Abstract
The aim of this study was to develop a nanostructured lipid carrier (NLC)-based hydrogel and study its potential for the topical delivery of 5-fluorouracil (5-FU). Precirol® ATO 5 (glyceryl palmitostearate) and Labrasol® were selected as the solid and liquid lipid phases, respectively. Poloxamer 188 and Solutol® HS15 (polyoxyl-15-hydroxystearate) were selected as surfactants. The developed lipid formulations were dispersed in 1% Carbopol® 934 (poly[acrylic acid]) gel medium in order to maintain the topical application consistency. The average size, zeta potential, and polydispersity index for the 5-FU-NLC were found to be 208.32±8.21 nm, −21.82±0.40 mV, and 0.352±0.060, respectively. Transmission electron microscopy study revealed that 5-FU-NLC was <200 nm in size, with a spherical shape. In vitro drug permeation studies showed a release pattern with initial burst followed by sustained release, and the rate of 5-FU permeation was significantly improved for 5-FU-NLC gel (10.27±1.82 μg/cm2/h) as compared with plain 5-FU gel (2.85±1.12 μg/cm2/h). Further, skin retention studies showed a significant retention of 5-FU from the NLC gel (91.256±4.56 μg/cm2) as compared with that from the 5-FU plain gel (12.23±3.86 μg/cm2) in the rat skin. Skin irritation was also significantly reduced with 5-FU-NLC gel as compared with 5-FU plain gel. These results show that the prepared 5-FU-loaded NLC has high potential to improve the penetration of 5-FU through the stratum corneum, with enormous retention and with minimal skin irritation, which is the prerequisite for topically applied formulations.
Keywords: nanostructured lipid carrier, topical delivery, controlled release, 5-fluorouracil, skin penetration, skin infection
Introduction
5-Fluorouracil (5-FU) is an antineoplastic agent used in the topical treatment of a number of diseases, including skin cancers and actinic keratosis.1 However, in each treatment, less-than-optimal delivery of 5-FU using conventional preparations such as creams and ointments have necessitated the use of more drastic measures to obtain therapeutic outcomes.2 Pathogenesis of most skin diseases, such as psoriasis, cutaneous premalignant/malignant lesions and basal cell carcinoma in the epidermis, dermis, and deeper skin layers, dictates that the drug delivery strategy should be customized to localize high drug concentrations within the epidermis and dermis.3 Currently, commercial formulations for topical 5-FU application are available in the form of solutions or creams at 0.5% 5-FU concentration for once-daily application (Carac®; Sanofi, Gentilly, France). Commercial topical formulations are associated with the limitation of very short retention time at the site of administration, resulting in very poor skin permeation and retention of drug.4 The commercial topical dosage form is also associated with a number of skin irritation reactions, including redness, dryness, burning pain of the upper layer of skin, and swelling, and these reactions may continue for ≥2 weeks after treatment.5 However, 5-FU is a highly soluble but poorly permeable drug, with low logP (0.89), and therefore, deep skin penetration is very difficult to achieve with conventional formulations.6
There has been increased interest during recent years in the use of topical vehicle systems that could modify drug permeation through the skin using permeation enhancers, but use of these chemical enhancers may be harmful, especially in chronic application, as many of them are irritants.4,7 Therefore, it is desirable to develop a topical vehicle system without chemical enhancers to facilitate drug permeation through the skin. One of the most promising techniques for enhancement of skin permeation of drugs is lipid-based nanocarrier delivery.
In recent years, solid lipid nanoparticles (SLNs) have garnered great importance as potential drug carriers for topical delivery due to their unique advantages and great versatility as compared to polymeric nanoparticles, nanoemulsions, liposomes, and so on.8–10 The SLNs are made of solid lipid material, which remains in the solid state at room temperature, protects the chemically labile drugs, and provides slow drug release to achieve controlled drug release profiles for prolonged time intervals. The lipid materials used for SLNs are biodegradable and biocompatible and provide better safety profile.10,11 Nanostructured lipid carriers (NLCs) are the second generation of SLNs and offer many advantages over SLNs. The solid lipid, when used alone for preparing SLNs, forms a perfect crystal lattice with limited space for accommodating the drugs. The NLCs are prepared by using both solid lipid and liquid lipid, which leads to special nanostructures with improved properties for therapeutic loading, alteration of the drug release profile, and stability.12,13
Furthermore, the NLCs ensure close contact with the stratum corneum owing to their unique lipid composition and smaller particle size, thereby enhancing drug flux through the skin to facilitate drug permeation.14 The nanosized particles can tightly adhere to the skin surface and transport the drugs in a more controlled fashion. They are also found to significantly increase skin hydration and exhibit occlusive properties due to reduction in the transepidermal water loss.14,15 The occlusive effect exerted by NLCs can improve skin hydration and promote the penetration of drugs.10 Additionally, drugs (such as 5-FU) may be encapsulated within the core so that the skin irritation reaction could be minimized following topical application.
Several research groups have worked on NLCs to improve the skin permeation efficacy of many drugs after topical application.16–18 For example, Guo et al16 developed a quercetin (QT)-loaded NLC formulation for topical delivery. The results showed that NLCs could significantly promote the permeation of QT and increase the amount of QT retained in the epidermis and dermis, showing the usefulness of NLCs as carriers for topical administration. More recently, Upendra and Neha19 prepared an NLC-based gel of clobetasol propionate for topical delivery for the treatment of eczema. Using the paw edema technique, they found that the anti-inflammatory activity of the NLC gel had a rapid onset of action, with prolonged duration of action, as compared with the marketed gel. In another study, Bharti et al17 prepared an NLC system for the topical delivery of terbinafine hydrochloride for the treatment of fungal infection, and they found that the NLC system could significantly promote the permeation of the drug and that the NLC gel efficiently reduced the fungal burden in a shorter duration of time as compared to marketed drug. Kumari et al18 developed NLCs for the topical delivery of azelic acid for the treatment of acne. The results showed that the developed NLCs have enormous potential to improve the penetration of azelaic acid through the stratum corneum, with utmost retention in the skin. However, the preparation of a 5-FU-loaded NLC formulation for topical application has not been extensively explored. Several colloidal formulations containing 5-FU (niosomes, ultradeformable liposomes, and so on) for skin application have been developed and their in vivo potential has been explored.20–22
Therefore, the aim of this study was to design and characterize an 5-FU-loaded NLC (5-FU-NLC) as potential carrier for the topical delivery of 5-FU. The optimized 5-FU-NLC batches were dispersed in 1% Carbopol® 934 (poly[acrylic acid]) gel to achieve increased contact time and get the semisolid consistency suitable for topical application.
The optimized 5-FU-NLC hydrogel batches were evaluated for their particle size distribution profiles, in vitro drug permeation through excised mouse skin, and in vivo drug retention in epidermis and dermis of mice. In addition, the skin irritation index of the 5-FU-NLC gel was also investigated to ensure the potential use of NLCs for the topical delivery of 5-FU.
Materials and methods
Materials
5-FU was purchased from MP Biomedicals, LLC, Santa Ana, CA, USA. Precirol® ATO 5 (glyceryl palmitostearate [PRE]) and Labrasol® (caprylocaproyl polyoxylglycerides [LBS]) were purchased from Gattefossé, Lyon, France. Tween 80 and Poloxamer 188 were purchased from EMD Millipore, Billerica, MA, USA, and Solutol® HS15 (Polyoxyl-15-hydroxystearate) was purchased from BASF, Ludwigshafen, Germany. High-performance liquid chromatography (HPLC)-grade acetonitrile was purchased from Thermo Fisher Scientific, Waltham, MA, USA. All other chemicals used were of analytical grade.
