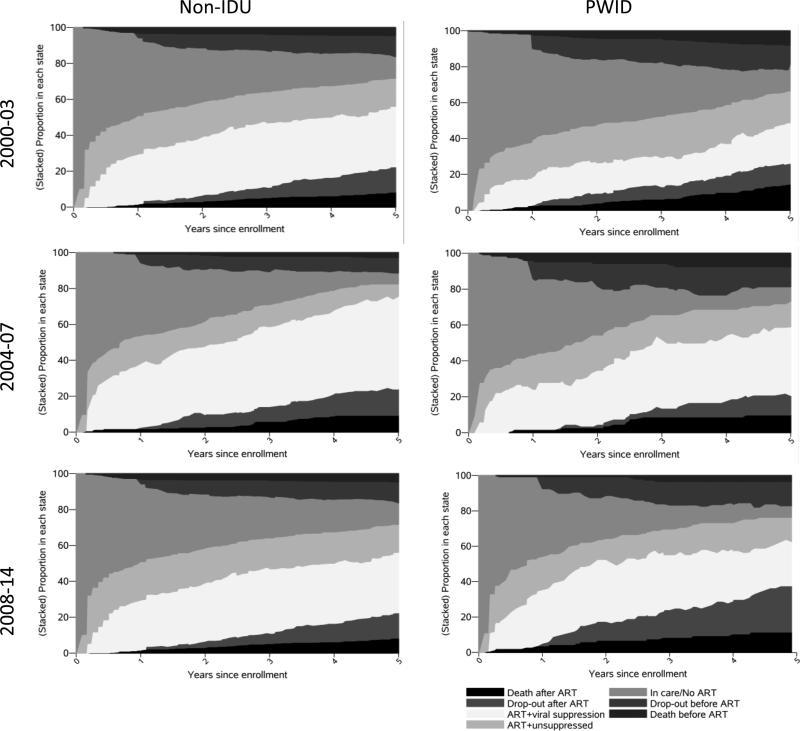
There were increases in the 5-year restricted mean time spent on ART and virally suppressed for both PWID and non-IDU from the 2000-2003 enrollment cohort (9.8 and 17.8 months, respectively) to the 2008-2014 enrollment cohort (17.6 and 28.0 months, respectively). However, differences in 5-year restricted mean time in each continuum stage comparing PWID with non-IDU were similar across all enrollment cohorts (Figure 2, Table 3). For example, the difference in months spent on ART and virally suppressed was −8.0 (95% CI: −10.9, −5.2) for the 2000-2003 enrollment cohort, and −10.3 (95% CI: −14.5, −6.1) for the 2008-2014 enrollment cohort. Thus while overall HIV care continuum outcomes improved, the disparity in outcomes between PWID and non-IDU did not.
Figure 2.
Proportion of ART-naïve, virally unsuppressed, HIV-infected PWID and non-IDU in each compartment of the HIV care continuum following enrollment in the Johns Hopkins HIV Clinical Cohort to 5 years follow-up, stratified by enrollment cohort
DISCUSSION
PWID and non-IDU spent only 23% and 37% of 10-years of follow-up on ART and virally suppressed. If we exclude years lost to death from the denominator, PWID and non-IDU spent only 28% and 41% of alive follow-up on ART and virally suppressed. If we exclude person-time lost to death or LTC (include only person-time in care), 56% (44.9/79.8 months) and 38% (27.9/73.3 months) of follow-up time was spent on ART and virally suppressed by non-IDU and PWID, respectively. In contrast, excluding persons who were dead or LTC from the denominator, 79% of non-IDU and 65% of PWID were on ART and virally suppressed at 10 years after clinic enrollment. In a traditional cross-sectional cascade analysis for 2009, 40% of non-IDU and 34% of PWID in the United States who were HIV-infected and who had initiated HIV-care were virally suppressed on ART [21]. While our analysis reached substantively similar conclusions for comparing non-IDU and PWID, the two quantities measure different constructs (person-time versus proportion of a population) in different populations (a clinical cohort versus a population). Recently, McNairy et al. compared the care continuum in four African countries using traditional continuum estimates and a new “comprehensive HIV care continuum” that shared several features with our approach (namely following persons longitudinally from when they are “at risk” for care continuum outcomes). Their conclusions comparing the four countries were substantively different depending on the approach used, demonstrating that longitudinal and traditional approaches yield different but complementary information [22].
