Abstract
Background
Optimal treatment and precise classification for anaplastic glioma are needed.
Methods
The objective for long-term follow-up of NOA-04 is to optimize the treatment sequence for patients with anaplastic gliomas. Patients were randomized 2:1:1 to receive the standard radiotherapy (RT) (arm A), procarbazine, lomustine and vincristine (PCV) (arm B1), or temozolomide (TMZ) (arm B2).
Results
Primary endpoint was time-to-treatment-failure (TTF), defined as progression after 2 lines of therapy or any time before if no further therapy was administered. Exploratory analyses examined associations of molecular marker status with TTF, progression-free survival (PFS), and overall survival (OS). At 9.5 (95% CI: 8.6–10.2) years, no difference between arms (A vs B1/B2) was observed: median TTF (4.6 [3.4–5.1] y vs 4.4 [3.3–5.3) y), PFS (2.5 [1.3–3.5] y vs 2.7 [1.9–3.2] y), and OS (8 [5.5–10.3] y vs 6.5 [5.4–8.3] y). Oligodendroglial versus astrocytic histology—but more so the subgroups according to CpG island methylator phenotype (CIMP) and 1p/19q co-deletion status—revealed a strong prognostic value of CIMPpos with (CIMPcodel) versus without 1p/19 co-deletion (CIMPnon-codel) versus CIMPneg. but no differential efficacy of RT versus chemotherapy for any of the endpoints. PFS was better for PCV- than for TMZ-treated patients with CIMPcodel tumors (HR B1 vs B2 0.39 [0.17–0.92], P = .031). In CIMPneg. tumors, hypermethylation of the O6-methyl-guanyl-DNA methyltransferase promoter (MGMT) provided a risk reduction for PFS with chemotherapy.
Conclusions
There is no differential activity of primary chemotherapy versus RT in any subgroup of anaplastic glioma. Molecular diagnosis is superior to histology.
Trial Registration: clinicaltrials.gov Identifier: NCT00717210.
Keywords: 1p/19q, anaplastic gliomas, CIMP, MGMT
Classification and treatment of anaplastic gliomas (World Health Organization [WHO] grade III) are intensely debated topics in neuro-oncology. Co-deletions of chromosomal arms 1p and 19q in the tumor tissue define a favorable prognostic group of patients with anaplastic, mostly oligodendroglial gliomas. These patients have been shown to benefit from combined radiochemotherapy (RT) with procarbazine, lomustine (CCNU) and vincristine (PCV) in 2 independent long-term subgroup analyses from cooperative group trials (EORTC 26951 and RTOG 9402) originating in the 1990s.1,2 In line with these results, patients with WHO grade II gliomas with risk factors (ie, less than total resection or age >40 years benefit considerably from RT-PCV compared with RT alone,3 although molecular subgroup information from this trial is awaited. Optimizing the standard of care for these patient groups is a major challenge given the younger age of these patient groups as well as their relatively long OS and the relevant risk of neurocognitive, functional, and quality-of-life impairment resulting from aggressive treatment of a tumor located in the brain,. To this end, a leading hypothesis in the international scientific debates and day-to-day management of these patients seems to be reducing the initial treatment to the potentially most relevant part (ie, alkylating chemotherapy) and thereby delaying RT. One necessary prerequisite for primary monotherapy with temozolomide (TMZ) or PCV and the deferred use of RT only for salvage is demonstration of comparable OS with monochemotherapy as compared with radiochemotherapy.
Anaplastic gliomas are developing into a disease best described not solely by histopathology but rather based on biomarkers. Mutations of the isocitrate dehydrogenase (IDH) genes 1 or 2, which lead to the glioma CpG island methylator phenotype (G-CIMP) assessed by methylation analyses, allow separation into 3 separate different disease entities in which the IDH-mutant tumors show a more benign course of disease4 that is biologically and prognostically further subdivided by 1p/19q status.5–8 IDH wild-type anaplastic gliomas, in contrast, by and large share biological and clinical similarities with glioblastoma.6–10 In these tumors, methylation of the promoter of O6-methylguanine-DNA methyltransferase (MGMT) is predictive of benefit from alkylating chemotherapy.11 By adding alpha-thalassemia/mental retardation syndrome X-linked (ATRX) status to these biomarkers, it seems possible to subgroup IDH-mutant anaplastic oligoastrocytoma with ATRX loss into the group of anaplastic astrocytoma. IDH-mutant anaplastic oligoastrocytomas with 1p/19q co-deletion, on the other hand, correspond molecularly and prognostically with anaplastic oligodendroglioma,12 thus eliminating the category of mixed gliomas.13,14 Taking this to the next level, we and others are proposing a molecularly based classification of anaplastic gliomas into 3 biologically and prognostically relevant subgroups that goes beyond the current WHO classification: (i) IDH wild-type (IDHwt–glioblastoma-like, which are called CIMPneg, to reflect biological relevance of the mutation), (ii) IDH mutant and 1p/19q intact (IDH mut/1p/19q intact–CIMPnon-codel), and (iii) IDH mutant and 1p/19q co-deleted (IDH mut/1p/19q codel–CIMPcodel).5,7,8
NOA-04 offers the unique opportunity to test the hypothesis that primary monochemotherapy with TMZ or PCV is superior to primary RT, specifically in the subgroup of patients with tumors harboring the IDH mutant and 1p/19q co-deleted genotype and allowing deferral of primary RT. The trial helps generate hypotheses on the potential differential efficacy of TMZ versus PCV. In addition, the present analysis with more events and a comprehensive molecular workup should provide mature and substantial data aiding day-to-day treatment as well as the development of new trial concepts.
