Summary
Human erythro-megakaryopoiesis does not occur in humanized mouse models, preventing the in vivo analysis of human hematopoietic stem cell (HSC) differentiation into these lineages in a surrogate host. Here we show that stably engrafted KIT-deficient NOD/SCID Il2rg−/−KitW41/W41 (NSGW41) mice support much improved human erythropoiesis and platelet formation compared with irradiated NSG recipients. Considerable numbers of human erythroblasts and mature thrombocytes are present in the bone marrow and blood, respectively. Morphology, composition, and enucleation capacity of de novo generated human erythroblasts in NSGW41 mice are comparable with those in human bone marrow. Overexpression of human erythropoietin showed no further improvement in human erythrocyte output, but depletion of macrophages led to the appearance of human erythrocytes in the blood. Human erythropoiesis up to normoblasts and platelet formation is fully supported in NSGW41 mice, allowing the analysis of human HSC differentiation into these lineages, the exploration of certain pathophysiologies, and the evaluation of gene therapeutic approaches.
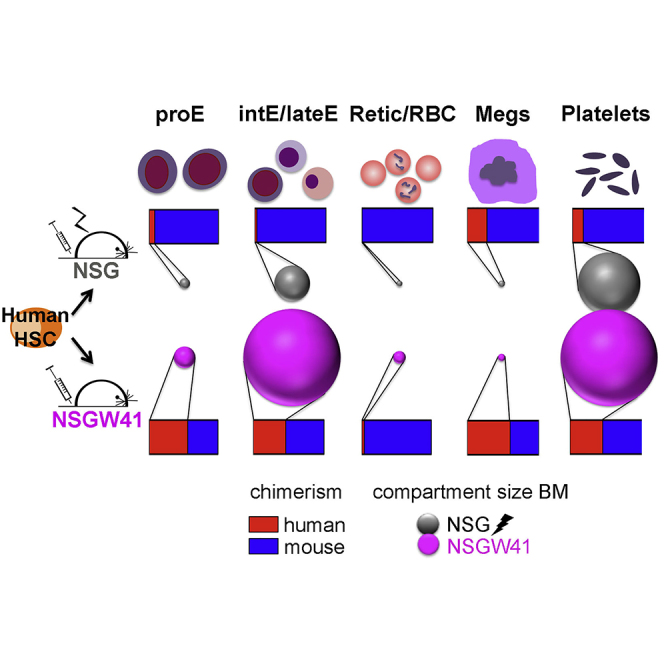
Graphical Abstract
Highlights
-
•
High engraftment of human erythro-megakaryocytic cells in NSGW41 mice
-
•
Complete maturation of human thrombocytes in vivo
-
•
Robust human erythropoiesis up to final nucleated RBC progenitors in vivo
In this article, Rahmig and colleagues report on highly improved reconstitution of human thrombocytes and erythroid cells in a KIT-deficient humanized mouse model, NSGW41 mice. They show that stable human HSC engraftment is sufficient to ensure continuous and robust output of human thrombocytic and erythroid cells. Enucleation of human normoblasts in mice occurs independently of human EPO.
Introduction
Erythrocytes are essential for oxygen supply, and anemia can have severe systemic consequences. The causes of anemia include iron deficiency/blood loss, cancer, infectious disease, and genetic disorders such as sickle cell anemia or thalassemia. Analysis of mechanisms leading to anemia is severely limited by the lack of appropriate in vivo models (Pishesha et al., 2014). Also, the generation of functional platelets in humanized mouse models is challenging. Abnormal platelet generation and function cause bleeding disorders and evoke thrombotic and cardiovascular diseases that are potential causes of mortality (Massberg et al., 2002). To date, the ex vivo generation of platelets from donor-independent or autologous sources has been hampered by an incomplete understanding of mechanisms promoting platelet differentiation and maturation (Karagiannis and Eto, 2015, Lambert et al., 2013). Finally, it is impossible to test for differentiation of these lineages in vivo (Mende et al., 2016), underlining the need for suitable in vivo models to study these processes.
Many immunodeficient mouse models receptive for transplantation of human hematopoietic stem cells (HSCs) have been developed over the last decade to support the engraftment and differentiation of specific hematopoietic lineages (Cosgun et al., 2014, Chen et al., 2009, Rongvaux et al., 2014). However, in all mouse models available to date human erythropoiesis is severely impaired. In contrast, human platelets can develop and mature in humanized mouse models, but their output is generally very low (Hu and Yang, 2012, Rongvaux et al., 2011, Suzuki et al., 2007). Clearance of human red blood cells (RBCs) by macrophages after infusion into CD47-deficient mice suggests that absence of RBCs and their immediate progenitors is due to signal-regulatory protein alpha (SIRPalpha)-independent phagocytic activity (Hu et al., 2011). Furthermore, macrophage depletion or overexpression of human interleukin-3 (IL-3) and human erythropoietin (hEPO) lead to the transient appearance of low numbers of human RBCs in the blood of humanized mice (Hu et al., 2011, Chen et al., 2009). Also, thrombocyte reconstitution in the blood can be further improved by treatment with clodronate liposomes or pegylated recombinant human megakaryocyte growth and development factor (Hu and Yang, 2012, Suzuki et al., 2007). However, due to the transient nature of the increase of human RBCs and human platelets in all models, the use of humanized mouse models to study human erythrocyte differentiation is severely limited.
