A syndrome-specific intervention to improve the management of community-acquired pneumonia in non-intensive care settings was associated with shorter treatment durations, less fluoroquinolone use, and a reduction in use of low-yield diagnostic tests.
Keywords: antimicrobial stewardship, community-acquired pneumonia, duration of therapy, levofloxacin, quality improvement
Abstract
Background. Syndrome-specific interventions are a recommended approach to antibiotic stewardship, but additional data are needed to understand their potential impact. We implemented an intervention to improve the management of inpatient community-acquired pneumonia (CAP) and evaluated its effects on antibiotic and resource utilization.
Methods. A stakeholder group developed and implemented a clinical practice guideline and order set for inpatient, non-intensive care unit CAP recommending a short course (5 days) of a fluoroquinolone-sparing antibiotic regimen in uncomplicated cases. Unless there was suspicion for complications or resistant pathogens, chest computed tomography (CT) and sputum cultures were discouraged. This was a retrospective preintervention postintervention study of patients hospitalized for CAP before (April 15, 2008–May 31, 2009) and after (July 1, 2011–July 31, 2012) implementation of the guideline. The primary comparison was the difference in duration of therapy during the baseline and intervention periods. Secondary outcomes included changes in use of levofloxacin, CT scans, and sputum culture.
Results. One hundred sixty-six and 84 cases during the baseline and intervention periods, respectively, were included. From the baseline to intervention period, the median duration of therapy decreased from 10 to 7 days (P < .0001). Prescription of levofloxacin at discharge decreased from 60% to 27% of cases (P < .0001). Use of chest CT and sputum culture decreased from 47% to 32% of cases (P = .02) and 51% to 31% of cases (P = .03), respectively. The frequency of clinical failure between the 2 periods was similar.
Conclusions. A syndrome-specific intervention for inpatient CAP was associated with shorter treatment durations and reductions in use of fluoroquinolones and low-yield diagnostic tests.
The White House National Action Plan for Combating Antibiotic-Resistant Bacteria outlines a set of strategies and goals to address the crisis of antimicrobial resistance [1]. Among these goals are, by 2020, to establish antibiotic stewardship programs in all hospitals and to reduce inpatient inappropriate antibiotic use by 20%. As one approach to reduce inappropriate antibiotic use, the Centers for Disease Control and Prevention (CDC), Infectious Diseases Society of America (IDSA), and Society for Healthcare Epidemiology of America (SHEA) recommend syndrome-specific antibiotic stewardship interventions [2, 3].
Community-acquired pneumonia (CAP) leads to approximately 1.1 million hospitalizations and 50 000 deaths annually in the United States [4] and is the most common indication for antibiotic therapy in US hospitals [5]. Previous studies have demonstrated there is substantial opportunity to reduce unnecessary antibiotic use among patients hospitalized with CAP [6–8]. Therefore, in many hospitals, CAP may represent a high-yield target for a syndrome-specific intervention to improve antibiotic use.
In cases of uncomplicated CAP, short courses of antibiotic therapy (≤7 days) have been shown to be effective [9–13]. However, at our institution and others, treatment durations are often prolonged for 10 or more days [6–8]. In addition to prolonged durations of therapy, the treatment of inpatient CAP often involves broad-spectrum antibiotic regimens. Respiratory fluoroquinolones, treatment options advocated by the IDSA and American Thoracic Society (ATS) [14], are efficacious for CAP but have broad activity against Gram-negative as well as Gram-positive pathogens and are associated with the development of antimicrobial resistance [15–19] and potentially severe adverse events [20–22]. Despite this, they are the most frequently prescribed antibiotics at the time of hospital discharge [6]. Thus, shortening treatment durations and optimizing antibiotic selection represent important opportunities to reduce unnecessary antibiotic exposure among patients hospitalized with CAP.
In addition to antibiotic use, there is opportunity to improve the diagnostic evaluation of CAP. At our institution, we observed frequent use of what appeared to be unnecessary or low-yield diagnostic tests; for example, early computed tomography (CT) scans of the chest despite an infiltrate being visible by chest radiograph and lack of concern for complications of pneumonia [6]. Furthermore, sputum specimens for culture were frequently of poor quality, delayed, or not able to obtained, resulting in a potential pathogen being identified in only 11% of cases when ordered.
To improve both antibiotic use and the diagnostic evaluation in cases of CAP, we developed a syndrome-specific antibiotic stewardship intervention that involved the multidisciplinary development and implementation of a clinical practice guideline. Adherence to guidelines for the management of CAP has been shown to be associated with reduced antibiotic use and improved clinical outcomes [23–27]; however, this has most often been demonstrated in clinical trials. The objectives of this intervention were to (1) reduce the duration of therapy in cases of uncomplicated CAP, (2) reduce overall levofloxacin use, and (3) prevent low-yield use of CT scans and sputum cultures in a clinical practice setting. In this study, we evaluate the effects of this intervention on antibiotic use, resource utilization, and outcomes.