Despite improvements in nearly all stages in the HIV care continuum across enrollment cohorts, there was an apparent increase in the restricted mean time spent LTC after ART initiation in 2008-2014. We believe that to be, in part, an artifact of the data, and in part, due to changing clinical practice. Clinical guidelines released in 2013 suggested that people who were adherent to ART and virally suppressed could be seen in clinic every 6 months and have viral load and CD4 cell count monitored every 6-12 months [23]. With traditional markers of being “in care” assessed less frequently, the potential for people who are on ART and virally suppressed to be classified as LTC will increase [13]. We do not have reason to suspect that missing visit data or changes in clinical management are differential according to history of IDU, however, and relative differences in restricted mean time should still lead to correct inference.
Once persons are virally suppressed their ability to transmit their infection is negligible [1, 24]. One strength of this analysis is that it yields a measure of person-time spent virally unsuppressed, which may give an idea of the relative potential impact of PWID and non-IDU on the HIV epidemic through their ability to transmit infection. If we assume that individuals LTC were not in care, on average PWID spent 6.02 of 10 years capable of transmitting infection (12.1+25.9+19.5+14.7 months/12 months/year, from table 3), and non-IDU spent 5.35 of 10 years capable of transmitting infection. Given the sample size in this analysis, that translates into 2744 and 5280 person-years at risk of transmission for PWID and non-IDU, respectively. If we instead assume that individuals lost-to-clinic were in care, on ART and virally suppressed elsewhere (i.e., transferred care to another clinic), on average PWID and non-IDU spent 3.78 and 2.92 of 10 years capable of transmitting infection, and contributed 1725 and 2879 total infectious person-years to the community. (These two assumptions represent the lower and upper bounds for infectious person-years.) Thus, although on average, an individual PWID spends more time capable of transmitting virus, greatest potential for transmission overall comes from non-IDU.
There is increasing appreciation of limitations of the care continuum as currently estimated [13, 25, 26] in that it does not capture patients’ transition through continuum stages nor follow the same population over time. Furthermore, there is movement towards developing a complementary, longitudinal assessment of the HIV care continuum [22, 25]. Our work is one of the first patient-centric, longitudinal presentations of the HIV care continuum and, while similar to other models, has unique strengths. Our approach summarizes patients’ experience across all of follow-up rather than at only one point in time. Furthermore, our approach corresponds directly to a public health priority: to reduce the amount of time people spend capable of transmitting HIV infection. Our approach is based on a multistate model framework. While it is relatively easy to implement, its utility for informing future mathematical models of the continuum may be limited compared to a more explicit multistate model approach. We compare our approach to other longitudinal continuum analyses in appendix B.
Our analysis remains limited in that we could not follow patients after LTC to appropriately stage them in the care continuum (other than for mortality). It is possible that patients LTC from the JHHCC entered HIV care elsewhere. Applying this approach to a population-based cohort may improve upon this potential misclassification error, but even population-based data may misclassify persons lost-to-follow-up as out of care if they migrate out of the catchment area [27].
We present a novel, patient-centric, longitudinal approach to estimating progression through the HIV care continuum. Information from this approach provides supplemental information for evaluating health systems’ success at retaining patients in care, initiating them on ART, and achieving viral suppression. PWID are not spending substantially more time LTC after enrollment in clinical care than non-IDU. Rather PWID in care are spending less time on ART, and once on ART, they spend less time virally suppressed. Efforts to increase viral suppression among PWID should focus on increasing ART initiation and improving adherence to therapy.