Patients and Methods
Patients, Evaluations, and Ethics
The NOA-04 trial (NCT00717210) for patients with newly diagnosed anaplastic gliomas compared the efficacy and safety of initial RT, followed by chemotherapy (TMZ or PCV) at progression or occurrence of unacceptable toxicity with the inverse sequence in patients with newly diagnosed anaplastic gliomas. Central pathology review demonstrated a high concordance between local and central histological diagnoses (κ = 0.7; 95% CI: 0.62–0.79). In the meantime, the diagnosis of mixed anaplastic oligoastrocytoma has been challenged and will be discouraged in the upcoming revision of the present WHO classification.15,16
In this trial, both sequences achieved similar results in the first analysis conducted after a median follow-up of 5.4 years.17 Median follow-up time for the present analysis is 9.5 years (95% CI: 8.6–10.2) (for assessment see Supplementary Patients and Methods). All patients consented to exploratory molecular analyses performed with the study data and materials. The original phase III trial was approved by the Ethics Committee at the University of Tuebingen, Germany, and subsequently by all local ethics committees of the participating clinical centers. NOA-04 enrolled patients after written informed consent including future molecular analyses at 39 sites in Germany (details in the Supplementary Patients and Methods). The primary endpoint, time-to-treatment-failure (TTF) was defined as progression after 2 lines of therapy, death, or at any time before if no further indicated therapy was administered.17
This trial is registered as an International Standard Randomized Controlled Trial at the German Cancer Trials Registry (ID 291) and with ClinicalTrials.gov (NCT00717210).
Role of the Funding Sources
The funding source (formerly Schering Plough and now Merck Sharp & Dohme) and the German Cancer Aid (grant number 110624) had no role in the study design, collection, analysis, or interpretation of the data or in writing the initial study report, manuscript, or this long-term outcome analysis. Access to the raw data was limited to W.W., C.M., B.W., A.v.D., G.R., and M.W. The corresponding author had full access to all data and the final responsibility for submitting the publication.
Statistics
After collection on case record forms with on-site support from a clinical project manager (P.R.) with a cutoff on December 31, 2014, forms of all long-term data were fed into the database at the coordinating study center in Heidelberg.
Kaplan-Meier estimator and Cox proportional hazards regression were performed to assess survival data. To compare the performance of Cox regression models, prediction error curves were generated using the R package pec,18 and analysis of deviance tables was computed. Processing and analysis of Illumina HumanMethylation 450k arrays as well as molecular subgrouping are described in Supplementary Methods. A P value <.05 was considered statistically significant without adaption for multiple tests. All tests were 2-sided. All tests were explorative as the long-term analysis was not the primary endpoint of the study. Analyses were carried out using R version 3.2.0 and Stata IC version 12.1 (StataCorp LP).
Results
Patient Characteristics
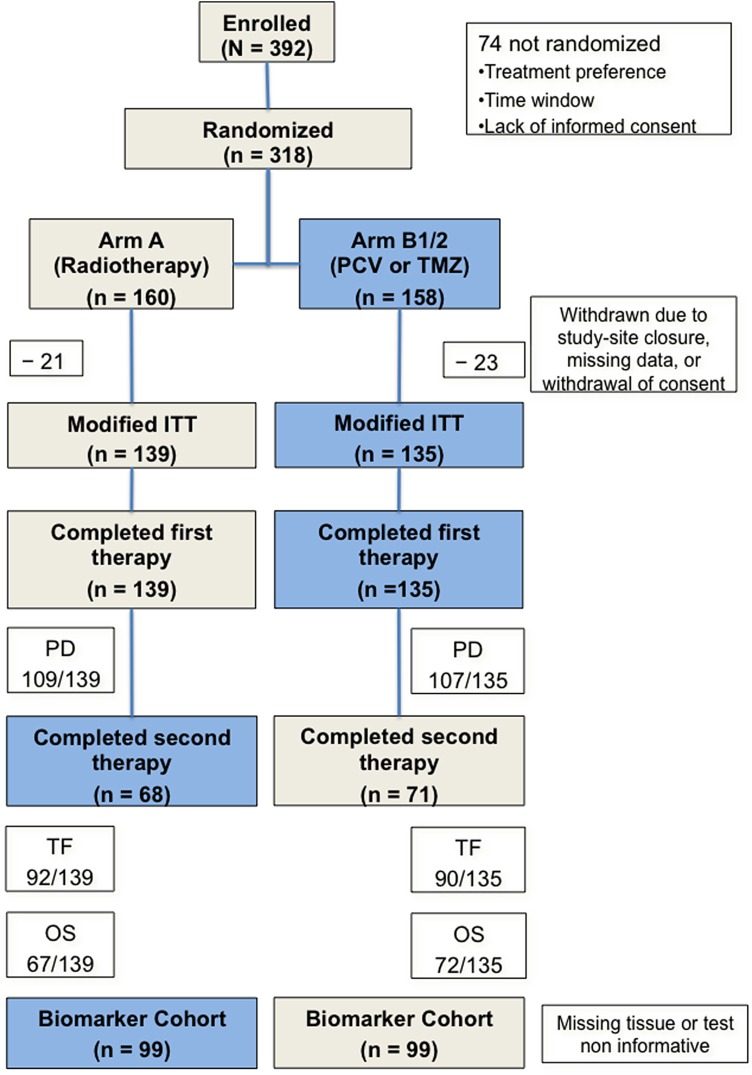
From June 1999 to February 2005, 318 patients with centrally confirmed anaplastic gliomas were randomized to receive RT (160 patients) or chemotherapy (78 PCV and 80 TMZ). The ITT population included 139 patients in Arm A and 135 in Arm B1/B2 (Fig. 1).
Fig. 1.
CONSORT diagram of patient disposition. Forty-four patients (21 in arm A; 23 in arm B1/B2) were excluded from the intention-to-treat analysis because of study site closure, missing data, or withdrawal of consent. The numbers of patients with an event, a documented progression (PD) at the time of the analysis (31.12.2014), reaching the primary endpoint treatment failure (TF), and death (OS), and the number of patients in the biomarker subset are indicated.