METHODS
Study Setting
Denver Health is an integrated public healthcare system with a 477-bed teaching hospital [28]. Patients with CAP are admitted to Medicine services (both teaching and nonteaching), the majority of which are staffed by Hospitalist physicians, and less commonly, Internal Medicine or Family Medicine physicians. Internal Medicine residents and medical students participate in the care of patients on the teaching services, whereas advanced practice providers assist with care of patients on nonteaching services. A formal antibiotic stewardship program has been in place at Denver Health since July 2008. Prospective audit with feedback to prescribers, a preauthorization requirement for restricted antibiotics, and the development of prescribing guidelines for common infections have been used as the core strategies to improve antibiotic use since the inception of the program [29].
Study Design and Population
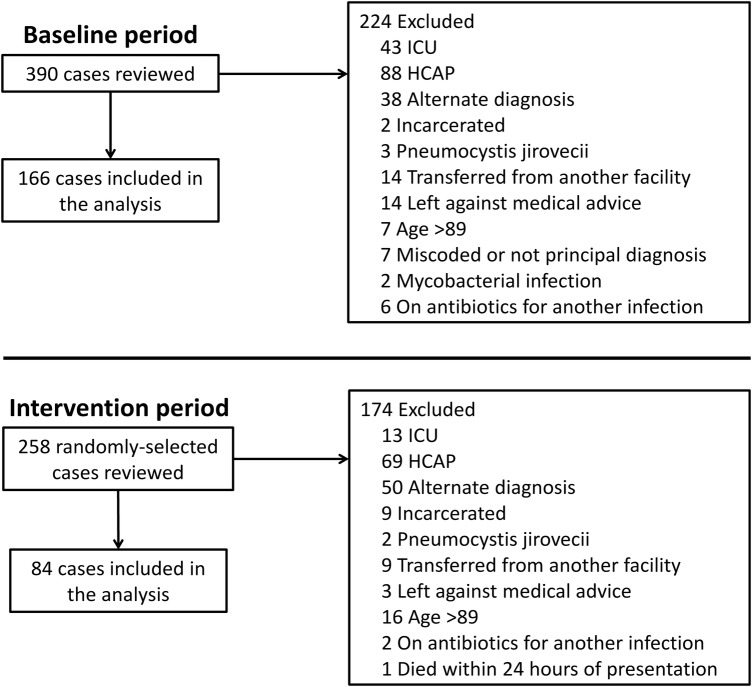
We performed a retrospective preintervention postintervention study comparing the management of patients hospitalized for CAP during periods before and after the intervention: April 15, 2008–May 31, 2009 (baseline period) and July 1, 2011–July 31, 2012 (intervention period). The findings from of the baseline period have been previously published [6]. We included patients 18–89 years of age who were admitted to a non-intensive care unit (ICU) medical ward with a principal diagnosis of CAP. Exclusion criteria included the following: ICU admission at the time of presentation, an alternative diagnosis for the respiratory illness, healthcare-associated pneumonia [30], Pneumocystis jirovecii or mycobacterial infection, pregnancy, incarceration, leaving against medical advice, receipt of antibiotics for an indication other than pneumonia, death within 24 hours of presentation, miscoded or not principal diagnosis, and transfer from an outside facility.
Study Definitions
Community-acquired pneumonia was defined according to IDSA/ATS guidance [14]. Severity of illness was determined by calculating each subject's CURB-65 score [31]. Clinical failure was a composite endpoint of any of the following during the hospitalization or within 30 days after discharge: (1) treatment failure, defined as a change in antibiotic therapy due to worsening signs or symptoms of infection or lack of clinical improvement; (2) in-hospital mortality; (3) recurrence, defined as signs or symptoms of infection after completion of therapy requiring re-initiation of antibiotics; (4) rehospitalization due to pulmonary infection; or (5) death during the follow-up period [6].
Intervention
After collection and analysis of the baseline period data, the antibiotic stewardship program convened a multidisciplinary meeting of stakeholders, including representatives from Infectious Diseases, Hospital Medicine, Pulmonary and Critical Care, Internal Medicine, Emergency Medicine, Pathology, Department of Patient Safety and Quality, Pharmacy, and the microbiology laboratory. The group reviewed the baseline period data describing current Denver Health management practices and reviewed the literature pertaining to the diagnosis and treatment of CAP. Based on these reviews, goals were set for the intervention. In April 2010, a draft institutional guideline for the management of non-ICU patients with CAP was circulated to the stakeholder group, revised based on feedback, and finalized in August 2010 (Supplementary Figure). Due to a major restructuring of the process by which guidelines are approved at our institution, formal approval of the clinical practice guideline was significantly delayed and ultimately occurred in June 2011, at which time the guideline was disseminated.