Supplementary Material
Acknowledgments
FUNDING SOURCES
This work was supported by NIH grants U01 DA036935 and P30 AI094189. The funders have had no influence on the design of this analysis or reporting of results.
APPENDIX A
Our goal was to estimate the proportion of the cohort in each of the h = 1,...,7 stages of the care continuum over time, stratified by history of IDU, x ∈ {0,1}. We represent this proportion as Gxh(t). To simplify notation, we suppress the subscript x where unnecessary. To obtain Gh(t) we first estimated time to transition between several of the states. There are many different transitions possible between states (figure 1), but we focused on estimating the time to nine continuum-related events. For each patient i = 1...N we measured time Tijkj from enrollment to administrative censoring on August 31, 2015 or at 10 years of follow-up, occurrence of a competing event (competing events for each outcome are listed in table 1), or occurrence of each of k = 1...Kj instances of j = 1...9 continuum-related events. Of the following nine events, those in bold correspond to transition into a continuum state (regardless of prior state); events in italics correspond to transition out of a continuum state (regardless of future state).
Death before ART initiation;
LTC before ART initiation;
No longer LTC before ART initiation (composite outcome of return-to-clinic and death prior to return-to-clinic);
ART initiation;
Viral suppression after ART initiation;
Viral failure after ART initiation and viral suppression (composite outcome including viral load measurement >400 copies/mL, death, or LTC);
LTC after ART initiation;
No longer LTC after ART initiation (composite outcome of return-to-clinic and death prior to return-to-clinic); and
Death after ART initiation.
ART initiation does not correspond to transition into one specific continuum state because we split ART initiators further into those who were in care and virally suppressed, in care and not virally suppressed, LTC, or dead. Events other than death could occur more once in the analysis. Kj for each event was based on the maximum number of events of type j in the data (table 1). All events have at least one competing event (table 1). For each of the ΣJKj events, in addition to Tijkj, we assigned an event indicator Δijkj that was equal to 1 for an event of type j, 2 for a competing event, and 0 for administrative censoring.
We estimated the cumulative incidence function for each event, R̂jkj(t), nonparametrically [14]. To estimate the proportion in each stage of the care continuum over time Ĝh(t), we added and subtracted cumulative incidence curves [16, 17] as follows:
Dead before ART initiation: ĜH=1(t)=R̂J=1(t)
LTC before ART initiation:
Dead after ART initiation: ĜH=3(t)=R̂J=9(t)
LTC after ART initiation:
On ART, virally suppressed:
On ART, not suppressed: ĜH=6(t)=R̂J=4(t) – ĜH=3(t) – ĜH=4(t) – ĜH=5(t)
In care, not on ART: ĜH=7(t)= 1 – R̂J=4(t) – ĜH=1(t) – ĜH=2(t)
By design, . Thus we can present the distribution of the cohort over time since enrollment as a set of stacked curves. The area between adjacent curves (or equivalently, the area under each individual curve graphed separately) is interpretable as the mean time that an average patient spends in each continuum stage over 10 years of follow-up. The area under the (cumulative incidence) curves for death are interpretable as the mean months of life lost over 10 years of follow-up. We estimate the restricted mean time in state h empirically as . When time is discrete, because the cumulative incidence functions are step-functions, this interval can be calculated as the Riemann sum where d indexes days of follow-up over the 10-year period. We estimated the difference in restricted mean time comparing PWID to non-IDU by . We present the distribution of total follow-up time across each of the care continuum stages and the proportion of the PWID and non-IDU in each stage of the care continuum at 10 years. This latter quantity is akin to traditional care continuum estimates in that it is a snapshot of population at a specific point in time. It is different from traditional care continuum estimates in that it is anchored to time since clinic enrollment, rather than to calendar time. We also compare inference with respect to the continuum experience of PWID versus non-IDU using differences in restricted mean time in each continuum stage, and differences in proportions in each continuum stage at 10 years.
