Abstract
Background.
A specific form of small-vessel vasculopathy—cerebral microbleeds (CMBs)—has been linked to various types of dementia in adults. We assessed the incidence of CMBs and their association with neurocognitive function in pediatric brain tumor survivors.
Methods.
In a multi-institutional cohort of 149 pediatric brain tumor patients who received cranial radiation therapy (CRT) between 1987 and 2014 at age <21 years and 16 patients who did not receive CRT, we determined the presence of CMBs on brain MRIs. Neurocognitive function was assessed using a computerized testing program (CogState). We used survival analysis to determine cumulative incidence of CMBs and Poisson regression to examine risk factors for CMBs. Linear regression models were used to assess effect of CMBs on neurocognitive function.
Results.
The cumulative incidence of CMBs was 48.8% (95% CI: 38.3–60.5) at 5 years. Children who had whole brain irradiation developed CMBs at a rate 4 times greater than those treated with focal irradiation (P < .001). In multivariable analysis, children with CMBs performed worse on the Groton Maze Learning test (GML) compared with those without CMBs (Z-score –1.9; 95% CI: –2.7, –1.1; P < .001), indicating worse executive function when CMBs are present. CMBs in the frontal lobe were associated with worse performance on the GML (Z-score –2.4; 95% CI: –2.9, –1.8; P < .001). Presence of CMBs in the temporal lobes affected verbal memory (Z-score –2.0; 95% CI: –3.3, –0.7; P = .005).
Conclusion.
CMBs are common and associated with neurocognitive dysfunction in pediatric brain tumor survivors treated with radiation.
Keywords: cerebral microbleeds, cranial radiation therapy, late effects of tumor therapy, neurocognitive function, pediatric brain tumor survivors.
Treatment with cranial radiation therapy (CRT) remains an integral part of pediatric brain tumor therapy despite its known associated morbidities, including endocrine, vascular, and neurological sequelae.1–6 Poor neurocognition is an important long-term side effect of CRT and has been associated with poor educational attainment, less full time employment, and decreased household income—especially if given at a young age.7 Affected neurocognitive domains include attention, processing speed, working memory, and executive function.6 , 8–10 However, despite similar CRT doses, some patients demonstrate only mild cognitive impairment, whereas others suffer from severe disabilities. Although other risk factors predictive of poor neurocognition besides CRT include tumor location, presence of hydrocephalus, and significant seizure burden,11–14 currently we cannot predict which individuals are more likely to have significant neurocognitive impairment.
The underlying biological mechanisms leading to radiotherapy-associated neurocognitive deficits have yet to be clearly delineated. Herein we report on the effects of a specific form of small-vessel vasculopathy, cerebral microbleeds (CMBs), as a potential biomarker for neurocognitive impairment in irradiated pediatric brain tumor survivors. CMBs have been associated with a variety of dementias in adult patients, including Alzheimer disease, frontotemporal dementia, and mild cognitive impairment,15–17 as well as in a healthy aging population.18 However, there are no published data regarding whether presence of CMBs is associated with worse neurocognitive function in children with brain tumors, or whether the presence of CMBs could serve as an early imaging biomarker for children at increased risk for neurocognitive decline.
In the current study, we examined the presence of CMBs in pediatric brain tumor patients enrolled in the Radiation-induced Arteriopathy (Rad-Art) study. Within this cohort we tested the hypotheses that (i) CMBs are common in children undergoing CRT and occur relatively early after CRT and (ii) the presence of CMBs is associated with worse neurocognitive function.
Importance of the Study
Cranial radiation therapy (CRT) remains an integral part of pediatric brain tumor therapy. However, deficits in attention, working memory, and executive function are important long-term side effects of CRT. Cerebral microbleeds (CMBs) have been associated with dementia in adult patients, and recently their presence has been demonstrated in brain tumor survivors. However, there are no published data on whether CMBs are associated with worse neurocognitive function in children with brain tumors. In this study, we found that CMBs are common in this population, with a cumulative incidence of 48% at 5 years. Moreover, CMBs were associated with worse performance in tests of working memory, executive function, and verbal memory. CMBs may serve as an early marker of neurocognitive decline and help guide targeted interventions. Future studies should investigate how CMB burden evolves over time in this population and whether CMBs serve as a marker of increased susceptibility to radiation damage.
Materials and Methods
Patient Characteristics
Patients are part of the ongoing Rad-Art study, which is a multisite cohort study of childhood cancer survivors that began enrollment in 2011. Patients in the study are followed with prospective brain and cerebrovascular imaging and neurocognitive assessments using a computerized testing tool (CogState).19 Inclusion criteria for case enrollment in Rad-Art are radiation therapy to the brain or neck, age ≤21 years at the time of radiation therapy, survival >1 year after diagnosis, and ability to undergo MRI. The study expanded in 2015 to include a comparison group of pediatric brain tumor patients who did not receive any radiation therapy. For the comparison group, diagnosis of the brain tumor must have occurred ≤21 years of age. Participating institutions include Benioff Children’s Hospital San Francisco and Oakland, Valley Children’s Hospital, and Washington University in St Louis. Appropriate institutional approvals were obtained, and participants consented to be part of this study.
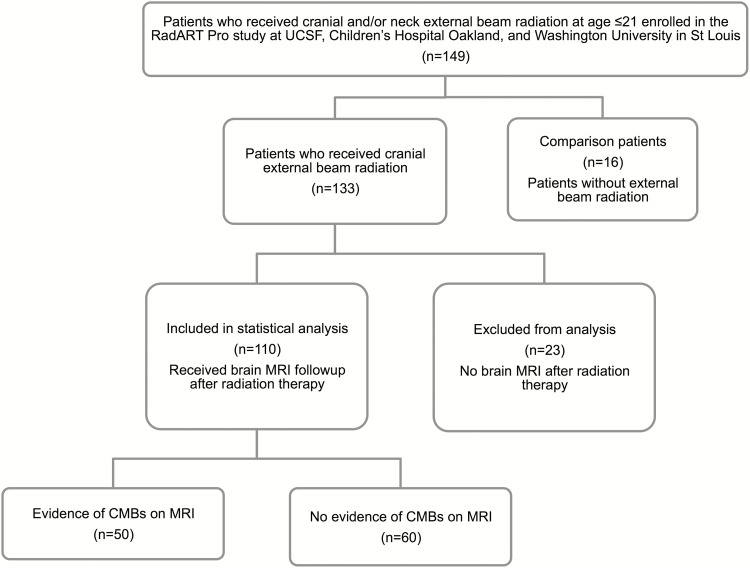
This analysis is based on 149 patients (n = 133 who received CRT, n = 16 comparison non-CRT patients) enrolled in the Rad-Art study between October 2011 and May 2015. Patients received CRT between 1987 and 2014. The final analysis included 126 out of 149 eligible patients (110 who received CRT and 16 comparison non-CRT patients). Patients without available MRI post-CRT were excluded (n = 23) (Fig. 1).