In the guideline, recommended empiric therapy included ceftriaxone plus azithromycin, with transition to oral azithromycin alone upon clinical improvement. A total treatment duration of 5 days was recommended in cases with an appropriate clinical response and no evidence of complications (eg, pleural space infection). Use of levofloxacin was recommended only in the setting of a severe allergy or other contraindication to β-lactam agents. If an infiltrate was evident on chest radiograph, use of chest CT was discouraged unless there was clinical suspicion of a complication. Due to the low yield of routine sputum culture at our institution, sputum culture was recommended only when there was suspicion for infection with Staphylococcus aureus or Gram-negative pathogens.
In June 2011, the guideline was disseminated to clinicians and pharmacists by electronic mail (including periodic e-mail reminders) and published on the hospital's antibiotic stewardship intranet site. The guidelines were additionally posted in physician work areas, particularly Hospitalist work areas because the majority of our patients admitted to the medicine service are managed by our Hospitalist group. Two Hospitalist physicians who were members of the stakeholder group promoted use of the guideline among their colleagues through discussion at staff meetings, resident noon conferences, and Family Medicine grand rounds. A computerized provider order entry (CPOE) admission order set was also developed incorporating the diagnostic and treatment recommendations in the guideline. For the proposed study, the intervention period was considered to start July 1, 2011, 2 weeks after the guideline was disseminated. There were no changes to the general structure or focus of the antibiotic stewardship program between the start of the baseline period and the end of the intervention period. In particular, ceftriaxone, azithromycin, and levofloxacin were unrestricted antibiotics. The Colorado Multiple Institutional Review Board reviewed the proposal and classified the work as quality improvement.
Data Collection
We identified potentially eligible cases from our healthcare data warehouse through the use of International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) codes for pneumonia: (481, 482.0, 482.1, 482.2, 482.30, 482.31, 482.32, 482.39, 482.40, 482.41, 482.42, 482.49, 482.82, 482.83, 482.84, 482.89, 482.9, 483.0, 483.1, 483.8, 485, 486). Cases from both periods were manually reviewed using a standardized data collection instrument. Individuals who did not have a principal discharge diagnosis of CAP were manually excluded before detailed chart review. For the baseline period, all identified cases during this timeframe were reviewed. For the intervention period, to reduce the burden of data collection, a randomly selected subset of cases during this timeframe was reviewed. We collected demographic, clinical, laboratory, microbiologic, and radiographic data and recorded comorbid conditions through a combination of ICD-9-CM codes and medical record review.
Antibiotics administered during the inpatient stay were recorded from the electronic medical administration record. Antibiotics prescribed at discharge were determined by review of pharmacy fill data and the hospital discharge summary. All clinical encounters occurring within 30 days after hospital discharge were reviewed to assess clinical outcomes.
Study Outcomes
The primary endpoint was the difference in the median length of antibiotic therapy between the baseline and intervention periods. Secondary outcomes included the change between the baseline and intervention periods in the proportion of patients who received levofloxacin, the proportion where chest CT or sputum culture were used, the incidence of clinical failure, and length of hospital stay.
Statistical Analysis
Descriptive statistics were used to describe clinical and demographic characteristics, microbiological and radiographic studies, and clinical outcomes. Continuous variables were summarized as mean or median (depending on distribution). Descriptive analyses of differences for continuous measures used the Mann-Whitney U test. Comparison of discrete data used the χ2 test for proportions or Fisher's exact test where appropriate. Analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC), with statistical significance assessed at the 5% level.
RESULTS
During the 2 periods, a total of 648 potential cases were identified by the ICD-9-CM searches. Three hundred ninety-eight cases were excluded for reasons shown in Figure 1 . A total of 250 patients were included for analysis: 166 from the baseline period and 84 from the intervention period.
Figure 1.
Study schematic.
Demographic and clinical characteristics of the included patients are shown in Table 1. The groups were similar with respect to baseline characteristics, except that there were significantly more patients with chronic obstructive pulmonary disease during the intervention period (27, 32%) compared with the baseline period (30, 18%) (P = .01). Severity of illness, as measured by the CURB-65 score and the presence of septic shock, multilobar infiltrates, and bacteremia, was also similar between the 2 groups.
Table 1.
