Abstract
The term “immune privilege” was originally coined to describe the suppression of inflammatory responses within organs protected by anatomic barriers, ie, the eyes, brain, placenta, and testes. However, cellular and metabolic processes, which orchestrate immune responses, also control inflammation within these sites. Our current understanding of tolerogenic mechanisms has extended the definition of immune privilege to include hair follicles, the colon, and cancer. By catabolizing tryptophan, cells expressing the enzyme indoleamine-2,3-dioxygenase produce kynurenine metabolites, which orchestrate local and systemic responses to control inflammation, thus maintaining immune privilege. This review highlights the double-edged role played by the kynurenine pathway (KP), which establishes and maintains immune-privileged sites while contributing to cancer immune escape. The identification of the underlying molecular drivers of the KP in immune-privileged sites and in cancer is essential for the development of novel therapies to treat autoimmunity and cancer and to improve transplantation outcomes.
Keywords: cancer, HIV, IDO, immune-privileged sites, kynurenine pathway, tryptophan
Introduction
Immune privilege was first observed by van Dooremaal, a Dutch ophthalmologist who, in 1873, transplanted murine skin into the eyes of rabbits and dogs in an attempt to study the formation of cataracts.1,2 He was astonished by the observation that the grafted tissue was not rejected following its transplantation into the anterior chamber of the eye. In 1948, Medawar3 reassessed the prolonged survival of foreign tissue grafted into the anterior chamber of the eye and coined the term “immune privilege”. He proposed that the apparent absence of lymphatic drainage protected the graft from immune rejection. In 1975, Kaplan et al.4 showed that antigen introduced into the anterior chamber of the eye was able to induce a systemic downregulation of the antigen-specific cellular response, a process he called immune deviation. It is now also known that immunoregulatory processes contribute to immune privilege. In addition to the eye, other immune-privileged sites have been described and include the brain, placenta, and testes. More recently, it was discovered that similar immunoregulatory processes operate within the hair follicle and the colon; as a result, these organs were identified as immune-privileged sites despite lacking anatomic barriers. Furthermore, immune-privileged sites can be categorized based on their cellular regenerative capacity, with the eyes and brain exhibiting limited capacity, while the other sites contain stem cells that can repair damaged tissue.5,6
Novel findings emerging from systems biology studies involving metabolomics and transcriptomics have led to an expansion of the concept of immune privilege.7 Based on the genome-wide transcriptome profiling of 23,843 genes, Doyle et al.8 identified three key immune mechanisms that are involved in testicular immune privilege; these mechanisms include an immunosuppressive cytokine milieu, the presence of proteins regulating leukocyte trafficking through controlled cell junctions, and the inhibition of complement activation. Shechter et al.9 recently proposed that the integrity of anatomic barriers within some immune-privileged sites is not absolute as shown by the existence of an epithelial “gate” beyond the endothelium vascular barrier in the testes; this gate allows selective cellular trafficking in response to inflammation. Furthermore, we and others have extended the concept of immune privilege to include chronically infected tissues and tissues in which cancer has escaped immune control; in both situations, immune privilege contributes to disease progression.10,11
In this review, we focus on the contribution of indoleamine 2,3-dioxygenase 1 (IDO1), and its immunosuppressive catabolite kynurenine (Kyn), to immune privilege in different anatomic sites and in the setting of cancer. Upon activation, IDO1 catabolizes the essential amino acid tryptophan (Trp) into metabolites collectively known as kynurenines (Kyns) (Fig. 1).11,12 IDO-expressing myeloid cells locally exert a profound inhibitory effect on T cells, which in turn suppress the proinflammatory processes that occur in response to tissue damage, infection, and cancer.13
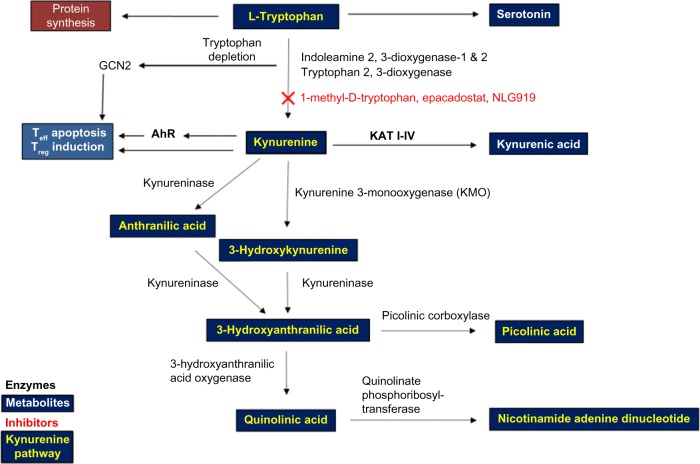
Figure 1.
A schematic representation of the key enzymes and metabolites in the kynurenine pathway.
Notes: The essential amino acid Trp is consumed via the diet and undergoes catabolism in the digestive tract. Besides its important role in protein synthesis and in serotonin production, Trp is catabolized in various body systems including in the colon by Lactobacilli, and in immune-privileged sites by multiple enzymes, to produce various metabolites. IDO1, IDO2, and TDO are the first rate-limiting enzymes involved in Trp catabolism and produce downstream metabolites collectively referred to as the kynurenines. Since the overproduction of Kyn and its ligation to AhR induce immunosuppressive cells, Kyn is an important therapeutic target. Serotonin, quinolinic acid, and kynurenic acid are Trp metabolites with important roles in normal brain function and in mood disorders. Nicotinamide adenine dinucleotide is an important cellular cofactor produced during Trp catabolism.
Abbreviations: AhR, aryl hydrocarbon receptor; GCN2, general control nonderepressible 2; IDO, indoleamine 2,3-dioxygenase; KAT, kynurenine aminotransferase; Teff, effector T cell; Treg, regulatory T cell; TDO, tryptophan 2,3-dioxygenase; Trp, tryptophan.
IDO is at the center of the immune synapse, which bridges innate and adaptive immune responses involving antigen-presenting cells (APCs), such as dendritic cells (DCs) and macrophages (MPs), and lymphocytes, such as effector T (Teff) cells, natural killer (NK) cells, and regulatory T (Treg) cells.
The kynurenine pathway (KP) contributes to immune privilege within organs and does so in conjunction with the adenosine/purinergic pathway and with immune checkpoints, such as cytotoxic T lymphocyte antigen-4 (CTLA-4) and programmed cell death-1 (PD-1).14,15 The aim of this review is to highlight the double-edged role played by the KP, which beneficially contributes to maintaining immune-privileged sites to protect them from inflammation-mediated damage, while detrimentally participating in cancer immune escape.
The KP Plays a Critical Role in Immune Privilege
l-Trp is one of the nine essential amino acids required by humans and has a distinctive indole functional group.12 Despite being the least abundant amino acid, Trp plays a considerable role in health and in disease given its roles in protein synthesis, serotonin production, and the KP (Fig. 1). The KP converts Trp into biologically active metabolites, including Kyn, kynurenic acid, 3-hydroxy Kyn, picolinic acid, quinolinic acid, and nicotinamide adenine dinucleotide (NAD). This process reduces Trp serum levels, which in turn decrease the availability of Trp, an amino acid required for microbial growth and cellular growth. These Trp metabolites and related enzymes have important and contrasting short-term antimicrobial and long-term immunosuppressive roles.11 The Trp-catabolizing enzymes implicated in the KP are found in numerous cell types, including endothelial cells, fibroblasts, and myeloid cells.
The KP involves three distinct rate-limiting enzymes, such as IDO1 and IDO2, which are inducible enzymes in the gut mucosa and in other tissues, and tryptophan 2,3-dioxygenase (TDO), which is constitutively expressed in the liver and plays a dominant role in health.16 TDO is also expressed at low levels in endothelial cells, neurons, astrocytes, and malignant cells, in conjunction with IDO1.17,18 TDO is upregulated by cellular stress, glucocorticoids, and Trp homeostasis. Conversely, IDO1 can be dramatically enhanced during inflammation, making it the most important of the three enzymes during inflammatory diseases.19 IDO2, which is not redundant with respect to IDO1 function, is present at a lower level than IDO1 and is expressed by similar cells as IDO1.20 In this review, the term IDO refers to IDO1 unless specified otherwise.
A variety of cells express IDO, including monocytes, MPs, DCs, endothelial cells, fibroblasts, and certain cancer cells.21,22 IDO is upregulated by inflammatory molecules, such as amyloid peptides and lipopolysaccharides, and by inflammatory cytokines, such as interleukin-1 (IL-1), interferon gamma (IFN-γ), and tumor necrosis factor alpha (TNF-α).23,24
IDO-deficient mice and mice treated with IDO inhibitors fail to tolerate the fetus during allogeneic pregnancy,25 experience colitis due to the failure of mucosal tolerance of the intestinal microbiota,26 and lose the ability to clear apoptotic cells.27 Furthermore, blocking or ablating IDO expression worsens inflammation in models of graft-versus-host disease,28 autoimmunity,29 and chronic conditions, such as chronic granulomatous disease and diabetes,30,31 illustrating the key role played by IDO in controlling inflammation. In all these models, IDO inhibition can represent an interesting target as it is induced by inflammation, while inhibition of CTLA-4 may be clinically beneficial at the expense of developing autoimmune conditions as its expression is constitutive in Treg cells and induced in conventional T cells.13,32
The KP contributes to immune privilege by the following four mechanisms: (1) by depleting Trp via the induction of the stress response kinase, general control nondepressible 2 (GCN2), and the suppression of mammalian target of rapamycin 1 (mTOR1) pathway, which senses amino acid withdrawal, a process inhibiting Teff cell function and growth (Fig. 2)33,34; (2) by the direct effect of Kyn on the aryl hydrocarbon receptor (AhR), which induces DC and MP differentiation; these cells in turn first induce inflammatory Th17 cells followed by a global reprogramming driving their conversion (transdifferentiation) to Treg cells upon the resolution of inflammation.35 In the context of chronic inflammation, the effect of Kyn on AhR is directed toward a predominant immune suppression; (3) by promoting the differentiation of CD4 T cells into Treg cells expressing CTLA-4 and via phosphatase and tensin homolog, a protein encoded by a tumor suppressor gene, which is mutated in several cancers36; and finally (4) by the Kyn-mediated inhibition of IL-2 signaling, which impairs memory CD4 T cell survival.37–39 Upon IDO activation, APCs capable of producing inflammatory cytokines such as IL-12 switch to produce inhibitory cytokines such as transforming growth factor beta (TGF-β) and IL−10.37 In addition, IDO can be triggered in the presence of apoptotic cells to induce self-tolerance and/or cancer persistence.40 Globally, IDO activation can transform the function of APCs and convert local T cell function from an immunogenic one to a tolerogenic one. The relative contribution of the KP to the various organs characterized by immune privilege will be described in the following sections.