Fig. 1.
Flow diagram of the recruitment and selection of the 149 study participants.
Data Collection
Trained research assistants used standardized data collection tools to perform chart abstraction at all 4 enrolling sites. Data integrity screens were performed to ensure internal consistency and correct data collection and input. We collected detailed demographic, clinical, and tumor and radiation related information for each subject. Variables extracted included age at tumor diagnosis and at initiation of radiation therapy, radiation dose and field, sex, race, type of chemotherapy if any, tumor type and location, tumor recurrence, surgical resection, highest level of parent education, type of MRI, as well as presence of seizures and ventriculo-peritoneal (VP) shunt. Type of MRI was recorded and classified as iron sensitive versus non–iron sensitive. Iron sensitive imaging was further classified into higher sensitivity, phase-sensitive (susceptibility-weighted imaging [SWI], including T2* susceptibility-weighted angiography), and lower sensitivity (gradient recalled echo imaging and fast-field echo imaging). Imaging was performed on both 1.5 and 3T scanners. Seizures and VP shunt were classified as dichotomous variables. CRT dose was quantified in cGy received and location was categorized into tumor bed, whole brain, or whole brain plus spine. CRT to ventricular field alone was classified as tumor bed only.
MRI Evaluation
A single board-certified radiologist with certificate of additional qualification in neuroradiology (B.T.) blinded to the identity and clinical characteristics of the patients reviewed all patients’ MRI pre- and postradiation to determine the presence, number, and location of CMBs. CMBs were defined as previously published, specifically as discrete foci of susceptibility (maximum diameter of 3mm) which did not correspond to perpendicular vessels, flow voids, or surgical cavity on consecutive slices.20 For all CMBs identified, previous scans were reviewed to determine first radiographic appearance of CMBs. Cavernous malformations and capillary telangiectasias were considered distinct lesions and not classified as CMBs. CMB location was classified into the following categories: frontal lobe, parietal lobe, temporal lobe, occipital lobe, basal ganglia, thalamus, internal capsule, cerebellum, or brainstem. MRI was performed on a clinical basis; imaging protocols differed according to institutional guidelines and year imaging was obtained. The minimum interval was 1 month and the longest interval was 120 months with a median interval of 12 months.
Neurocognitive Outcomes
Neurocognitive outcomes were assessed using a computerized neurocognitive assessment tool: CogState, which offers an easy-to-administer screen for cognitive impairment that has been validated in multiple neuropsychological and cognitive studies.19 , 21 , 22 Rad-Art patients >5 years old were given the option to undergo the CogState battery on a yearly basis. Patients received age-appropriate batteries (CogState battery for ages 5–9 and 10+). The raw scores of the tests were converted into age-adjusted Z-scores, and Z-scores were used as the basis for analysis in order to account for the variability in participants’ age. The battery tests selected for the Rad-Art study included the detection test (psychomotor function), Groton Maze Learning test (GML) (executive function), international shopping list (verbal learning and delayed recall), identification task (attention), and one-back (working memory). Details of these battery tasks have been described previously (Supplementary Table 1).23–25 All ages had age-appropriate norms for each test included in the battery except for patients ages 5–9 years for the international shopping list tests; patients ages 5–9 were therefore excluded from analysis of these tests. A total of 10 patients were excluded. CogState testing administration was standardized across all sites and was administered by trained research assistants in designated interruption-free locations. Patients did not receive CogState testing on days of neuro-imaging due to concern for the effects of anesthesia on performance.
Radiation Dosimetry Overlays
To determine the specific amount of radiation received by brain regions that later developed CMBs, we overlaid radiation dosimetry plans upon patients’ MR sequences with CMBs. Only radiation dosimetry plans for patients with CMBs who received CRT at Benioff Children’s Hospital San Francisco and Oakland between 2009 and 2012 were available. Plans for patients who received CRT earlier or at outside institutions were not included in this analysis due to unavailability of these plans. MRIs revealing CMBs were aligned with and overlaid upon the subject’s radiation oncology dosimetry treatment plans using Velocity AI image registration software (v2.7, Varian Medical Systems) to match voxel intensity values.26–28 Patients’ MRIs were first registered to their radiation planning CT scan using a rigid automatic match based on bony anatomy. The alignment of the registration was then visually evaluated and manual adjustment was performed to correct any errors in alignment. The dosimetry plan was then overlaid onto the registered MRI, and the median radiation dose (Gy) received at each CMB location was recorded. Further details of the registration process have been reported previously.29 , 30
Statistical Analyses
Pearson’s chi-square test was used to compare categorical demographic, tumor, and clinical characteristics of patients with CMBs with those without. These characteristics included type of cancer, chemotherapy exposure, exposure to anti-angiogenic therapy, sex, race, and type of MRI. The Mann–Whitney test was used to analyze continuous characteristics including maximum brain radiation dose (cGy) and age (y). Kaplan–Meier survival analysis techniques were used to determine the cumulative incidence of CMBs. The observation period for all patients began at the end of radiation therapy. “First appearance” of CMBs was defined as the midway point between the date of the first MRI scan demonstrating CMB and the date of the previous MRI. Patients without CMBs were censored from the analysis at the time of their most recent MRI. The period of observation for patients who did not receive CRT was defined as the period from tumor diagnosis to most recent MRI.
We used univariate Poisson regression to identify predictors of CMB development. Variables associated with CMB development (P < .05) were then included in a multivariable model. Race, sex, and age at CRT were included as a priori variables. Linear regression models were used to assess the effect of CMBs on multiple domains of neurocognitive function as assessed by CogState battery subcategory scores (Supplementary Table 1). Standardized Z-scores for all age groups were used for our regression analysis. Variables found to be associated with neurocognitive function in univariate linear regression (P < .20) were included in a multivariate regression model. Race, sex, age at CRT, time from CRT to CogState testing, tumor recurrence or growth, and surgery were included as a priori variables in the multivariable analysis. Statistical significance was defined as P < .05.