Methods
Preparation of 5-FU-NLC
The 5-FU-NLC was prepared by the hot homogenization method using a high-pressure homogenizer.23 Briefly, the lipid phase was prepared by mixing the required amounts of PRE and LBS, and the resultant mixture was heated up to 70°C to melt the solid lipid completely. The aqueous phase was prepared by adding the desired amounts of Poloxamer 188 and Polyoxyl-15-hydroxystearate into 50 mL deionized water, and the resultant mixture was heated up to 70°C with continuous stirring to dissolve the surfactant completely. The accurate amount of drug was dissolved in the aqueous phase. The melted lipid phase was slowly added into the aqueous phase under high homogenization at the speed of 8,000 rpm (ULTRA-TURRAX Basic T25; IKA, Wilmington, NC, USA) for 2 minutes to form a primary emulsion. The resultant emulsion was passed through a high-pressure homogenizer (EmulsiFlex-C3; Avestin, Ottawa, ON, Canada) at 1,500 bars, the resultant mixture was subjected to 5–6 repeat cycles, and the final product was cooled to room temperature to form 5-FU-NLC. The prepared NLC mixture was placed in an ultrafiltration tube (Amicon Ultra-4 filtration unit; EMD Millipore, Billerica, MA, USA) with a molecular weight cutoff of 30,000 Da, and centrifuged for 10 minutes at 3,000× g (Centrifuge 5702; Eppendorf AG, Hamburg, Germany). Lipid nanoparticles free of unloaded drug were obtained. 5-FU-NLCs of different compositions were prepared using the same method, as tabulated in Table 1.
Table 1.
Formulation composition for the preparation of 5-FU-loaded NLC
| Formulation | Drug (%, w/w) | Lipid (%, w/w)
|
Surfactants (%, w/w)
|
Water (%, w/w) | ||
|---|---|---|---|---|---|---|
| PRE | LBS | P188 | HS15 | |||
| 5-FU-NLC1 | 0.50 | 2.0 | 1.0 | 1.0 | 1.0 | 94.50 |
| 5-FU-NLC2 | 0.50 | 2.0 | 1.0 | 0 | 2.0 | 94.50 |
| 5-FU-NLC3 | 0.50 | 2.0 | 1.0 | 2.0 | 0 | 94.50 |
| 5-FU-NLC4 | 0.50 | 2.0 | 1.0 | 2.0 | 2.0 | 92.50 |
| 5-FU-NLC5 | 0.50 | 2.0 | 1.0 | 0 | 4.0 | 92.50 |
| 5-FU-NLC6 | 0.50 | 2.0 | 1.0 | 4.0 | 0 | 92.50 |
| 5-FU-NLC7 | 0.50 | 2.5 | 1.0 | 1.0 | 1.0 | 94.0 |
| 5-FU-NLC8 | 0.50 | 2.5 | 1.0 | 0 | 2.0 | 94.0 |
| 5-FU-NLC9 | 0.50 | 2.5 | 1.0 | 2.0 | 0 | 94.0 |
| 5-FU-NLC10 | 0.50 | 3.0 | 1.5 | 1.0 | 1.0 | 93.0 |
| 5-FU-NLC11 | 0.50 | 3.0 | 1.5 | 0 | 2.0 | 93.0 |
| 5-FU-NLC12 | 0.50 | 3.0 | 1.5 | 2.0 | 0 | 93.0 |
| Placebo-NLC | 0 | 2.0 | 1.0 | 1.0 | 1.0 | 95.0 |
Abbreviations: 5-FU, 5-fluorouracil; HS15, Solutol® HS15 (polyoxyl-15-hydroxy stearate); LBS, Labrasol® (caprylocaproyl polyoxylglycerides); NLC, nano structured lipid carrier; P188, Poloxamer 188; PRE, Precirol® ATO 5 (glyceryl palmitostearate).
Determination of entrapment efficiency (EE)
The EE of optimized 5-FU-NLC was determined using the ultrafiltration method.8 The amount of drug entrapped was measured based on the free drug concentration in the NLC. Briefly, the sample was placed in an Amicon Ultra-4 filtration unit with molecular weight cutoff of 30,000 Da (EMD Millipore) and centrifuged for 10 minutes at 3,000× g (Centrifuge 5702; Eppendorf AG). The filtrate was analyzed using HPLC (HPLC 1200 series; Agilent Technologies, Santa Clara, CA, USA) with a Phenomenex C18 analytical column (5 μm, 4.6×150 mm), with the ultraviolet detector set at 260 nm.24 The EE was calculated based on the following equation:
Particle size and zeta potential analysis
The particle size, zeta potential, and polydispersity index (PDI) of the optimized NLC formulations were determined using the Zetasizer Nano ZS (Malvern Instruments, Malvern, UK). Prior to the measurement, the instrument was calibrated using latex standard to ensure measurement accuracy.
Transmission electron microscopy (TEM) studies
The shape and surface appearance of the developed NLC formulations were determined using a TEM instrument (Hitachi 7100S; Hitachi, Tokyo, Japan). A drop of the diluted formulations was placed on a carbon-coated copper grid, stained with aqueous solution of 2% uranyl acetate, and examined using TEM.
Preparation of 5-FU-NLC-loaded hydrogels
The hydrogels were prepared using different concentrations of poly(acrylic acid) (0.5%, 1.0%, 1.5%, and 2.0% w/v). The desired amount of poly(acrylic acid) was dispersed in purified water and 5% v/v glycerol was used as the hydrating agent in the aqueous dispersion of poly(acrylic acid). The mixture was stirred for 10 minutes at 1,000 rpm and the aqueous dispersion was neutralized with triethanolamine until topical application consistency was obtained; the hydrogels were further allowed to equilibrate for 24 hours at room temperature. The freshly prepared 5-FU-NLC gels were incorporated in poly(acrylic acid) hydrogels using a Remi stirrer (Remi Lab World, Mumbai, Maharashtra, India) at ~1,000 rpm for 2 minutes to obtain the gels containing a final concentration of 0.5% 5-FU-NLC.
Measurement of viscosity of 5-FU-NLC gel
Viscosity determinations of the prepared 5-FU-NLC gel were carried out on a cone (0.8°) and plate geometry viscometer (Brookfield-AMETEK, Middleboro, MA, USA) using a CP40 spindle.25 The viscosity of the in situ gelling solutions was measured at different angular velocities at a temperature of 37°C±1°C. A typical run comprised changing of the angular velocity (shear rate in rotations per minute) from 0.5 to 100 rpm at a controlled ramp speed. After 6 seconds at 0.5 rpm, the velocity was increased to 100 rpm with a similar wait at each speed. The hierarchy of angular velocity was reversed (100–0.5 rpm) with a similar wait of 6 seconds. Rheograms were plotted using viscosity versus shear rate to study the behavior of gels.
Determination of drug content and pH of 5-FU-NLC gel
To determine the drug content, 5-FU-NLC gel equivalent to 10 mg of 5-FU was measured and dissolved in 20 mL of methanol in a 50 mL volumetric flask. The solution was filtered through a 0.45 μm-sized membrane and the 5-FU content in the filtrate was analyzed by HPLC. The pH values of the optimized 5-FU-NLC hydrogel and placebo gel were measured using a digital pH meter (pH Tutor Bench Meter; Thermo Fisher Scientific). Briefly, 0.5 g of 5-FU-NLC gel formulation was uniformly dispersed in distilled water, and the pH of the dispersion was measured using the pH meter. All the experiments were performed in triplicate.
Determination of spreadability of 5-FU-NLC gel
The spreadability test was determined using the parallel plate method as reported earlier.11 Briefly, 0.1 g of gel was placed within a circle of 1 cm diameter premarked on a glass plate, over which a second glass plate was placed. A weight of 200 g was allowed to rest on the upper glass plate for 5 minutes. The increase in the diameter due to gel spreading was noted.