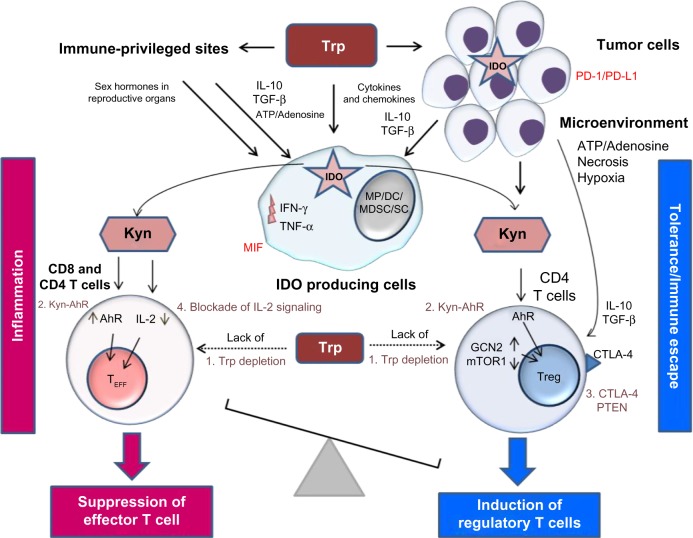
Figure 2.
The role of the kynurenine pathway in inducing tolerance in immune-privileged sites, in tumors, and in the tumor microenvironment.
Notes: The production of kynurenine by the IDO enzymatic activity of tumor cells and of APCs leads to immune tolerance in immune-privileged sites and in the tumor microenvironment. Kyn plays a major role by inducing Treg cells, which can also be directly induced by the cytokines and chemokines produced by tumor cells. The cytokines and chemokines produced by tumor cells can also stimulate APCs to activate the KP, which contributes further to tumor development. The four major mechanisms of the KP that influence the immune response are as follows: (1) Trp depletion followed by GCN2 induction and mTOR1 suppression; (2) activation of AhR by Kyn; (3) Treg induction through expression of CTLA-4 and PTEN; and (4) Kyn-mediated blockade of IL-2. Other factors implicated in immune tolerance include tumor cell expression of PD-1/PD-L1 and macrophage expression of MIF. Dotted lines represent decreased Trp concentrations.
Abbreviations: AhR, aryl hydrocarbon receptor; APCs, antigen-presenting cells; DC, dendritic cell; GCN2, general control nonderepressible 2; IDO, indoleamine 2,3-dioxygenase; IFN-γ, interferon gamma; IL-32, interleukin-32; KP, kynurenine pathway; Kyn, kynurenine; MDSC, myeloid-derived suppressor cell; MP, macrophage; MIF, macrophage migration inhibitory factor; mTOR1, mammalian target of rapamycin 1; SC, Sertoli cell; Teff, effector T cell; TNF-α, tumor necrosis factor alpha; Treg, regulatory T cell; Trp, tryptophan.
The KP as a Guardian of the Eyes and Brain
The eye and the central nervous system (CNS) are formed from the neuroepithelium during embryonic development, and they each have a nonexpandable anatomic barrier.9 Since these barriers do not permit swelling in the case of inflammation and these sites have limited cellular regenerative capacity, immune privilege is a necessary protective feature.
The eyes
The concept of immune privilege originated from experimental animal models in which allografts were accepted by the eyes but rejected by the skin.41,42 The eye is anatomically divided into the following three layers: the outer or fibrous layer (the cornea/sclera), the middle or vascular layer (the uvea-iris, ciliary body, and choroid), and the inner or sensorineural layer containing the blood–retina barrier. The blood–retina barrier contributes to immune privilege in the eye.
Over the last decade, the contribution of the IDO expressed by the human corneal cells lining the anterior chamber of the eye to ocular immune privilege has gained considerable attention.2,43,44 IDO is expressed in many ocular tissues and contributes to the immune privilege of corneal allografts.2 In vitro studies have shown that T cells cocultured with iris and ciliary body cells acquire Treg activity induced by IDO and TGF-β expression.40,45
Other ocular tissues such as the uvea, the conjunctiva, and the periocular fascia contain IDO-expressing MPs and tolerogenic DCs secreting immunosuppressive cytokines; these cytokines inhibit Teff cells and induce Treg cells.41 Other mechanisms involved in inducing immune privilege within the eye include programmed cell death ligand 1 (PD-L1), macrophage migration inhibitory factor (MIF), TNF-related apoptosis-inducing ligand (TRAIL), and Fas ligand (FasL), all of which also inhibit T cell proliferation and survival and increase Treg cell numbers (Table 1), which was recently reviewed by Niederkorn et al.2 and Benhar et al.46 In addition, NK and γδ T cells were found to contribute to immune privilege in the murine eye, as did the reduced expression of MHC molecules.47–50
Table 1.
A summary of selected studies which have examined the contributions of the kynurenine pathway and other factors to immune-privileged sites and to cancer and its microenvironment.
| FACTOR | IMMUNE PRIVILEGED SITE | TUMOR AND ITS MICROENVIRONMENT | MECHANISM OF ACTION | ||||
|---|---|---|---|---|---|---|---|
| EYE | BRAIN | PLACENTA | TESTES | HAIR FOLLICLE | |||
| IDO/TDO Kynurenine |
X43,44 | X55 | X25 | X77,83 | X90 | X13,106 | Tryptophan is catabolized into kynurenine by IDO/TDO to induce Treg cells. |
| CTLA-4 | X125 | X126 | X127 | ND | ND | X102,128 | Inhibition of T cell activation |
| PD-L1 | X129 | X130 | X68 | X87,131 | ND | X102,132 | Inhibition of T cell proliferation |
| TGF-β | X133 | X133 | X133 | X134 | X90 | X102,135 | Inhibition of NK cell activity; production of Treg |
| MIF | X136 | X137 | X71 | X138 | X90,139 | X140 | Inhibition of NK cell activity |
| MHC class I and other non-classical MHC molecules | X42 | ND | X63,141 | X141 | X88–90 | X102 | Inhibition of NK cell activity |
| FasL | X142 | X143 | X144 | X145 | ND | X102,135 | Induction of T cell apoptosis |
| TRAIL | X146,147 | X143 | X63 | ND | ND | X147 | Inhibition of T cell proliferation |
Abbreviations: CTLA-4, cytotoxic T lymphocyte-associated protein 4; FasL, Fas ligand; IDO, indoleamine 2,3-dioxygenase; MIF, macrophage migration inhibitory factor; MHC, major histocompatibility complex; ND, No data available; PD-L1, programmed cell death-ligand 1; TDO, tryptophan 2,3-dioxygenase; TGF-β, transforming growth factor beta; TRAIL, TNF-related apoptosis-inducing ligand.
The brain
In 1913, Goldmann51 was the first to report the existence of a blood–brain barrier (BBB) using dyes devised by Ehrlich.52 Goldmann observed that systemically injected trypan blue dye failed to penetrate the brains of animals as readily as it penetrated other tissues. Although, the term “BBB” was first used as early as 1900 by Ehrlich’s student Lewandowski, it was not until the 1960s that the actual existence of the BBB was proven by the elucidation of its cellular structure.53,54
Several types of neural cells are known to produce KP metabolites along with varying levels of KP enzymes. In 2001, Guillemin et al.55 were the first to report that astrocytes produced significant quantities of Kyn, which, by inducing T cell suppression, plays a protective role against neuronal excitotoxic-induced cell death.56–58 Kwidzinski et al.59 showed that microglial cells, which are able to inhibit T cell responses by both Trp depletion and Treg cell induction, increase IDO expression upon IFN-γ stimulation in vitro and in vivo. Using an experimental murine autoimmune encephalomyelitis (EAE) model, which is the result of a breach in immune tolerance to CNS antigens and resembles multiple sclerosis in humans, Kwidzinski et al.60 also showed that the administration of the IDO inhibitor 1-methyl-d-tryptophan exacerbated this condition. Similarly, Yan et al.29 have shown that in IDO-deficient mice, enhanced inflammatory Th1 and Th17 responses exacerbated EAE. Conversely, the administration of 3-hydroxyanthranilic acid, another downstream KP metabolite, was associated with an increased proportion of Treg cells and with an inhibition of the Th1/Th17 response, which reduced EAE severity.
In multiple sclerosis patients, IDO gene expression and activity are predictive of relapse.61 These findings indicate that during inflammation, the KP orchestrates self-protective mechanisms, limiting antigen-specific immune responses in the CNS. Other factors implicated in brain immune privilege have been recently reviewed by Louveau et al.62 and are summarized in Table 1.
Maternal Acceptance of the Fetus: Transgenerational Tolerance
In a pregnant woman, the fetus is a semihaplotypic allograft that is nonetheless tolerated by the maternal immune system.63 As initially reported in 1961 by Medawar,64 the immune tolerance exerted by the placenta protects the fetus from maternal immune responses directed toward paternally inherited antigens. In rare cases, spontaneous abortions occur due to a break in immune tolerance induced by placental inflammation.