Results
Median follow-up period was 3.6 years for all patients who received CRT (interquartile range [IQR], 1.4–6.8) and 5.3 years for comparison patients (IQR, 1.8–7.3). Median age at initiation of radiation therapy was 8.6 years (IQR, 5.7–12.7).
Baseline clinical and demographic characteristics of patients receiving CRT were not significantly different from those not receiving CRT (Supplementary Table 2). As expected, tumor type differed among those treated with CRT compared with those who did not: the majority of patients without CRT had a diagnosis of low-grade glioma or craniopharyngioma, while the majority of those with CRT had a diagnosis of medulloblastoma and ependymoma.
Cerebral Microbleeds
Fifty out of 110 children who were treated with CRT had evidence of CMBs on MRIs (Fig. 2). No patients had CMBs present on MR scans prior to CRT. None of the 16 comparison patients who had not received CRT had any evidence of CMBs at last follow-up, despite the fact that they were more likely to have the more sensitive imaging for CMB detection available (P = .007) and similar follow-up times (follow-up times of patients who received CRT versus comparison group: 3.6 vs 5.3 y, P = .287).
Fig. 2.
Representative example of CMBs. CMBs are seen on iron sensitive SWI (A, C) but not conventional (B, D) T1 MRI sequences. Characteristic CMBs appear as discrete foci of hypointensity on iron sensitive imaging. (A, B) A 13-year-old patient with a pineal germinoma treated at 9 years with 2400 cGy of craniospinal irradiation and boost of 4500 cGy. Images obtained at 48 months post-CRT. (C, D) An 11-year-old patient with medulloblastoma treated at 7 years with 3600 cGy of craniospinal irradiation and 5500 cGy boost to tumor bed. Images obtained at 42 months post-CRT.
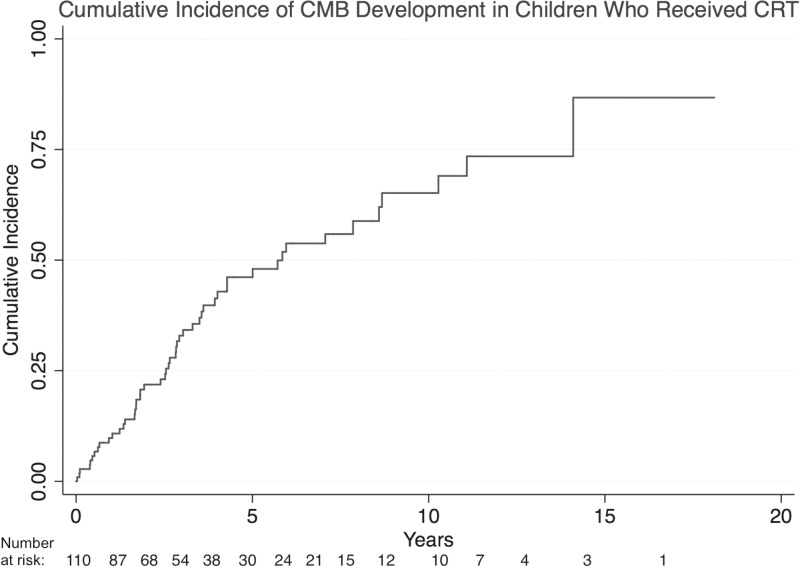
Children who received CRT developed CMBs with a cumulative incidence of 10.8% (95% CI: 6.1–18.7) at 1-year post-CRT and 48.8% (38.3–60.5) at 5 years (Fig. 3). Median latency to CMB development was 2.6 years postradiation (IQR, 1.3–4.3 y). The median length of follow-up did not differ significantly between patients with CMBs and those without (3.2 vs 3.9 y, P = .313). As expected, patients with CMBs were more likely to have the more iron sensitive imaging available; however, the total number of patients with no iron sensitive imaging in this cohort was very small (total n = 7) (Table 1).
Fig. 3.
Cumulative incidence of development of CMBs in 110 brain tumor survivors who received cranial radiation therapy.
Table 1.
Baseline clinical and demographic characteristics of patients who received cranial radiation therapy with CMBs compared with those without CMBs
|
CMBs
(n = 50) |
No CMBs
(n = 60) |
P-value a | |
|---|---|---|---|
| Female (%) | 26 (52) | 24 (48) | .577 |
| Race (%) | .51 | ||
| White | 40 (80) | 46 (77) | |
| Black | 4 (8) | 8 (13) | |
| Asian | 5 (10) | 3 (5) | |
| Other | 1 (2) | 3 (5) | |
| Age at radiation therapy, median (range) | 7.6 (1–21) | 9.6 (1–20) | .126 |
| Adjuvant chemotherapy (%) | 46 (92) | 46 (77) | .3 |
| Anti-angiogenic chemotherapy (%) | 7 (14) | 7 (12) | 1. |
| Cancer type (%) | .253 | ||
| Medulloblastoma | 19 (38) | 11 (19) | |
| Ependymoma | 9 (18) | 11 (19) | |
| Germinoma | 4 (8) | 7 (12) | |
| High grade glioma | 4 (8) | 8 (13) | |
| Low grade glioma | 4 (8) | 10 (17) | |
| Other | 10 (20) | 13 (22) | |
| Iron sensitive imaging (ISI) | .17 | ||
| (ISI) b (%) | 1 (2) | 6 (10) | |
| No ISS | 18 (36) | 32 (53) | |
| GRE/FFE | 31 (62) | 22 (37) | |
| SWI | |||
| Radiation (%) | .64 | ||
| Craniospinal or whole brain | 29 (60) | 23 (41) | |
| Tumor bed only | 20 (40) | 33 (59) | |
| Maximum brain radiation, cGy median (range) | 5580 (2400–6260) | 5400 (2700–6674) | .93 |
| VP shunt (%) | 16 (32) | 16(27) | .54 |
| Seizure disorder (%) | 8 (16) | 10 (13) | .925 |
| Surgical resection | 7 (12) | 7 (16) | .792 |
a P-values are Pearson’s chi-square test for categorical variables and the Mann‒Whitney U-test for continuous variables.
bISS, iron sensitive sequencing. ISI includes SWI and T2* susceptibility-weighted angiography, gradient-recalled echo (GRE), fast-field echo (FFE), and multiplanar gradient recalled imaging which utilize the difference in magnetic susceptibility of blood, iron, and calcification to detect the presence of iron with high sensitivity.