To get adjusted estimates, we followed the approach described above, but weighted each observation by the inverse probability that the individual reported IDU as a probable route of HIV acquisition X, conditional on covariates L [18, 19]. Weights were constructed as where the vector L contains all the baseline covariates listed in the methods section. Probabilities were estimated using logistic regression. All continuous covariates were entered into the model using restricted quadratic splines with knots at the 5th, 35th, 65th, and 95th percentiles [28]. HIV viral load was log-transformed before creating splines.
Appendix Figure 1.
HIV care continuum states and allowable movement through the continuum under the proposed model
* Note that individuals were allowed to move directly from being lost-to-clinic prior to ART initiation or in care, not on ART initiation into being on ART and virally suppressed; this could have occurred if observation of ART initiation and viral suppression occurred simultaneously, e.g. if person was virally suppressed on mono- or dual- therapy and then initiated ART, or if viral suppression occurred in the same month as ART initiation among ART-naïve persons.
Appendix Table 1.
HIV care continuum time-to-event outcomes, definitions, and competing risks for those outcomes
| Outcome* | Definition | Competing event(s) | Max. # of instances† |
|---|---|---|---|
| Death before ART initiation | Death date (prior to ART initiation date) | ART initiation | 1 |
| Loss to clinic before ART initiation | 12 months since most recent CD4 cell count, viral load measurement, or clinical encounter date (prior to ART initiation date) | ART initiation; Death before ART initiation | 4 |
| No longer lost to clinic before ART initiation | First CD4 cell count, viral load measurement, clinical encounter, or ART initiation after a 12 month gap | ART initiation; Death before ART initiation | 4 |
| ART initiation | ART initiation date | Death before ART initiation | 1 |
| Viral suppression on ART | Date viral load tested ≤400 copies/mL, after ART initiation date | Death before ART initiation; Death after ART initiation | 12 |
| Loss of viral suppression on ART | Date viral load tested >400 copies/mL or date lost to clinic or death date, after viral suppression | Death before ART initiation; Death after ART initiation | 11 |
| Loss to clinic after ART initiation | 12 months since most recent encounter date (after ART initiation date) | Death before ART initiation; Death after ART initiation | 5 |
| No longer lost to clinic after ART initiation | First CD4 cell count, viral load measurement or clinical encounter after a 12 month gap after ART initiation | Death before ART initiation; Death after ART initiation | 4 |
| Death after ART initiation | Death date (after ART initiation date) | Death before ART initiation | 1 |
The outcome listed here (top to bottom) corresponds to R̂J = 1,..., R̂J = 9, respectively in Appendix A
Maximum number of instances the outcome did occur (i.e., re-entry into state) and corresponds to K in Appendix A
APPENDIX B
McNairy et al. proposed a similar longitudinal approach to the continuum in which they use survival methods to report the proportion of patient outcomes at 3, 6 and 12 months after enrollment into HIV care that were optimal, sub-optimal, or poor [22]. Haber et al. used a similar approach, summarizing the distribution of the population 2 years after enrollment [29]. A strength McNairy's and our approach is that both consider outcomes of patients who do not initiate ART. Our approach has an additional advantage over both prior longitudinal approaches in that it provides a means for summarizing the distribution of person-time across all of follow-up, rather than requiring the user select a single follow-up time and report the distribution of the population across continuum stages at a cross-sectional point in time after enrollment. Our approach is based in a multistate model framework [16, 17] yet does not require fitting multistate models. The work of Lee et al. [30] and framework by Powers & Miller [26] approach the continuum from a more formal multistate model framework, which may prove useful for future mathematical models of the continuum.
Footnotes
CONFLICTS OF INTEREST
CRL reports receiving a speaker honorarium from Gilead Sciences, Inc. for work unrelated to this analysis. No others have any conflicts of interest to report.
