Knowledge of the duration of colonization with KPC is essential for infection control measures. We found that only 17% of LTACH patients lost colonization within four weeks. Half of the KPC-positive patients were still carriers when readmitted after nine months.
Keywords: carbapenemase, duration of colonization, KPC, LTACH
Abstract
Background. High prevalence of Klebsiella pneumoniae carbapenemase (KPC)-producing Enterobacteriaceae has been reported in long-term acute care hospitals (LTACHs), in part because of frequent readmissions of colonized patients. Knowledge of the duration of colonization with KPC is essential to identify patients at risk of KPC colonization upon readmission and to make predictions on the effects of transmission control measures.
Methods. We analyzed data on surveillance isolates that were collected at 4 LTACHs in the Chicago region during a period of bundled interventions, to simultaneously estimate the duration of colonization during an LTACH admission and between LTACH (re)admissions. A maximum-likelihood method was used, taking interval-censoring into account.
Results. Eighty-three percent of patients remained colonized for at least 4 weeks, which was the median duration of LTACH stay. Between LTACH admissions, the median duration of colonization was 270 days (95% confidence interval, 91–∞).
Conclusions. Only 17% of LTACH patients lost colonization with KPC within 4 weeks. Approximately half of the KPC-positive patients were still carriers when readmitted after 9 months. Infection control practices should take prolonged carriage into account to limit transmission of KPCs in LTACHs.
The emergence of carbapenem-resistant Enterobacteriaceae (CRE) significantly reduces antibiotic treatment options, because carbapenems are the first choice and sometimes last resort antibiotics for infections caused by extended-spectrum β-lactamase-producing Enterobacteriaceae [1]. Four types of carbapenemases exist: Ambler class A, B, C, and D, of which Klebsiella pneumoniae carbapenemase (KPC) enzymes are the most frequent class A carbapenemases. Klebsiella pneumoniae carbapenemase-producing Enterobacteriaceae were first discovered in the United States in an isolate collected in 1996 and have since then spread across the country and the world. Klebsiella pneumoniae carbapenemases are now endemic in several states in the United States [2].
Prevalence of KPC is especially high in long-term acute care hospitals (LTACHs), where a convergence of risk factors in the patient population results in frequent colonization and infection with multidrug-resistant organisms. Vulnerable patients with multiple comorbidities, high rates of medical device use, intensive antibiotic exposure, and long hospital stays require frequent hands-on care, allowing bacteria to spread easily [3, 4]. Patients in LTACHs have high readmission rates, creating a feedback loop of reintroducing pathogens if carriage persists long enough [3, 5–7]. For instance, in a survey of 7 LTACHs and 24 intensive care units (ICUs) in Chicago, Illinois in 2011, carriage with KPC was detected in each LTACH, with an overall prevalence of 30.4% among LTACH patients compared with 3.3% of short-stay hospital ICU patients [8].
The persistence of KPC carriage has been analyzed in 4 studies, and in each a declining trend in the fraction of positive isolates over time from first KPC detection was reported [9–12]. Only 1 study [12] calculated a median duration of colonization, which was found to be 295 days. However, no study has focused on LTACHs. Knowledge of the duration of colonization with KPC is essential to identify patients at risk of KPC carriage when readmitted and to control the spread of KPC in LTACHs. Therefore, we determined the duration of colonization with KPC during admission and between discharge and readmission in a cohort of patients from 4 LTACHs in Chicago, Illinois.
METHODS
Data Collection
Data from the study of Hayden et al [13] were used for the current study. Data were collected from November 2011 until June 2013 in 4 LTACHs in the Chicago, Illinois region. These 4 LTACHs had a total mean daily census of 247 and a median length of stay of 24 days. During the study period, a bundled intervention was implemented [13]. This infection control bundle involved daily bathing of all LTACH patients with 2% chlorhexidine gluconate (CHG) cloths (Sage Products, Inc.), education of the medical staff on KPC and infection prevention, adherence monitoring (focusing on hand hygiene), patient and staff cohorting, and surveillance screening. Surveillance screening for KPC comprised rectal swab culture screening on admission and every other week for all LTACH patients. The LTACH staff was instructed to discontinue screening patients once they were found to be positive; however, this policy was not always adhered to by staff. These data are informative for the duration of colonization. More information on study design, patient characteristics, and results of pre- and postintervention periods can be found in the paper written by Hayden et al [13].