Predictors of cerebral microbleed development
In the univariate analysis, whole brain irradiation, exposure to chemotherapy, presence of iron sensitive imaging, and older age at CRT were associated with increased rate of CMB development (Table 2). Dose of radiation therapy was not significantly associated with CMB development. In the multivariate analysis, children with whole brain irradiation developed CMBs at a rate 4.3 times greater than those treated with focal radiation only (P ≤ .001, 95% CI: 3.1–5.9) (Table 2). In order to assess if anti-angiogenic therapy affects CMB development, we assessed the rate of CMBs in children being treated with regimens including the anti-angiogenic drug bevacizumab (n = 14). Children exposed to bevacizumab developed CMBs at a rate 6.2 times greater than those not exposed to chemotherapy (95% CI: 3.3, 11.7; P < .001). By contrast, children exposed to chemotherapy regimens not including bevacizumab developed CMBs at a lesser rate than those exposed to chemotherapy including bevacizumab (Table 2).
Table 2.
Multivariate Poisson regression analysis of predictors of CMBs
| Univariate Analysis Multivariate Analysis | ||||
|---|---|---|---|---|
| Characteristic | Incidence Rate Ratioa | P-value | Incidence Rate Ratiob | P-value |
| Radiation | ||||
| Whole brain CRT vs focal | 3.0 (2.3, 4.1) | <.001 | 4.3 (3.1, 5.9) | <.001 |
| Exposure to chemotherapy | ||||
| Any chemotherapy vs none | 4.2 (2.4, 7.2) | <.001 | 3.4 (2.0, 6.1) | <.001 |
| Any chemotherapy + bevacizumab vs none | 8.5 (4.5, 15.9) | <.001 | 6.2 (3.3, 11.7) | <.001 |
| Age at CRT, each additional 10 y | 1.8 (1.3, 2.4) | <.001 | 1.6 (1.2, 2.1) | .003 |
| Iron sensitive imaging c | ||||
| T2 FFE/GRE/MPGR vs. T2 b0 | 23.0 (3.2, 165.5) | .002 | 35.6 (4.9, 259.3) | <.001 |
| SWI vs T2 b0 | 46.5 (6.5, 331.0) | <.001 | 90.7 (12.6, 652.9) | <.001 |
| Maximum brain radiation, each additional Gy | 0.97 (0.78, 1.2) | .824 | ||
aUnivariate Poisson analysis.
bMultivariate Poisson regression analysis; race and gender were included as a priori factors.
cWe defined a participant having iron sensitive images if the MRI included the following sequences: SWI, T2* susceptibility-weighted angiography, gradient-recalled echo (GRE), fast-field echo (FFE), or multiplanar gradient recalled (MPGR) imaging. T2b0 was used as the least iron sensitive imaging and participants with T2b0 imaging only were considered to not have iron sensitive sequencing.
Number and location of cerebral microbleeds
In the 50 patients with CMBs, the total number of CMBs in a single patient ranged from 1 to 49 (median = 3; IQR, 1.5). The most common locations for CMBs were the temporal lobe (26 patients, 52%), parietal lobe, and cerebellum (25 patients each, 50%), followed by frontal and occipital lobes (22 patients each, 44%). Relatively few patients had lesions in the basal ganglia (5 patients, 10%) and brainstem (2 patients, 4%). No patients had CMBs in the thalamus or internal capsule. Of the 50 patients with CMBs, 30 had follow-up MRI available 1 year after the study that first demonstrated CMBs. Of these 30 patients, 17 (57%) had no interval change in the number of CMBs, while 13 (43%) had interval increase in the number of CMBs, ranging from 1 to 43 more CMBs.
Radiation dosimetry plans
A subset of 20 patients with CMBs (40%) had radiation dosimetry plans available. These 20 patients did not differ from the other 30 patients with CMBs with respect to either baseline patient, tumor, or treatment-related characteristics (data not shown). The majority of CMBs (63%) were in locations that received over 50 Gy of radiation, while 11% were in locations that received 40–49 Gy, 17% were in locations that received 30–39 Gy, and only 9% were in locations that received 20–29 Gy. The median time from CRT to CMB detection in this group was 2.5 years (range 8 mo–10 y).
Cerebral Microbleeds and Neurocognitive Function
Of the 110 patients who received CRT, 105 were eligible to complete the CogState assessment of neurocognitive function. Of these, 67 (64%) completed the optional assessment (66% of patients with CMBs and 56% of those without) at time of enrollment in the study. Only 15 (22%) of these 67 patients completed 2 or more assessments; therefore, we limited analysis to initial CogState assessment at time of enrollment. Patients who completed the assessment were not different from those who did not in terms of age at radiation, tumor type, or other baseline variables (data not shown). The median age at CogState administration was 14 years (IQR, 10–19; range 5–26 y), and the median time from CRT to CogState testing was 4.2 years (IQR, 2.3–6.9; range 0.5–16 y). In our univariate analysis of factors predicting neurocognitive performance, variables that met the P-value cutoff of .2 for inclusion in the multivariate model included CMB presence, presence of VP shunt, seizure disorder, and highest level of education achieved by patients’ mothers. Time from CRT to CogState testing, tumor recurrence or growth, surgery, age at CRT, race, and sex were included as a priori variables in the multivariable analysis. In our multivariable analysis, presence of CMBs was associated with worse executive function (GML: Z-score −1.9; 95% CI: −2.7, −1.1; P < .001), especially when CMBs were located within the frontal lobes (GML: decrease in Z-score by 2.4 points compared with patients without CMBs in the frontal lobe; P < .001; 95% CI: −2.9, −1.8). (Table 3).
Table 3.