Demographic and Clinical Characteristicsa
| Demographics/Clinical Characteristics | Baseline Period (n = 166) | Intervention Period (n = 84) | Total Cohort (n = 250) | P Value |
|---|---|---|---|---|
| Age, mean (standard deviation) | 53 (15) | 50 (19) | 52 (17) | .16 |
| Male | 91 (55) | 50 (59) | 141 (56) | .5 |
| Comorbid conditions | ||||
| Current smoking | 85 (51) | 42 (50) | 127 (51) | .9 |
| Alcohol abuse | 41 (25) | 22 (27) | 63 (26) | .8 |
| Diabetes mellitus | 31 (19) | 12 (16) | 43 (18) | .4 |
| COPD | 30 (18) | 27 (32) | 55 (22) | .01 |
| Asthma | 26 (16) | 7 (8) | 33 (13) | .1 |
| Cardiovascular disease | 20 (12) | 7 (8) | 27 (11) | .4 |
| HIV infection | 11 (7) | 11 (13) | 22 (9) | .09 |
| CURB-65 score | .7 | |||
| 0 | 55 (33) | 30 (35) | 85 (34) | |
| 1 | 67 (40) | 32 (32) | 99 (40) | |
| 2 | 32 (19) | 13 (16) | 45 (18) | |
| 3 | 11 (7) | 9 (11) | 20 (8) | |
| 4 | 1 (1) | 0 | 1 (0.4) | |
| Septic shock | 2 (1) | 2 (2) | 4 (2) | .5 |
| Multilobar infiltrate | 68 (41) | 32 (38) | 100 (40) | .7 |
| Bacteremia | 9 (5) | 3 (4) | 12 (5) | .5 |
Abbreviations: COPD, chronic obstructive pulmonary disease; HIV, human immunodeficiency virus.
a Data presented as n (%) unless otherwise noted.
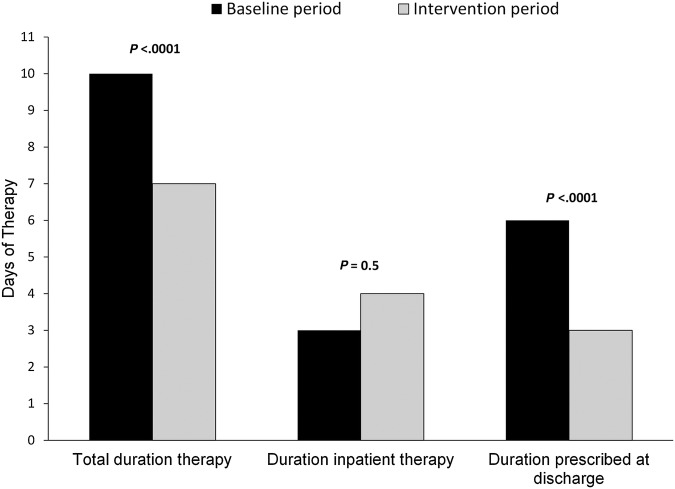
The median total duration of therapy decreased from 10 days during the baseline period (interquartile range [IQR], 8–11 days) to 7 days (IQR, 5–8 days) during the intervention period (P < .0001) (Figure 2). This was largely driven by a decrease in the duration of therapy prescribed at hospital discharge during the intervention period (median 6 days [IQR, 4–7 days] vs 3 days [IQR, 1–5 days]; P < .0001).
Figure 2.
Comparison of duration of antibiotic therapy before and during the intervention.
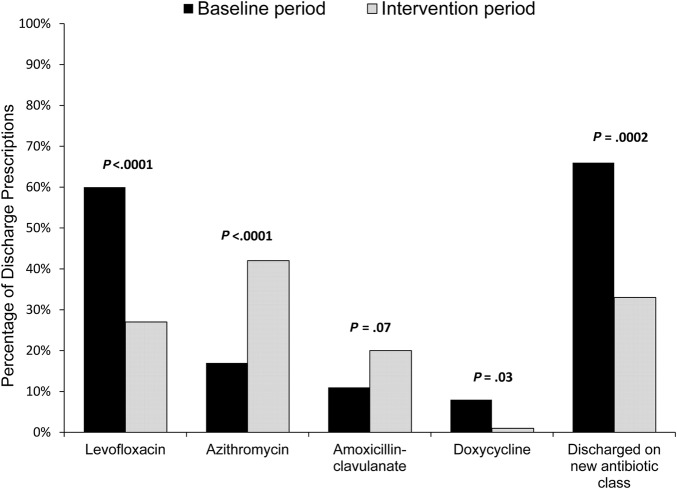
Initial inpatient therapy consisted of ceftriaxone plus azithromycin in 147 (89%) and 76 (90%) cases during the baseline and intervention periods, respectively (P = .6). At the time of hospital discharge, a new antibiotic class was significantly less likely to be prescribed during the intervention period (Figure 3). Prescriptions for levofloxacin decreased from 99 (60%) cases at baseline to 23 (27%) (P < .0001) during the intervention period. In total, any exposure to a fluoroquinolone (inpatient or discharge prescription) decreased from 107 (64%) cases at baseline to 38 (45%) (P = .004) during the intervention period; the average duration of fluoroquinolone therapy decreased from 4.4 days (standard deviation [SD] = 3.9 days) to 2.1 days (SD = 2.9 days) (P < .0001), respectively. In accordance with the guideline, prescription of azithromycin at hospital discharge increased (29 [17%] vs 35 [42%], P < .0001).