The project was considered to be a quality improvement program by the LTACHs and not research. The study was reviewed and approved by the institutional review board of Rush University Medical Center, and informed consent was waived.
Microbiology
Rectal swab specimens were screened for KPC in a central laboratory using an ertapenem disk screen method. Screen-positive samples were tested by a polymerase chain reaction assay to confirm the presence of bla KPC [13, 14].
Data Analysis
We used results of testing surveillance screening samples to simultaneously estimate the duration of KPC colonization during an LTACH admission and between LTACH (re)admissions. All patients with a KPC-positive culture and at least 1 follow-up culture were included. For the duration of colonization during LTACH stay, the date of the first positive culture was taken as the start of colonization. Readmissions were allowed to be in the same or a different study-LTACH. Patients who were discharged for at least 1 day were regarded as readmissions when they returned to the same or to a different LTACH. All cultures of all KPC-positive patients were included, until the first culture of the second admission in the study period. The latter should have been taken within 3 days of readmission. To estimate the duration of colonization between LTACH (re)admissions, the date of discharge was taken as the starting point.
Because the length of the time periods (length of stay in an LTACH and time between admissions) was variable and the exact time of possible clearance of KPC in these intervals was unknown, a maximum likelihood analysis was used to estimate the survival curve and to take interval-censoring into account [15, 16]. With this method, the most likely day of clearance within the interval was calculated for patients who had lost KPC colonization.
Negative cultures in between positive cultures were regarded as false-negative and influenced the estimate of the sensitivity. Negative cultures after the last positive culture and negative cultures upon readmission were either true negative or false negative, depending on the estimate for the sensitivity. In these cases, sensitivity was interpreted as the probability of identifying a KPC-positive isolate, when assuming that patients were colonized at least until their last positive culture. A 100% specificity was assumed. A more precise, non-parametric approach was used as well as an assumption of an exponential distribution for the survival curve, which is often used in mathematical models. The likelihood ratio test was used to calculate 95% confidence intervals (CIs). Analyses were done in Mathematica 9.0 (Mathematica, Version 9.0; Wolfram Research, Inc., Champaign, IL).
RESULTS
Duration of Colonization During Long-Term Acute Care Hospital Stay
In total, 542 surveillance cultures were available from 247 patients, with a median number of 2 (interquartile range [IQR], 2–2) cultures per patient. Of these, 379 cultures were taken during a patient's first LTACH stay in the study period. The median time from admission to the first positive culture was 1 day (IQR, 1–17). Fifty-seven patients (23.1%) were from LTACH A, 54 patients (21.9%) were from LTACH B, 102 patients (41.3%) were from LTACH C, and 34 patients (13.8%) were from LTACH D. This distribution is similar to the study by Hayden et al [13]. Intermitted carriage was found in 25 patients (10.1%). This corresponds with the overall sensitivity of the screening test for KPC, which was estimated to be 89.3% (95% CI, 83.4–94.4).
All patients contributed to the estimate of the duration of colonization during LTACH stay. One hundred ten patients had at least 1 culture after the first positive culture during the first LTACH stay; of this group, 26 patients were readmitted during the study period. There were 137 positive patients who did not have a subsequent culture during their first LTACH stay but were readmitted. These 137 patients also contribute to the estimate on the duration of colonization in an LTACH, eg, patients who had a positive culture result at readmission did not lose their colonization during the first admission episode.
Eighty-three percent of the patients were still KPC-positive after 4 weeks (Figure 1), the median duration of LTACH stay [13]. The calculated median duration of colonization with KPC during an LTACH stay was 165 days (95% CI, 70–∞). When assuming an exponential curve for the colonization duration, a median duration of colonization of 205 days (95% CI, 101–1150) was found. The number of patients admitted longer than 1 month was low, hence the CIs are wide.
Figure 1.