Multivariate linear regression analysis of neurocognitive function for patients with CMBs compared with those without CMBs
| CMB Location | Executive Function (Groton Maze Learning)a |
P-value, R2, β |
Verbal Memory (Shopping list)a |
P-value, R2, β |
Working Memory (One back)a |
P-value, R2, β |
Verbal Memory (shopping list delayed recall)a |
P-value, R2, β |
Psychomotor Function (Detection)a |
P-value, R2, β |
|---|---|---|---|---|---|---|---|---|---|---|
| Frontal (n = 16) |
-2.4 (-2.9, -1.8) | <.001, R2=0.79 β= -0.51 | -2.0 (-2.2, -1.7) | .020, R2=0.81 β= -0.31 | -1.2 (-2.0, -0.2) | .019, R2=0.52 β= -0.39 | 0.5 (-0.4, 1.4) | .254 | -2.6 (-5.4, 0.2) | .069 |
| Temporal (n = 17) |
-1.3 (-2.3, -0.2) | .032 R2=0.60 β=-0.32 |
-0.1 (-1.0, 0.8) | .789 | -0.6 (-1.5, 0.4) | .286 | -2.0 (-3.3, -0.7) | .005, R2=0.80 β= -0.39 |
-1.1 (-4.4, 2.2) | .488 |
| Parietal (n = 16) |
-2.1 (-3.1, -1.2) | <.001 R2=0.69 β=-0.46 |
-0.2 (-0.5, 0.1) | .225 | -0.2 (-2.3, 0.1) | .721 | 0.8 (-0.4, 2.1) | .172 | -1.5 (-4.5, 1.4) | .299 |
| Occipital (n = 16) |
-1.5 (-2.7, -0.4) | .012 R2=0.59 β=-0.30 |
-0.8 (-1.6, -0.1) | .039 R2=0.82 β=-0.28 |
-0.4 (-1.5, 0.7) | .427 | 0.2 (-0.8, 1.4) | .656 | -2.9 (-5.9, -0.3) | .040, R2=0.61 β= -0.35 |
| Cerebellar (n = 20) |
-1.8 (-2.8, -0.8) | .001 R2=0.59 β=-0.47 |
-0.25 (-1.0, 0.5) | .501 | -0.2 (-1.2, 0.9) | .748 | 0.1 (-1.4, 1.6) | .900 | -2.1 (-5.2, 0.12) | .061 |
| Any CMB | -1.8 (-2.6, -1.0) | <.001 R2=0.71 β=-0.47 |
-0.6 (-1.2, -0.3) | .042 R2=0.82 β=-0.25 |
-0.53 (-1.5, 0.4) | .236 | 0.3 (-0.8, 1.4) | .599 | -2.3 (-5.1, 0.5) | .101 |
| No CMBs, median (IQR) |
0.5 (-1.3, 0.8)b | -0.3 (-0.8, 0.4) | -0.7 (-2.0, 0.4) | -1.0 (-2.3, -0.1) | -0.8 (-1.6, 0.6) |
aDifference in age-corrected Z-scores (95% CI).
bMedian (IQR).
The multivariate linear regression was adjusted for mother’s highest level of education, presence of seizures, and presence of VP shunt. Race, gender, age at radiation therapy, time from radiation, surgery, tumor location, and tumor recurrence or growth were included as a priori factors.
In assessments of other aspects of neurocognitive function, including delayed recall, verbal learning, attention, and working memory, patients with CMBs continued to perform worse than patients without CMBs, with a specific effect of the location of CMBs on these different neurocognitive tasks. Patients with CMBs in the occipital lobe took the longest on the detection test of visual selective attention and psychomotor function (Z-score 2.9 points less compared with children without CMBs in the occipital lobe [95% CI: –5.9, –0.3; P = .040]). On the “one back test” of working memory, patients with CMBs in the frontal lobe made the most mistakes (Z-score –1.2 points less than patients without CMBs in the frontal lobe, 95% CI: –2.0, –0.2; P = .019). On the test of verbal learning, patients with CMBs in the temporal lobes performed the worst (Z-score 2 points worse than those without CMBs in the temporal lobes, 95% CI: −3.3, −0.7; P = .005).
Discussion
Our analysis of 149 pediatric brain tumor patients followed by the Rad-Art study is the first to demonstrate that CMBs develop frequently in children who received CRT and that presence of CMBs is correlated with worse neurocognitive function in this population.
The prevalence of CMBs in a general pediatric population has not been reported. However, in the prospective Rotterdam population-based cohort study of 3979 adult subjects, CMB prevalence in subjects ages 45–50 was only 6.5%, while it was 35.7% in subjects over 80 years,31 suggesting that CMBs are uncommon in younger populations. Previous cross-sectional studies of CMBs in pediatric brain tumor patients found prevalence rates ranging from 29% to 100%.32–34 Yeom et al showed that 44% (n = 93) of children with medulloblastoma receiving CRT had evidence of focal hemosiderin deposition (including CMBs and cavernous malformations) with a median follow-up time of 5.8 years,32 similar to another report that in 100 children who received CRT, 33% had evidence of CMBs or cavernous malformations.34 However, Peters et al found that 100% of children with medulloblastoma (n = 7) who received CRT developed CMBs at a median follow-up of 21 months.33 The difference in these results likely reflects differences in MRI sensitivity: Yeom utilized T2* gradient recalled echo imaging with 4- to 5-mm slices, while Peters utilized a more sensitive SWI sequence with 1-mm slices.34 In our study of children with various types of iron sensitive imaging (6% with no SWI, 45% with lower-sensitivity SWI, and 49% with high-sensitivity SWI), the cumulative incidence of CMBs in patients who underwent CRT was 48.8% at 5 years, while the cumulative incidence in comparison patients at 5 years was 0%.