Figure 3.
Comparison of antibiotics prescribed at hospital discharge before and during the intervention.
With the exception of 3 individuals during the baseline period, all patients had a chest radiograph. An infiltrate was present on chest radiograph somewhat less frequently during the intervention period (81% of cases) compared with the baseline period (88% of cases) (P = .2). Despite this, chest CT was performed less frequently during the intervention period (47% vs 32%, P = .02).
Blood cultures were obtained with similar frequency between the 2 periods (81% vs 74% of cases, P = .2). However, the proportion of patients in whom sputum cultures were obtained decreased from 51% at baseline to 31% during the intervention period (P = .03). There was no change in the proportion of sputum cultures ordered where adequate specimens were obtained (38% vs 38%, P = .13).
The composite outcome of clinical failure occurred in 7% and 10% of cases in the baseline and intervention periods, respectively (P = .53) (Table 2). Although there were no statistically significant differences between periods among the individual components of the composite outcome, there was a trend toward more frequent rehospitalizations due to pulmonary infection during the intervention period (2 [1%] vs 5 [6%], P = .12). Four of the 5 patients rehospitalized for pulmonary infection during the intervention period had initially received therapy that was not concordant with the clinical practice guideline recommendations. Hospital length of stay was similar between the 2 periods.
Table 2.
Clinical Outcomesa
| Clinical Outcome | Baseline Period (n = 166) | Intervention Period (n = 84) | Total Cohort (n = 250) | P Value |
|---|---|---|---|---|
| Clinical failure | 12 (7) | 8 (10) | 20 (8) | .53 |
| In-hospital mortality | 2 (1) | 0 | 2 (0.8) | .55 |
| Treatment failure | 8 (5) | 3 (4) | 11 (4) | .76 |
| Recurrence | 3 (2) | 3 (4) | 6 (2) | .41 |
| Rehospitalization within 30 d due to pulmonary infection | 2 (1) | 5 (6) | 8 (3) | .12 |
| Death within 30 d after discharge | 0 | 0 | 0 | – |
| Medical ward to ICU transfer >24 h after admission | 7 (4) | 7 (8) | 14 (6) | .24 |
| Rehospitalization within 30 d | 11 (7) | 8 (10) | 19 (8) | .41 |
| Length of hospital stay, median days (IQR) | 4 (3–5) | 4 (3–6) | 4 (3–5) | .15 |
Abbreviations: ICU, intensive care unit; IQR; interquartile range.
a Data presented as n (%) unless otherwise noted.
DISCUSSION
This syndrome-specific antibiotic stewardship intervention to improve the evaluation and treatment of inpatient, non-ICU CAP was associated with intended changes in prescribing practices, including significant reductions in the total duration of antibiotic therapy, use of levofloxacin, and the prescription of a new antibiotic class at the time of hospital discharge. The intervention was also associated with significant reductions in use of chest CT and sputum cultures, studies that were deemed to be low yield in the initial workup of uncomplicated CAP.
Previous studies have demonstrated that CAP can be effectively treated with short courses (≤7 days) of antibiotic therapy [10, 13]; however, clinicians frequently prescribe longer durations [6, 7]. The intervention under study was associated with a 30% reduction in duration of therapy (from a median of 10 days to 7 days). Although our guideline recommended a treatment duration of 5 days for uncomplicated infections, we suspect we did not observe a reduction in the median to 5 days because this study included complicated as well as uncomplicated cases, uptake of the prescribing guidance among providers was incomplete, and the clinical response to therapy was likely delayed in some cases leading to extension of therapy. Our result is identical to that of a distinct antibiotic stewardship program intervention described by Avdic et al [7], where prospective identification of patients hospitalized with CAP and the provision of recommendations for the duration of therapy was associated with a decrease in the median duration of therapy from 10 to 7 days. This demonstrates that there is not a single correct approach to syndrome-specific antibiotic stewardship and supports the CDC and IDSA/SHEA recommendation that programs should tailor interventions to existing resources and hospital infrastructure [2, 3]. It is worth noting that, on an ongoing basis, our intervention likely required less personnel time and may therefore represent an effective approach for hospitals without the resources to perform daily antibiotic stewardship activities. This has particular relevance because the Centers for Medicare and Medicaid Services (CMS) and The Joint Commission (TJC) have proposed regulations to require all hospitals to engage in antibiotic stewardship [32, 33].
