Abstract
Tumor recurrence after surgical resection of NSCLC obstructs long-term disease-free survival in approximately 50% of cases. Our data suggest that combining circulating tumor cell enumeration (as single cells or clusters) in tumor-draining pulmonary vein and peripheral blood (assessed by CellSearch) at the time of NSCLC surgery better identifies those patients at higher risk for lung cancer recurrence than does peripheral circulating tumor cell number alone.
Keywords: Early stage, NSCLC, Surgery, Circulating tumor cells, Recurrence
Introduction
Surgical resection of NSCLC (approximately 88%) can produce long-term survival. However, tumor recurrence occurs in approximately 50% of cases. Circulating tumor cells (CTCs) are assumed to be the predominant cause of metastatic dissemination, and evidence supports the tumor-initiating properties of (a subset of) CTCs.1, 2 The CellSearch platform (Janssen Diagnostics LLC, Raritan, NJ) exploits expression of cell surface epithelial markers to capture, enrich, and enumerate CTCs. The detection of CTCs in peripheral blood is associated with a worse prognosis in NSCLC.3, 4 However, the total number of CTCs detected by CellSearch in early-stage NSCLC is low. Pulmonary veins drain blood directly from the lungs. Proximity to primary tumor and blood draw upstream of capillary bed filtering implies that pulmonary vein sampling may be advantageous to assess the association of CTC prevalence with disease outcome. To test this hypothesis, we performed a pilot study of CellSearch CTC enumeration, comparing blood sampling from peripheral and tumor-draining pulmonary veins at the time of tumor resection. We hypothesized that patients with detectable pulmonary vein CTCs would have a higher risk for disease recurrence.
Methods
This was a single-center, prospective study of patients undergoing a curative-intent operation for stage I to IIIA NSCLC (University Hospital of South Manchester, Manchester, UK). Patients with a history of malignancy within 2 years were excluded. Blood samples (10 mL) were taken immediately before surgery from a peripheral vein and intraoperatively (10 mL) from the cancer-draining pulmonary vein before tumor resection or vessel ligation to minimize artifactual elevation in CTC number.5 CellSearch processing and analysis followed previously published protocols.6, 7 CTC enumeration was expressed as number of cells per 7.5 mL of blood. Circulating tumor microemboli (CTMs) were defined as three or more contiguous CTCs in a cluster.8
Primary and secondary outcome measures were disease-free survival (DFS) and overall survival (OS), respectively. Patients found to have unresectable disease during the operation were not included in the survival analysis. DFS and OS were measured from the day of the operation until either the diagnosis of lung cancer recurrence or death. In patients with no evidence of recurrence, DFS was censored at the date of death or last follow-up. Deaths within 30 days of the operation were not included in the analysis. Peripheral vein CTC status was predefined as positive at one or more CTCs per 7.5 mL of blood.3 Pulmonary vein CTC status was an exploratory end point, and high was defined according to the upper quartile of the CTC count. Kaplan-Meier curves were used to demonstrate DFS and OS for each CTC category and compared using log-rank tests. A Cox proportional hazard regression analysis with a backward conditional regression approach was used to define the optimal multivariate model to determine independent risk factors associated with DFS and OS. Log2 transformation was carried out if nonlinearity was found.
The study was granted ethical approval by the North West 7 Research Ethics Committee (reference no. 10/H1008/79), and all participants gave written informed consent before study participation.
Results
Of the 33 patients who underwent an operation, 30 had a complete resection. Three patients found intraoperatively to have unresectable disease were not included in the analysis. The clinical parameters of the study cohort (n = 30) are detailed in Table 1. Median study follow-up was 22.0 months (range 1–52). Lung cancer recurrence affected 10 of 30 patients (33%), most commonly (in seven of 10 cases) at distant sites. Almost half of the study population died (13 of 30 patients [43%]); one patient who died within 30 days of having the operation was excluded. Cause of death was not recorded; however, nine of 10 patients with recurrent disease (90%) died compared with three of 19 (16%) patients without disease recurrence (p = 0.0002), suggesting that lung cancer was the predominant cause.