In our study, craniospinal or whole brain irradiation was the main risk factor for the development of CMBs. While CMBs appeared in patients who received focal irradiation, they were more likely to develop in patients receiving whole brain/craniospinal irradiation. Additionally, we did not find an association between radiation dose and CMB development. However, in our more detailed CRT dosimetry analysis of 20 patients, we demonstrated that while some CMBs occur in areas receiving lower doses (20–40 Gy), they most commonly appear in areas receiving high dose (>50 Gy). This discrepancy most likely reflects that we used an approximation to assess the radiation dose based on the overall prescribed dose reported in the radiation oncology reports, while in actuality patients received varying doses of radiation to varying volumes of brain. Overall our results suggest that increased radiation dose is associated with higher risk of CMBs and that craniospinal irradiation or whole brain irradiation contributes to the development of CMBs by increasing the volume of brain at risk. This is in concordance with previous reports that showed that whole brain irradiation is associated with higher incidence of late cerebrovascular complications including CMBs.32 , 34
Younger age at radiation has been linked to poorer neurocognitive function.35 We found that older age at radiation is a predictor of CMB development and that both CMB development and younger age at CRT were associated with worse neurocognitive function in a range of domains, including executive function, working memory, visual processing speed, and verbal memory. These results suggest that younger age at time of radiation therapy and CMB presence are both independently associated with decreased neurocognitive function through different biological mechanisms.6 , 35 , 36 Additionally, we found that CMBs were associated with poorer neurocognitive outcomes even in patients who were evaluated years after first CMB appearance, suggesting that the detrimental effects of CMBs are long-lasting. Given the prevalence of CMBs in elderly populations with dementia, our results support the concern that survivors of CNS tumors may experience accelerated CNS aging, as reported previously.37
We found that location of CMBs is associated with domain-specific deficits. Frontal lobe CMBs were most highly associated with poor executive function and poor working memory, consistent with the major role of the frontal lobe in mediating those functions.38 , 39 Similarly, occipital lobe CMBs were associated with poor performance on the detection task, which measures visual selective attention, mediated by the occipital lobe.41 , 42 CMBs in the temporal and frontal lobes were associated with poor performance in verbal learning, consistent with the implicated role of both lobes in learning and memory.43–45 While Yeom et al reported no correlation between IQ or need for special education and presence of focal hemosiderin deposits in childhood medulloblastoma patients, assessment of a global cognitive score such as IQ might not be sensitive enough to detect task-specific differences.9 , 32 , 46 , 47 The CogState battery includes assessment of specific cognitive domains and therefore may have allowed us to capture more subtle effects of CMBs on neurocognition. Additionally, our study allowed for examination of the relationship between neurocognitive functional decline and CMBs in specific brain areas. Finally, the correlation between CMB presence and neurocognitive function remained significant even after controlling for other factors known to be associated with neurocognitive function, including highest level of parental education or presence of VP shunt or seizure disorder.48, 49
We found that treatment with the anti-angiogenic drug bevacizumab was associated with an increased rate of CMB formation in children. By contrast, administration of the anti-angiogenic drug enzastaurin with CRT was associated with decreased rate of additional CMB formation in adults.50 The authors hypothesized that anti-angiogenic therapy may confer a possible radioprotective effect on microvasculature by decreasing capillary permeability and cytokine release.51 Although we examined a different anti-angiogenic agent, our results support the alternative hypothesis that anti-angiogenic therapies may increase the susceptibility of microvasculature to bleeding after radiation by impairing platelet-endothelial function and vascular repair.52 Anti-angiogenic therapies have been associated with increased risk of hemorrhage in adult brain tumor and other cancer patients.53–55 Our results suggest that bevacizumab therapy may impair vascular endothelial growth factor–mediated endothelial repair after CRT and increase risk of CMB formation.
Limitations of this study include that CMB development was often assessed retrospectively, and time to development was therefore an estimate based on midpoint between the last normal and first abnormal MRI. Additionally, patients had disparate interval imaging schedules. Some patients had imaging every year, whereas others had imaging every 3 months. Imaging interval also changed in the same patients from year to year based on time from brain tumor treatment. Further, there was a lack of standardized MRI with a specific SWI protocol as well as variation in scanner strength across institutions and across time, and more sensitive imaging techniques developed over time. We attempted to control for these differences by reporting on the number of patients who received recent high quality SWI compared with patients who received no SWI or less sensitive T2* imaging. Additionally, although we attempted to control for potential neurologic comorbidities affecting neurocognitive outcome by including variables such as seizure disorder, VP shunt, mothers’ education level, underlying genetic diagnoses (neurofibromatosis 1 and 2), and tumor location and surgery, residual confounding by other host factors may exist.
Unfortunately, there are currently no interventions that have been shown to prevent the development of neurocognitive deficits in survivors of pediatric brain tumors. However, recent studies have shown promising initial results in ameliorating such deficits. For example, computerized cognitive training and cognitive remediation programs, which are modeled after traditional brain injury rehabilitation techniques, have been found to improve scores in various domains, including working memory in patients with known cognitive deficits.56, 57 Additionally, methylphenidate has demonstrated utility in improving attention and social skills in brain tumor survivors.58 Additional studies are needed to determine the efficacy of these interventions in patients with CMBs.
Future directions include following the Rad-Art cohort to determine how CMB presence evolves over time, whether CMBs continue to develop years after radiation, and whether they continue to grow or remain stable in size. Based on adult studies, the presence of CMBs may indicate an increased susceptibility to radiation-induced damage and possibly increased likelihood of late stroke.59 We also plan to follow the longitudinal development and changes in neurocognitive outcomes in our cohort.
In conclusion, CMBs are common in survivors of pediatric brain tumors who received CRT and are associated with neurocognitive deficits. They may hold promise as an early marker for neurocognitive decline and help facilitate targeted intervention.
Supplementary material
Supplementary material is available online at Neuro-Oncology (http://neuro-oncology.oxfordjournals.org).
Funding
This work was supported by a donation from the LaRoche family (to H.F., S.M.), and the National Center for Advancing Translational Sciences, National Institutes of Health, through UCSF-CTSI grant number KL2TR000143 (to S.M.).
Supplementary Material
Acknowledgments
We thank the patients and families for their participation in our study.