The maternal vasculature does not penetrate the fetal parenchyma, while the placenta serves as the fetomaternal barrier.65 The contribution of the KP to maternal immune tolerance of the fetus was discovered in 1998 by Munn et al.25 It had been previously shown that the complement system also contributes to fetal tolerance.66 The placenta is not considered as a strict anatomic barrier since the inhibition of Trp metabolism was observed to induce potent fetal T cell responses in mice, indicating that the KP contributes significantly to immune tolerance of the fetus. In 2004, Aluvihare et al.67 showed that IDO-myeloid dependent induced Treg cells contributed to maternal tolerance of the fetus. Moreover, IDO expression has been observed in extravillous trophoblasts and in chorionic villi within the placenta.68 Importantly, Zenclussen et al.69 showed that the adoptive transfer of pregnancy-induced Treg cells prevented fetal rejection in a murine abortion model. In humans, Somerset et al.70 showed that normal human pregnancy is associated with an increased number of immunosuppressive Treg cells, thus confirming the observations made in the murine model.
Other factors associated with immune privilege within the gravid uterus include the expression of inhibitory PD-L1 and CTLA-4 by both Treg cells and trophoblast giant cells, the expression of MIF by trophoblasts, the inhibition of NK cell cytotoxicity, and the upregulation of apoptosis-inducing FasL expression in the human endometrium (Table 1).63,68,71 Furthermore, in the pregnant uterus, MPs with an anti-inflammatory M2 phenotype secrete immunosuppressive IL-10 and prostaglandin E2, in addition to IDO expression.72,73 Moreover, the hormonal control of the innate and adaptive immune responses plays a particularly important immunosuppressive role, as has been previously reviewed by Wira et al.74 It has also been shown that the placental expression of the nonclassical MHC class I E, F, and G molecules, which can activate certain inhibitory receptors expressed on cells of lymphoid and myeloid origin, contributes to maternal–fetal tolerance.75
The KP Contributes to Testicular Immune Privilege
The testes are an immune-privileged site that tolerate new germ cell autoantigens generated during puberty, a period of immune competence (Fig. 3).76
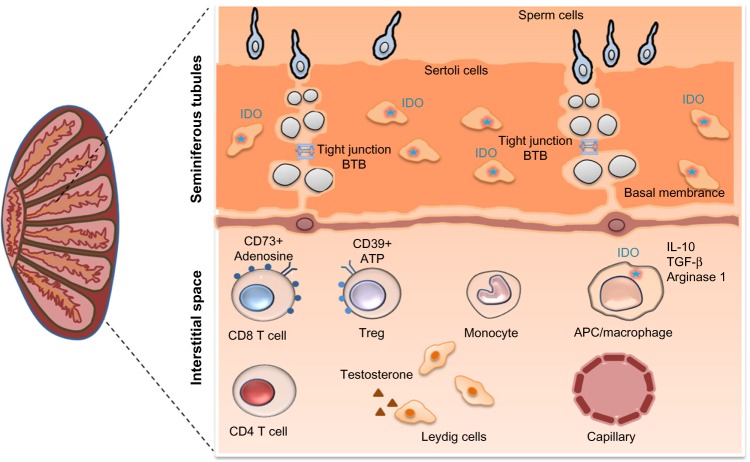
Figure 3.
The kynurenine pathway enzyme IDO plays a central role in testicular immune privilege.
Notes: IDO expression by Sertoli cells and by macrophages contributes to testicular immune privilege, resulting in tolerance to spermatozoa and to infectious agents. Tissue-resident macrophages express several immunosuppressive molecules, including IDO, arginase-1, IL-10, and TGF-β, which may further induce the production of Treg cells. The BTB plays an important role in the trafficking of immune cells and in the transport of drugs into the testes. The BTB is a physical barrier between the blood or lymph vessels and the lumen of the seminiferous tubules of the testis.
Abbreviations: ATP, adenosine triphosphate; BTB, blood–testis barrier; IDO, indoleamine 2,3-dioxygenase; IL-10, interleukin-10; TGF-β, transforming growth factor beta; Treg, regulatory T cell.
Similarly to the eyes and CNS, testicular immune privilege was initially attributed to the presence of a barrier, specifically the blood–testis barrier (BTB).76 The BTB limits the passage of antibodies into the testes, in addition to limiting the access of germ cell antigens to testicular interstitial immune cells. In 1980, Yoshida et al.77 reported that rodent epididymal and testicular extracts had high IDO activity. Later, Hansen et al.78 demonstrated that, unlike the inflammation-induced expression of IDO that occurs in nearly all mammalian tissues, the expression of IDO in the testes is constitutive. Testicular immune privilege has been extensively characterized in rodents in which attenuated innate and adaptive immune responses have been shown to be associated with reduced T cell numbers, except for IDO-myeloid-induced Treg cells whose numbers are increased, and with the enhanced production of several anti-inflammatory cytokines including IL-10 and TGF-β.76,79,80
Sertoli cells (SCs) are found in the testes, where their main function is to provide local immune tolerance and nourishment to developing germ cells. SCs were used as a therapeutic strategy in autoimmune diabetes to self-protect and coprotect allogeneic and xenogeneic pancreatic grafts from immune destruction in different experimental settings. In a murine cotransplantation model, Fallarino et al.81 showed that inhibiting the IDO activity abrogates the ability of porcine SCs to protect pancreatic islet allografts. Furthermore, efficient Trp metabolism was linked to the protective effects of SCs, an effect that involved the IDO- and TGF-β-dependent emergence of autoantigen-specific Treg cells.
SCs, also called “mother cells”, provide myeloid and lymphoid immune tolerances to protect germ cells in the testes and cotransplanted cells at ectopic sites. This is further supported by Jrad-Lamine et al.82 who showed that the level of IDO expression in the murine epididymis is higher compared to that in tissues in which IDO expression is induced.
Understanding human testicular immune privilege in health and in disease has been limited by the difficulty of sampling testicular tissue. Recently, we were the first to assess the complex immunological milieu of the human testes in healthy and in human immunodeficiency virus (HIV)-infected adults who had elected to undergo gender reassignment surgery.83 Healthy testes expressed significantly higher levels of IDO compared to blood, as determined by quantitative PCR. Interestingly, we also observed the contribution of another immunometabolic pathway, the adenosine–adenosine triphosphate (ATP) pathway, to testicular immune privilege. This signaling pathway is characterized by its location on the extracellular surface of the cell membrane. In contrast to ATP, which acts on immune cells to promote inflammation, its metabolite adenosine functions as an anti-inflammatory molecule. The extracellular ectoenzymes CD39 and CD73, which are expressed mainly on T cells, dephosphorylate extracellular ATP, generating adenosine monophosphate (AMP) and immunosuppressive adenosine, which induces immune tolerance by blocking IL-2 signaling.84,85 Of interest, we observed significantly higher frequencies of adenosine-dependent, immunosuppressive CD39+ Treg cells and CD73+ memory CD8 T cells in the testes than in the blood. We also observed that both the KP and adenosine-ATP immunosuppressive pathway are involved in testicular immune privilege.83
Other factors that contribute to testicular immune privilege include the secretion of immunosuppressive IL-10 and TGF-β by MPs, and by peritubular, Sertoli, and testosterone-secreting Leydig cells,76,79,80 which was recently reviewed by Cutolo86 and Li et al.87 (Table 1).
The Hair Follicle and Colon: Immune-Privileged Sites Without Anatomic Barriers
In 2005, Paus et al described immune privilege-like mechanisms in hair follicles; these mechanisms included IDO expression by tolerogenic APCs, low-level expression of MHC class I, and high-level expression of TGF-β and IL-10.88–90 Hair follicles are, therefore, organs with distinctive immunologic characteristics, which was recently reviewed by Paus and Bertolini.89 Alopecia areata, an autoimmune disease resulting from the loss of immune privilege within the hair follicles, is characterized by T cell cytotoxicity toward the follicles, which causes transient, nonscarring hair loss.91 Encouragingly, the use of Ruxolitinib, a JAK2 inhibitor, blocks CD8 T cell cytotoxic function, leading to an improvement of alopecia.
Another organ without anatomic barriers is the intestinal mucosa, which encompasses epithelial cells, the lamina propria, and the mucosal immune system within the gut.92 This organ, which has the largest surface area of all of the organs, separates the human body from the outside world. Since the intestine must balance the need to tolerate food and commensal flora while controlling invading pathogens, the intestine can be considered to be an unconventional immune-privileged site. The KP was recently shown to play a significant role in establishing intestinal immune privilege. In 2013, Zelante et al.93 showed that Trp catabolites generated by certain Lactobacilli species contribute to mucosal tolerance of the commensal flora, known as the microbiota. Catabolites such as Kyns, which are AhR ligands, promote local IL-22 production by innate lymphoid cells type 2, resulting in protective intestinal homeostasis. Furthermore, Vujkovic-Cvijin et al.94 recently reported that the Lactobacilli present in stool were associated with IDO upregulation, which contributed to the tolerance of the microbiota by the gut mucosa in simian immunodeficiency virus (SIV)-infected macaques.
Recently, Lamas et al.95 determined that mice lacking the CARD9 gene were more susceptible to colitis due to the defective activation of the gene for IL-22, a cytokine involved in gut homeostasis and repair. Furthermore, Kyn generated by the catabolism of Trp by Lactobacillus increased the production of IL-22 via ligation to AhR. To establish the causal role of the microbiota in colitis, CARD9−/− germ-free mice were supplemented with Lactobacillus strains capable of metabolizing Trp; this restored IL-22 expression and AhR ligand production, which decreased susceptibility to colitis. Stool samples from patients having inflammatory bowel syndrome (IBD) showed a reduced activation of AhR compared to those form healthy controls. These results illustrate the important link between host inflammatory genes, IBD, Trp metabolites, and Lactobacilli, with the production of AhR agonists in colon homeostasis.