In vitro skin permeation studies
Swiss albino mice (8–9 weeks old) weighing 25–30 g were used for this study. All the experimental animals were housed in cages and allowed access to food and water ad libitum. Mice skins were obtained by peeling the skin from the underlying cartilage tissue of the sacrificed animal.26 The skin was washed with normal saline and then dried between two filter papers. The skin was then checked carefully using a magnifying glass to ensure whether any surface irregularities such as tiny holes or cervices are present in the portion. Then, a 2.4 cm2 area of the excised skin samples was clamped between the receptor and the donor chamber of a Franz diffusion cell with the stratum corneum facing the donor chamber. Then, 0.5 mL of the 5-FU-NLC gel (5 mg/mL) or 5-FU plain gel (5 mg/mL) was placed into the donor chamber. The receptor chamber was filled with 15 mL of phosphate-buffered saline (PBS), pH 7.4. The receptor medium was maintained at 37°C±0.5°C and stirred at 500 rpm throughout the experiment. Subsequently, 0.5 mL of the sample was collected from the receptor medium of each cell every hour for 12 hours and then immediately replaced by the same amount of preheated PBS. The collected samples were filtered through a 0.45 μm pore size cellulose membrane filter and analyzed using HPLC as described earlier.27 This study and the protocol of the animal study were approved by the Research Ethics Committee at the International Medical University, Malaysia. All animals were treated in accordance with the Association for Assessment and Accreditation of Laboratory Animal Care International’s guidelines for animal care and use/ethics.
Permeation data analysis
The cumulative amount of drug permeating through the skin (in micrograms per centimeter squared) was plotted against time (in hours) for each formulation. The drug flux (permeation rate) at steady state (Jss), log time (LT), and permeability coefficient (KP) were calculated from the slope of the linear portion of the graph using the following equation:28
where Jss is the drug flux at steady state and C0 is the initial drug concentration in the donor cell. The significance of the results was checked statistically at P<0.05 applying a one-way analysis of variance test. Post hoc multiple comparisons were carried out using the least squares difference test.
In vivo skin retention studies
Male Swiss albino mice (8–9 weeks old) weighing 25–30 g were used for this study. The animals were divided into 2 groups, each group consisting of 5 animals; the animals were allowed 1 week to get accustomed to laboratory conditions. Twenty-four hours prior to the experiment, the hairs on the dorsal surface of the skin of the mice were removed using a razor and the skin was washed with physiological saline solution. 5-FU-NLC gel (0.5 mL of 5 mg/mL solution) or 5-FU plain gel (5 mg/mL) was applied with gentle rubbing on the dorsal surface of the skin. The animals were sacrificed at 2, 4, 8, and 12 hours after dorsal administration, and the skin was carefully stripped. The excised skin samples were thoroughly washed with physiological saline solution and cleaned with methanol. Subsequently, the excised skin was placed in a water bath at 60°C for 1 minute to separate the epidermis and dermis layers. The epidermis and dermis layers were homogenized separately for 5 minutes using a tissue homogenizer.16 The 5-FU content in the skin homogenate was analyzed by HPLC.
Skin irritation studies
Wistar albino rats (16 weeks) weighing 200–250 g were used in this part of the study.24 The animals were maintained under standard laboratory conditions, with standard temperature of 25°C±1°C and relative humidity of 60%. They had free access to a standard laboratory diet, with water given ad libitum. Prior to the application of the formulations onto the animals, the hairs on the dorsal surface of the rats were removed by clipping. Five rats were assigned to each treatment group: Group 1 received placebo formulation (negative control), Group 2 received sodium lauryl sulfate (SLS) solution (5% w/v, positive control), Group 3 received the prepared 5-FU-NLC gel formulation, and Group 4 received 5-FU plain gel. A small amount (0.5 g) of each sample was applied onto the dorsal surface of the rats daily for 6 days using the reported method.16 The rats were observed and erythema scores ranging from 0 to 4 were allocated depending on the degree of erythema, as follows: 0: no erythema; 1: slight erythema (barely perceptible light pink); 2: moderate erythema (dark pink); 3: moderate-to-severe erythema (light red); and 4: severe erythema (extreme redness).
Statistical analysis
The differences in drug permeation levels between 5-FU plain gel and 5-FU-NLC gels in both in vitro and in vivo experiments were statistically analyzed by one-way analysis of variance, with posttest Dunnett’s post hoc multiple comparisons test. Statistically significant differences between groups were defined as P<0.05.
Results and discussion
Preparation of 5-FU-NLC
The 5-FU-NLC formulations were prepared by the hot homogenization method with different concentrations of lipids, surfactants, cosurfactants, and oil. The hot homogenization method involves the mixing of the hot oil and the water phase together to form a preemulsion using high-speed stirring, followed by passage through a high-pressure homogenizer at pressure of 1,500 bars to break down the particle into nanosized ones.29,30 The resultant sample was passed through for 5 cycles in order to obtain particle size <200 nm with uniform size distribution. It was observed that there was no significant reduction in the particle size of NLCs when the number of cycles was increased further. Surfactants have an important role in the stabilization of emulsions during preparation of 5-FU-NLCs. Poloxamer 188 is a hydrophilic surfactant with hydrophilic–lipophilic balance (HLB) value between 24 and 29 and was selected as one of the surfactants for the preparation of NLC. The HLB value of polyoxyl-15-hydroxystearate is between 14 and 16, which was selected as the second surfactant for the formulation.31 PRE and LBS were used as the solid and liquid lipids, respectively. The HLB values of PRE and LBS are 4.2 and 14, respectively, and these are commonly used in formulations for their surfactant properties.30 The combination of solid and liquid lipids showing the most promising results (ie, smaller particle size and higher loading of liquid lipid) was around 65:35 of PRE/LBS. The maximum lipid concentrations of both solid and liquid lipids that allow a stable nanodispersion was selected and used for further analysis. The composition of the optimized 5-FU-NLC formulations is shown in Table 1.
Particle size and PDI
Determining the particle size is of great importance in nanostructured formulations as the small particle size contributes to a greater interfacial area, which can then provide better drug partitioning and absorption at the skin surface. However, there is no consensus in the literature on the exact size range of nanoformulations.32 The average particle size and zeta potential of the optimized 5-FU-NLC formulation were determined by photon correlation spectroscopy analysis using Zetasizer. The average particle size and zeta potential of the 5-FU-NLC were found to be 209.21±5.85 and −21.32±1.2 mV, respectively (Table 2). When the concentration of surfactant (HS15 and P188) was increased from 2% to 4%, there was a reduction in the particle size. A similar type of result was obtained by Liu et al32 during the preparation of isotretinoin-loaded SLNs, and the results suggested that when the surfactant concentration (TW80) increased, the particle size decreased accordingly.33 This could be attributed to the higher concentration of surfactant that covers the surface of the lipid phase, resulting in a reduction of the particle size.34
Table 2.