Table 1.
Clinical Characteristics of the Study Population with Resectable Disease (n = 30) Stratified according to Pulmonary Vein and Peripheral Vein CTC Status
| Strata | All | Pulmonary Vein CTC Status |
p Value |
Peripheral Vein CTC Status |
p Value |
||
|---|---|---|---|---|---|---|---|
| High | Low | Positive | Negative | ||||
| Total | |||||||
| n (%) | 30 | 7 (23.3) | 23 (76.7) | — | 6 (22.2) | 21 (77.8) | — |
| Age | |||||||
| y ± SD | 67.5 ± 7.1 | 67.7 ± 10.6 | 67.5 ± 5.9 | 0.94 | 71.7 ± 8.8 | 65.9 ± 6.3 | 0.08 |
| Male sex | |||||||
| n (%) | 16 (53.3) | 4 (57.1) | 12 (52.2) | 0.82 | 4 (66.7) | 11 (52.4) | 0.66 |
| Smoking status (%) | |||||||
| Current | 16 (53.3) | 6 (85.7) | 10 (43.5) | 0.09 | 2 (33.3) | 12 (57.4) | 0.39 |
| Former | 14 (46.7) | 1 (14.3) | 13 (56.5) | 4 (66.7) | 9 (42.9) | ||
| Never | 0 | 0 | 0 | 0 | 0 | ||
| Smoking (± SD) | |||||||
| Age started | 15.2 ± 2.5 | 14.3 ± 1.4 | 15.5 ± 2.7 | 0.26 | 15.3 ± 1.9 | 15.1 ± 2.7 | 0.85 |
| Cigs/d | 23.2 ± 12.9 | 23.3 ± 12.2 | 23.2 ± 13.4 | 0.99 | 16.6 ± 10.1 | 25.4 ± 14.0 | 0.21 |
| Duration | 43.8 ± 12.8 | 51.1 ± 10.7 | 41.5 ± 12.7 | 0.08 | 45.5 ± 14.3 | 41.7 ± 12.5 | 0.53 |
| Pack-years | 51.8 ± 36.4 | 60.5 ± 32.9 | 48.8 ± 37.8 | 0.47 | 38.7 ± 29.3 | 54.6 ± 40.5 | 0.42 |
| FEV1 (± SD) | |||||||
| Liters | 1.91 ± 0.72 | 1.96 ± 0.42 | 1.89 ± 0.79 | 0.84 | 1.99 ± 0.83 | 1.82 ± 0.70 | 0.62 |
| %pred | 73.4 ± 20.3 | 79.0 ± 20.5 | 71.9 ± 20.5 | 0.50 | 71.0 ± 18.0 | 70.4 ± 19.4 | 0.95 |
| FVC (± SD) | |||||||
| Liters | 3.09 ± 0.82 | 3.28 ± 0.44 | 3.03 ± 0.90 | 0.53 | 3.38 ± 0.96 | 2.91 ± 0.68 | 0.20 |
| %pred | 98.1 ± 17.4 | 104.2 ± 16.1 | 96.5 ± 17.8 | 0.39 | 95.7 ± 13.2 | 96.0 ± 17.8 | 0.97 |
| FEV1/FVC | |||||||
| Ratio | 58.8 ± 17.8 | 59.8 ± 9.0 | 58.4 ± 20.2 | 0.87 | 56.8 ± 14.5 | 58.6 ± 19.9 | 0.86 |
| Alcohol | |||||||
| U/wk | 16.9 ± 35.6 | 8.3 ± 14.2 | 19.6 ± 40.0 | 0.47 | 3.7 ± 8.0 | 15.0 ± 31.1 | 0.39 |
| Pathological stage (%) | |||||||
| I | 12 (40.0) | 1 (14.3) | 11 (47.8) | 0.09 | 2 (33.3) | 10 (47.6) | 0.31 |
| II | 11 (36.7) | 5 (71.4) | 6 (26.1) | 1 (16.7) | 7 (33.3) | ||
| III | 7 (23.3) | 1 (14.3) | 6 (26.1) | 3 (50.0) | 4 (19.0) | ||