REFERENCES
- 1.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. The New England journal of medicine. 2011;365(6):493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Group ISS, Lundgren JD, Babiker AG, et al. Initiation of Antiretroviral Therapy in Early Asymptomatic HIV Infection. N Engl J Med. 2015;373(9):795–807. doi: 10.1056/NEJMoa1506816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gardner EM, McLees MP, Steiner JF, Del Rio C, Burman WJ. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2011;52(6):793–800. doi: 10.1093/cid/ciq243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greenberg AE, Hader SL, Masur H, Young AT, Skillicorn J, Dieffenbach CW. Fighting HIV/AIDS in Washington, D.C. Health affairs. 2009;28(6):1677–87. doi: 10.1377/hlthaff.28.6.1677. [DOI] [PubMed] [Google Scholar]
- 5.Hallett TB, Eaton JW. A side door into care cascade for HIV-infected patients? J Acquir Immune Defic Syndr. 2013;63(Suppl 2):S228–32. doi: 10.1097/QAI.0b013e318298721b. [DOI] [PubMed] [Google Scholar]
- 6.Lucas GM, Griswold M, Gebo KA, Keruly J, Chaisson RE, Moore RD. Illicit drug use and HIV-1 disease progression: a longitudinal study in the era of highly active antiretroviral therapy. Am J Epidemiol. 2006;163(5):412–20. doi: 10.1093/aje/kwj059. [DOI] [PubMed] [Google Scholar]
- 7.Moore RD, Keruly JC, Chaisson RE. Differences in HIV disease progression by injecting drug use in HIV-infected persons in care. J Acquir Immune Defic Syndr. 2004;35(1):46–51. doi: 10.1097/00126334-200401010-00006. [DOI] [PubMed] [Google Scholar]
- 8.Hall HI, Gray KM, Tang T, Li J, Shouse L, Mermin J. Retention in care of adults and adolescents living with HIV in 13 U.S. areas. J Acquir Immune Defic Syndr. 2012;60(1):77–82. doi: 10.1097/QAI.0b013e318249fe90. [DOI] [PubMed] [Google Scholar]
- 9.Rebeiro P, Althoff KN, Buchacz K, et al. Retention among North American HIV-infected persons in clinical care, 2000-2008. J Acquir Immune Defic Syndr. 2013;62(3):356–62. doi: 10.1097/QAI.0b013e31827f578a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanna DB, Buchacz K, Gebo KA, et al. Trends and disparities in antiretroviral therapy initiation and virologic suppression among newly treatment-eligible HIV-infected individuals in North America, 2001-2009. Clin Infect Dis. 2013;56(8):1174–82. doi: 10.1093/cid/cit003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Egger M, May M, Chene G, et al. Prognosis of HIV-1-infected patients starting highly active antiretroviral therapy: a collaborative analysis of prospective studies. Lancet. 2002;360(9327):119–29. doi: 10.1016/s0140-6736(02)09411-4. [DOI] [PubMed] [Google Scholar]
- 12.Sterne JA, Hernan MA, Ledergerber B, et al. Long-term effectiveness of potent antiretroviral therapy in preventing AIDS and death: a prospective cohort study. Lancet. 2005;366(9483):378–84. doi: 10.1016/S0140-6736(05)67022-5. [DOI] [PubMed] [Google Scholar]
- 13.Lesko CR, Sampson LA, Miller WC, et al. Measuring the HIV care continuum using public health surveillance data in the United States. J Acquir Immune Defic Syndr. 2015 doi: 10.1097/QAI.0000000000000788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cole SR, Lau B, Eron JJ, et al. Estimation of the standardized risk difference and ratio in a competing risks framework: application to injection drug use and progression to AIDS after initiation of antiretroviral therapy. Am J Epidemiol. 2015;181(4):238–45. doi: 10.1093/aje/kwu122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lau B, Cole SR, Gange SJ. Competing risk regression models for epidemiologic data. Am J Epidemiol. 