Physical characteristics and particle size distribution of prepared 5-FU-NLC formulation batches (n=3)
| Formulation | Average particle size (nm) | PDI | ZP (mV) | EE (%) |
|---|---|---|---|---|
| 5-FU-NLC1 | 231.21±5.85 | 0.188±0.042 | −21.32±1.2 | 73.91±2.82 |
| 5-FU-NLC2 | 209.34±6.83 | 0.363±0.055 | −22.51±1.1 | 71.45±2.45 |
| 5-FU-NLC3 | 213.45±6.32 | 0.331±0.052 | −18.34±0.6 | 73.52±3.12 |
| 5-FU-NLC4 | 211.23±4.92 | 0.412±0.065 | −21.67±0.7 | 82.42±3.14 |
| 5-FU-NLC5 | 305.82±5.56 | 0.512±0.045 | −22.82±1.0 | 84.91±2.12 |
| 5-FU-NLC6 | 212.45±5.31 | 0.510±0.025 | −19.34±0.7 | 86.62±1.94 |
| 5-FU-NLC7 | 221.23±8.52 | 0.378±0.064 | −21.22±0.9 | 79.32±1.87 |
| 5-FU-NLC8 | 263.23±6.42 | 0.412±0.043 | −24.91±0.7 | 83.13±2.21 |
| 5-FU-NLC9 | 309.21±7.12 | 0.347±0.026 | −18.92±0.6 | 81.25±1.43 |
| 5-FU-NLC10 | 310.59±5.29 | 0.412±0.048 | −19.38±0.5 | 85.82±2.92 |
| 5-FU-NLC11 | 216.94±4.68 | 0.332±0.023 | −19.45±1.2 | 85.62±1.23 |
| 5-FU-NLC12 | 210.73±6.42 | 0.345±0.015 | −17.34±0.6 | 83.51±1.31 |
| Placebo-NLC | 202.45±3.84 | 0.368±0.123 | −17.25±1.3 | 0 |
Note: Data presented as mean ± standard deviation.
Abbreviations: 5-FU, 5-fluorouracil; NLC, nanostructured lipid carrier; PDI, polydispersity index; ZP, zeta potential; EE, entrapment efficiency.
PDI measures the size distribution of the nanoparticles in a sample. It has been reported that monodispersed samples with uniform particle size distribution have a PDI range from 0.1 to 0.7, whereas samples with broad size distribution have a PDI value >0.7.35 In our study, the PDI value of all the batches of NLC formulation was found to be between 0.188 and 0.512, suggesting that they were all monodispersed with uniform particle size distribution. The zeta potential represents the stability of the nanodispersion. It has been reported that nanodispersion with a minimum zeta potential of <−60 mV has excellent stability, and that with a minimum zeta potential of <−30 mV has physical stability.35 All the NLC formulations developed in this study were shown to have zeta potential values between −17.34 mV and −24.91 mV, as shown in Table 2. The small particle size, as well as uniform size distribution with optimum zeta potential, of the developed 5-FU-NLCs is suitable for the development of nanomedicines. It was observed that as the concentration of total lipids increases, the particle size of 5-FU-NLC decreases, as shown in Table 2. When the concentration of PRE was increased from 2% to 3%, the particle size of NLC was decreased.
Estimation of EE
EE studies were carried out to determine the amount of 5-FU entrapped in the NLC by breaking them down by heating and then extracting the drug. Several factors, including types of lipids and the surfactant concentration, affect the optimal EE of NLCs.30 The EE of the optimized 5-FU-NLC formulations ranged from 71.45%±2.45% to 86.62%±1.94%, and the results are shown in Table 2. It was noticed that an increase in the concentration of total lipids resulted in increased EE. The higher lipid content could prevent the escape of drug to the outer milieu by effectively enclosing the surfactant, which could be the possible reason behind the increase in the EE of NLC. There was also increase in the EE of NLC with increased concentration of surfactant, as shown in Table 2. This could be due to the presence of a sufficient amount of surfactant, which helps to solubilize the drug and stabilize the drug molecule entrapped within the lipid matrix and at the surface of the NLC. Similar findings were reported in our previous study,23 and Liu et al32 also reported similar results, observing that increasing the soybean lecithin concentration resulted in an increase in the isotretinoin EE of NLCs. However, in this study, it was observed that the influence of surfactant concentration on the EE was not significant.
TEM studies
The TEM image revealed that the 5-FU-NLC particles were spherical in shape, with a size range between 150 and 200 nm, as shown in Figure 1. The measurement using Zetasizer (photon correlation spectroscopy) does not measure the actual size but only estimates the size of the nanoparticles based on the scattered light and intensity.36 Therefore, TEM analysis is useful to confirm the results from photon correlation spectroscopy and also to investigate the morphology of 5-FU-NLC. The TEM image also reveals that the developed 5-FU-NLC had a smooth surface and exhibited no surface drug crystals (Figure 1).
Figure 1.
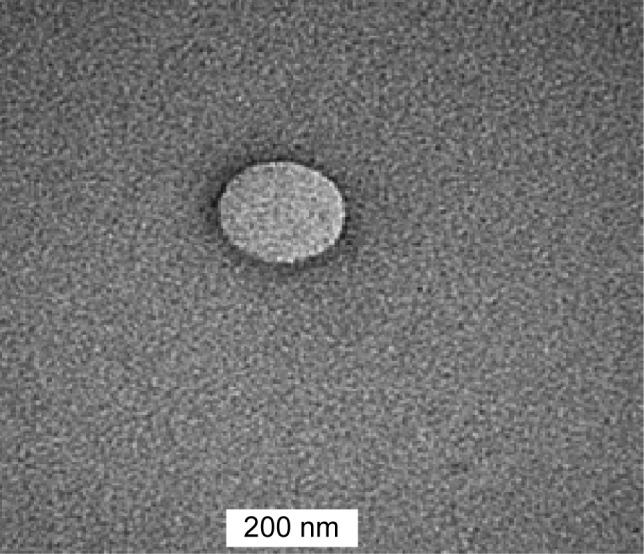
Transmission electron micrograph of 5-FU-loaded nanostructured lipid carrier.
Abbreviation: 5-FU, 5-fluorouracil.
Physicochemical characterization of 5-FU-NLC-based hydrogels
The physicochemical properties, such as pH, drug content, and spreadability, of different concentrations of 5-FU-NLC-loaded poly(acrylic acid) (1%, 1.5%, and 2%) were studied and the results are presented in Table 3. Based on the results, 1% poly(acrylic acid) hydrogel was selected as suitable for the preparation of 5-FU-NLC formulation and for further studies. The pH of the 5-FU-NLC-based 1% poly(acrylic acid) gel was found to be 5.81±0.15 (Table 3), which was within the acceptable limits for topical applications. The drug content of 5-FU-NLC formulation was found to be 99.29%±2.21%. The spreadability plays an important role in patient compliance and helps in uniform application of gel to the skin.37 The spreadability values of various concentrations of poly(acrylic acid) (1%, 1.5%, and 2%) gel are shown in Table 4. In fact, it would be more comfortable if the NLC hydrogel can be spread easily on application over inflamed or diseased skin.37 The therapeutic efficacy of a topical formulation can also depend upon its spreadability value. As shown in Table 3, the spreadability value of 1% poly(acrylic acid) gel loaded with 5-FU-NLC (6.52±0.31 cm) was comparatively low with respect to the 1.5% and 2% formulations of poly(acrylic acid) gel and it would be considered suitable for topical application.
Table 3.
Physiochemical characteristics of optimized 5-FU-NLC gel (mean ± SD, n=3)
| Serial number | Carbopol® 940 (%) | Drug content (%) | pH | Spreadability (cm) |
|---|---|---|---|---|
| 1 | 1.0 | 99.29±2.21 | 5.81±0.15 | 6.52±0.31 |
| 2 | 1.5 | 98.21±2.42 | 6.12±0.12 | 8.12±0.25 |
| 3 | 2.0 | 97.97±1.64 | 5.72±0.14 | 10.26±0.34 |
Abbreviations: Carbopol® 940, poly(acrylic acid); 5-FU, 5-fluorouracil; NLC, nanostructured lipid carrier; SD, standard deviation.
Table 4.