| IV | 0 | 0 | 0 | 0 | 0 | ||
| Disease (%) | |||||||
| Squam | 21 (70.0) | 5 (71.4) | 15 (65.2) | 0.25 | 5 (83.3) | 16 (76.2) | 0.88 |
| Adeno | 8 (26.7) | 1 (14.3) | 7 (30.4) | 1 (16.7) | 4 (26.1) | ||
| Tumor size | |||||||
| mm ± SD | 39.7 ± 29.2 | 45.6 ± 29.4 | 37.9 ± 29.6 | 0.55 | 52.5 ± 34.6 | 37.9 ± 28.5 | 0.30 |
| PET/CT | |||||||
| SUV | 12.0 ± 6.8 | 11.5 ± 5.3 | 12.1 ± 7.3 | 0.83 | 10.9 ± 5.1 | 13.0 ± 7.3 | 0.52 |
| Received Adj chemo | |||||||
| n (%) | 12 (40) | 2 (28.6) | 10 (43.5) | 0.67 | 3 (50.0) | 8 (38.1) | 0.66 |
| Experienced Recurrence | |||||||
| n (%) | 10 (33.3) | 4 (57.1) | 6 (26.1) | 0.18 | 4 (66.7) | 5 (23.8) | 0.14 |
| Dead | |||||||
| n (%) | 13 (44.8) | 4 (57.1) | 9 (40.9) | 0.67 | 4 (66.7) | 7 (35.0) | 0.35 |
Note: For pulmonary vein circulating tumor cell status, values of ≥18 CTCs per 7.5 mL of blood were considered high. For peripheral vein circulating tumor cell status, values of ≥1 CTCs per 7.5 mL of blood were assumed for +ve values.
CTC, circulating tumor cell; Cigs, cigarettes; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; U, unit(s); Squam, squamous cell carcinoma; Adeno, adenocarcinoma; PET/CT, positron emission tomography/computed tomography; SUV, standardized uptake value; Adj chemo, adjuvant chemotherapy.
CTC and CTM Prevalence
Peripheral blood was taken from 27 of 30 patients (90%) and a pulmonary vein sample was obtained from the whole study cohort. CTCs (≥1 CTC per 7.5 mL of blood) were more commonly detected in the pulmonary vein (in 13 of 30 patients [43%], range 1–3093 CTCs per 7.5 mL of blood) than in the peripheral blood (in six of 27 patients [22%], range 1–4 CTCs per 7.5 mL of blood) (p = 0.018) and total CTC number was greater in the pulmonary vein (p = 0.002). Only two of 27 patients (7%) had CTCs detected in the peripheral and pulmonary vein samples; both died after lung cancer recurrence within 8 months of having their operation. CTMs were detected only in pulmonary vein blood (six of 30 patients (20%), range 1–37 CTMs per 7.5 mL of blood). The numbers of pulmonary vein CTCs and CTMs were significantly correlated (r = 0.51, p = 0.002).