Sixty-four percent of patients were younger than aged 50 years, 80% had complete or partial resection, 90% had a KPS ≥ 80%, and 52% had an anaplastic astrocytoma (AA). Arms were balanced for clinical features, histology, and corticosteroid use (Table 1). Data on the status of IDH, 1p/19q, and O6-methylguanine-DNA-methyltransferase (MGMT) were available for 74%, 72%, and 74% of cases, respectively. There was no difference in the baseline characteristics between the full and biomarker cohorts. Since the initial report in 2009,17 molecular analyses on 115 samples using 450 k methylation arrays have been performed,5,19 and a comprehensive molecular classification into 3 groups has been proposed: IDH wild-type (CIMPneg.), IDH mutant and 1p/19q intact (CIMPnon-codel), and IDH mutant and 1p/19q co-deleted (CIMPcodel).5
Table 1.
Baseline patient characteristics
| PCV or TMZ (n = 135) | Radiotherapy (n = 139) | |
|---|---|---|
| Median age (range), y | 42 (20–77) | 44 (23–74) |
| Sex (female/male), n | 61/74 | 55/84 |
| Local/centrala histopathology, n | ||
| Anaplastic astrocytoma | 66/74 | 65/70 |
| Anaplastic oligoastrocytoma | 41/44 | 41/47 |
| Anaplastic oligodendroglioma | 27/17 | 33/22 |
| κ-value (concordance between local and reference histopathology) | 0.7 (0.62–0.79) | |
| Median KPS (range) [%] | 90 (70–100) | 90 (70–100) |
| Median Mini-Mental State Examination score (out of 30) (range) | 30 (21–30) | 30 (21–30) |
| Resection, n | ||
| Complete | 47 | 53 |
| Partial | 57 | 61 |
| Biopsy | 31 | 25 |
| Median time from surgery to study treatment (range) [days] | 28 (9–111) | 46 (7–175) |
| Steroids, n | 33 | 27 |
| 1p/19q codel, n | ||
| Yes | 32 | 37 |
| No | 65 | 63 |
| Missing | 38 | 39 |
| MGMT promoter, n | ||
| Methylated | 73 | 74 |
| Unmethylated | 28 | 28 |
| Missing | 34 | 37 |
| IDH, n | ||
| Mutated | 73 | 71 |
| Wild-type | 30 | 28 |
| Missing | 32 | 40 |
| ATRX | ||
| Lost | 21 | 23 |
| Expressed | 46 | 43 |
| Missing | 68 | 73 |
| TERT promoter | ||
| Mutated | 29 | 19 |
| Wild-type | 40 | 52 |
| Missing | 66 | 68 |
| Molecular group | ||
| CIMPcodel | 33 | 35 |
| CIMPnon-codel | 36 | 36 |
| CIMPneg | 30 | 28 |
| Missing | 36 | 40 |
Abbreviations: PCV, procarbazine, lomustine and vincristine; TMZ, temozolomide; y, years.
aPatients were assessed centrally prior to inclusion into NOA-04. Diagnoses from both local and central pathology are given for information.
Clinical Efficacy
At a median follow-up of 9.5 years (95% CI: 8.6–10.2), 78% (arm A) and 79% (arms B1/B2) progression events have been observed. The primary endpoint TTF has been reached by 66% and 67% of patients, respectively. About half of the patients have died in both arms (48% in arm A and 53% in arms B1/B2). Data are relevantly more mature as the initial publication had a maximal follow-up of 4.5 years, and a TTF event was documented for 42.7% of patients.
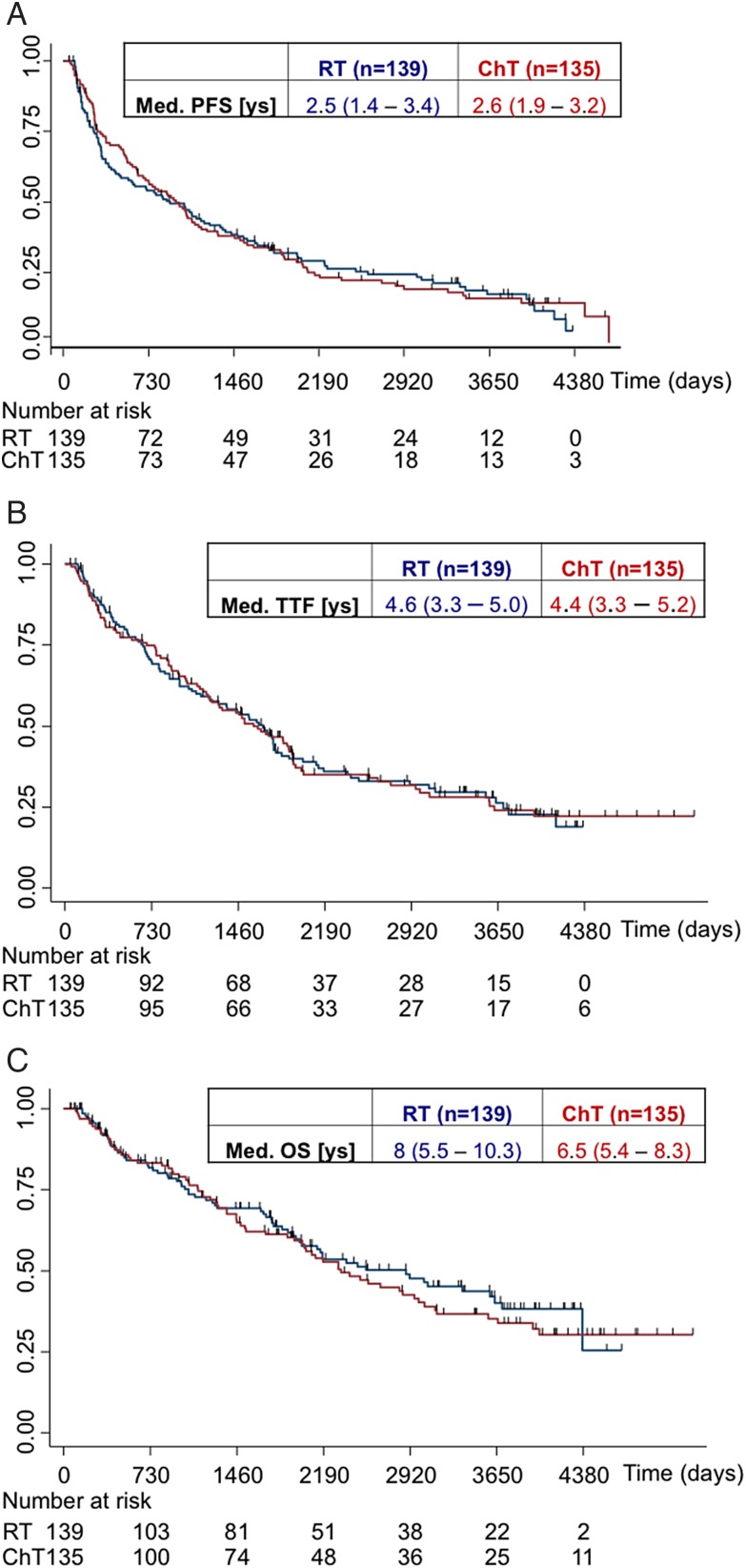