In vitro permeation parameters of 5-FU from optimized nanoemulsions (mean ± SD, n=3)
| Formulations | SSF (Jss) (μg/cm2/h) | CAP 12 hours (μg/cm2) | LT (hours) | CP (cm/h) |
|---|---|---|---|---|
| 5-FU-NLC gel | 10.27±1.82 | 92.45±4.85 | 1.92±0.132 | 6.24±2.84 |
| 5-FU plain gel | 2.85±1.12 | 23.26±2.64 | 0.85±0.154 | 1.85±0.86 |
Abbreviations: CAP, cumulative amounts of drug permeated; CP, permeation coefficient; 5-FU, 5-fluorouracil; Jss, drug flux at steady state; LT, log time; NLC, nanostructured lipid carrier; SD, standard deviation; SSF, steady-state flux.
Rheological properties of 5-FU-NLC gel formulation
The rheological properties of the solutions are of importance in view of their proposed topical application. In the selection of concentration of polymer, a compromise is sought between a sufficiently high concentration for the formation of gel of satisfactory strength for use as a delivery vehicle, and a sufficiently low concentration to maintain an acceptable viscosity that ensures optimal consistency for topical application.25 In order to study the effect of the type of hydrogel on the physicochemical properties of semisolid formulations, the rheological flow patterns were determined for hydrogels containing 5-FU-NLC and only 5-FU. For studying the rheological behavior of the NLC formulations, viscosity measurements were made at several shear rates.37 The viscosity of the NLC formulation gradually decreased as the shear rate was increased from 0.5 rpm to 100 rpm, which characterizes shear thinning behavior. From the rheograms obtained for 5-FU-NLC- and 5-FU-loaded hydrogel formulations, their behavior followed a pseudoplastic system (Figure 2).
Figure 2.
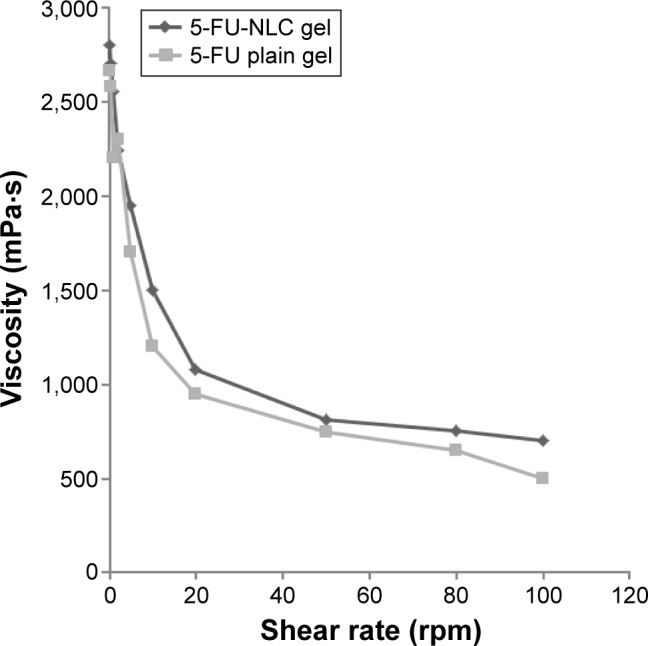
Rheological behavior of 5-FU-NLC gel and 5-FU plain gel under different shear rates.
Abbreviations: 5-FU, 5-fluorouracil; NLC, nanostructured lipid carrier.
In vitro skin permeation studies
Formulation batch 5-FU-NLC4 was selected to study the in vitro skin permeation profiles, and the results of in vitro skin permeation of 5-FU-loaded NLC gel and 5-FU-loaded plain gel are presented in Figure 3. The in vitro permeation of 5-FU from the NLC gel through the mouse skin showed a biphasic pattern of drug release, with an initial phase of slow drug release for 4 hours, followed by fast release up to 12 hours. The release pattern for NLC depends on the nature of the lipid matrix, the surfactant concentration, and the production temperature. This is explained by earlier studies describing the partitioning of the drug in both aqueous and lipid phases.38–40 During the formulation, the solubility of the drug is increased when the temperature increases (70°C–80°C) in the presence of a surfactant in the aqueous phase. During the cooling phase, the drug is repartitioned into the lipid phase, and the solid lipid recrystallizes and forms a solid lipid core.41 Greater amounts of the drug are entrapped in the core lipid matrix and lower amounts of the drug are deposited at the shell and/or the surface of the lipid nanoparticles.42 Therefore, the formulation contains less amount of drug on the surface and the outer shell of the NLC contributes to the initial slow release; moreover, the drug present in the core of the lipid matrix contributes to the second fast release phase.
Figure 3.
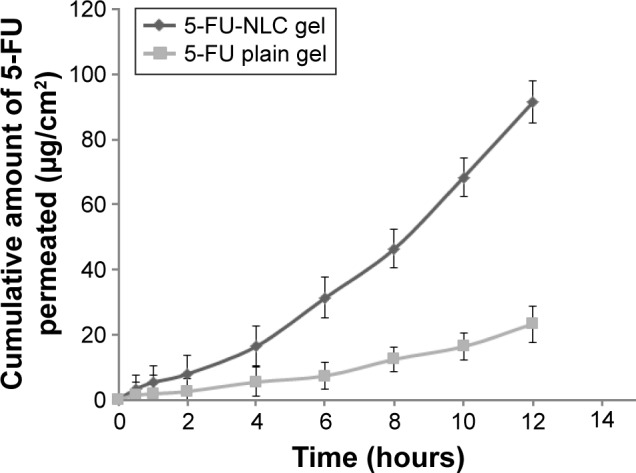
Percutaneous permeation profiles of drug from 5-FU-NLC gel and 5-FU-loaded plain gel through the excised rat skins (mean ± SD; n=5).
Abbreviations: 5-FU, 5-fluorouracil; NLC, nanostructured lipid carrier; SD, standard deviation.
The cumulative amounts of 5-FU released from the NLC gel and plain gel formulations at 12 hours after dosing were 91.25±4.25 μg/cm2 and 12.19±23.19 μg/cm2, respectively. This result clearly indicated that the amount of 5-FU penetrating through the mouse skin from the NLC gel was significantly (P<0.05) much higher (3.9-fold higher) than the amount of 5-FU permeating from the 5-FU plain gel at 12 hours. The enhanced skin permeation of the 5-FU loaded in the NLC hydrogel is mainly due to the increased surface area and smaller size of the particles that interface with skin corneocytes, superior skin occlusion characteristics, and more effective hydration of the stratum corneum as compared with other dosage forms.43 In addition to this, the LBS used in the NLC acts as a surfactant, which may loosen or fluidize the lipid bilayers of the stratum corneum, thus contributing to the enhanced skin permeation.44
In vitro skin permeation parameters, such as steady-state flux (SSF), cumulative amounts of drug permeated (CAP), LT, and permeation coefficient (CP) of 5-FU-NLC gel and 5-FU-loaded plain gel were calculated from in vitro permeation data and are represented in Table 4. The SSF, CAP, LT, and CP values of 5-FU-NLC gel were found to be 10.27±1.82 μg/cm2/h, 92.4±4.85 μg/cm2, 1.92±0.132 hours, and 6.24±2.84 cm/h, respectively, whereas the values of the permeation parameters (SSF, CAP, LT, and CP) of 5-FU-loaded plain gel were found to be 2.85±1.12 μg/cm2/h, 23.26±2.64 μg/cm2, 0.85±0.154 hours, and 1.85±0.86 cm/h, respectively.