CTCs and Survival
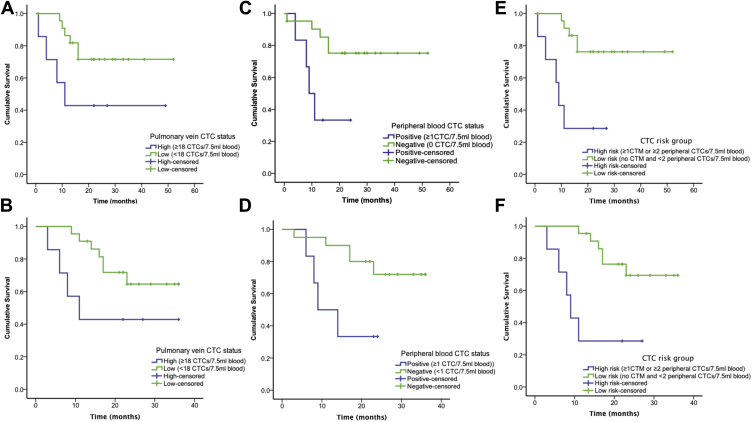
Patients were stratified according to high (≥18 CTCs per 7.5 mL of blood) or low (<18 CTCs per 7.5 mL of blood) pulmonary vein CTC groups. Differences approaching significance in DFS (p = 0.055) and 3-year OS (p = 0.082) were observed (Fig. 1A and B). Clinical characteristics stratified according to these groups are detailed in Table 1. CTMs were identified in six of seven of those in the high-CTC group (85.7%) compared with in none of 23 of the low-CTC group (p = 0.00001). The presence of CTMs (≥1 CTM per 7.5 mL of blood) was associated with a significant reduction in DFS (p = 0.011) and OS (p = 0.024). The detection of any CTCs (≥1 CTC per 7.5 mL of blood) in peripheral blood was associated with reduced DFS (p = 0.011) and OS (p = 0.037) (Fig. 1C and D).
Figure 1.
Survival in patients after curative-intent surgical resection of NSCLC. (A) Disease-free survival (DFS) and (B) 3-year overall survival (OS) according to pulmonary vein circulating tumor cell (CTC) status. (C) DFS and (D) 3-year OS according to peripheral vein CTC detection. (E) DFS and (F) 3-year OS stratified according to high- and low-risk CTC groups. CMT, circulating tumor microemboli.
Cox Multivariate Modeling of Survival
Variables included in the model were age, pathological stage, and log2-transformed CTC and CTM numbers in pulmonary and/or peripheral vein blood as continuous variables. Both peripheral vein (p = 0.01) and pulmonary vein CTC number (p = 0.001) remained independent risk factors (after Bonferroni correction) for lung cancer recurrence along with pathological stage (p = 0.00006). The hazard ratio (HR) for lung cancer recurrence for every doubling of CTC count was 1.51 (95% confidence interval [CI]: 1.17–1.94) for pulmonary vein and 5.26 (95% CI: 1.48–18.68) for peripheral vein CTCs. Pulmonary vein CTC count (p = 0.01) and pathological stage (p = 0.001) were independent risk factors for death; the association with peripheral vein CTC number was of borderline significance (p = 0.026) after Bonferroni correction. The HR for death for every doubling of CTC count was 1.33 (95% CI: 1.06–1.67) for pulmonary and 3.73 (95% CI: 1.17–11.93) for peripheral blood.
Overall CTC Status
Patients were categorized in a high-risk group if they had CTMs detected (≥1 CTM per 7.5 mL of blood) or two or more CTCs per 7.5 mL of peripheral blood. These measures were chosen to define the highest-risk group because of increased specificity for lung cancer recurrence (in six of nine patients [66.7%]) compared with cutoff values of one or more CTC per 7.5 mL of peripheral blood or 18 or more CTCs per 7.5 mL of pulmonary vein blood (in six of 11 patients [54.5%]). Lung cancer recurred in five of seven of these high-risk patients (71.4%) compared with in five of 23 low-risk patients (21.7%) (p = 0.026). Deaths occurred in five of seven high-risk patients (71.4%) and eight of 22 low-risk patients (36.4%) (p = 0.19). Kaplan-Meier analysis demonstrated a significant difference in DFS (p = 0.001) and OS (p = 0.002) (Fig. 1E and F). The HR for lung cancer recurrence was 8.44 (95% CI: 2.09–34.05) (p = 0.003) and that for death was 7.53 (95% CI: 1.77–32.04) (p = 0.006) in the high-risk group after multivariate analysis. Overall, these data show that pulmonary vein CTC/CTM enumeration provides additional information on risk for disease recurrence compared with CTC enumeration from the peripheral circulation alone.