Other factors involved in intestinal immune privilege have been reported and include FasL, TRAIL, thymic stromal lymphopoietin, prostaglandin E2, retinoic acid, TGF-β, and IL-10 (Table 1).96–98 These substances contribute to the induction of tolerogenic DCs. Treg cells also contribute to immune privilege as reported by Coquerelle et al.99 who showed that in a murine colitis model, administration of anti-CTLA-4 monoclonal antibody induced the development and/or expansion of IL-4- and IL-10-producing Treg cells in an IDO-dependent manner, which improved the colitis.
Overall, the findings of these various studies involving different animal models highlight the therapeutic potential of KP-associated immune privilege.
Cancer and its Microenvironment: Kyn is the Enemy Within
In 2008, Mellor and Munn10 extended the definition of immune privilege to include cancer and its microenvironment. In the early stage of tumor development, proteins encoded by mutated genes are thought to be the major source of antigens in cancer cells, which are able to circumvent tumor-specific immune responses owing to the immune-privileged tumor or its microenvironment.15,100,101
IDO can be expressed by tumor cells and by myeloid cells surrounding the tumor; these myeloid cells include APCs, tumor-associated macrophages (TAMs), and myeloid-derived suppressor cells (MDSCs), which mediate an acquired immune tolerance toward the tumor, thereby thwarting host immune responses. It was recently recognized that the tumor’s metabolic microenvironment plays a major role in maintaining immune responses by causing a shift from mitochondrial oxidative phosphorylation, an efficient way to generate ATP, to a less efficient aerobic glycolysis pathway in cytotoxic T cells (CTLs).102 This metabolic reprogramming or Warburg effect within the tumor and its microenvironment leads initially to a local immune exhaustion involving CTLs and NK cells, which will become systemic when metastasis develops. Such cancer-related immune exhaustion has recently been shown to be influenced by the gut microbiota in a distal nondigestive tumor.103 Furthermore, cytokines and chemokines secreted by the hypoxic and necrotic zones of the tumor microenvironment attract TAMs; the presence of high numbers of TAMs expressing high levels of IDO and arginase-1 is a phenomenon linked to immune escape and to faster disease progression.
The tumor microenvironment also accumulates immature and tolerogenic tumor-infiltrating APCs expressing low levels of costimulatory proteins such as CD80 and CD86; thus, these APCs lack the ability to stimulate naive T cells.102 The migration of these APCs into the draining lymph nodes in which the APCs present tumor antigens to tumor-specific T cells subsequently results in their failure to activate T cells. In mice, MDSCs infiltrate the tumor microenvironment and promote T cell dysfunction and Treg cell generation through the expression of IDO and arginase-1.104 Targeting MDSCs with monoclonal antibodies has been shown to restore the tumoricidal function of tumor-infiltrating T lymphocytes in mice.
In 2003, Uyttenhove et al.105 were the first to describe the constitutive expression of IDO by either the tumor cells or the APCs in the tumor microenvironment of various human cancers including cancers of the lung, pancreas, stomach, prostate, endometrium, and ovaries. They also showed that the expression of IDO by immunogenic murine tumor cells prevented their rejection by preimmunized mice, thereby inducing immune privilege. This effect was associated with the inhibition of specific T cell responses at the tumor site, an effect that was partly reversed by the administration of an IDO inhibitor. Subsequently, a number of studies reported an important role for the KP in several other cancers.106–109
Another immunometabolic pathway involving adenosine and ATP was recently described.85,110 Pericellular adenosine in the tumor microenvironment suppresses antitumor T cells by binding to the adenosine A2 A receptor, which activates cyclic adenosine monophosphate/protein kinase A signaling; this pathway subsequently inhibits IL-2 production in T cells, thereby suppressing the function of Teff cells. Interestingly, we have recently shown in vitro that a physiological concentration of Kyn (5 µM) was sufficient to reduce the ability of memory CD4 T cells to respond to IL-2,39 therefore identifying a mechanism that can be therapeutically targeted.
Other factors contributing to immune privilege within tumors include the following immune checkpoints and immunosuppressive cytokines: (1) immune checkpoints like PD-L1 and/or PD-L2, CTLA-4, and Fas, which are expressed at higher levels in the tumor and its microenvironment are the targets of several immune interventions; and (2) the influence of cytokines which was first reported in 1989 by Werner et al.111 who showed that the influence of IFN-γ on IDO expression in cell culture using various human cells. Litzenburger et al.112 established a link between AhR and the IL-6/STAT3 axis in driving IDO expression. However, Li et al.113 have shown that the long-term maintenance of IDO expression was found to be independent of IFN-γ, IL-10, TGF-β, TNF-α, and IL-6, contrasting with IDO enzymatic activity and IFN-γ-induced AhR expression. These immune checkpoint molecules and cytokines have been recently reviewed by Galluzzi and Kroemer,114 and their inhibition by therapeutic antibodies is under intense clinical evaluation (Table 1).
KP-Associated Immune Privilege as an Immunotherapeutic Target
Given the dual role of the KP in immune tolerance and in cancer immune escape, this pathway is being investigated as an immunomodulator in autoimmune conditions and in cancer. Encouragingly, due to its upstream effect on the antigen-presenting milieu, IDO is nonredundant with more distal T cell checkpoints. Thus, blocking IDO concurrently with CTLA-4/PD-1 might confer additive benefits.
Recently, pharmacological, genetic, and immunological methods targeting IDO in a number of animal tumor models have shown therapeutic benefits.115 Furthermore, several pharmacological IDO inhibitors are currently undergoing clinical evaluation.116–118 In particular, indoximod, a competitive inhibitor of IDO, was shown in a Phase I/II study to induce tumor responses in individuals with metastatic solid tumors.118,119 A Phase 1, dose-escalation study of epacadostat (INCB024360) in patients with advanced solid tumors has been initiated to assess the tolerability and efficacy of this novel IDO inhibitor, which is a specific IDO1 enzymatic inhibitor, versus indoximod, which acts through multiple targets within the KP.120 The ongoing clinical studies of IDO inhibitors have been recently summarized.115,118
Finally, studies of combination therapy conducted by Wainwright et al.121 showed that simultaneously blocking the expression of IDO, CTLA-4, and PD-L1 in a murine model of malignant glioma, a brain tumor with poor prognosis, provided significant therapeutic benefits. These encouraging preclinical results suggest that this combinatorial immunosuppressive strategy should be assessed in patients with glioma. Furthermore, while combining antibodies to CTLA-4 and PD-1 induced cumulative toxicities in human trials, preliminary data in patients with metastatic melanoma indicate that combining IDO inhibitors with either CTLA-4 inhibitor or PD-1 inhibitor is very effective and does not cause additive immune toxicity.122,123
Preclinical studies of IDO2 and TDO inhibitors as cancer treatments are also ongoing.118,124 The results of these studies are expected to provide novel insights into the contribution of the KP to immune-privileged sites and to cancer.
Conclusion
The KP has unique features distinguishing it from those of other immunosuppressive processes that are also involved in inducing immune privilege. The KP, which affects cell growth and proliferation, functions in conjunction with immunometabolic processes that induce tolerance by bridging myeloid and lymphoid cellular interactions. IDO, the major KP enzyme, has emerged as a key player in the establishment and maintenance of immune privilege within the eye, brain, placenta, and testes, and also appears to be involved in tumor immune escape. It is expected that current and future studies investigating immunotherapeutic approaches that control the activity of KP enzymes such as IDO and TDO, and of key metabolites such as Kyn and 3-hydroxykynurenine, will lead to the development of new treatments, which will improve the clinical outcomes of transplant, autoimmune, and cancer patients.
Acknowledgments
The authors are grateful to Angie Massicotte for administrative assistance.
Abbreviations
- AhR
aryl hydrocarbon receptor
- APCs
antigen-presenting cells
- CTLA-4
cytotoxic T lymphocyte-associated protein-4
- DCs
dendritic cells
- GCN2
general control nonderepressible 2
- HIV
human immunodeficiency virus
- IDO
indoleamine 2,3-dioxygenase
- IFN-γ
interferon gamma
- MDSC(s)
myeloid-derived suppressor cell(s)
- MP
macrophage
- MIF
macrophage migration inhibitory factor
- mTOR1
mammalian target of rapamycin 1
- NK
natural killer
- KP
kynurenine pathway
- Kyn
kynurenine
- NAD
nicotinamide adenine dinucleotide
- PD-1
programmed cell death-1
- TAMs
tumor-associated macrophages
- TDO
tryptophan 2,3-dioxygenase
- Trp
tryptophan
- TNF-α
tumor necrosis factor alpha
- TGF-β
transforming growth factor beta
- Teff
effector T
- Treg
regulatory T
Footnotes
ACADEMIC EDITOR: Gilles Guillemin, Editor in Chief
PEER REVIEW: Seven peer reviewers contributed to the peer review report. Reviewers’ reports totaled 1856 words, excluding any confidential comments to the academic editor.
FUNDING: This work was supported by the Fonds de la Recherche Québec-Santé (FRQ-S): Réseau SIDA/Maladies infectieuses and Thérapie cellulaire; the Canadian Institutes of Health Research (CIHR; grant MOP 103230); the Vaccines & Immunotherapies Core of the CIHR Canadian HIV Trials Network (CTN; grant CTN 257); the Canadian Foundation for AIDS Research (CANFAR; grant 02-512); and the Canadian HIV Cure Enterprise Team Grant (HIG-133050) awarded by the CIHR in partnership with CANFAR. BR is supported by the Gustave Roussy Philanthropia course of excellence in oncology and the Townsend Haematology McGill University Research Fellowship. VM is supported by an FRQ-S Postdoctoral Fellowship Award. J-PR is the holder of the Louis Lowenstein Chair in Hematology and Oncology, McGill University. The authors confirm that the funder had no influence over the study design, content of the article, or selection of this journal.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE). Provenance: the authors were invited to submit this paper.