In order to investigate the mode of drug release from the NLC formulations, the in vitro permeation data were analyzed using the following mathematical models: zero-order kinetic model (Q = K0⋅t); first-order kinetic model [ln (100 − Q) = lnQ0 − K1t]; and Higuchi equation (Q = KH⋅t1/2). Examination of the correlation coefficient (R2) value indicated that the drug permeation followed a diffusion-controlled mechanism from the 5-FU-NLC gel, as the R2 value for Higuchi’s square root of time (0.9784) was higher in comparison to the zero-order (0.8213) and the first-order (0.8672) kinetic models, as shown in Table 5. Furthermore, to understand the drug release mechanism, the data were fitted to Korsmeyer–Peppas exponential model,25 Mt/M∞ = Ktn, where Mt/M∞ is the fraction of drug released after time t, “K” is the kinetic constant, and n is the release exponent that characterizes the drug transport mechanism: Fickian diffusion when n≤0.5, non-Fickian transport when 0.45<n<0.89, case II transport when n=0.89, and super case II transport when n>0.89. According to the calculated values of the release exponent, n, which was found to be 0.6450, 5-FU permeation from the NLC formulation can be inferred to follow a non-Fickian diffusion mechanism (Table 5).
Table 5.
Drug release kinetics parameters of 5-FU-NLC gel and 5-FU plain gel obtained from in vitro data on permeation through excised rat skin (mean ± SD, n=3)
| Batches | Zero order
|
First order
|
Higuchi
|
Korsmeyer–Peppas
|
||||
|---|---|---|---|---|---|---|---|---|
| K0 (μg/h) | R2 | K1 (h−1) | R2 | KH (h−0.5) | R2 | n | R2 | |
| 5-FU-NLC gel | 1.1240 | 0.8213 | 2.8324 | 0.8672 | 3.1280 | 0.9784 | 0.6450 | 0.9586 |
| 5-FU plain gel | 0.8726 | 0.6126 | 1.2483 | 0.8824 | 1.9240 | 0.8235 | 6.742 | 0.8972 |
Abbreviations: 5-FU, 5-fluorouracil; K0, zero-order release constant; K1, first-order rate constant; KH, Higuchi release constant; n, release exponent in Korsmeyer–Peppas model; NLC, nanostructured lipid carrier; R2, squared correlation coefficient; SD, standard deviation.
In vivo skin retention studies
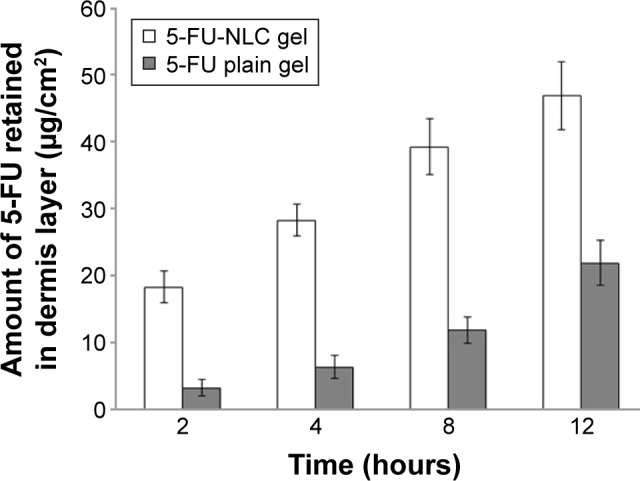
The purpose of this study was to target the drug into the skin with controlled release for prolonged duration. The cumulative amounts of 5-FU retained in the epidermis and dermis of the mouse skin from the 5-FU-NLC and 5-FU plain gel at 2, 4, 8, and 12 hours after administration are shown in Figures 4 and 5, respectively. The amount of 5-FU retained in the epidermis from the 5-FU-NLC-based gel was found to be 68.12±4.45 μg/cm2, which is significantly (P<0.05) higher as compared to 24.32±2.42 μg/cm2 from the 5-FU plain gel, at the end of 12 hours (Figure 4). As expected, a similar type of results was obtained from the in vivo retention study in the dermis layer as well (Figure 5). The amount of 5-FU retained in the dermis from the 5-FU plain gel was much lower (21.82±2.24 μg/cm2) in comparison with that retained from the 5-FU-NLC-based gel (46.45±3.12 μg/cm2) at 12 hours after administration. The results of in vivo retention studies clearly indicate that the drug penetration potential of the 5-FU-NLC-based hydrogel into the epidermis and dermis layers was enhanced 2.78 and 2.09 times (P<0.05) that of the 5-FU plain hydrogel formulation, respectively. In this NLC formulation, the drug was well dispersed within the lipid matrix and, furthermore, the NLC was incorporated into the poly(acrylic acid) gel, which adhered to the skin, further increasing the contact time.
Figure 4.
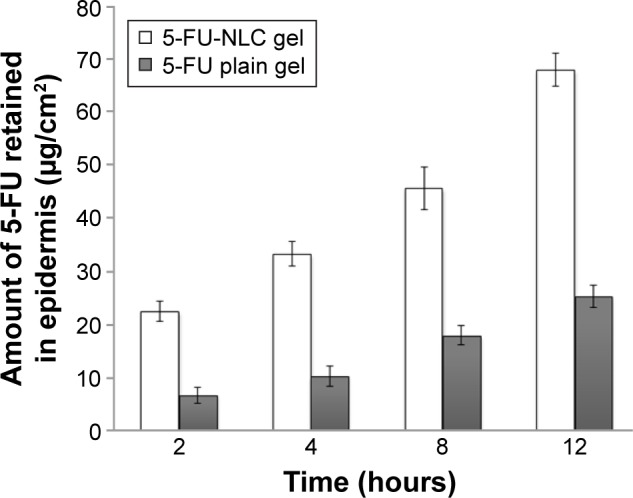
The amount of 5-FU retained in the epidermal layer of rat skin at 2, 4, 8, and 12 hours after application of 5-FU-NLC gel and 5-FU-loaded plain gel (mean ± SD; n=6).
Abbreviations: 5-FU, 5-fluorouracil; NLC, nanostructured lipid carrier; SD, standard deviation.
Figure 5.
The amount of 5-FU retained in the dermis layer of rat skin at 2, 4, 8, and 12 hours after application of 5-FU-NLC gel and 5-FU-loaded plain gel (mean ± SD; n=5).
Abbreviations: 5-FU, 5-fluorouracil; NLC, nanostructured lipid carrier; SD, standard deviation.
Therefore, the enhanced dermal retention of 5-FU from the NLC formulation was attributed to the increased contact with corneocytes, skin retention, and sustained-release properties of NLCs. Furthermore, the small particle size of NLCs enables closer contact with the superficial junctions of the corneocyte clusters and furrows present between the corneocyte islands and favors the accumulation of the drug for several hours, which could improve the hydration of the stratum corneum and enhance the penetration of the drug, as described in previous studies.17,43,44 In addition, the liquid lipid (LBS) used in the NLC formulation as percutaneous absorption enhancers could also facilitate the permeation of drug into the skin.43,44 In addition, the drug from the NLC-based drug delivery system can be targeted to the skin with reduced systemic access and side effects in comparison with conventional formulations such as cream, gel, and so on. Furthermore, the NLCs were formulated using lipids that are solid at ambient temperature and their incorporation into the gel may induce structural changes in the particle structure due to evaporation of water, resulting in the transition of the lipid matrix into a highly ordered structure, causing drug expulsion. From the result, it can be noted that NLCs may play an important role in enhancing the permeation of 5-FU into the skin with sustained drug release, as well as in targeting of the drug to the skin.16,25
Skin irritation studies
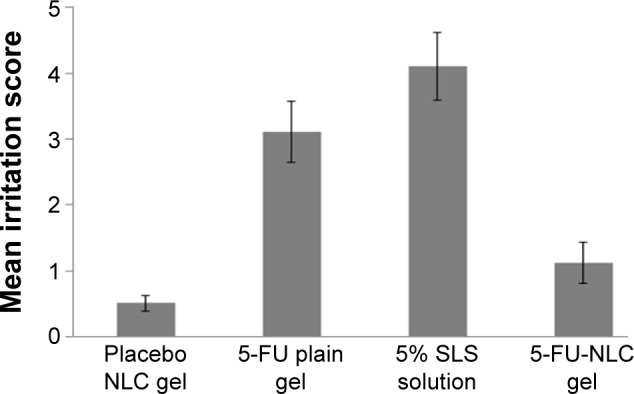
The safety of and skin irritation caused by 5-FU-NLC formulations were also studied. Erythema is caused by increased blood flow in the dermis and it can be considered a tool to monitor the response of that layer to topical preparations.5,28 In this study, 5% w/v of SLS was used as a standard irritant to compare with the formulations. The mean erythema scores of placebo NLC gel, 5-FU-NLC gel, 5-FU plain gel, and 5% w/v SLS solution at 12 hours after application are presented in Figure 6. Results indicate that erythema caused by SLS had a much higher score (=4) as compared to 5-FU-NLC-based gel (=1) and 5-FU plain gel (=3) containing 0.5% 5-FU. It was noticed from the result that the mean irritation potential of 5-FU from NLC-based gel formulations was significantly (P<0.05) reduced compared with that of 5-FU plain gel formulation. The 5-FU is encapsulated in lipid matrix in NLC, and moreover, the direct exposure of the drug to the skin for long duration was avoided; this could be the reason why the irritation potential of 5-FU was reduced to a level much lower than that of 5-FU plain gel. In fact, it has been reported that 5-FU is associated with dermatitis, erythema, pain, and desquamation of the skin of palms and soles.5 The results of this study clearly indicate that the optimized NLC formulation was well tolerated by the mouse skin and produced minimal irritation. It was concluded from the results that the optimized 5-FU-NLC formulation was safe to be used for topical drug delivery as compared to conventional formulations such as gel, cream, and so on.