Discussion
This study assessed the prognostic significance of CTCs and the feasibility of pulmonary vein sampling at the time of surgical resection NSCLC. Significantly more CellSearch-enriched CTCs were detected in pulmonary blood than in matched peripheral blood, and the presence of pulmonary vein CTCs was an independent risk factor for lung cancer recurrence and death. CTMs were also detected in samples of pulmonary vein blood, and patients with detectable CTMs or two or more peripheral vein CTCs represented a high-risk subgroup with an eightfold increased risk for disease recurrence and sevenfold increased risk for death.
An important aspect of the study was the timing of pulmonary vein sampling. Blood was taken before tumor manipulation, vessel ligation, or lung resection to avoid as much as possible artifactual elevation of CTC number.5 The association of pulmonary vein CTC count with disease recurrence and reduced OS suggests that this may be a biologically important cell population reflecting tumor biology rather than being a consequence of surgery alone. This approach was safe with no complications reported. The detection of CTMs has been reported to increase the risk for lung cancer recurrence after resection.3 We have previously described the prognostic significance in SCLC of CellSearch-detected CTMs8 and postulate that CTMs have an increased ability to survive in the circulation.9 In vivo models also support evidence that CTMs have increased malignant potential.10, 11
Three patients suffered disease recurrence despite negative CTC detection by CellSearch. Coexpression of epithelial cell adhesion molecules and cytokeratins is a requirement for CTC capture and assignment using the CellSearch platform. Although this subpopulation of CTCs is biologically important as evidenced by the association between CellSearch CTC detection and prognosis in numerous studies,3, 4, 12, 13, 14 it may not provide a comprehensive assessment of total CTC burden. Subpopulations of CTCs that lose their epithelial phenotype (e.g., epithelial-to-mesenchymal transition) may become undetectable by CellSearch. To more fully assess CTC heterogeneity, the use of marker-independent platforms is required; however, no other platform has been through the vigorous validation process required for routine use in the clinic.
Conclusions and Future Directions
To confirm our findings and explore the potential for CTCs to be used in the clinical management of early-stage NSCLC, a larger validation study is required. In addition to the potential role of CTCs as a prognostic/predictive biomarker, isolation and genetic analysis of individual CTCs may shed light on tumor biology and the metastatic process. Whether CTCs are representative of the cells responsible for distant metastatic spread and whether they predict the genetic landscape of recurrent disease are important areas for future research, which we are addressing as part of the TRACERx study.15
Acknowledgments
The study was supported by core funding from Cancer Research UK (grant reference C5759/A20971) and was based in the Clinical and Experimental Pharmacology Group at the Cancer Research UK Manchester Institute. The study also received funding from the North West Lung Centre Charity. The study was designed by Drs. Crosbie, Shah, and Booton. Patient recruitment was undertaken by Drs. Crosbie, Shah, and Krysiak. Circulating tumor cell analysis was performed in Professor Dive’s laboratory with study oversight by Professors Blackhall and Dive. Sample processing and analysis was supervised by Drs. Morris and Tugwood. Drs. Zhou and Crosbie performed the statistical analysis. All authors contributed to manuscript development and approved the final draft.
Footnotes
Disclosure: The authors declare no conflict of interest.