We generated mouse strains suitable for humanization by introducing loss-of-function KIT receptors (W41 or Wv alleles) into NOD/SCID Il2rg−/− (NSG; NSGW41, NSGWv) or BALB/c Rag2−/− Il2rg−/− (BRgWv) mice (Cosgun et al., 2014). These mice efficiently support stable engraftment of human hematopoietic stem and progenitor cells (HSPCs) in the long term without the need for previous conditioning therapy, and were instrumental for the study of effects of cell physiological processes on human HSC function in vivo (Mende et al., 2015). A second striking feature is the remarkably increased engraftment of human myeloid cells in bone marrow (BM) and spleen, evidencing that stable stem cell engraftment is sufficient to ensure continuous output of human myeloid cells (Cosgun et al., 2014, Rahmig et al., 2015). In BRgWv mice, low frequencies of human erythroid cells were detected in the BM (Cosgun et al., 2014), suggesting that RBC generation may occur in humanized KIT-mutant recipient mice. Here, we show the efficient differentiation of human donor HSPCs into erythroblasts/normoblasts and megakaryocytes in humanized NSGW41 recipients compared with conventional NSG recipients, suggesting that growth factors responsible for these differentiation paths are compatible across species. Mature human RBCs are absent and low numbers of human platelets are found in the blood, but macrophage depletion transiently restores erythrocyte and platelet repopulation. The entire differentiation from human HSCs into RBCs and platelets is recapitulated in the murine BM, providing an in vivo setting that allows for the study of the regulation of RBC and platelet formation in health and disease.
Results and Discussion
Human Erythropoiesis in NSGW41 Recipients
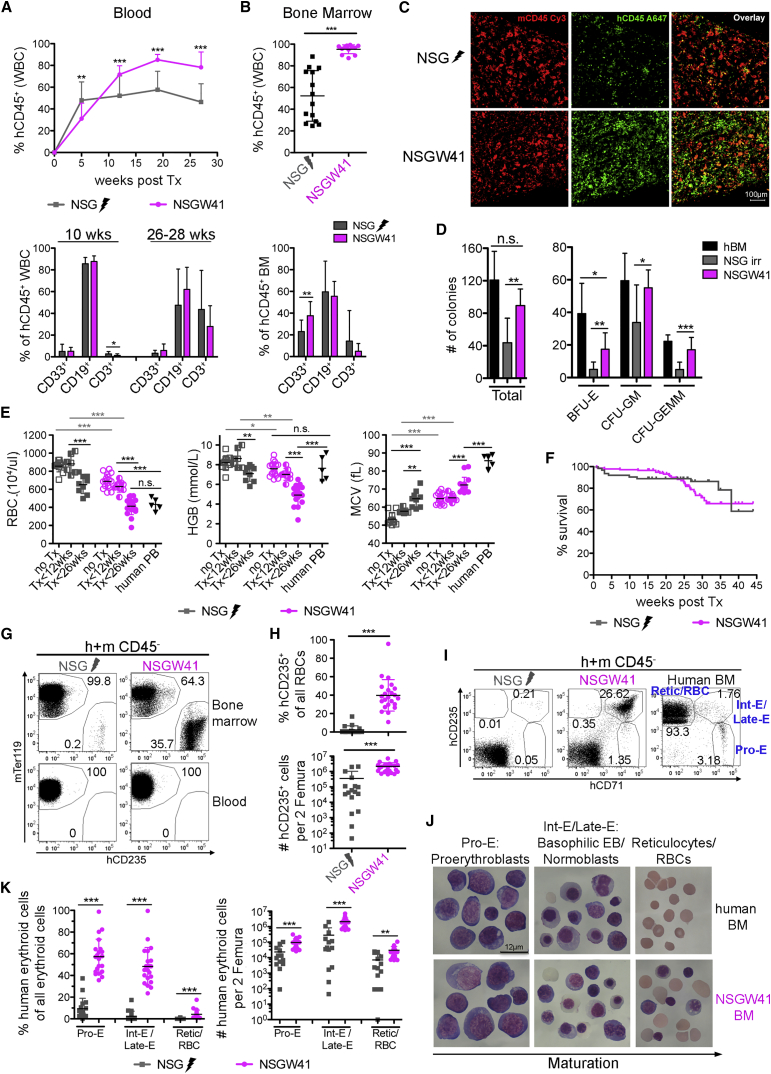
Transplantation of human CD34-enriched cord blood (CB) cells results in enhanced and uniform engraftment of human leukocytes in the blood (Figure 1A) and BM (Figures 1B and 1C) of NSGW41 recipients compared with irradiated NSG mice. Colony formation confirmed the presence of increased numbers of lineage-specific progenitors in humanized NSGW41 mice compared with controls (Figure 1D), evidencing improved engraftment of human HSPCs. To test whether cell-intrinsic impairment of murine erythropoiesis (Sharma et al., 2007) provides an advantage for human RBC differentiation, we compared blood cell parameters between non-transplanted and humanized mice (Figure 1E). Non-transplanted NSGW41 mice display a macrocytic anemia (Sharma et al., 2007). After humanization, RBC parameters decreased in both recipients but were more pronounced in NSGW41 mice. Despite the severe anemia in NSGW41 mice, the survival between both humanized mouse strains was comparable (Figure 1F). Neither conditioned NSG nor unconditioned NSGW41 mice showed human RBCs in the circulation (Figure 1G). In contrast, the BM of NSGW41 mice was highly repopulated