The unadjusted HR for PFS in arms B1/B2 versus arm A was 0.97 (95% CI: 0.74–1.26; log-rank P = .8), for TTF 0.99 (95% CI: 0.74–1.33; log-rank P = .97), and for the OS 1.11 (95% CI: 0.8–1.55; log-rank P = .53). Median PFS (2.7 [1.9–3.2] y vs 2.5 [1.3–3.5] y), TTF (4.4 [3.3–5.3] y vs 4.6 [3.4–5.1] y), and OS (6.5 [5.4–8.3] y vs 8 [5.5–10.3] y) demonstrated no difference between arms (B1/B2 vs A), although the median OS favors arm A (Fig. 2). No difference in PFS between patients treated with PCV versus TMZ was observed (median PFS: 2.52 [1.62–4.02] y vs 2.71 [1.57–3.62] y). As opposed to the protocol plan, rechallenge with chemotherapy was not commonly done (<20%) in arms B and C, precluding a detailed assessment.
Fig. 2.
Principal efficacy outcomes per treatment. Data of progression-free survival (PFS; (panel A), time-to-treatment failure (TTF; panel B), and overall survival (OS; panel C) were analyzed by treatment arm.
Prognostic and Predictive Factors
The major prognostic factors defined for adult glioma patients were confirmed (Supplementary material, Fig. 1). Efficacy analyses were performed in the different histopathological subgroups (astrocytic vs oligodendroglial) and according to biomarkers assessed for a total of 198 patients (Fig. 1).
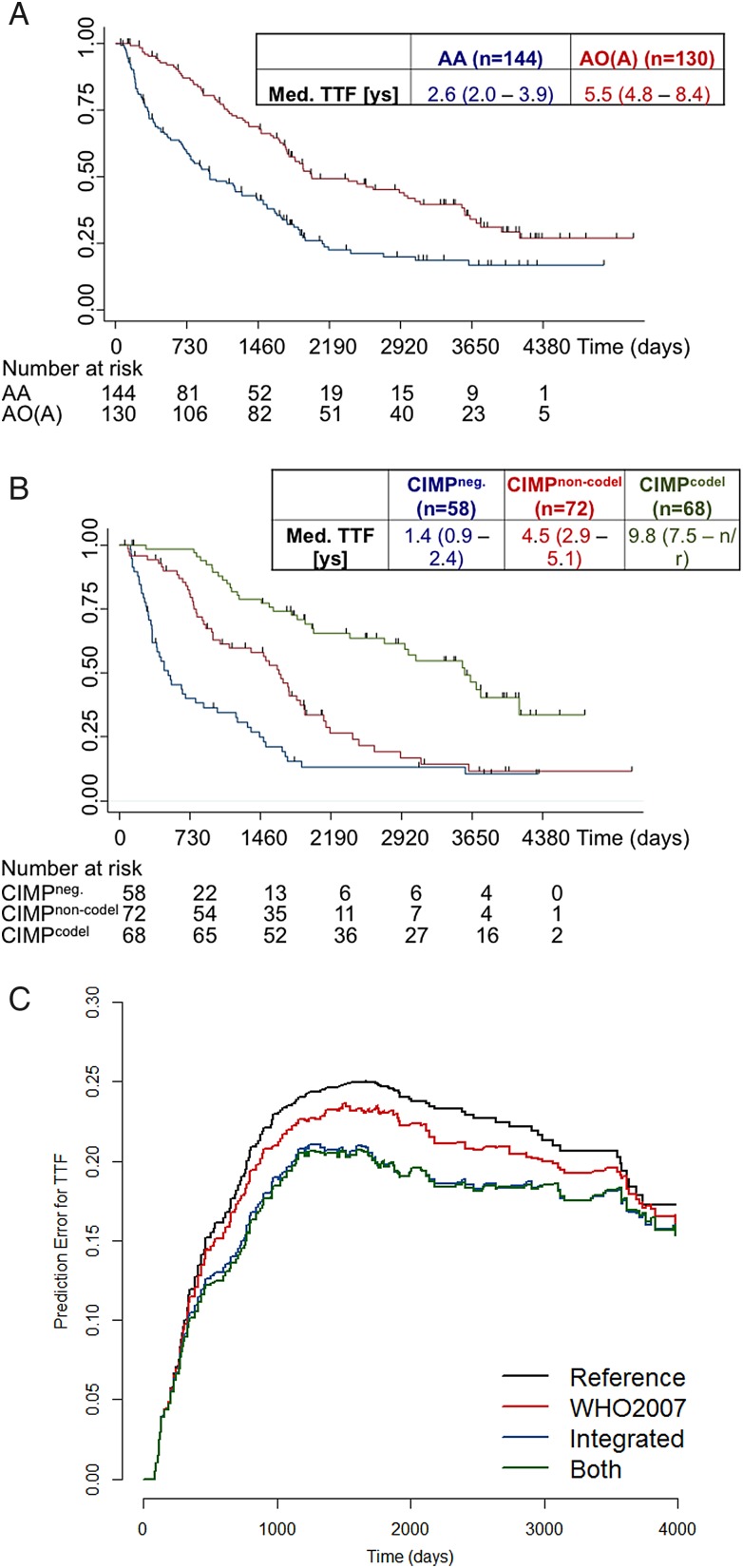
PFS, TTF, and OS were longer in patients with oligodendroglial gliomas compared with those having astrocytic anaplastic gliomas (Fig. 3A and Table 2). Importantly, molecular classification of samples based on IDH and 1p/19q status into 3 subgroups was prognostically superior to histological classification for PFS, TTF, and OS (Fig. 3B and C): In multivariate Cox models including both histology and molecular diagnosis, only the latter was associated with survival (Tables 3 and 4). However, no evidence was found to indicate that size of the treatment effect was influenced by histology or molecular status as indicated by the test of interaction between treatment and subgroup factor. In none of these molecular subgroups did initial RT in arm A or initial chemotherapy in arms B1/B2 demonstrate differential efficacy for PFS, TTF, and OS (Supplementary material, Fig. 2 and Table 2, Supplementary material, Fig. 3). The present long-term analysis confirmed the marked prognostic relevance of the IDH mutation regardless of the treatment arm for PFS (Table 2). While the positive prognostic impact of 1p/19q codeletion was confirmed, there was no signal to suggest a greater benefit from primary chemotherapy (Table 2 and Supplementary material, Fig. 2, Tables 5 and 6), and alpha-thalassemia/mental retardation syndrome X-linked or human telomerase reverse transcriptase were neither prognostic nor predictive (Supplementary material, Fig. 4). When comparing TMZ and PCV in the subgroups, patients with CIMPcodel tumors had better PFS and a trend toward better TTF with PCV versus TMZ, whereas