In addition to shorter treatment durations, our intervention was associated with marked changes in antibiotic selection. The IDSA/ATS guideline for the management of CAP states that at the time of discharge, transition from β-lactam and macrolide combination therapy to a highly orally bioavailable agent such as a fluoroquinolone is not necessary [14]. Our intervention was associated with more than a 50% relative reduction in the prescription of fluoroquinolones at the time of discharge, a substantial reduction in overall fluoroquinolone use, and less frequent prescription of a new antibiotic class at discharge. Fluoroquinolone use is associated with the emergence of resistance among both Gram-negative and Gram-positive pathogens [19, 34], is considered a high-risk agent for the development of Clostridium difficile infection [21, 22, 35], and can delay the diagnosis of pulmonary tuberculosis [36]. Furthermore, fluoroquinolones can cause severe and potentially permanent side effects, leading the US Food and Drug Administration to recently approve labeling changes to enhance warnings about these risks [20]. Thus, reducing fluoroquinolone exposure in favor of azithromycin at the time of hospital discharge, as achieved with this intervention, may represent one effective approach to prevent these unintended consequences.
Although the goal at the outset of this intervention was to improve antibiotic therapy in cases of inpatient CAP, our baseline period data highlighted several opportunities to reduce low-yield use of diagnostic tests. Chest CT is not routinely recommended by the IDSA/ATS for the diagnosis of CAP [14]. In clinical scenarios where there is not suspicion for a complication of pneumonia, a chest CT infrequently provides clinically useful information and may lead to unnecessary evaluation of incidental findings, increases exposure to radiation [37, 38], and increases costs. Accordingly, our clinical practice guideline encouraged clinicians to limit the use of chest CT to cases with suspected complications, and this was associated with a significant decline in utilization of this test. Similarly, our recommendation to limit use of sputum culture to cases with an increased risk of antibiotic-resistant pathogens is consistent with IDSA/ATS guidance and was associated with a significant reduction in overall use of sputum cultures. Together, the changes in use of chest CT and sputum culture highlight that syndrome-specific interventions may not only be effective tools to improve stewardship of antibiotics but also healthcare resources.
Although there was not a significant difference in the composite endpoint of clinical failure, we observed a trend toward more frequent rehospitalizations for pulmonary infections during the intervention period. The significance of this finding is uncertain, particularly given the overall low number of adverse clinical outcomes. Of the 5 individuals who were rehospitalized during the intervention period, 4 did not receive antibiotic therapy concordant with the clinical practice guideline. Furthermore, in vitro resistance to macrolides among Streptococcus pneumoniae is substantially more frequent than resistance to fluoroquinolones at our institution. However, during the intervention period when azithromycin alone was recommended to complete oral therapy, we did not identify any treatment failures, recurrent infections, or rehospitalizations associated with a macrolide-resistant S pneumoniae isolate. Therefore, although the finding of increased rehospitalizations due to pulmonary infection during the intervention period deserves further exploration, it seems unlikely that the shorter antibiotic courses or increased macrolide use were causative factors.
CONCLUSIONS
Our study had several limitations. First, the intervention was performed at a single site and therefore is not generalizable. Second, the retrospective preintervention postintervention design was subject to period effect. It is important to note that because the baseline period data were collected before the intervention (to inform the intervention), and because of the extensive delay in obtaining formal institutional approval of the clinical practice guideline, there was a substantial time gap between the baseline period and the start of the intervention. Because this was a quality improvement project, we did not have the resources to collect data during this gap and during the intervention period. Despite this, we are not aware of factors other than the intervention that may explain the observed changes in both diagnosis and treatment during the intervention period. In particular, there were no changes in the activities or focus of the antibiotic stewardship program that would have accounted for the changes in antibiotic use. In addition, no new diagnostic tests (eg, rapid diagnostic panels, procalcitonin) were introduced between the baseline and intervention periods that might have impacted use of sputum cultures. Furthermore, the consistent direction of the results in favor of the intervention for multiple prespecified goals of the intervention are highly suggestive that the changes were due to the intervention itself. It is nonetheless important to note that this study does not prove the changes in management were a result of the intervention. Third, although the 2 groups were relatively similar with respect to clinical characteristics and severity of illness, selection bias could have contributed to differences between the groups that affected study outcomes. Fourth, the relatively short study period and small sample size precluded interrupted time series analysis and a more robust comparison of differences in clinical outcomes associated with the intervention. Finally, it is not known from this study whether the intervention would lead to persistent effects over a longer time period.
Overall, our findings demonstrate that a syndrome-specific antibiotic stewardship intervention to improve the management of non-ICU CAP was associated with substantial changes in antibiotic use and use of diagnostic tests. This work has important implications in light of the recently proposed CMS and TJC requirements that hospitals actively participate in antibiotic stewardship [32, 33], because syndrome-specific interventions such as this may be an effective, low-resource approach to improve use of antibiotics and healthcare resources while satisfying these regulatory requirements.