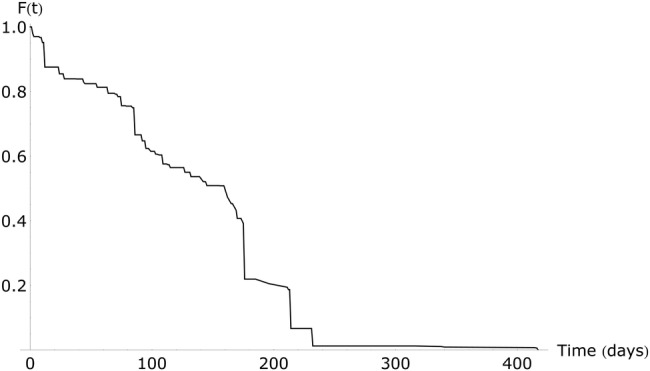
Duration of colonization with Klebsiella pneumoniae carbapenemase-producing bacteria during long-term acute care hospital admission, non-parametric approach. Abbreviation: F(t), fraction still colonized.
Duration of Colonization Between Discharge and Readmission
In all, 163 of the 251 patients (64.9%) were readmitted during the study period and had a culture taken within 3 days. The median time between discharge and readmission was 28 days (IQR, 14–72.5). More than half of these patients were still colonized at readmission. Using these data, the model estimated the median duration of colonization with KPC between LTACH admissions to be 270 days (95% CI, 91–∞) when using a non-parametric approach (Figure 2) and 367 days (95% CI, 175–2143) when assuming an exponential distribution of the survival curve.
Figure 2.
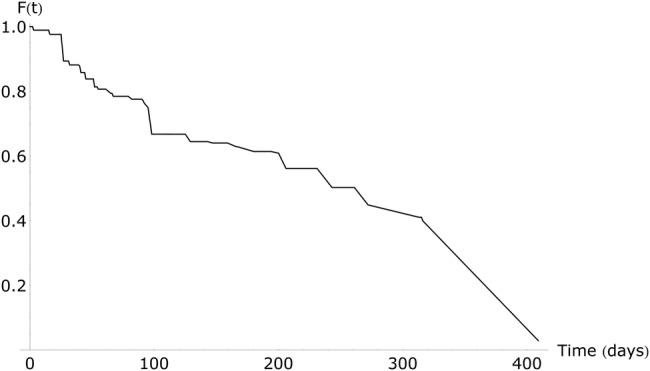
Duration of colonization with Klebsiella pneumoniae carbapenemase-producing bacteria between discharge and readmission, non-parametric approach. Abbreviation: F(t), fraction still colonized.
We also conducted the analyses with all available cultures, ie, including cultures taken during the second and subsequent admissions (data not shown). The graphs changed, indicating that the patient populations were different. The number of patients with multiple admissions during the study period was small (n = 64 having 3 admission with cultures taken, n = 25 having 4 admissions with cultures taken), leading to very wide CIs and precluding meaningful interpretation.
DISCUSSION
During a median 1-month LTACH stay, only 17% of patients appeared to lose colonization with KPC. Based on this result, the commonly made assumption that patients who are found to be KPC carriers remain colonized throughout their hospitalization appears reasonable, and it seems justified to isolate KPC-positive patients for the duration of their hospital stay.
From the second analysis, we conclude that approximately half of KPC-positive LTACH patients who are readmitted after 9 months are still colonized. This has an impact on infection control strategies for KPC carriers. Colonized patients likely require isolation or other infection control precautions for a prolonged period. However, we had no information on where patients resided between admissions. These could be diverse locations with variable antibiotic and other selective pressures, which might influence the estimates we calculated. Furthermore, we had readmission culture information for only a small fraction of the total study population. This is probably because the study protocol specified not to screen previously known KPC-positive patients [13].
It might seem counterintuitive that the median duration of colonization is longer between LTACH admissions than during LTACH admission. First, note that the CIs overlap. Only a limited number of cultures were available for patients staying longer than 1 month, as can be seen from the wide CIs. Furthermore, in between admissions, LTACH patients usually have frequent healthcare contact and can come into contact with high antibiotic pressure, both of which are risk factors for KPC colonization [3]. The LTACH patients who are readmitted to an LTACH may thus comprise a particularly high-risk population for colonization, because they carry KPC-producing bacteria for a long time.