OS was not different but did show a limited number of events. This difference was not visible in CIMPnon-codel or CIMPneg tumor patients (Supplementary material, Fig. 5). The data consistently suggested a superior PFS with RT compared with TMZ (Table 6), while RT and PCV conferred a similar outcome. MGMT promoter methylation was associated with improved PFS in chemotherapy-treated patients only in the subgroup of patients with tumors harboring wild-type IDH status. In patients with IDH-mutant tumors (either with or without 1p/19q codeletion), an unmethylated MGMT promoter was rare (15% in CIMPnon-codel and 9% in CIMPcodel) and neither prognostic nor predictive (Supplementary material, Table 2).
Fig. 3.
Efficacy outcomes according to histological and molecular subgroups. Data for time-to-treatment failure (TTF) according to histology (panel A) or molecular subtypes (panel B) and prediction error curves for TTF in Cox regression models including histology only, molecular classification only, or molecular classification and histology (both) (panel C) are depicted.
Table 2.
Differential treatment efficacy according to histology and molecular markers
| PCV/TMZ | Radiotherapy | |
|---|---|---|
| Histology, AO(A) vs AA [Hazard ratiosa] | ||
| PFS | 0.51 (95% CI: 0.35–0.76; P = .001) | 0.44 (95% CI: 0.3–0.65; P < .001) |
| TTF | 0.48 (95% CI: 0.31–0.73; P < .001) | 0.5 (95% CI: 0.33–0.76; P < .001) |
| OS | 0.49 (95% CI: 0.3–0.78; P = .003) | 0.37 (95% CI: 0.22–0.61; P < .001) |
| CIMPNon-Codel vs CIMPneg. | ||
| PFS | 0.57 (95% CI: 0.34–0.97; P = .041) | 0.59 (95% CI: 0.34–1.0; P = .052) |
| TTF | 0.46 (95% CI: 0.26–0.81; P = .007) | 0.55 (95% CI: 0.31–0.97; P = .04) |
| OS | 0.41 (95% CI: 0.22–0.77; P = .006) | 0.63 (95% CI: 0.33–1.2; P = .17) |
| CIMPCodel vs CIMPneg. | ||
| PFS | 0.22 (95% CI: 0.12–0.41; P < .001) | 0.25 (95% CI: 0.14–0.48; P < .001) |
| TTF | 0.26 (95% CI: 0.14–0.49; P < .0001) | 0.22 (95% CI: 0.11–0.43; P < .0001) |
| OS | 0.24 (95% CI: 0.12–0.48; P < .001) | 0.14 (95% CI: 0.06–0.36; P < .001) |
| IDH mutated vs wild-type | ||
| PFS | 0.33 (95% CI: 0.21–0.54; P < .001) | 0.38 (95% CI: 0.23–0.62; P < .001) |
| TTF | 0.34 (95% CI: 0.21–0.55; P < .0001) | 0.34 (95% CI: 0.2–0.58; P < .0001) |
| OS | 0.31 (95% CI: 0.18–0.53; P < .001) | 0.34 (95% CI: 0.18–0.64; P = .001) |
| 1p/19q codeleted vs non-codeleted | ||
| PFS | 0.29 (95% CI: 0.17–0.48; P < .001) | 0.34 (95% CI: 0.2–0.58; P < .001) |
| TTF | 0.39 (95% CI: 0.22–0.68; P < .001) | 0.32 (95% CI: 0.18–0.57; P < .0001) |
| OS | 0.35 (95% CI: 0.19–0.67; P = .001) | 0.24 (95% CI: 0.11–0.49; P < .001) |
Abbreviations: AA, anaplastic astrocytoma; AO(A), anaplastic oligodendroglioma/oligoastrocytoma; CI, confidence interval; OS, overall survival; PFS, progression-free survival: TTF, time-to-treatment failure; PCV, procarbazine, lomustine and vincristine; TMZ, temozolomide.
arisk reduction in the comparison.