Supplementary Data
Supplementary material is available online at Open Forum Infectious Diseases online (http://OpenForumInfectiousDiseases.oxfordjournals.org/).
Acknowledgments
Financial support. This work was supported by the Department of Patient Safety and Quality, Denver Health and the National Institute of Allergy and Infectious Diseases, National Institutes of Health (grant K23 AI099082; to T. C. J.).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. National Action Plan for Combating Antibiotic-Resistant Bacteria. March 2015. Available at: https://www.whitehouse.gov/sites/default/files/docs/national_action_plan_for_combating_antibotic-resistant_bacteria.pdf Accessed 6 April 2015.
- 2. Pollack LA, Srinivasan A. Core elements of hospital antibiotic stewardship programs from the Centers for Disease Control and Prevention. Clin Infect Dis 2014; 59 (Suppl 3):S97–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Barlam TF, Cosgrove SE, Abbo LM et al. Executive Summary: Implementing an antibiotic stewardship program: guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis 2016; 62:e51–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Centers for Disease Control and Prevention. National and Global Impact of Pneumonia. 2010. Available at: http://www.cdc.gov/Features/Pneumonia/ Accessed 6 June 2014.
- 5. Magill SS, Edwards JR, Beldavs ZG et al. Prevalence of antimicrobial use in US acute care hospitals, May-September 2011. JAMA 2014; 312:1438–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jenkins TC, Stella SA, Cervantes L et al. Targets for antibiotic and healthcare resource stewardship in inpatient community-acquired pneumonia: a comparison of management practices with National Guideline Recommendations. Infection 2013; 41:135–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Avdic E, Cushinotto LA, Hughes AH et al. Impact of an antimicrobial stewardship intervention on shortening the duration of therapy for community-acquired pneumonia. Clin Infect Dis 2012; 54:1581–7. [DOI] [PubMed] [Google Scholar]
- 8. Yogo N, Haas MK, Knepper BC et al. Antibiotic prescribing at the transition from hospitalization to discharge: a target for antibiotic stewardship. Infect Control Hosp Epidemiol 2015; 36:474–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Siegel RE, Alicea M, Lee A et al. Comparison of 7 versus 10 days of antibiotic therapy for hospitalized patients with uncomplicated community-acquired pneumonia: a prospective, randomized, double-blind study. Am J Ther 1999; 6:217–22. [DOI] [PubMed] [Google Scholar]
- 10. el Moussaoui R, de Borgie CA, van den Broek P et al. Effectiveness of discontinuing antibiotic treatment after three days versus eight days in mild to moderate-severe community acquired pneumonia: randomised, double blind study. BMJ 2006; 332:1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tellier G, Niederman MS, Nusrat R et al. Clinical and bacteriological efficacy and safety of 5 and 7 day regimens of telithromycin once daily compared with a 10 day regimen of clarithromycin twice daily in patients with mild to moderate community-acquired pneumonia. J Antimicrob Chemother 2004; 54:515–23. [DOI] [PubMed] [Google Scholar]
- 12. Dunbar LM, Wunderink RG, Habib MP et al. High-dose, short-course levofloxacin for community-acquired pneumonia: a new treatment paradigm. Clin Infect Dis 2003; 37:752–60. [DOI] [PubMed] [Google Scholar]
- 13. Dimopoulos G, Matthaiou DK, Karageorgopoulos DE et al. Short- versus long-course antibacterial therapy for community-acquired pneumonia: a meta-analysis. Drugs 2008; 68:1841–54. [DOI] [PubMed] [Google Scholar]
- 14. Mandell LA, Wunderink RG, Anzueto A et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis 2007; 44 (Suppl 2):S27–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chen DK, McGeer A, de Azavedo JC et al. Decreased susceptibility of Streptococcus pneumoniae to fluoroquinolones in Canada. Canadian Bacterial Surveillance Network. N Engl J Med 1999; 341:233–9. [DOI] [PubMed] [Google Scholar]
- 16. MacDougall C, Powell JP, Johnson CK et al. Hospital and community fluoroquinolone use and resistance in Staphylococcus aureus and Escherichia coli in 17 US hospitals. Clin Infect Dis 2005; 41:435–40. [DOI] [PubMed] [Google Scholar]
- 17. Ambrose PG, Bast D, Doern GV et al. Fluoroquinolone-resistant Streptococcus pneumoniae, an emerging but unrecognized public health concern: is it time to resight the goalposts? Clin Infect Dis 2004; 39:1554–6. [DOI] [PubMed] [Google Scholar]
- 18. Lautenbach E, Strom BL, Nachamkin I et al. Longitudinal trends in fluoroquinolone resistance among Enterobacteriaceae isolates from inpatients and outpatients, 1989–2000: differences in the emergence and epidemiology of resistance across organisms. Clin Infect Dis 2004; 38:655–62. [DOI] [PubMed] [Google Scholar]
- 19. Johnson L, Sabel A, Burman WJ et al. Emergence of fluoroquinolone resistance in outpatient urinary Escherichia coli isolates. Am J Med 2008; 121:876–84. [DOI] [PubMed] [Google Scholar]
- 20. U.S. Food and Drug Administration. FDA News Release: FDA updates warnings for fluoroquinolone antibiotics. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm513183.htm Accessed 9 August 2016.