with human erythroid progenitor cells (Figures 1G and 1H) that were present only at low frequencies in the donor cells (Figure S1D), suggesting that stable human HSC engraftment supports an increased output of cells of the erythroid lineage. Consistent with this interpretation is the fact that the transplantation of sorter-purified human HSCs also results in the engraftment of human erythroblasts in the BM of KIT-mutant recipient mice (Koichi Akashi, personal communication).
Figure 1.
Improved Human Erythroid Engraftment in NSGW41 Mice
(A and B) Human leukocyte chimerism (top) and composition of human graft (bottom): (A) in the blood at indicated time points, five experiments, 2–5 mice per experiment; and (B) in the BM of NSG and NSGW41 recipients 24–32 weeks after humanization, five experiments, 2–4 mice per experiment.
(C) Immunofluorescence of human and mouse CD45+ cells in bone sections of NSG and NSGW41 recipients. Scale bar, 100 μm.
(D) Colony assays. Two experiments, 2–5 mice per experiment, two technical replicates each.
(E) Blood parameters of non-transplanted (open) and transplanted NSG and NSGW41 mice early (<12 weeks, half-filled) and late (>26 weeks, closed) after humanization. There is no discrimination between human and mouse parameters. Five experiments, 2–8 mice per experiment.
(F) Survival plot after humanization. Twenty experiments, 3–16 mice per experiment.
(G) RBC chimerism.
(H) Frequency (top) and number per two femura (bottom) of CD235+ cells within all erythrocytes. Seven experiments, 2–9 mice per experiment.
(I) Human erythroid precursors.
(J) Giemsa-stained cells sorted as shown in (I). Scale bar, 12 μm.
(K) Frequencies (left) and numbers (right) of cells of human origin within all erythroid cells of the indicated differentiation stage in humanized mice. Seven experiments, 2–9 mice per experiment.
Data represent means ± SD. ∗p = 0.05–0.01, ∗∗p = 0.01–0.001, ∗∗∗p < 0.001; n.s., not significant. See also Figure S1.
To assess for a specific block of differentiation due to a potential incompatibility for human RBC differentiation in the murine environment, we quantified erythroid precursors (pro-E, Int-E/Late-E, Retic/RBC, Figures 1I and 1J). All differentiation stages were present, and increased numbers of human RBC progenitors were detected in humanized NSGW41 compared with NSG recipients (Figures S1C and 1K), suggesting that human erythropoiesis is strongly supported in NSGW41 mice. Engraftment of human erythroblasts/normoblasts in NSGW41 occurred at the expense of endogenous erythroid progenitors (Figures S1A and S1B), resulting in a more pronounced anemia in NSGW41 mice. We conclude that stable human HSC engraftment is a prerequisite for continuous human erythropoiesis in mice, and commitment to the erythroid lineage and differentiation up to nucleated erythroid progenitor stages is supported by the murine microenvironment in NSGW41 recipients.