Table 3.
Multivariate Cox regression of histology and molecular classification for time-to-treatment failure
| Factor | Hazard Ratio (95% CI) | P value |
|---|---|---|
| Histology, AO(A) vs AA | 0.7 (0.48–1.02) | .065 |
| CIMPNon-Codel vs CIMPneg. | 0.5 (0.34–0.75) | .001 |
| CIMPCodel vs CIMPneg. | 0.25 (0.15–0.40) | <.001 |
Abbreviation: CI, confidence interval.
Table 4.
Integrated Brier scores for time-to-treatment failure
| Factor | Integrated Brier score |
|---|---|
| Reference | 0.199 |
| Histology | 0.187 |
| Molecular classification | 0.168 |
| Histology + molecular classification | 0.167 |
Table 5.
Hazard ratios for chemotherapy versus radiotherapy in CIMPcodel
| Hazard Ratio | 95% CI | P value | |
|---|---|---|---|
| PFS, ChT vs RT | 1.30 | 0.70–2.38 | .416 |
| TTF, ChT vs RT | 1.35 | 0.68–2.70 | .381 |
| OS, ChT vs RT | 0.46 | 0.86–5.56 | .101 |
Abbreviations: ChT, chemotherapy;HR, hazard ratio; OS, overall survival; PFS, progression-free survival; RT, radiotherapy;
Table 6.
Efficacy of arms A, B1 and B2 in CIMPcodel
| PCV (n = 17) | TMZ (n = 16) | Radiotherapy (n = 35) | P (log-rank), PCV vs TMZ | |
|---|---|---|---|---|
| Median PFS in years (95% CI) | 9.4 (3.18–n/r) | 4.46 (2.01–7.8) | 8.67 (6.14–11.05) | .0254 |
| Median TTF in years (95% CI) | n/r (3.34–n/r) | 5.26 (3.05–n/r) | 10.12 (8.4–n/r) | .0646 |
| Median OS in years (95% CI) | n/r (8.19–n/r) | 8.09 (3.77–n/r) | n/r (9.95–n/r) | .0689 |
Abbreviations: CI, confidence interval; n/r, not reached OS, overall survival; PFS, progression-free survival; PCV, procarbazine, lomustine and vincristine; TMZ, temozolomide; TTF, time-to-treatment failure.
Discussion
Summary
NOA-04 long-term data do not support a differential efficacy of primary TMZ monotherapy or PCV polychemotherapy versus RT in any of the histological or molecular subgroups of anaplastic glioma. Specifically, the patients with the best prognosis (ie, patients with CIMPcodel anaplastic gliomas) do not seem to benefit selectively from one of the therapies, (Table 6).
Embedding NOA-04 Into Current Trial Data
In light of the data from 3 randomized trials in subsets of patients with WHO grades II and III gliomas showing a superiority of chemoradiotherapy over RT alone,1–3 the present results of at best equal clinical outcome with chemotherapy versus RT do not support the use of primary monochemotherapy in this disease subgroup (Supplementary material, Table 1) and even suggest that TMZ monotherapy may confer a worse outcome (Table 6). Also, OS in NOA-04, although not statistically different, numerically favors first-line RT (Fig. 2C). Further, data from these other randomized trials1–3 contradict the general sentiment that monochemotherapy may be a sufficient treatment, although prospectively controlled data are missing and the field is still divided into believers of primary monochemotherapy and combined chemoradiation and assessed in an ongoing trial (NCT00887146). In the large retrospective analysis,20 no difference had been reported in the median OS of patients with 1p/19q-codeleted gliomas who had been initially treated with chemotherapy alone (median: 10.5 y) versus those treated with chemoradiotherapy (median: 8.4 y). It is interesting to speculate that the inferior outcome of TMZ in the best-prognosis patients in NOA-04 within the limitations of the small numbers is also supporting the current standard of care of chemoradiation with PCV.
The performance of TMZ/PCV in NOA-04 is not explained by obvious principal differences in the patient cohorts between EORTC 26951/RTOG 9402 and NOA-04 after focusing on the intended patient cohorts with anaplastic oligodendroglial tumors of the EORTC and RTOG trials (which both demonstrated superiority of chemoirradiation with PCV over RT alone). Despite this and despite the limitations of cross-trial comparisons, it is surprising that the RT-treated patients with CIMPcodel anaplastic gliomas showed a better course of disease in NOA-04 than in the international trials (Supplementary material, Table 1). An explanation for the relatively high RT efficacy may lay in the limitation to stringently define these patients despite molecular markers. A limitation of NOA-04 even with this long-term evaluation (median follow up time 9.5 y [95% CI: 8.6–10.2]) is the still-immature OS with <50% events being observed overall and an especially low event rate in the best prognosis CIMPcodel group (29% deceased).