Conflict of interest statement. The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1. Roddy E, Mueller S. Late effects of treatment of pediatric central nervous system tumors. J Child Neurol. 2015. [DOI] [PubMed] [Google Scholar]
- 2. Fullerton HJ, Stratton K, Mueller S, et al. Recurrent stroke in childhood cancer survivors. Neurology. 2015;85(12):1056–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mueller S, Sear K, Hills NK, et al. Risk of first and recurrent stroke in childhood cancer survivors treated with cranial and cervical radiation therapy. Int J Radiat Oncol, Biol, Phys. 2013;86(4):643–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Packer RJ, Gurney JG, Punyko JA, et al. Long-term neurologic and neurosensory sequelae in adult survivors of a childhood brain tumor: childhood cancer survivor study. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2003;21(17):3255–3261. [DOI] [PubMed] [Google Scholar]
- 5. Merchant TE, Conklin HM, Wu S, et al. Late effects of conformal radiation therapy for pediatric patients with low-grade glioma: prospective evaluation of cognitive, endocrine, and hearing deficits. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2009;27(22):3691–3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Armstrong GT. Long-term survivors of childhood central nervous system malignancies: the experience of the Childhood Cancer Survivor Study. European journal of paediatric neurology: EJPN: official journal of the European Paediatric Neurology Society. 2010;14(4):298–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ellenberg L, Liu Q, Gioia G, et al. Neurocognitive status in long-term survivors of childhood CNS malignancies: a report from the Childhood Cancer Survivor Study. Neuropsychology. 2009;23(6):705–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mulhern RK, Palmer SL, Reddick WE, et al. Risks of young age for selected neurocognitive deficits in medulloblastoma are associated with white matter loss. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2001;19(2):472–479. [DOI] [PubMed] [Google Scholar]
- 9. Reeves CB, Palmer SL, Reddick WE, et al. Attention and memory functioning among pediatric patients with medulloblastoma. J Pediatr Psychol. 2006;31(3):272–280. [DOI] [PubMed] [Google Scholar]
- 10. Robinson KE, Kuttesch JF, Champion JE, et al. A quantitative meta-analysis of neurocognitive sequelae in survivors of pediatric brain tumors. Pediatr Blood Cancer. 2010;55(3):525–531. [DOI] [PubMed] [Google Scholar]
- 11. Radcliffe J, Packer RJ, Atkins TE, et al. Three- and four-year cognitive outcome in children with noncortical brain tumors treated with whole-brain radiotherapy. Ann Neurol. 1992;32(4):551–554. [DOI] [PubMed] [Google Scholar]
- 12. Reimers TS, Ehrenfels S, Mortensen EL, et al. Cognitive deficits in long-term survivors of childhood brain tumors: Identification of predictive factors. Med Pediatr Oncol. 2003;40(1):26–34. [DOI] [PubMed] [Google Scholar]
- 13. Aarsen FK, Paquier PF, Arts WF, et al. Cognitive deficits and predictors 3 years after diagnosis of a pilocytic astrocytoma in childhood. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2009;27(21):3526–3532. [DOI] [PubMed] [Google Scholar]
- 14. Reimers TS, Mortensen EL, Nysom K, et al. Health-related quality of life in long-term survivors of childhood brain tumors. Pediatr Blood Cancer. 2009;53(6):1086–1091. [DOI] [PubMed] [Google Scholar]
- 15. Werring DJ, Gregoire SM, Cipolotti L. Cerebral microbleeds and vascular cognitive impairment. J Neurol Sci. 2010;299(1–2):131–135. [DOI] [PubMed] [Google Scholar]
- 16. Yates PA, Villemagne VL, Ellis KA, et al. Cerebral microbleeds: a review of clinical, genetic, and neuroimaging associations. Front Neurol. 2014;4:205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kirsch W, McAuley G, Holshouser B, et al. Serial susceptibility weighted MRI measures brain iron and microbleeds in dementia. J Alzheimers Dis: JAD. 2009;17(3):599–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Poels MM, Ikram MA, van der Lugt A, et al. Cerebral microbleeds are associated with worse cognitive function: the Rotterdam Scan Study. Neurology. 2012;78(5):326–333. [DOI] [PubMed] [Google Scholar]
- 19. Hammers D, Spurgeon E, Ryan K, et al. Validity of a brief computerized cognitive screening test in dementia. J Geriatr Psychiatry Neurol. 2012;25(2):89–99. [DOI] [PubMed] [Google Scholar]
- 20. Greenberg SM, Vernooij MW, Cordonnier C, et al. Cerebral microbleeds: a guide to detection and interpretation. Lancet Neurol. 2009;8(2):165–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Maruff P, Thomas E, Cysique L, et al. Validity of the CogState brief battery: relationship to standardized tests and sensitivity to cognitive impairment in mild traumatic brain injury, schizophrenia, and AIDS dementia complex. Archives of clinical neuropsychology: the official journal of the National Academy of Neuropsychologists. 2009;24(2):165–178. [DOI] [PubMed] [Google Scholar]
- 22. Pietrzak RH, Maruff P, Mayes LC, et al. An examination of the construct validity and factor structure of the Groton Maze Learning test, a new measure of spatial working memory, learning efficiency, and error monitoring. Archives of clinical neuropsychology: the official journal of the National Academy of Neuropsychologists. 2008;23(4):433–445. [DOI] [PubMed] [Google Scholar]
- 23. Collie A, Darekar A, Weissgerber G, et al. Cognitive testing in early-phase clinical trials: development of a rapid computerized test battery and application in a simulated phase I study. Contemp Clin Trials. 2007;28(4):391–400. [DOI] [PubMed] [Google Scholar]
- 24. Mollica CM, Maruff P, Vance A. Development of a statistical approach to classifying treatment response in individual children with ADHD. Hum Psychopharmacolo. 2004;19(7):445–456. [DOI] [PubMed] [Google Scholar]
- 25. Snyder PJ, Werth J, Giordani B, et al. A method for determining the magnitude of change across different cognitive functions in clinical trials: the effects of acute administration of two different doses alprazolam. Hum Psychopharmacolo. 2005;20(4):263–273. [DOI] [PubMed] [Google Scholar]
- 26. Liang Y, Bydder M, Yashar CM, et al. Prospective study of functional bone marrow-sparing intensity modulated radiation therapy with concurrent chemotherapy for pelvic malignancies. Int J Radiat Oncol, Biol, Phys. 2013;85(2):406–414. [DOI] [PubMed] [Google Scholar]
- 27. Elstrom UV, Wysocka BA, Muren LP, et al. Daily kV cone-beam CT and deformable image registration as a method for studying dosimetric consequences of anatomic changes in adaptive IMRT of head and neck cancer. Acta oncologica. 2010;49(7):1101–1108. [DOI] [PubMed] [Google Scholar]