The major strengths of this study, which support the importance and generalizability of our findings, include the growing problem of CRE, the need to improve our understanding of the epidemiologic challenges for control of this problem, the large number of patients assessed, the evaluation of patients at several different healthcare facilities, the culture techniques, and the longitudinal prospectively collected data.
The infection control bundle that was implemented during the entire study period might have influenced our results. In a previous study, it was found that KPC skin colonization was reduced after CHG bathing of patients [17]. If CHG bathing also reduces rectal colonization, our results may represent an underestimation of the duration of colonization. On the other hand, because we did not perform molecular subtyping of KPC isolates, we cannot be sure that a patient carried the same strain of KPC each time that KPC was detected. However, we do know most (90%) of the KPC-positive isolates were K pneumoniae [18]. (Re)Acquisition of a new strain was not taken into account, which might have resulted in an overestimation of the duration of colonization [19]. This will especially be true for the estimate of the duration of colonization between admissions, because no surveillance cultures were taken in that time period. One way to overcome this uncertainty is to compare whole genome sequences of the isolates, to determine the relatedness between subsequent isolates from the same patient. Antibiotic use might also have influenced the results. Unfortunately, no prescription data were available for the included patients.
Both patients colonized on admission as well as patients acquiring colonization during LTACH stay are included in the model. They are treated as equivalent groups; however, this assumption might not be completely correct. Patients colonized on admission might represent a population with a history of more hospital admissions and thus more contact with KPC-producing bacteria. Yet, the influence of this on the duration of colonization is not known.
A longer follow-up period and more frequent screening of all patients, including those known to be positive for KPC, would allow a more firm estimate of the colonization duration with smaller CIs. However, our estimate for the median duration of colonization with KPC between admissions is similar to the estimate by Zimmerman et al [12], despite the different populations. Although no estimate for the overall duration of colonization is given in Feldman et al [10], the graphs presented there overlap with our results. Lübbert et al [9] found a slightly shorter duration of colonization of 6 months, but they acknowledge that long-term carriage is possible. Furthermore, the sensitivity of the screening test was not perfect, indicating that a single negative fecal screening in this study is not sufficient to rule out persistent colonization.
The differences between the median durations of colonization as estimated using a non-parametric approach versus an exponential approach were small and 95% CIs overlapped. This indicates that an exponential distribution, which is easy to use in mathematical models for transmission, might appropriately reflect the duration of colonization with KPC.
CONCLUSIONS
In conclusion, carriage of KPC in LTACH patients can persist for a long time. Infection control practices should take this into account to limit transmission of KPCs in LTACHs.
Acknowledgments
Financial support. This work was supported by the following: Netherlands Organization of Scientific Research (VICI NWO Grant 918.76.611 [to M. J. M. B.] and Priority Medicines Antimicrobial Resistance grant 205100013 [to M. R. H. and M. C. J. B.]); funding from the European Community (RGNOSIS Integrated project [FP7/2007-2013] under grant agreement no. 282512 [to M. C. J. B. and M. J. M. B.]); the US Centers for Disease Control and Prevention (grant numbers U54CK000161 [to R. A. W.] and 200-2011-M-41103 [to M. K. H.]); funding from a Fulbright Scholarship (to M. R. H.); and an unrestricted gift from the Foglia Family Foundation (to R. A. W.).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Nordmann P, Cuzon G, Naas T. The real threat of Klebsiella pneumoniae carbapenemase-producing bacteria. Lancet Infect Dis 2009; 9:228–36. [DOI] [PubMed] [Google Scholar]