NOA-04 allows investigation of possible differential efficacy of PCV and TMZ. In the retrospective series reported by Lassman et al.,20 there was a positive signal for OS for PCV in 1p/19q codeleted patients, which was supported by the PFS data in the CIMPcodel patients of NOA-04 (Tables 5 and 6 and Supplementary material, Fig. 5) with the TTF and OS data not yet mature for final assessments (Supplementary material, Fig. 5) and no difference between the 2 chemotherapies in the patients with CIMPnon-codel or CIMPne. The current CODEL trial is conducted under the assumption that chemoradiotherapy using TMZ or PCV will have similar efficacy but a favorable safety profile and potentially other patient-related outcome measures such as neurocognitive function and health-related quality of life for TMZ. Whereas lower toxicity has already been reported in the first report of NOA-04,17 the present long-term analysis provides the first randomized data to suggest that there might be a superiority of PCV alone over TMZ alone in the best-prognosis subset of patients, although no sign of superiority of PCV alone over RT alone was seen (Tables 5 and 6). In the prematurely stopped RTOG 9813 trial for anaplastic astrocytoma, RT + TMZ seemed no different for OS compared with RT + nitrosourea.21
In NOA-04, molecular diagnosis is superior to histology in terms of biological and clinical separation of different prognostic groups (Table 2 and Fig. 3B and C). For optimal management of patients with anaplastic gliomas, there is a clinical need for reliable information on IDH, 1p/19q, MGMT, and ATRX status.22,23 Of note, ATRX is not prognostic for the full dataset (Supplementary material, Fig. 4A), but ATRX loss helps to identify the better-prognosis anaplastic astrocytoma (P = .02) (Supplementary material, Fig. 4B).12 There is accumulating evidence that the IDH mutation molecularly defines a tumor group with the molecular characteristics of low-grade or anaplastic diffuse gliomas. On the other hand, IDH wild-type WHO grade III gliomas, specifically anaplastic astrocytomas, match the poor clinical course of glioblastoma and often exhibit methylation and mutation profiles similar to glioblastoma.6–10 In looking at this subgroup with molecular pathology and course of disease more closely resembling glioblastoma, initial treatment may be given as if for WHO grade IV gliomas. This is also supported by initial releases on the CATNON data (looking at patients with non-codeleted anaplastic gliomas), which advocated adding TMZ to the therapy of patients originally randomized into the arm of primary monoradiotherapy.
Other than for classification purposes, IDH mutations serve as the target for immunotherapy (IDH1 R132H)24 within the trial NCT02454634 and targeted approaches with inhibitors aiming to block the generation of 2-hydroxygutarate.25 Trials with IDH inhibitors are NCT02073994 with AG-120, NCT02273739 with AG-221 and NCT02381886 with IDH305.
The 1p/19q co-deletion is predictive for a better outcome with combined chemoradiotherapy with PCV. The ongoing CODEL trial may show whether the same efficacy with less toxicity can be achieved with chemoradiotherapy using TMZ instead of PCV (NCT00887146).
NOA-04 has also helped to better define the use of MGMT status in anaplastic gliomas. In the first publication, we had determined the mere prognostic role for MGMT. The present long-term analysis confirms the hypothesis11 that MGMT promoter methylation is a predictive biomarker for benefit from alkylating chemotherapy in CIMPneg. gliomas only.
Limitations
Shortcomings of the present trial are the limited number of samples for biomarker analyses, which resulted in limited patient numbers in the biological subgroups, although the results reached statistical significance. For prognostic factors, interpretation of the impact of resection needs to be done within the limitations of an assessment, which not necessarily had to be done on the basis of an early postoperative MRI, which should be standard in today's trials. Another limitation impacting interpretation of the data is that 50% of the patients are still alive, which obviously precludes final statements on survival-related questions. This hopeful finding also indicates that many patients with anaplastic gliomas, particularly those with IDH-mutant tumors, are experiencing long-term survival today.
Conclusion
NOA-04 shows that primary monochemotherapy is not superior to primary RT. The trial allows the definition of biologically and clinically relevant subgroups, not only for oligodendroglial but for all anaplastic gliomas. Data in the 1p/19q co-deleted subgroup are supporting (i) the use of primary chemoradiotherapy (most likely using PCV in clinical routine) and (ii) the concept of the current CODEL trial, while (iii) not demonstrating superior efficacy of monochemotherapy compared with monoradiotherapy. Ultimately NOA-04, in conjunction with the concurrent trials,1–3 would support closing the book on primary monochemotherapy in these molecularly defined tumor subgroups.
Author Contributions
The concept of the trial was developed by M.W. and W.W. in collaboration with the German Neurooncology Working Group (NOA) in the German Cancer Society. All updated data were collected by P.R. and W.W. and reviewed by M.W. The statistical analyses were performed by B.W. The article was written by W.W., B.W., and M.W. with support from all co-authors. All authors reviewed and approved the manuscript.
Supplementary material
Acknowledgments
We are indebted to the patients and their families for agreeing to participate in this trial as well as to the nurses and data managers for their collaboration. We would like to thank Julius Schuth, PharmD, and Detlef Hecker, MD, for their valuable scientific advice during conduct of the study. The molecular analyses have been supported by the German Cancer Research Center and German Cancer Aid (grant number 110624). Data from this manuscript were presented at the ASCO Meeting 2015, on June 2 2015. Publication of biomarker subset details not involving this long-term analysis are referenced where appropriate in the manuscript. Merck Sharp & Dohme (clinical trial). German Cancer Aid (Deutsche Krebshilfe, “Molecular classification of anaplastic gliomas in the NOA-04 trial“, project 110624). This report has been presented as abstract 2001 at ASCO 2015 by W. Wick.
Conflict of interest statement: M.W. has received honoraria from MSD and Merck Serono. W.W. has participated in a speakers bureau for MSD. W.W. and M.W. have received research funding from MSD. M.W. has received research funding from Merck Serono and Novocure. W.W. has received research funding from Apogenix, Boehringer Ingelheim, Genentech Roche, and Pfizer. M.W. has a consultant relationship with BMS, Celldex, Genentech/Roche, Merck Serono, MSD, and Novocure. W.W. has a consultant relationship with BMS, Celldex, and Genentech/Roche.