Author Contributions
Conceived and designed the review: J-PR, VM. Wrote the first draft of the article: J-PR, VM. Contributed to the writing of the article: J-PR, BR, GMG, VM. Jointly developed the structure and arguments for the article and contributed to the figures and table: VM, BR, GMG, J-PR. Made the critical revisions and approved the final version: J-PR, GMG. All authors reviewed and approved the final article.
REFERENCES
- 1.van Dooremaal JC. Die Entwicklung der in fremden Grund versetzten lebenden Geweba. Albrecht Von Graefes. Arch Ophthalmol. 1873;19:358–73. [Google Scholar]
- 2.Niederkorn JY. Ocular immune privilege and ocular melanoma: parallel universes or immunological plagiarism? Front Immunol. 2012;3:148. doi: 10.3389/fimmu.2012.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Medawar PB. Immunity to homologous grafted skin; the fate of skin homografts transplanted to the brain, to subcutaneous tissue, and to the anterior chamber of the eye. Br J Exp Pathol. 1948;29(1):58–69. [PMC free article] [PubMed] [Google Scholar]
- 4.Kaplan HJ, Stevens TR, Streilein JW. Transplantation immunology of the anterior chamber of the eye. I. An intra-ocular graft-vs-host reaction (immunogenic anterior uveitis) J Immunol. 1975;115(3):800–4. [PubMed] [Google Scholar]
- 5.Li L, Baroja ML, Majumdar A, et al. Human embryonic stem cells possess immune-privileged properties. Stem Cells. 2004;22(4):448–56. doi: 10.1634/stemcells.22-4-448. [DOI] [PubMed] [Google Scholar]
- 6.Chen HF, Yu CY, Chen MJ, et al. Characteristic expression of major histocompatibility complex and immune privilege genes in human pluripotent stem cells and their derivatives. Cell Transplant. 2015;24(5):845–64. doi: 10.3727/096368913X674639. [DOI] [PubMed] [Google Scholar]
- 7.Kuri-Cervantes L, Fourati S, Canderan G, Sekaly RP. Systems biology and the quest for correlates of protection to guide the development of an HIV vaccine. Curr Opin Immunol. 2016;41:91–7. doi: 10.1016/j.coi.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 8.Doyle TJ, Kaur G, Putrevu SM, et al. Immunoprotective properties of primary Sertoli cells in mice: potential functional pathways that confer immune privilege. Biol Reprod. 2012;86(1):1–14. doi: 10.1095/biolreprod.110.089425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shechter R, London A, Schwartz M. Orchestrated leukocyte recruitment to immune-privileged sites: absolute barriers versus educational gates. Nat Rev Immunol. 2013;13(3):206–18. doi: 10.1038/nri3391. [DOI] [PubMed] [Google Scholar]
- 10.Mellor AL, Munn DH. Creating immune privilege: active local suppression that benefits friends, but protects foes. Nat Rev Immunol. 2008;8(1):74–80. doi: 10.1038/nri2233. [DOI] [PubMed] [Google Scholar]
- 11.Mehraj V, Routy JP. Tryptophan catabolism in chronic viral infections: handling uninvited guests. Int J Tryptophan Res. 2015;8:41–8. doi: 10.4137/IJTR.S26862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moffett JR, Namboodiri MA. Tryptophan and the immune response. Immunol Cell Biol. 2003;81(4):247–65. doi: 10.1046/j.1440-1711.2003.t01-1-01177.x. [DOI] [PubMed] [Google Scholar]
- 13.Munn DH, Mellor AL. IDO in the tumor microenvironment: inflammation, counter-regulation, and tolerance. Trends Immunol. 2016;37(3):193–207. doi: 10.1016/j.it.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Antonioli L, Pacher P, Vizi ES, Hasko G. CD39 and CD73 in immunity and inflammation. Trends Mol Med. 2013;19(6):355–67. doi: 10.1016/j.molmed.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pitt JM, Vetizou M, Daillere R, et al. Resistance mechanisms to immune-checkpoint blockade in cancer: tumor-intrinsic and -extrinsic factors. Immunity. 2016;44(6):1255–69. doi: 10.1016/j.immuni.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 16.Ball HJ, Jusof FF, Bakmiwewa SM, Hunt NH, Yuasa HJ. Tryptophan-catabolizing enzymes—party of three. Front Immunol. 2014;5:485. doi: 10.3389/fimmu.2014.00485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guillemin GJ, Cullen KM, Lim CK, et al. Characterization of the kynurenine pathway in human neurons. J Neurosci. 2007;27(47):12884–92. doi: 10.1523/JNEUROSCI.4101-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Platten M, von Knebel Doeberitz N, Oezen I, Wick W, Ochs K. Cancer immunotherapy by targeting IDO1/TDO and their downstream effectors. Front Immunol. 2014;5:673. doi: 10.3389/fimmu.2014.00673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ott M, Litzenburger UM, Rauschenbach KJ, et al. Suppression of TDO-mediated tryptophan catabolism in glioblastoma cells by a steroid-responsive FKBP52-dependent pathway. Glia. 2015;63(1):78–90. doi: 10.1002/glia.22734. [DOI] [PubMed] [Google Scholar]
- 20.Fatokun AA, Hunt NH, Ball HJ. Indoleamine 2,3-dioxygenase 2 (IDO2) and the kynurenine pathway: characteristics and potential roles in health and disease. Amino Acids. 2013;45(6):1319–29. doi: 10.1007/s00726-013-1602-1. [DOI] [PubMed] [Google Scholar]
- 21.Hissong BD, Byrne GI, Padilla ML, Carlin JM. Upregulation of interferon-induced indoleamine 2,3-dioxygenase in human macrophage cultures by lipopolysaccharide, muramyl tripeptide, and interleukin−1. Cell Immunol. 1995;160(2):264–9. doi: 10.1016/0008-8749(95)80037-j. [DOI] [PubMed] [Google Scholar]
- 22.Currier AR, Ziegler MH, Riley MM, Babcock TA, Telbis VP, Carlin JM. Tumor necrosis factor-alpha and lipopolysaccharide enhance interferon-induced antichlamydial indoleamine dioxygenase activity independently. J Interferon Cytokine Res. 2000;20(4):369–76. doi: 10.1089/107999000312306. [DOI] [PubMed] [Google Scholar]
- 23.Babcock TA, Carlin JM. Transcriptional activation of indoleamine dioxygenase by interleukin 1 and tumor necrosis factor alpha in interferon-treated epithelial cells. Cytokine. 2000;12(6):588–94. doi: 10.1006/cyto.1999.0661. [DOI] [PubMed] [Google Scholar]
- 24.Werner-Felmayer G, Werner ER, Fuchs D, Hausen A, Reibnegger G, Wachter H. Tumour necrosis factor-alpha and lipopolysaccharide enhance interferon-induced tryptophan degradation and pteridine synthesis in human cells. Biol Chem Hoppe Seyler. 1989;370(9):1063–9. doi: 10.1515/bchm3.1989.370.2.1063. [DOI] [PubMed] [Google Scholar]
- 25.Munn DH, Zhou M, Attwood JT, et al. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science. 1998;281(5380):1191–3. doi: 10.1126/science.281.5380.1191. [DOI] [PubMed] [Google Scholar]
- 26.Matteoli G, Mazzini E, Iliev ID, et al. Gut CD103+ dendritic cells express indoleamine 2,3-dioxygenase which influences T regulatory/T effector cell balance and oral tolerance induction. Gut. 2010;59(5):595–604. doi: 10.1136/gut.2009.185108. [DOI] [PubMed] [Google Scholar]
- 27.Ravishankar B, Liu H, Shinde R, et al. Tolerance to apoptotic cells is regulated by indoleamine 2,3-dioxygenase. Proc Natl Acad Sci U S A. 2012;109(10):3909–14. doi: 10.1073/pnas.1117736109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu Y, Giver CR, Sharma A, et al. IFN-gamma and indoleamine 2,3-dioxygenase signaling between donor dendritic cells and T cells regulates graft versus host and graft versus leukemia activity. Blood. 2012;119(4):1075–85. doi: 10.1182/blood-2010-12-322891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yan Y, Zhang GX, Gran B, et al. IDO upregulates regulatory T cells via tryptophan catabolite and suppresses encephalitogenic T cell responses in experimental autoimmune encephalomyelitis. J Immunol. 2010;185(10):5953–61. doi: 10.4049/jimmunol.1001628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Romani L, Fallarino F, De Luca A, et al. Defective tryptophan catabolism underlies inflammation in mouse chronic granulomatous disease. Nature. 2008;451(7175):211–5. doi: 10.1038/nature06471. [DOI] [PubMed] [Google Scholar]
- 31.Fallarino F, Volpi C, Zelante T, et al. IDO mediates TLR9-driven protection from experimental autoimmune diabetes. J Immunol. 2009;183(10):6303–12. doi: 10.4049/jimmunol.0901577. [DOI] [PubMed] [Google Scholar]
- 32.Linsley PS, Bradshaw J, Greene J, Peach R, Bennett KL, Mittler RS. Intracellular trafficking of CTLA-4 and focal localization towards sites of TCR engagement. Immunity. 1996;4(6):535–43. doi: 10.1016/s1074-7613(00)80480-x. [DOI] [PubMed] [Google Scholar]
- 33.Munn DH, Sharma MD, Baban B, et al. GCN2 kinase in T cells mediates proliferative arrest and anergy induction in response to indoleamine 2,3-dioxygenase. Immunity. 2005;22(5):633–42. doi: 10.1016/j.immuni.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 34.Fallarino F, Grohmann U, You S, et al. The combined effects of tryptophan starvation and tryptophan catabolites down-regulate T cell receptor zeta-chain and induce a regulatory phenotype in naive T cells. J Immunol. 2006;176(11):6752–61. doi: 10.4049/jimmunol.176.11.6752. [DOI] [PubMed] [Google Scholar]