References
- 1.Baccelli I., Schneeweiss A., Riethdorf S. Identification of a population of blood circulating tumor cells from breast cancer patients that initiates metastasis in a xenograft assay. Nat Biotechnol. 2013;31:539–544. doi: 10.1038/nbt.2576. [DOI] [PubMed] [Google Scholar]
- 2.Hodgkinson C.L., Morrow C.J., Li Y. Tumorigenicity and genetic profiling of circulating tumor cells in small-cell lung cancer. Nat Med. 2014;20:897–903. doi: 10.1038/nm.3600. [DOI] [PubMed] [Google Scholar]
- 3.Hofman V., Ilie M.I., Long E. Detection of circulating tumor cells as a prognostic factor in patients undergoing radical surgery for non-small-cell lung carcinoma: comparison of the efficacy of the CellSearch assay and the isolation by size of epithelial tumor cell method. Int J Cancer. 2011;129:1651–1660. doi: 10.1002/ijc.25819. [DOI] [PubMed] [Google Scholar]
- 4.Krebs M.G., Sloane R., Priest L. Evaluation and prognostic significance of circulating tumor cells in patients with non-small-cell lung cancer. J Clin Oncol. 2011;29:1556–1563. doi: 10.1200/JCO.2010.28.7045. [DOI] [PubMed] [Google Scholar]
- 5.Hashimoto M., Tanaka F., Yoneda K. Significant increase in circulating tumour cells in pulmonary venous blood during surgical manipulation in patients with primary lung cancer. Interact Cardiovasc Thorac Surg. 2014;18:775–783. doi: 10.1093/icvts/ivu048. [DOI] [PubMed] [Google Scholar]
- 6.Allard W.J., Matera J., Miller M.C. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res. 2004;10:6897–6904. doi: 10.1158/1078-0432.CCR-04-0378. [DOI] [PubMed] [Google Scholar]
- 7.Riethdorf S., Fritsche H., Muller V. Detection of circulating tumor cells in peripheral blood of patients with metastatic breast cancer: a validation study of the CellSearch system. Clin Cancer Res. 2007;13:920–928. doi: 10.1158/1078-0432.CCR-06-1695. [DOI] [PubMed] [Google Scholar]
- 8.Hou J.M., Krebs M.G., Lancashire L. Clinical significance and molecular characteristics of circulating tumor cells and circulating tumor microemboli in patients with small-cell lung cancer. J Clin Oncol. 2012;30:525–532. doi: 10.1200/JCO.2010.33.3716. [DOI] [PubMed] [Google Scholar]
- 9.Krebs M.G., Metcalf R.L., Carter L., Brady G., Blackhall F.H., Dive C. Molecular analysis of circulating tumour cells-biology and biomarkers. Nat Rev Clin Oncol. 2014;11:129–144. doi: 10.1038/nrclinonc.2013.253. [DOI] [PubMed] [Google Scholar]
- 10.Fidler I.J. Metastasis: quantitative analysis of distribution and fate of tumor emboli labeled with 125 I-5-iodo-2′-deoxyuridine. J Natl Cancer Inst. 1970;45:773–782. [PubMed] [Google Scholar]
- 11.Liotta L.A., Saidel M.G., Kleinerman J. The significance of hematogenous tumor cell clumps in the metastatic process. Cancer Res. 1976;36:889–894. [PubMed] [Google Scholar]
- 12.Cohen S.J., Punt C.J., Iannotti N. Prognostic significance of circulating tumor cells in patients with metastatic colorectal cancer. Ann Oncol. 2009;20:1223–1229. doi: 10.1093/annonc/mdn786. [DOI] [PubMed] [Google Scholar]
- 13.Cristofanilli M., Budd G.T., Ellis M.J. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004;351:781–791. doi: 10.1056/NEJMoa040766. [DOI] [PubMed] [Google Scholar]
- 14.de Bono J.S., Scher H.I., Montgomery R.B. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin Cancer Res. 2008;14:6302–6309. doi: 10.1158/1078-0432.CCR-08-0872. [DOI] [PubMed] [Google Scholar]
- 15.Jamal-Hanjani M., Hackshaw A., Ngai Y. Tracking genomic cancer evolution for precision medicine: the lung TRACERx study. PLoS Biol. 2014;12:e1001906. doi: 10.1371/journal.pbio.1001906. [DOI] [PMC free article] [PubMed] [Google Scholar]