References
- 1.van den Bent MJ, Brandes AA, Taphoorn MJ et al. Adjuvant procarbazine, lomustine, and vincristine chemotherapy in newly diagnosed anaplastic oligodendroglioma: long-term follow-up of EORTC brain tumor group study 26951. J Clin Oncol. 2013;31(3):344–350. [DOI] [PubMed] [Google Scholar]
- 2.Cairncross G, Wang M, Shaw E et al. Phase III trial of chemoradiotherapy for anaplastic oligodendroglioma: long-term results of RTOG 9402. J Clin Oncol. 2013;31(3):337–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buckner JC, Pugh SL, Shaw EG et al. Phase III study of radiation therapy (RT) with or without procarbazine, CCNU, and vincristine (PCV) in low-grade glioma: RTOG 9802 with Alliance, ECOG, and SWOG. J Clin Oncol. 2014;32:5s (suppl; abstr 2000). [Google Scholar]
- 4.Hartmann C, Hentschel B, Wick W et al. Patients with IDH1 wild type anaplastic astrocytomas exhibit worse prognosis than IDH1-mutated glioblastomas, and IDH1 mutation status accounts for the unfavorable prognostic effect of higher age: implications for classification of gliomas. Acta Neuropathol. 2010;120(6):707–718. [DOI] [PubMed] [Google Scholar]
- 5.Wiestler B, Capper D, Sill M et al. Integrated DNA methylation and copy-number profiling identify three clinically and biologically relevant groups of anaplastic glioma. Acta Neuropathol. 2014;128(4):561–571. [DOI] [PubMed] [Google Scholar]
- 6.Weller M, Weber RG, Willscher E et al. Molecular classification of diffuse cerebral WHO grade II/III gliomas using genome- and transcriptome-wide profiling improves stratification of prognostically distinct patient groups. Acta Neuropathol. 2015;129(5):679–693. [DOI] [PubMed] [Google Scholar]
- 7.Suzuki H, Aoki K, Chiba K et al. Mutational landscape and clonal architecture in grade II and III gliomas. Nat Genet. 2015;47(5):458–468. [DOI] [PubMed] [Google Scholar]
- 8.Cancer Genome Atlas Research Network. Comprehensive, integrative genomic analysis of diffuse lower-grade gliomas. N Engl J Med. 2015;372(26):2481–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reuss DE, Mamatjan Y, Schrimpf D et al. IDH mutant diffuse and anaplastic astrocytomas have similar age at presentation and little difference in survival: a grading problem for WHO. Acta Neuropathol. 2015a;129(6):867–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reuss DE, Kratz A, Sahm F et al. Adult IDH wild type astrocytomas biologically and clinically resolve into other tumor entities. Acta Neuropathol. 2015b;130(3):407–417. [DOI] [PubMed] [Google Scholar]
- 11.Wick W, Meisner C, Hentschel B et al. Prognostic or predictive value of MGMT promoter methylation in gliomas depends on IDH1 mutation. Neurology. 2013;81(17):1515–1522. [DOI] [PubMed] [Google Scholar]
- 12.Wiestler B, Capper D, Holland-Letz T et al. ATRX loss refines the classification of anaplastic gliomas and identifies a subgroup of IDH mutant astrocytic tumors with better prognosis. Acta Neuropathol. 2013;126(3):443–451. [DOI] [PubMed] [Google Scholar]
- 13.Sahm F, Reuss D, Koelsche C et al. Farewell to oligoastrocytoma: in situ molecular genetics favor classification as either oligodendroglioma or astrocytoma. Acta Neuropathol. 2014;128(4):551–559. [DOI] [PubMed] [Google Scholar]
- 14.Reuss DE, Sahm F, Schrimpf D et al. ATRX and IDH1-R132H immunohistochemistry with subsequent copy number analysis and IDH sequencing as a basis for an “integrated” diagnostic approach for adult astrocytoma, oligodendroglioma and glioblastoma. Acta Neuropathol. 2015;129(1):133–146. [DOI] [PubMed] [Google Scholar]
- 15.Louis DN, Ohgaki H, Wiestler OD et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114(5):97–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Louis DN, Perry A, Burger P et al. International Society Of Neuropathology–Haarlem consensus guidelines for nervous system tumor classification and grading. Brain Pathol. 2014;24(6):429–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wick W, Hartmann C, Engel C et al. for the Neurooncology Working Group (NOA) of the German Cancer Society. NOA-04 randomized phase III trial of sequential radiochemotherapy of anaplastic glioma with PCV or temozolomide. J Clin Oncol. 2009;27(35):5874–5880. [DOI] [PubMed] [Google Scholar]
- 18.Mogensen UB, Ishwaran H, Gerds TA. Evaluating random forests for survival analysis using prediction error curves. J Stat Softw. 2012;50(11):1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wiestler B, Capper D, Hovestadt V et al. Assessing CpG island methylator phenotype, 1p/19q codeletion, and MGMT promoter methylation from epigenome-wide data in the biomarker cohort of the NOA-04 trial. Neuro Oncol. 2014;16(12):1630–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lassman AB, Iwamoto FM, Cloughesy TF et al. International retrospective study of over 1000 adults with anaplastic oligodendroglial tumors. Neuro Oncol. 2011;13(6):649–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chang SM, Zhang P, Cairncross JG et al. Results of NRG oncology/RTOG 9813: A phase III randomized study of radiation therapy (RT) and temozolomide (TMZ) versus RT and nitrosourea (NU) therapy for anaplastic astrocytoma (AA). J Clin Oncol. 2015;33 (suppl; abstr 2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weller M, Wick W. Neuro-oncology in 2013: Improving outcome in newly diagnosed malignant glioma. Nat Rev Neurol. 2014;10(2):68–70. [DOI] [PubMed] [Google Scholar]
- 23.Weller M, Wick W, Aldape K et al. Glioma. Nat Rev Dis Primers. 2015;1:15017 doi:10.1038/nrdp.2015.17 [DOI] [PubMed] [Google Scholar]
- 24.Schumacher T, Bunse L, Pusch S et al. A vaccine targeting mutant IDH1 induces antitumor immunity. Nature. 2014;512(7514):324–327. [DOI] [PubMed] [Google Scholar]
- 25.Rohle D, Popovici-Muller J, Palaskas N et al. An inhibitor of mutant IDH1 delays growth and promotes differentiation of glioma cells. Science. 2013;340(6132):626–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.