- 35.Gagliani N, Amezcua Vesely MC, Iseppon A, et al. Th17 cells transdifferentiate into regulatory T cells during resolution of inflammation. Nature. 2015;523(7559):221–5. doi: 10.1038/nature14452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sharma MD, Shinde R, McGaha TL, et al. The PTEN pathway in Tregs is a critical driver of the suppressive tumor microenvironment. Sci Adv. 2015;1(10):e1500845. doi: 10.1126/sciadv.1500845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ravishankar B, Liu H, Shinde R, et al. The amino acid sensor GCN2 inhibits inflammatory responses to apoptotic cells promoting tolerance and suppressing systemic autoimmunity. Proc Natl Acad Sci U S A. 2015;112(34):10774–9. doi: 10.1073/pnas.1504276112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Quintana FJ, Murugaiyan G, Farez MF, et al. An endogenous aryl hydrocarbon receptor ligand acts on dendritic cells and T cells to suppress experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A. 2010;107(48):20768–73. doi: 10.1073/pnas.1009201107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dagenais-Lussier X, Aounallah M, Mehraj V, et al. Kynurenine reduces memory CD4 T-cell survival by interfering with interleukin-2 signaling early during HIV-1 infection. J Virol. 2016;90(17):7967–79. doi: 10.1128/JVI.00994-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fallarino F, Grohmann U, Vacca C, et al. T cell apoptosis by tryptophan catabolism. Cell Death Differ. 2002;9(10):1069–77. doi: 10.1038/sj.cdd.4401073. [DOI] [PubMed] [Google Scholar]
- 41.Forrester JV, Xu H, Lambe T, Cornall R. Immune privilege or privileged immunity? Mucosal Immunol. 2008;1(5):372–81. doi: 10.1038/mi.2008.27. [DOI] [PubMed] [Google Scholar]
- 42.Niederkorn JY. The induction of anterior chamber-associated immune deviation. Chem Immunol Allergy. 2007;92:27–35. doi: 10.1159/000099251. [DOI] [PubMed] [Google Scholar]
- 43.Beutelspacher SC, Pillai R, Watson MP, et al. Function of indoleamine 2,3-dioxygenase in corneal allograft rejection and prolongation of allograft survival by over-expression. Eur J Immunol. 2006;36(3):690–700. doi: 10.1002/eji.200535238. [DOI] [PubMed] [Google Scholar]
- 44.Ryu YH, Kim JC. Expression of indoleamine 2,3-dioxygenase in human corneal cells as a local immunosuppressive factor. Invest Ophthalmol Vis Sci. 2007;48(9):4148–52. doi: 10.1167/iovs.05-1336. [DOI] [PubMed] [Google Scholar]
- 45.Yoshida M, Takeuchi M, Streilein JW. Participation of pigment epithelium of iris and ciliary body in ocular immune privilege. 1. Inhibition of T-cell activation in vitro by direct cell-to-cell contact. Invest Ophthalmol Vis Sci. 2000;41(3):811–21. [PubMed] [Google Scholar]
- 46.Benhar I, London A, Schwartz M. The privileged immunity of immune privileged organs: the case of the eye. Front Immunol. 2012;3:296. doi: 10.3389/fimmu.2012.00296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sonoda KH, Exley M, Snapper S, Balk SP, Stein-Streilein J. CD1-reactive natural killer T cells are required for development of systemic tolerance through an immune-privileged site. J Exp Med. 1999;190(9):1215–26. doi: 10.1084/jem.190.9.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kaplan HJ, Streilein JW. Immune response to immunization via the anterior chamber of the eye. I. F. lymphocyte-induced immune deviation. J Immunol. 1977;118(3):809–14. [PubMed] [Google Scholar]
- 49.Streilein JW. Ocular immune privilege: the eye takes a dim but practical view of immunity and inflammation. J Leukoc Biol. 2003;74(2):179–85. doi: 10.1189/jlb.1102574. [DOI] [PubMed] [Google Scholar]
- 50.Streilein JW, Arancibia-Caracamo C, Osawa H. The role of minor histocompatibility alloantigens in penetrating keratoplasty. Dev Ophthalmol. 2003;36:74–88. doi: 10.1159/000067655. [DOI] [PubMed] [Google Scholar]
- 51.Goldmann EE. Vitalfarbungen am Zentralnervensystem. Beitrag zur Physio-Pathologie des Plexus Chorioideus und der Hirnhaute. Abh. Preuss. Akad. Wiss. Physik. -Math.; 1913. [Google Scholar]
- 52.Ehrlich P. Das Sauerstoff-Bedürfniss des Organismus: eine farbenanalytische Studie. A. Hirschwald; Berlin: 1885. [Google Scholar]
- 53.Abbott NJ. Dynamics of CNS barriers: evolution, differentiation, and modulation. Cell Mol Neurobiol. 2005;25(1):5–23. doi: 10.1007/s10571-004-1374-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Carson MJ, Doose JM, Melchior B, Schmid CD, Ploix CC. CNS immune privilege: hiding in plain sight. Immunol Rev. 2006;213:48–65. doi: 10.1111/j.1600-065X.2006.00441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guillemin GJ, Kerr SJ, Smythe GA, et al. Kynurenine pathway metabolism in human astrocytes: a paradox for neuronal protection. J Neurochem. 2001;78(4):842–853. doi: 10.1046/j.1471-4159.2001.00498.x. [DOI] [PubMed] [Google Scholar]
- 56.Ting KK, Brew BJ, Guillemin GJ. Effect of quinolinic acid on human astrocytes morphology and functions: implications in Alzheimer’s disease. J Neuroinflammation. 2009;6:36. doi: 10.1186/1742-2094-6-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Guillemin GJ, Brew BJ, Noonan CE, Takikawa O, Cullen KM. Indoleamine 2,3 dioxygenase and quinolinic acid immunoreactivity in Alzheimer’s disease hippocampus. Neuropathol Appl Neurobiol. 2005;31(4):395–404. doi: 10.1111/j.1365-2990.2005.00655.x. [DOI] [PubMed] [Google Scholar]
- 58.Guillemin GJ, Smythe G, Takikawa O, Brew BJ. Expression of indoleamine 2,3-dioxygenase and production of quinolinic acid by human microglia, astrocytes, and neurons. Glia. 2005;49(1):15–23. doi: 10.1002/glia.20090. [DOI] [PubMed] [Google Scholar]
- 59.Kwidzinski E, Bunse J, Kovac AD, et al. IDO (indolamine 2,3-dioxygenase) expression and function in the CNS. Adv Exp Med Biol. 2003;527:113–8. doi: 10.1007/978-1-4615-0135-0_13. [DOI] [PubMed] [Google Scholar]
- 60.Kwidzinski E, Bunse J, Aktas O, et al. Indolamine 2,3-dioxygenase is expressed in the CNS and down-regulates autoimmune inflammation. FASEB J. 2005;19(10):1347–9. doi: 10.1096/fj.04-3228fje. [DOI] [PubMed] [Google Scholar]
- 61.Lovelace MD, Varney B, Sundaram G, et al. Current evidence for a role of the kynurenine pathway of tryptophan metabolism in multiple sclerosis. Front Immunol. 2016;7:246. doi: 10.3389/fimmu.2016.00246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Louveau A, Harris TH, Kipnis J. Revisiting the mechanisms of CNS immune privilege. Trends Immunol. 2015;36(10):569–77. doi: 10.1016/j.it.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pang PC, Haslam SM, Dell A, Clark GF. The human fetoembryonic defense system hypothesis: twenty years on. Mol Aspects Med. 2016;51:71–88. doi: 10.1016/j.mam.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 64.Medawar PB. Immunological tolerance. Nature. 1961;189:14–7. doi: 10.1038/189014a0. [DOI] [PubMed] [Google Scholar]
- 65.Erlebacher A. Mechanisms of T cell tolerance towards the allogeneic fetus. Nat Rev Immunol. 2013;13(1):23–33. doi: 10.1038/nri3361. [DOI] [PubMed] [Google Scholar]
- 66.Holmes CH, Simpson KL, Wainwright SD, et al. Preferential expression of the complement regulatory protein decay accelerating factor at the fetomaternal interface during human pregnancy. J Immunol. 1990;144(8):3099–105. [PubMed] [Google Scholar]
- 67.Aluvihare VR, Kallikourdis M, Betz AG. Regulatory T cells mediate maternal tolerance to the fetus. Nat Immunol. 2004;5(3):266–271. doi: 10.1038/ni1037. [DOI] [PubMed] [Google Scholar]
- 68.Guleria I, Sayegh MH. Maternal acceptance of the fetus: true human tolerance. J Immunol. 2007;178(6):3345–51. doi: 10.4049/jimmunol.178.6.3345. [DOI] [PubMed] [Google Scholar]
- 69.Zenclussen AC, Gerlof K, Zenclussen ML, et al. Abnormal T-cell reactivity against paternal antigens in spontaneous abortion: adoptive transfer of pregnancy-induced CD4+CD25+ T regulatory cells prevents fetal rejection in a murine abortion model. Am J Pathol. 2005;166(3):811–22. doi: 10.1016/S0002-9440(10)62302-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Somerset DA, Zheng Y, Kilby MD, Sansom DM, Drayson MT. Normal human pregnancy is associated with an elevation in the immune suppressive CD25+ CD4+ regulatory T-cell subset. Immunology. 2004;112(1):38–43. doi: 10.1111/j.1365-2567.2004.01869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Arcuri F, Cintorino M, Carducci A, et al. Human decidual natural killer cells as a source and target of macrophage migration inhibitory factor. Reproduction. 2006;131(1):175–82. doi: 10.1530/rep.1.00857. [DOI] [PubMed] [Google Scholar]
- 72.Gustafsson C, Mjosberg J, Matussek A, et al. Gene expression profiling of human decidual macrophages: evidence for immunosuppressive phenotype. PLoS One. 2008;3(4):e2078. doi: 10.1371/journal.pone.0002078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Moffett A, Loke C. Immunology of placentation in eutherian mammals. Nat Rev Immunol. 2006;6(8):584–94. doi: 10.1038/nri1897. [DOI] [PubMed] [Google Scholar]