2009;170(2):244–56. doi: 10.1093/aje/kwp107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Edwards JK, Cole SR, Adimora A, Fine J, Martin J, Eron J. Illustration of a measure to combine viral suppression and viral rebound in studies of HIV therapy. J Acquir Immune Defic Syndr. 2015;68(2):241–4. doi: 10.1097/QAI.0000000000000423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gouskova NA, Cole SR, Eron JJ, Fine JP. Viral suppression in HIV studies: combining times to suppression and rebound. Biometrics. 2014;70(2):441–8. doi: 10.1111/biom.12140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robins J, Hernan MA, Brumback B. Marginal Structural Models and Causal Inference in Epidemiology. Epidemiology. 2000;11(5):550–60. doi: 10.1097/00001648-200009000-00011. [DOI] [PubMed] [Google Scholar]
- 19.Sato T, Matsuyama Y. Marginal structural models as a tool for standardization. Epidemiology. 2003;14(6):680–6. doi: 10.1097/01.EDE.0000081989.82616.7d. [DOI] [PubMed] [Google Scholar]
- 20.Efron B, Tibshirani R. An introduction to the bootstrap. Chapman & Hall; New York: 1993. [Google Scholar]
- 21.Hall HI, Frazier EL, Rhodes P, et al. Differences in human immunodeficiency virus care and treatment among subpopulations in the United States. JAMA internal medicine. 2013;173(14):1337–44. doi: 10.1001/jamainternmed.2013.6841. [DOI] [PubMed] [Google Scholar]
- 22.McNairy ML, Lamb MR, Abrams EJ, et al. Use of a Comprehensive HIV Care Cascade for Evaluating HIV Program Performance: Findings From 4 Sub-Saharan African Countries. J Acquir Immune Defic Syndr. 2015;70(2):e44–51. doi: 10.1097/QAI.0000000000000745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aberg JA, Gallant JE, Ghanem KG, Emmanuel P, Zingman BS, Horberg MA. Primary care guidelines for the management of persons infected with HIV: 2013 update by the HIV Medicine Association of the Infectious Diseases Society of America. Clinical Infectious Diseases. 2013 doi: 10.1093/cid/cit757. [DOI] [PubMed] [Google Scholar]
- 24.Quinn TC, Wawer MJ, Sewankambo N, et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. N Engl J Med. 2000;342(13):921–9. doi: 10.1056/NEJM200003303421303. [DOI] [PubMed] [Google Scholar]
- 25.Haber N, Pillay D, Porter K, Barnighausen T. Constructing the cascade of HIV care: methods for measurement. Curr Opin HIV AIDS. 2016;11(1):102–8. doi: 10.1097/COH.0000000000000212. [DOI] [PubMed] [Google Scholar]
- 26.Powers KA, Miller WC. Building on the HIV Cascade: A Complementary “HIV States and Transitions” Framework for Describing HIV Diagnosis, Care, and Treatment at the Population Level. J Acquir Immune Defic Syndr. 2015;69(3):341–7. doi: 10.1097/QAI.0000000000000611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buskin SE, Kent JB, Dombrowski JC, Golden MR. Migration Distorts Surveillance Estimates of Engagement in Care: Results of Public Health Investigations of Persons Who Appear to be Out of HIV Care. Sex Transm Dis. 2014;41(1):35–40. doi: 10.1097/OLQ.0000000000000072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Howe CJ, Cole SR, Westreich DJ, Greenland S, Napravnik S, Eron JJ., Jr. Splines for trend analysis and continuous confounder control. Epidemiology. 2011;22(6):874–5. doi: 10.1097/EDE.0b013e31823029dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haber N, Naidu K, Pillay D, Barnighausen T. HIV System Assessment with Longitudinal Treatment Cascade in Kwazulu-Natal, South Africa. Population Association of America; San Diego, CA: 2015. [Google Scholar]
- 30.Lee H, Genberg BL, Hogan J, Braitstein P. State Space Modeling of the HIV Care Cascade in Sub-Saharan Africa.. Joint Statistical Meeting; Seattle, WA. 2015. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.