Figure 6.
Mean irritation indexes of placebo NLC gel, 5-FU plain gel, 5% w/v SLS solution and 5-FU-NLC gel (mean ± SD; n=5).
Abbreviations: 5-FU, 5-fluorouracil; NLC, nanostructured lipid carrier; SD, standard deviation; SLS, sodium lauryl sulfate.
Conclusion
In this study, 5-FU-loaded NLC-based gel formulations were successfully developed by high-pressure homogenization technique using PRE as the solid lipid, LBS as the liquid lipid, Poloxamer 188 as the stabilizer, and polyoxyl-15-hydroxystearate as a surfactant. The obtained results confirmed the potential of NLCs as carriers for topical administration. In vitro skin permeation studies showed that 5-FU-NLC could obviously increase the permeation of 5-FU into the skin and in vivo retention studies confirmed that the amount of drug retained in the epidermis and dermis from the 5-FU-NLC gel was significantly enhanced as compared to that from the 5-FU-loaded plain gel. Furthermore, skin irritation studies revealed that the irritation potential of 5-FU from NLC was significantly reduced as compared to that of the 5-FU plain gel. Therefore, it was concluded that 5-FU-NLC-based hydrogels have great potential for the topical delivery of 5-FU for safe and better treatment of various skin diseases including skin cancer. However, further studies, including extensive pharmacokinetic studies, histopathological studies, and toxicity studies, are needed to fully explore these formulations.
Acknowledgments
Dr Rajinikanth Siddalingam is grateful to the School of Pharmacy, International Medical University, Kuala Lumpur, Malaysia, for providing financial support for this study.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Tsuji T, Sugai T. Topically administered fluorouracil in vitiligo. Arch Dermatol. 1983;119(9):722–727. [PubMed] [Google Scholar]
- 2.Saif MW, Choma A, Salamone SJ, Chu E. Pharmacokinetically guided dose adjustment of 5-fluorouracil: a rational approach to improving therapeutic outcomes. J Natl Cancer Inst. 2009;101(22):1543–1552. doi: 10.1093/jnci/djp328. [DOI] [PubMed] [Google Scholar]
- 3.Kong M, Chen XG, Kwon DK, Park HJ. Investigations on skin permeation of hyaluronic acid based nanoemulsions as transdermal carrier. Carboohydr Polym. 2011;86(2):837–843. [Google Scholar]
- 4.Khandavilli S, Panchagnula R. Nanoemulsions as versatile formulations for paclitaxel delivery: peroral and dermal delivery studies in rats. J Invest Dermatol. 2007;127(1):154–162. doi: 10.1038/sj.jid.5700485. [DOI] [PubMed] [Google Scholar]
- 5.Aliaa N, ElMeshad I, Ibrahim MT. Transdermal delivery of an anticancer drug via W/O emulsions based on alkyl polyglycosides and lecithin: design, characterization, and in vivo evaluation of the possible irritation potential in rats. AAPS PharmSciTech. 2011;12(1):1–9. doi: 10.1208/s12249-010-9557-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu F, Xiao YY, Ping QN, Yang C. Water in oil microemulsions for transdermal delivery of fluorouracil. Yao Xue Xue Bao. 2009;44(5):540–547. [PubMed] [Google Scholar]
- 7.Gupta RR, Jain SK, Varshney M. AOT water-in-oil microemulsions as a penetration enhancer in transdermal drug delivery of 5-fluorouracil. Colloids Surf B Biointerfaces. 2005;41(1):25–32. doi: 10.1016/j.colsurfb.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 8.Pardeike J, Schwabea K, Müller RH. Influence of nanostructured lipid carriers (NLC) on the physical properties of the Cutanova nanorepair Q10 cream and the in vivo skin hydration effect. Int J Pharm. 2010;396(1–2):166–173. doi: 10.1016/j.ijpharm.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 9.Mitri K, Shegokar R, Gohla S, Anselmi C, Müller RH. Lipid nanocarriers for dermal delivery of lutein: preparation, characterization, stability and performance. Int J Pharm. 2011;414(1–2):267–275. doi: 10.1016/j.ijpharm.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 10.Nikoli S, Keck CM, Anselmi C, Müller RH. Skin photoprotection improvement: synergistic interaction between lipid nanoparticles and organic UV filters. Int J Pharm. 2011;414(1–2):276–284. doi: 10.1016/j.ijpharm.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 11.Cirri M, Bragagni M, Menni N, Mura P. Development of a new delivery system consisting drug- in cyclodextrin- in nanostructured lipid carriers; for ketoprofen topical delivery. Eur J Phram Biopharm. 2011;32(4):21–32. doi: 10.1016/j.ejpb.2011.07.015. [DOI] [PubMed] [Google Scholar]
- 12.Souto EB, Wissing SA, Barbosa CM, Muller RH. Development of a controlled release formulation based on SLN and NLC for topical clotrimazole delivery. Int J Pharm. 2004;271(1):71–77. doi: 10.1016/j.ijpharm.2004.02.032. [DOI] [PubMed] [Google Scholar]
- 13.Radtke M, Souto EB, Muller RH. Nanostructured lipid carriers: a novel generation of solid lipid drug carriers. Pharm Technol Eur. 2005;17(4):45–50. [Google Scholar]
- 14.Tiwari S, Mistry P, Patel V. SLNs based on co-processed lipids for topical delivery of terbinafine hydrochloride. J Pharm Drug Dev. 2014;1(6):604. [Google Scholar]
- 15.Vaghasiya H, Kumar A, Sawant K. Development of solid lipid nanoparticles based controlled release system for topical delivery of terbinafine hydrochloride. Eur J Pharm Sci. 2014;2(1):1–12. doi: 10.1016/j.ejps.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 16.Guo C, Yang C, Li Q, Tan Q, Liu W, Zhai G. Development of a quercetin-loaded nanostructured lipid carrier formulation for topical delivery. Int J Pharm. 2012;430(1–2):392–398. doi: 10.1016/j.ijpharm.2012.03.042. [DOI] [PubMed] [Google Scholar]
- 17.Bharti G, Fazil M, Saba K, Ali A, Baboota S, Javed A. Nanostructured lipid carrier system for topical delivery of terbinafine hydrochloride. Bull Fac Pharm (Cairo Univ) 2015;55(2):147–159. [Google Scholar]
- 18.Kumari S, Deepti P, Neelam P, Viney L. Nanostructured lipid carriers for topical delivery of an anti-acne drug: characterization and ex vivo evaluation. Pharm Nanotechnol. 2016;3(2):122–133. [Google Scholar]
- 19.Upendra N, Neha G. Nanostructured lipid carrier (NLC)-based gel was developed as a potential topical system for clobetasol propionate (CP) topical delivery for the treatment of eczema. Drug Deliv Transl Res. 2016;6(3):289–298. [Google Scholar]