- 74.Wira CR, Rodriguez-Garcia M, Patel MV. The role of sex hormones in immune protection of the female reproductive tract. Nat Rev Immunol. 2015;15(4):217–30. doi: 10.1038/nri3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Slukvin II, Lunn DP, Watkins DI, Golos TG. Placental expression of the nonclassical MHC class I molecule Mamu-AG at implantation in the rhesus monkey. Proc Natl Acad Sci U S A. 2000;97(16):9104–9. doi: 10.1073/pnas.97.16.9104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jacobo P, Guazzone VA, Theas MS, Lustig L. Testicular autoimmunity. Autoimmun Rev. 2011;10(4):201–4. doi: 10.1016/j.autrev.2010.09.026. [DOI] [PubMed] [Google Scholar]
- 77.Yoshida R, Nukiwa T, Watanabe Y, Fujiwara M, Hirata F, Hayaishi O. Regulation of indoleamine 2,3-dioxygenase activity in the small intestine and the epididymis of mice. Arch Biochem Biophys. 1980;203(1):343–51. doi: 10.1016/0003-9861(80)90185-x. [DOI] [PubMed] [Google Scholar]
- 78.Hansen AM, Ball HJ, Mitchell AJ, Miu J, Takikawa O, Hunt NH. Increased expression of indoleamine 2,3-dioxygenase in murine malaria infection is predominantly localised to the vascular endothelium. Int J Parasitol. 2004;34(12):1309–19. doi: 10.1016/j.ijpara.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 79.De Rose R, Fernandez CS, Hedger MP, Kent SJ, Winnall WR. Characterisation of macaque testicular leucocyte populations and T-lymphocyte immunity. J Reprod Immunol. 2013;100(2):146–56. doi: 10.1016/j.jri.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 80.Winnall WR, Muir JA, Hedger MP. Rat resident testicular macrophages have an alternatively activated phenotype and constitutively produce interleukin-10 in vitro. J Leukoc Biol. 2011;90(1):133–43. doi: 10.1189/jlb.1010557. [DOI] [PubMed] [Google Scholar]
- 81.Fallarino F, Luca G, Calvitti M, et al. Therapy of experimental type 1 diabetes by isolated Sertoli cell xenografts alone. J Exp Med. 2009;206(11):2511–26. doi: 10.1084/jem.20090134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jrad-Lamine A, Henry-Berger J, Gourbeyre P, et al. Deficient tryptophan catabolism along the kynurenine pathway reveals that the epididymis is in a unique tolerogenic state. J Biol Chem. 2011;286(10):8030–42. doi: 10.1074/jbc.M110.172114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jenabian MA, Costiniuk CT, Mehraj V, et al. Immune tolerance properties of the testicular tissue as a viral sanctuary site in ART-treated HIV-infected adults. AIDS. 2016 Sep 24; doi: 10.1097/QAD.0000000000001282. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 84.Nikolova M, Carriere M, Jenabian MA, et al. CD39/adenosine pathway is involved in AIDS progression. PLoS Pathog. 2011;7(7):e1002110. doi: 10.1371/journal.ppat.1002110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jenabian MA, Seddiki N, Yatim A, et al. Regulatory T cells negatively affect IL-2 production of effector T cells through CD39/adenosine pathway in HIV infection. PLoS Pathog. 2013;9(4):e1003319. doi: 10.1371/journal.ppat.1003319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cutolo M. Androgens in rheumatoid arthritis: when are they effectors? Arthritis Res Ther. 2009;11(5):126. doi: 10.1186/ar2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Li N, Wang T, Han D. Structural, cellular and molecular aspects of immune privilege in the testis. Front Immunol. 2012;3:152. doi: 10.3389/fimmu.2012.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Paus R, Nickoloff BJ, Ito T. A ‘hairy’ privilege. Trends Immunol. 2005;26(1):32–40. doi: 10.1016/j.it.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 89.Paus R, Bertolini M. The role of hair follicle immune privilege collapse in alopecia areata: status and perspectives. J Investig Dermatol Symp Proc. 2013;16(1):S25–S27. doi: 10.1038/jidsymp.2013.7. [DOI] [PubMed] [Google Scholar]
- 90.Meyer KC, Klatte JE, Dinh HV, et al. Evidence that the bulge region is a site of relative immune privilege in human hair follicles. Br J Dermatol. 2008;159(5):1077–85. doi: 10.1111/j.1365-2133.2008.08818.x. [DOI] [PubMed] [Google Scholar]
- 91.Pieri L, Guglielmelli P, Vannucchi AM. Ruxolitinib-induced reversal of alopecia universalis in a patient with essential thrombocythemia. Am J Hematol. 2015;90(1):82–3. doi: 10.1002/ajh.23871. [DOI] [PubMed] [Google Scholar]
- 92.Rooks MG, Garrett WS. Gut microbiota, metabolites and host immunity. Nat Rev Immunol. 2016;16(6):341–52. doi: 10.1038/nri.2016.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zelante T, Iannitti RG, Cunha C, et al. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin−22. Immunity. 2013;39(2):372–85. doi: 10.1016/j.immuni.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 94.Vujkovic-Cvijin I, Swainson LA, Chu SN, et al. Gut-resident Lactobacillus abundance associates with IDO1 inhibition and Th17 dynamics in SIV-infected Macaques. Cell Rep. 2015;13(8):1589–97. doi: 10.1016/j.celrep.2015.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lamas B, Richard ML, Leducq V, et al. CARD9 impacts colitis by altering gut microbiota metabolism of tryptophan into aryl hydrocarbon receptor ligands. Nat Med. 2016;22(6):598–605. doi: 10.1038/nm.4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Singh B, Read S, Asseman C, et al. Control of intestinal inflammation by regulatory T cells. Immunol Rev. 2001;182:190–200. doi: 10.1034/j.1600-065x.2001.1820115.x. [DOI] [PubMed] [Google Scholar]
- 97.Izcue A, Coombes JL, Powrie F. Regulatory T cells suppress systemic and mucosal immune activation to control intestinal inflammation. Immunol Rev. 2006;212:256–71. doi: 10.1111/j.0105-2896.2006.00423.x. [DOI] [PubMed] [Google Scholar]
- 98.Rescigno M. The intestinal epithelial barrier in the control of homeostasis and immunity. Trends Immunol. 2011;32(6):256–64. doi: 10.1016/j.it.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 99.Coquerelle C, Oldenhove G, Acolty V, et al. Anti-CTLA-4 treatment induces IL-10-producing ICOS+ regulatory T cells displaying IDO-dependent anti-inflammatory properties in a mouse model of colitis. Gut. 2009;58(10):1363–73. doi: 10.1136/gut.2008.162842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Joyce JA, Fearon DT. T cell exclusion, immune privilege, and the tumor microenvironment. Science. 2015;348(6230):74–80. doi: 10.1126/science.aaa6204. [DOI] [PubMed] [Google Scholar]
- 101.Munn DH, Mellor AL. Indoleamine 2,3 dioxygenase and metabolic control of immune responses. Trends Immunol. 2013;34(3):137–43. doi: 10.1016/j.it.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Speiser DE, Ho PC, Verdeil G. Regulatory circuits of T cell function in cancer. Nat Rev Immunol. 2016;16(10):599–611. doi: 10.1038/nri.2016.80. [DOI] [PubMed] [Google Scholar]
- 103.Zitvogel L, Ayyoub M, Routy B, Kroemer G. Microbiome and anticancer immunosurveillance. Cell. 2016;165(2):276–87. doi: 10.1016/j.cell.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 104.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9(3):162–74. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Uyttenhove C, Pilotte L, Theate I, et al. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat Med. 2003;9(10):1269–74. doi: 10.1038/nm934. [DOI] [PubMed] [Google Scholar]
- 106.Munn DH, Sharma MD, Hou D, et al. Expression of indoleamine 2,3-dioxygenase by plasmacytoid dendritic cells in tumor-draining lymph nodes. J Clin Invest. 2004;114(2):280–90. doi: 10.1172/JCI21583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Curti A, Pandolfi S, Valzasina B, et al. Modulation of tryptophan catabolism by human leukemic cells results in the conversion of CD25- into CD25+ T regulatory cells. Blood. 2007;109(7):2871–7. doi: 10.1182/blood-2006-07-036863. [DOI] [PubMed] [Google Scholar]
- 108.Mansfield AS, Heikkila PS, Vaara AT, von Smitten KA, Vakkila JM, Leidenius MH. Simultaneous Foxp3 and IDO expression is associated with sentinel lymph node metastases in breast cancer. BMC Cancer. 2009;9:231. doi: 10.1186/1471-2407-9-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Moretti S, Menicali E, Voce P, et al. Indoleamine 2,3-dioxygenase 1 (IDO1) is up-regulated in thyroid carcinoma and drives the development of an immunosuppressant tumor microenvironment. J Clin Endocrinol Metab. 2014;99(5):E832–40. doi: 10.1210/jc.2013-3351. [DOI] [PubMed] [Google Scholar]
- 110.Regateiro FS, Cobbold SP, Waldmann H. CD73 and adenosine generation in the creation of regulatory microenvironments. Clin Exp Immunol. 2013;171(1):1–7. doi: 10.1111/j.1365-2249.2012.04623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Werner ER, Werner-Felmayer G, Fuchs D, Hausen A, Reibnegger G, Wachter H. Parallel induction of tetrahydrobiopterin biosynthesis and indoleamine 2,3-dioxygenase activity in human cells and cell lines by interferon-gamma. Biochem J. 1989;262(3):861–6. doi: 10.1042/bj2620861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Litzenburger UM, Opitz CA, Sahm F, et al. Constitutive IDO expression in human cancer is sustained by an autocrine signaling loop involving IL-6, STAT3 and the AHR. Oncotarget. 2014;5(4):1038–51. doi: 10.18632/oncotarget.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Li Q, Harden JL, Anderson CD, Egilmez NK. Tolerogenic phenotype of IFN-gamma-induced IDO+ dendritic cells is maintained via an autocrine IDO-kynurenine/AhR-IDO loop. J Immunol. 2016;197(3):962–70. doi: 10.4049/jimmunol.1502615. [DOI] [PubMed] [Google Scholar]