- 2. Munoz-Price LS, Poirel L, Bonomo RA et al. Clinical epidemiology of the global expansion of Klebsiella pneumoniae carbapenemases. Lancet Infect Dis 2013; 13:785–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Munoz-Price LS. Long-term acute care hospitals. Clin Infect Dis 2009; 49:438–43. [DOI] [PubMed] [Google Scholar]
- 4. Gould CV, Rothenberg R, Steinberg JP. Antibiotic resistance in long-term acute care hospitals: the perfect storm. Infect Control Hosp Epidemiol 2006; 27:920–5. [DOI] [PubMed] [Google Scholar]
- 5. Cooper BS, Medley GF, Stone SP et al. Methicillin-resistant Staphylococcus aureus in hospitals and the community: stealth dynamics and control catastrophes. Proc Natl Acad Sci U S A 2004; 101:10223–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Prabaker K, Lin MY, McNally M et al. Transfer from high-acuity long-term care facilities is associated with carriage of Klebsiella pneumoniae carbapenemase-producing Enterobacteriaceae: a multihospital study. Infect Control Hosp Epidemiol 2012; 33:1193–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Papadimitriou-Olivgeris M, Marangos M, Fligou F et al. Risk factors for KPC-producing Klebsiella pneumoniae enteric colonization upon ICU admission. J Antimicrob Chemother 2012; 67:2976–81. [DOI] [PubMed] [Google Scholar]
- 8. Lin MY, Lyles-Banks RD, Lolans K et al. The importance of long-term acute care hospitals in the regional epidemiology of Klebsiella pneumoniae carbapenemase-producing Enterobacteriaceae . Clin Infect Dis 2013; 57:1246–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lübbert C, Lippmann N, Busch T et al. Long-term carriage of Klebsiella pneumoniae carbapenemase-2-producing K. pneumoniae after a large single-center outbreak in Germany. Am J Infect Control 2014; 42:376–80. [DOI] [PubMed] [Google Scholar]
- 10. Feldman N, Adler A, Molshatzki N et al. Gastrointestinal colonization by KPC-producing Klebsiella pneumoniae following hospital discharge: duration of carriage and risk factors for persistent carriage. Clin Microbiol Infect 2013; 19:E190–6. [DOI] [PubMed] [Google Scholar]
- 11. Schechner V, Kotlovsky T, Tarabeia J et al. Predictors of rectal carriage of carbapenem-resistant Enterobacteriaceae (CRE) among patients with known CRE carriage at their next hospital encounter. Infect Control Hosp Epidemiol 2011; 32:497–503. [DOI] [PubMed] [Google Scholar]
- 12. Zimmerman FS, Assous MV, Bdolah-Abram T et al. Duration of carriage of carbapenem-resistant Enterobacteriaceae following hospital discharge. Am J Infect Control 2013; 41:190–4. [DOI] [PubMed] [Google Scholar]
- 13. Hayden MK, Lin MY, Lolans K et al. Prevention of colonization and infection by Klebsiella pneumoniae carbapenemase-producing Enterobacteriaceae in long-term acute-care hospitals. Clin Infect Dis 2014; 60:1153–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lolans K, Calvert K, Won S et al. Direct ertapenem disk screening method for identification of KPC-producing Klebsiella pneumoniae and Escherichia coli in surveillance swab specimens. J Clin Microbiol 2010; 48:836–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Goggins WB, Finkelstein DM. A proportional hazards model for interval-censored failure time data. Biometrics 2000; 56:940–3. [DOI] [PubMed] [Google Scholar]
- 16. Haverkate MR, Derde LP, Brun-Buisson C et al. Duration of colonization with antimicrobial-resistant bacteria after ICU discharge. Intensive Care Med 2014; 40:564–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lin MY, Lolans K, Blom DW et al. The effectiveness of routine daily chlorhexidine gluconate bathing in reducing Klebsiella pneumoniae carbapenemase-producing Enterobacteriaceae skin burden among long-term acute care hospital patients. Infect Control Hosp Epidemiol 2014; 35:440–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Haverkate MR, Bootsma MC, Weiner S et al. Modeling spread of KPC-producing bacteria in long-term acute care hospitals in the Chicago Region, USA. Infect Control Hosp Epidemiol 2015; 36:1148–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bart Y, Paul M, Eluk O et al. Risk factors for recurrence of carbapenem-resistant Enterobacteriaceae carriage: case-control study. Infect Control Hosp Epidemiol 2015; 36:936–41. [DOI] [PubMed] [Google Scholar]