- 28. Kim H, Huq MS, Houser C, et al. Mapping of dose distribution from IMRT onto MRI-guided high dose rate brachytherapy using deformable image registration for cervical cancer treatments: preliminary study with commercially available software. J Contemp Brachytherapy. 2014;6(2):178–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kessler ML. Image registration and data fusion in radiation therapy. Br J Radiol. 2006;79 Spec No 1:S99–108. [DOI] [PubMed] [Google Scholar]
- 30. Maintz JB, Viergever MA. A survey of medical image registration. Med Image Anal. 1998;2(1):1–36. [DOI] [PubMed] [Google Scholar]
- 31. Poels MM, Vernooij MW, Ikram MA, et al. Prevalence and risk factors of cerebral microbleeds: an update of the Rotterdam scan study. Stroke; a journal of cerebral circulation. 2010;41(10 Suppl):S103–106. [DOI] [PubMed] [Google Scholar]
- 32. Yeom KW, Lober RM, Partap S, et al. Increased focal hemosiderin deposition in pediatric medulloblastoma patients receiving radiotherapy at a later age. J Neurosurg Pediatr. 2013;12(5):444–451. [DOI] [PubMed] [Google Scholar]
- 33. Peters S, Pahl R, Claviez A, et al. Detection of irreversible changes in susceptibility-weighted images after whole-brain irradiation of children. Neuroradiology. 2013;55(7):853–859. [DOI] [PubMed] [Google Scholar]
- 34. Passos J, Nzwalo H, Marques J, et al. Late cerebrovascular complications after radiotherapy for childhood primary central nervous system tumors. Pediatr Neurol. 2015;53(3):211–215. [DOI] [PubMed] [Google Scholar]
- 35. Duffner PK. Risk factors for cognitive decline in children treated for brain tumors. European journal of paediatric neurology: EJPN: official journal of the European Paediatric Neurology Society. 2010;14(2):106–115. [DOI] [PubMed] [Google Scholar]
- 36. Ullrich NJ, Embry L. Neurocognitive dysfunction in survivors of childhood brain tumors. Semin pediatr Neurol. 2012;19(1):35–42. [DOI] [PubMed] [Google Scholar]
- 37. Schuitema I, Deprez S, Van Hecke W, et al. Accelerated aging, decreased white matter integrity, and associated neuropsychological dysfunction 25 years after pediatric lymphoid malignancies. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2013;31(27):3378–3388. [DOI] [PubMed] [Google Scholar]
- 38. Smith EE, Jonides J. Storage and executive processes in the frontal lobes. Science. 1999;283(5408):1657–1661. [DOI] [PubMed] [Google Scholar]
- 39. Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. [DOI] [PubMed] [Google Scholar]
- 40. Ogawa S, Tank DW, Menon R, et al. Intrinsic signal changes accompanying sensory stimulation: functional brain mapping with magnetic resonance imaging. Proc Natl Acad Sci U S A. 1992;89(13):5951–5955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Luck SJ, Ford MA. On the role of selective attention in visual perception. Proc Natl Acad Sci U S A. 1998;95(3):825–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mangun GR. Neural mechanisms of visual selective attention. Psychophysiology. 1995;32(1):4–18. [DOI] [PubMed] [Google Scholar]
- 43. Savage CR, Deckersbach T, Heckers S, et al. Prefrontal regions supporting spontaneous and directed application of verbal learning strategies: evidence from PET. Brain: a journal of neurology. 2001;124(Pt 1):219–231. [DOI] [PubMed] [Google Scholar]
- 44. Alvarez P, Squire LR. Memory consolidation and the medial temporal lobe: a simple network model. Proc Natl Acad Sci U S A. 1994;91(15):7041–7045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Squire LR, Stark CE, Clark RE. The medial temporal lobe. Annu Rev Neurosci. 2004;27:279–306. [DOI] [PubMed] [Google Scholar]
- 46. Mulhern RK, Reddick WE, Palmer SL, et al. Neurocognitive deficits in medulloblastoma survivors and white matter loss. Ann Neurol. 1999;46(6):834–841. [DOI] [PubMed] [Google Scholar]
- 47. Palmer SL, Armstrong C, Onar-Thomas A, et al. Processing speed, attention, and working memory after treatment for medulloblastoma: an international, prospective, and longitudinal study. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2013;31(28):3494–3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Noble KG, McCandliss BD, Farah MJ. Socioeconomic gradients predict individual differences in neurocognitive abilities. Dev Sci. 2007;10(4):464–480. [DOI] [PubMed] [Google Scholar]
- 49. Ardila A, Rosselli M, Matute E, et al. The influence of the parents’ educational level on the development of executive functions. Dev Neuropsychol. 2005;28(1):539–560. [DOI] [PubMed] [Google Scholar]
- 50. Lupo JM, Molinaro AM, Essock-Burns E, et al. The effects of anti-angiogenic therapy on the formation of radiation-induced microbleeds in normal brain tissue of patients with glioma. Neuro oncology. 2016;18(1):87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Matuschek C, Bolke E, Nawatny J, et al. Bevacizumab as a treatment option for radiation-induced cerebral necrosis. Strahlentherapie und Onkologie: Organ der Deutschen Rontgengesellschaft ... [et al]. 2011;187(2):135–139. [DOI] [PubMed] [Google Scholar]
- 52. Kamba T, McDonald DM. Mechanisms of adverse effects of anti-VEGF therapy for cancer. Br J Cancer. 2007;96(12):1788–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hapani S, Sher A, Chu D, et al. Increased risk of serious hemorrhage with bevacizumab in cancer patients: a meta-analysis. Oncology. 2010;79(1–2):27–38. [DOI] [PubMed] [Google Scholar]
- 54. Khasraw M, Holodny A, Goldlust SA, et al. Intracranial hemorrhage in patients with cancer treated with bevacizumab: the Memorial Sloan-Kettering experience. Annals of oncology: official journal of the European Society for Medical Oncology / ESMO. 2012;23(2):458–463. [DOI] [PubMed] [Google Scholar]
- 55. Seet RC, Rabinstein AA, Lindell PE, et al. Cerebrovascular events after bevacizumab treatment: an early and severe complication. Neurocrit Care. 2011;15(3):421–427. [DOI] [PubMed] [Google Scholar]
- 56. Butler RW, Copeland DR, Fairclough DL, et al. A multicenter, randomized clinical trial of a cognitive remediation program for childhood survivors of a pediatric malignancy. J Consult Clin Psychol. 2008;76(3):367–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Conklin HM, Ogg RJ, Ashford JM, et al. Computerized cognitive training for amelioration of cognitive late effects among childhood cancer survivors: a randomized controlled trial. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2015;33(33):3894–3902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Conklin HM, Reddick WE, Ashford J, et al. Long-term efficacy of methylphenidate in enhancing attention regulation, social skills, and academic abilities of childhood cancer survivors. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2010;28(29):4465–4472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lupo JM, Chuang CF, Chang SM, et al. 7-Tesla susceptibility-weighted imaging to assess the effects of radiotherapy on normal-appearing brain in patients with glioma. Int J Radiat Oncol, Biol, Phys. 2012;82(3):e493–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.