- 21. Stevens V, Dumyati G, Fine LS et al. Cumulative antibiotic exposures over time and the risk of Clostridium difficile infection. Clin Infect Dis 2011; 53:42–8. [DOI] [PubMed] [Google Scholar]
- 22. Slimings C, Riley TV. Antibiotics and hospital-acquired Clostridium difficile infection: update of systematic review and meta-analysis. J Antimicrob Chemother 2014; 69:881–91. [DOI] [PubMed] [Google Scholar]
- 23. McCabe C, Kirchner C, Zhang H et al. Guideline-concordant therapy and reduced mortality and length of stay in adults with community-acquired pneumonia: playing by the rules. Arch Intern Med 2009; 169:1525–31. [DOI] [PubMed] [Google Scholar]
- 24. Dean NC, Bateman KA, Donnelly SM et al. Improved clinical outcomes with utilization of a community-acquired pneumonia guideline. Chest 2006; 130:794–9. [DOI] [PubMed] [Google Scholar]
- 25. Yealy DM, Auble TE, Stone RA et al. Effect of increasing the intensity of implementing pneumonia guidelines: a randomized, controlled trial. Ann Intern Med 2005; 143:881–94. [DOI] [PubMed] [Google Scholar]
- 26. Mortensen EM, Restrepo M, Anzueto A et al. Effects of guideline-concordant antimicrobial therapy on mortality among patients with community-acquired pneumonia. Am J Med 2004; 117:726–31. [DOI] [PubMed] [Google Scholar]
- 27. Fleming NS, Ogola G, Ballard DJ. Implementing a standardized order set for community-acquired pneumonia: impact on mortality and cost. Jt Comm J Qual Patient Saf 2009; 35:414–21. [DOI] [PubMed] [Google Scholar]
- 28. Gabow P, Eisert S, Wright R. Denver Health: a model for the integration of a public hospital and community health centers. Ann Intern Med 2003; 138:143–9. [DOI] [PubMed] [Google Scholar]
- 29. Jenkins TC, Knepper BC, Shihadeh K et al. Long-term outcomes of an antimicrobial stewardship program implemented in a hospital with low baseline antibiotic use. Infect Control Hosp Epidemiol 2015; 36:664–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. American Thoracic Society and Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med 2005; 171:388–416. [DOI] [PubMed] [Google Scholar]
- 31. Lim WS, van der Eerden MM, Laing R et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax 2003; 58:377–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Centers for Medicare and Medicaid Services. Proposed Rules: Hospital and Critical Access Hospital Changes to Promote Innovation, Flexibility, and Improvement in Patient Care. Available at: https://www.federalregister.gov/public-inspection Accessed 16 June 2016.
- 33. The Joint Commission. Proposed Standard for Antimicrobial Stewardship in AHC, CAH, HAP, NCC, and OBS. Available at: https://jointcommission.az1.qualtrics.com/ControlPanel/ Accessed 15 June 2016.
- 34. Chen IL, Lee CH, Su LH et al. Antibiotic consumption and healthcare-associated infections caused by multidrug-resistant gram-negative bacilli at a large medical center in Taiwan from 2002 to 2009: implicating the importance of antibiotic stewardship. PLoS One 2013; 8:e65621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zilberberg MD, Reske K, Olsen M et al. Risk factors for recurrent Clostridium difficile infection (CDI) hospitalization among hospitalized patients with an initial CDI episode: a retrospective cohort study. BMC Infect Dis 2014; 14:306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dooley KE, Golub J, Goes FS et al. Empiric treatment of community-acquired pneumonia with fluoroquinolones, and delays in the treatment of tuberculosis. Clin Infect Dis 2002; 34:1607–12. [DOI] [PubMed] [Google Scholar]
- 37. Berrington de Gonzalez A, Mahesh M, Kim KP et al. Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Intern Med 2009; 169:2071–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Meer AB, Basu PA, Baker LC et al. Exposure to ionizing radiation and estimate of secondary cancers in the era of high-speed CT scanning: projections from the Medicare population. J Am Coll Radiol 2012; 9:245–50. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.