- 114.Galluzzi L, Kroemer G. A four-lane highway to cancer. Nat Rev Mol Cell Biol. 2016;17(7):398. doi: 10.1038/nrm.2016.73. [DOI] [PubMed] [Google Scholar]
- 115.Vacchelli E, Aranda F, Eggermont A, et al. Trial watch: IDO inhibitors in cancer therapy. Oncoimmunology. 2014;3(10):e957994. doi: 10.4161/21624011.2014.957994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Soliman HH, Jackson E, Neuger T, et al. A first in man phase I trial of the oral immunomodulator, indoximod, combined with docetaxel in patients with metastatic solid tumors. Oncotarget. 2014;5(18):8136–46. doi: 10.18632/oncotarget.2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Colman H, Mott F, Spira AI, et al. A phase 1b/2 study of the combination of the IDO pathway inhibitor indoximod and temozolomide for adult patients with temozolomide-refractory primary malignant brain tumors: Safety analysis and preliminary efficacy of the phase 1b component. ASCO Meeting; Chicago. 2015. [Google Scholar]
- 118.Buque A, Bloy N, Aranda F, et al. Trial Watch-Small molecules targeting the immunological tumor microenvironment for cancer therapy. Oncoimmunology. 2016;5(6):e1149674. doi: 10.1080/2162402X.2016.1149674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Soliman H, Ringash J, Jiang H, et al. Phase II trial of palliative radiotherapy for hepatocellular carcinoma and liver metastases. J Clin Oncol. 2013;31(31):3980–6. doi: 10.1200/JCO.2013.49.9202. [DOI] [PubMed] [Google Scholar]
- 120.Khleif S, Munn DH, Nyak-Kapoor A, et al. First-in-human phase 1study of the novel indoleamine-2,3-dioxygenase (IDO) inhibitor NLG−919. ASCO Meeting; Chicago. 2014. [Google Scholar]
- 121.Wainwright DA, Chang AL, Dey M, et al. Durable therapeutic efficacy utilizing combinatorial blockade against IDO, CTLA-4, and PD-L1 in mice with brain tumors. Clin Cancer Res. 2014;20(20):5290–301. doi: 10.1158/1078-0432.CCR-14-0514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zakharia Y, Drabick JJ, Khleif S, et al. Updates on phase1b/2 trial of the indoleamine 2,3-dioxygenase pathway (IDO) inhibitor indoximod plus checkpoint inhibitors for the treatment of unresectable stage 3 or 4 melanoma. ASCO Meeting; Chicago. 2016. [Google Scholar]
- 123.Gangadhar TC, Hamid O, Smith DC, et al. Preliminary results from a phase I/II study of epacadostat (incb024360) in combination with pembrolizumab in patients with selected advanced cancers. J Immunother Cancer. 2015;3(suppl 2):O7. [Google Scholar]
- 124.Zhai L, Spranger S, Binder DC, et al. Molecular pathways: targeting IDO1 and other tryptophan dioxygenases for cancer immunotherapy. Clin Cancer Res. 2015;21(24):5427–33. doi: 10.1158/1078-0432.CCR-15-0420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sugita S, Streilein JW. Iris pigment epithelium expressing CD86 (B7-2) directly suppresses T cell activation in vitro via binding to cytotoxic T lymphocyte- associated antigen 4. J Exp Med. 2003;198(1):161–71. doi: 10.1084/jem.20030097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gimsa U, ØRen A, Pandiyan P, et al. Astrocytes protect the CNS: antigen-specific T helper cell responses are inhibited by astrocyte-induced upregulation of CTLA-4 (CD152) J Mol Med (Berl) 2004;82(6):364–72. doi: 10.1007/s00109-004-0531-6. [DOI] [PubMed] [Google Scholar]
- 127.Kaufman KA, Bowen JA, Tsai AF, Bluestone JA, Hunt JS, Ober C. The CTLA-4 gene is expressed in placental fibroblasts. Mol Hum Reprod. 1999;5(1):84–7. doi: 10.1093/molehr/5.1.84. [DOI] [PubMed] [Google Scholar]
- 128.Waterhouse P, Penninger JM, Timms E, et al. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science. 1995;270(5238):985–8. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- 129.Hori J, Wang M, Miyashita M, et al. B7-H1-induced apoptosis as a mechanism of immune privilege of corneal allografts. J Immunol. 2006;177(9):5928–35. doi: 10.4049/jimmunol.177.9.5928. [DOI] [PubMed] [Google Scholar]
- 130.Magnus T, Schreiner B, Korn T, et al. Microglial expression of the B7 family member B7 homolog 1 confers strong immune inhibition: implications for immune responses and autoimmunity in the CNS. J Neurosci. 2005;25(10):2537–46. doi: 10.1523/JNEUROSCI.4794-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Dal Secco V, Riccioli A, Padula F, Ziparo E, Filippini A. Mouse Sertoli cells display phenotypical and functional traits of antigen-presenting cells in response to interferon gamma. Biol Reprod. 2008;78(2):234–42. doi: 10.1095/biolreprod.107.063578. [DOI] [PubMed] [Google Scholar]
- 132.Kuang DM, Zhao Q, Peng C, et al. Activated monocytes in peritumoral stroma of hepatocellular carcinoma foster immune privilege and disease progression through PD-L1. J Exp Med. 2009;206(6):1327–37. doi: 10.1084/jem.20082173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wilbanks GA, Streilein JW. Fluids from immune privileged sites endow macrophages with the capacity to induce antigen-specific immune deviation via a mechanism involving transforming growth factor-beta. Eur J Immunol. 1992;22(4):1031–6. doi: 10.1002/eji.1830220423. [DOI] [PubMed] [Google Scholar]
- 134.Mullaney BP, Skinner MK. Transforming growth factor-beta (beta 1, beta 2, and beta 3) gene expression and action during pubertal development of the seminiferous tubule: potential role at the onset of spermatogenesis. Mol Endocrinol. 1993;7(1):67–76. doi: 10.1210/mend.7.1.8446109. [DOI] [PubMed] [Google Scholar]
- 135.Houston A, Bennett MW, O’Sullivan GC, Shanahan F, O’Connell J. Fas ligand mediates immune privilege and not inflammation in human colon cancer, irrespective of TGF-beta expression. Br J Cancer. 2003;89(7):1345–51. doi: 10.1038/sj.bjc.6601240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Apte RS, Niederkorn JY. Isolation and characterization of a unique natural killer cell inhibitory factor present in the anterior chamber of the eye. J Immunol. 1996;156(8):2667–73. [PubMed] [Google Scholar]
- 137.Bacher M, Meinhardt A, Lan HY, et al. MIF expression in the rat brain: implications for neuronal function. Mol Med. 1998;4(4):217–30. [PMC free article] [PubMed] [Google Scholar]
- 138.Meinhardt A, Bacher M, McFarlane JR, et al. Macrophage migration inhibitory factor production by Leydig cells: evidence for a role in the regulation of testicular function. Endocrinology. 1996;137(11):5090–5. doi: 10.1210/endo.137.11.8895383. [DOI] [PubMed] [Google Scholar]
- 139.Ito T, Ito N, Saatoff M, et al. Maintenance of hair follicle immune privilege is linked to prevention of NK cell attack. J Invest Dermatol. 2008;128(5):1196–206. doi: 10.1038/sj.jid.5701183. [DOI] [PubMed] [Google Scholar]
- 140.Simpson KD, Templeton DJ, Cross JV. Macrophage migration inhibitory factor promotes tumor growth and metastasis by inducing myeloid-derived suppressor cells in the tumor microenvironment. J Immunol. 2012;189(12):5533–40. doi: 10.4049/jimmunol.1201161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ryan AF, Grendell RL, Geraghty DE, Golos TG. A soluble isoform of the rhesus monkey nonclassical MHC class I molecule Mamu-AG is expressed in the placenta and the testis. J Immunol. 2002;169(2):673–83. doi: 10.4049/jimmunol.169.2.673. [DOI] [PubMed] [Google Scholar]
- 142.Yamagami S, Kawashima H, Tsuru T, et al. Role of Fas-Fas ligand interactions in the immunorejection of allogeneic mouse corneal transplants. Transplantation. 1997;64(8):1107–11. doi: 10.1097/00007890-199710270-00004. [DOI] [PubMed] [Google Scholar]
- 143.Choi C, Benveniste EN. Fas ligand/Fas system in the brain: regulator of immune and apoptotic responses. Brain Res Brain Res Rev. 2004;44(1):65–81. doi: 10.1016/j.brainresrev.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 144.Kauma SW, Huff TF, Hayes N, Nilkaeo A. Placental Fas ligand expression is a mechanism for maternal immune tolerance to the fetus. J Clin Endocrinol Metab. 1999;84(6):2188–94. doi: 10.1210/jcem.84.6.5730. [DOI] [PubMed] [Google Scholar]
- 145.Bellgrau D, Gold D, Selawry H, Moore J, Franzusoff A, Duke RC. A role for CD95 ligand in preventing graft rejection. Nature. 1995;377(6550):630–2. doi: 10.1038/377630a0. [DOI] [PubMed] [Google Scholar]
- 146.Wang S, Boonman ZF, Li HC, et al. Role of TRAIL and IFN-gamma in CD4+ T cell-dependent tumor rejection in the anterior chamber of the eye. J Immunol. 2003;171(6):2789–96. doi: 10.4049/jimmunol.171.6.2789. [DOI] [PubMed] [Google Scholar]
- 147.Lee HO, Herndon JM, Barreiro R, Griffith TS, Ferguson TA. TRAIL: a mechanism of tumor surveillance in an immune privileged site. J Immunol. 2002;169(9):4739–44. doi: 10.4049/jimmunol.169.9.4739. [DOI] [PubMed] [Google Scholar]