- 20.Cosco D, Paolino D, Maiuolo J, et al. Ultradeformable liposomes as multidrug carrier of resveratrol and 5-fluorouracil for their topical delivery. Int J Pharm. 2015;489(1–2):1–10. doi: 10.1016/j.ijpharm.2015.04.056. [DOI] [PubMed] [Google Scholar]
- 21.Khan MA, Pandit J, Sultana Y, et al. Novel carbopol-based transfersomal gel of 5-fluorouracil for skin cancer treatment: in vitro characterization and in vivo study. Drug Deliv. 2015;22(6):795–802. doi: 10.3109/10717544.2014.902146. [DOI] [PubMed] [Google Scholar]
- 22.Hussain A, Samad A, Ramzan M, Ahsan MN, Ur Rehman Z, Ahmad FJ. Elastic liposome-based gel for topical delivery of 5-fluorouracil: in vitro and in vivo investigation. Drug Deliv. 2016;23(4):1115–1129. doi: 10.3109/10717544.2014.976891. [DOI] [PubMed] [Google Scholar]
- 23.Lim WM, Rajinikanth PS, Mallikarjun C, Kang YB. Formulation and delivery of itraconazole to the brain using nanolipid carrier system. Int J Nanomed. 2014;9(1):2117–2126. doi: 10.2147/IJN.S57565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ana Cristina DM, Najeh MK, Rubiana MM. Development and validation of an HPLC method for the determination of fluorouracil in polymeric nanoparticles. Braz J Pharm Sci. 2013;49(1):117–126. [Google Scholar]
- 25.Rajinikanth PS, Mishra B. Floating in situ gelling system for stomach site-specific delivery of clarithromycin to eradicate H Pylori. J Control Release. 2008;4(1):33–41. doi: 10.1016/j.jconrel.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 26.Shakeel F, Baboota S, Ahuja A, Ali J, Aqil M, Shafiq S. Nanoemulsions as vehicles for transdermal delivery of aceclofenac. AAPS Pharm-SciTech. 2007;8(4):104. doi: 10.1208/pt0802042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vipasha D, Shiwang S, Subheet J, Sandeep A, Arvind S. Formulation characterization and evaluation of new topical 5-FU by drug entrapment in oleic acid vesicles. Am J Pharm Res. 2011;1(2):1–15. [Google Scholar]
- 28.Reeta RG, Swantrant KJ, Varshneya M. AOT water-in-oil microemulsions as a penetration enhancer in transdermal drug delivery of 5-fluorouracil. Colloids Surf B Biointerfaces. 2005;41(1):25–32. doi: 10.1016/j.colsurfb.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 29.Severino P, Andreani T, Macedo AS, et al. Current state-of-art and new trends on lipid nanoparticles (SLN and NLC) for oral drug delivery. J Drug Deliv. 2012;2012:750891. doi: 10.1155/2012/750891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Noack A, Hause G, Mader K. Physicochemical characterization of curcuminoid-loaded solid lipid nanoparticles. Int J Pharm. 2012;423(2):440–451. doi: 10.1016/j.ijpharm.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 31.Kaur IP, Bhandari R, Bhandari S, Kakkar V. Potential of solid lipid nanoparticles in brain targeting. J Control Release. 2008;27(2):97–109. doi: 10.1016/j.jconrel.2007.12.018. [DOI] [PubMed] [Google Scholar]
- 32.Liu J, Hu W, Chen H, Ni Q, Xu H, Yang X. Isotretinoin-loaded solid lipid nanoparticles with skin targeting for topical delivery. Int J Pharm. 2007;328(2):191–195. doi: 10.1016/j.ijpharm.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 33.Das S, Ng WK, Kanaujia P, Kim S, Tan RB. Formulation design, preparation and physicochemical characterizations of solid lipid nanoparticles containing a hydrophobic drug: effects of process variables. Colloids Surf B Biointerfaces. 2011;88(1):483–489. doi: 10.1016/j.colsurfb.2011.07.036. [DOI] [PubMed] [Google Scholar]
- 34.Helgason T, Awad TS, Kristbergsson K, McClements DJ, Weiss J. Effect of surfactant surface coverage on formation of solid lipid nanoparticles (SLN) J Colloid Interface Sci. 2009;334(1):75–81. doi: 10.1016/j.jcis.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 35.Muller RH, Mader K, Gohla S. Solid lipid nanoparticles (SLN) for controlled drug delivery – a review of the state of the art. Eur J Pharm Biopharm. 2000;50(1):161–177. doi: 10.1016/s0939-6411(00)00087-4. [DOI] [PubMed] [Google Scholar]
- 36.Silva AC, Santos D, Ferreira DC, Souto EB. Minoxidil-loaded nanostructured lipid carriers (NLC): characterization and rheological behaviour of topical formulations. Pharmazie. 2009;64(3):177–182. [PubMed] [Google Scholar]
- 37.Joshi M, Patravale V. Nanostructure lipid carrier (NLC) based gel of celecoxib. Int J Pharm. 2008;346(1–2):124–132. doi: 10.1016/j.ijpharm.2007.05.060. [DOI] [PubMed] [Google Scholar]
- 38.Zhao YJ, Brown MB, Jones SA. Pharmaceutical foams: are they the answer to the dilemma of topical nanoparticles? Nanomedicine. 2010;6(2):227–236. doi: 10.1016/j.nano.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 39.Patel D, Dasgupta S, Dey S, Ramani YR, Ray S, Mazumder B. Nanostructured lipid carriers (NLC)-based gel for the topical delivery of aceclofenac: preparation, characterization, and in vivo evaluation. Sci Pharm. 2012;80(3):749–764. doi: 10.3797/scipharm.1202-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xie S, Zhu L, Dong Z, et al. Preparation, characterization and pharmacokinetics of enrofloxacin-loaded solid lipid nanoparticles: influences of fatty acids. Colloids Surf B Biointerfaces. 2011;83(2):382–387. doi: 10.1016/j.colsurfb.2010.12.014. [DOI] [PubMed] [Google Scholar]
- 41.Priyanka K, Sathali AA. Preparation and evaluation of montelukast sodium loaded solid lipid nanoparticles. J Young Pharm. 2012;4(3):129–137. doi: 10.4103/0975-1483.100016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schwarz JC, Baisaeng N, Hoppel M, Löw M, Keck CM, Valenta C. Ultra-small NLC for improved dermal delivery of coenyzme Q10. Int J Pharm. 2013;447(1–2):213–217. doi: 10.1016/j.ijpharm.2013.02.037. [DOI] [PubMed] [Google Scholar]
- 43.Fang JY, Fang CL, Liu CH, Su YH. Lipid nanoparticles as vehicles for topical psoralen delivery: solid lipid nanoparticles (SLN) versus nanostructured lipid carriers (NLC) Eur J Pharm Biopharm. 2008;70(2):633–640. doi: 10.1016/j.ejpb.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 44.Feldman LD, Ajani JA. Fluorouracil-associated dermatitis of the hands and feet. (1985) JAMA. 1985;254(24):3479–3485. [PubMed] [Google Scholar]