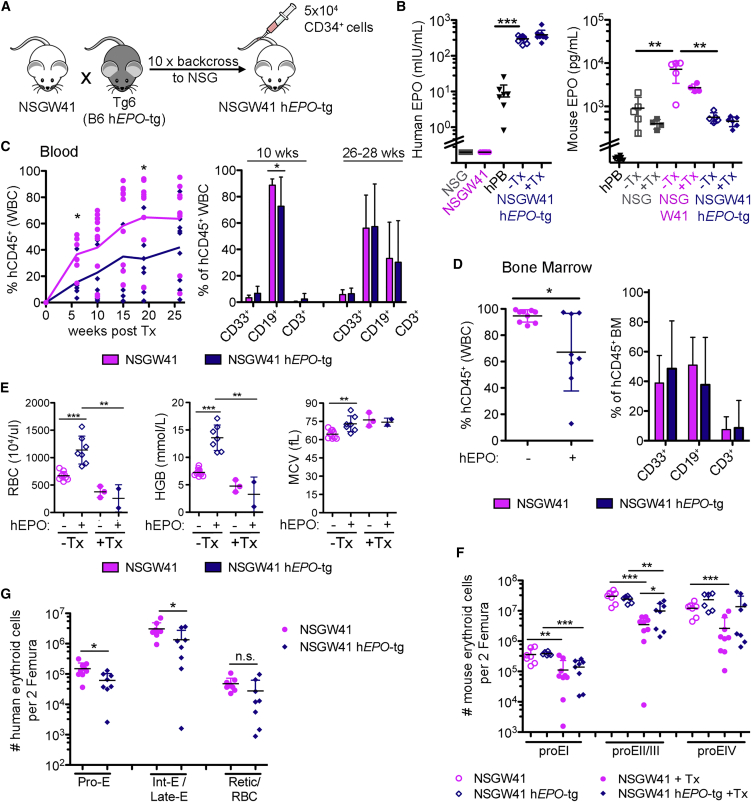
Overexpression of Human Erythropoietin Fails to Enhance Human RBC Engraftment
The injection of hEPO- and IL-3-encoding plasmids into humanized NSG mice results in the appearance of low frequencies of human CD235+ erythrocytes in the blood (Chen et al., 2009). To test whether the overexpression of hEPO (Waskow et al., 2002, Waskow et al., 2004) in NSGW41 mice increases RBC reconstitution, we generated NSGW41 hEPO-transgenic (hEPO-tg) mice (Figure 2A). Increased plasma hEPO levels were confirmed before and after humanization (Figure 2B). NSGW41 mice, comparable with Kit mutants in other genetic backgrounds (Waskow et al., 2004), displayed 8-fold higher mouse EPO levels in response to the endogenous anemia (Geissler et al., 1981). hEPO levels in NSGW41 hEPO-tg mice were ∼50-fold elevated compared with human blood. Human donor cell chimerism was reduced in the blood and BM of NSGW41 hEPO-tg mice compared with NSGW41 mice (Figures 2C and 2D), suggesting that hEPO induces differentiation of donor HSPCs and prevents their efficient engraftment. However, hEPO had no effect on the composition of human white blood cells (Figures 2C and 2D). RBC parameters were significantly increased in non-transplanted NSGW41 hEPO-tg mice, evidencing the functionality of the surplus hEPO, but after humanization RBC counts and hemoglobin contents still significantly decreased in both mouse strains (Figure 2E). The number of murine erythroblasts/normoblasts decreased equally in humanized NSGW41 hEPO-tg or NSGW41 mice (Figures 2F and S1B). Regardless, the engraftment of human erythroblasts in BM of humanized NGSW41 hEPO-tg mice was reduced compared with NSGW41 mice (Figure 2G) and no mature human RBCs were found in the blood of recipients. This evidences a lack of support for human erythroid engraftment by hEPO overexpression. EPO supports RBC differentiation in mice and humans at late stages during erythroid differentiation by promoting erythroblast proliferation and inhibiting apoptosis (Kaushansky, 2006, Waskow et al., 2004). However, intermediate and late stages of erythroid differentiation are particularly well repopulated by cells of human origin in NSGW41 mice (Figures 1G–1K), suggesting a defect at or beyond the enucleation of human RBC precursors.
Figure 2.
Overexpression of Human Erythropoietin in NSGW41 Mice
(A) Generation of NSGW41 hEPO-tg mice.
(B) Human (left) and murine (right) blood EPO levels in indicated mice and human controls (hPB). human EPO: two experiments, 3–6 replicates each; mouse EPO: one experiment, 4–6 replicates each.
(C) Human leukocyte chimerism (left) and donor cell composition in the blood (right). Four experiments, 2–4 mice per experiment.
(D) BM leukocyte chimerism (left) and graft composition (right) 25–32 weeks after humanization. Four experiments, 1–3 mice per experiment.
(E) Blood parameters in NSGW41 and NSGW41 hEPO-tg mice with (31 weeks, one experiment, 2–3 mice) and without humanization (7–10 mice).
(F) Mouse RBC precursors per two femura. Four experiments, 1–3 mice per experiment.
(G) Human RBC precursors per two femura. Four experiments, 1–3 mice per experiment.
Data represent means ± SD. ∗p = 0.05–0.01, ∗∗p = 0.01–0.001, ∗∗∗p < 0.001; n.s., not significant.
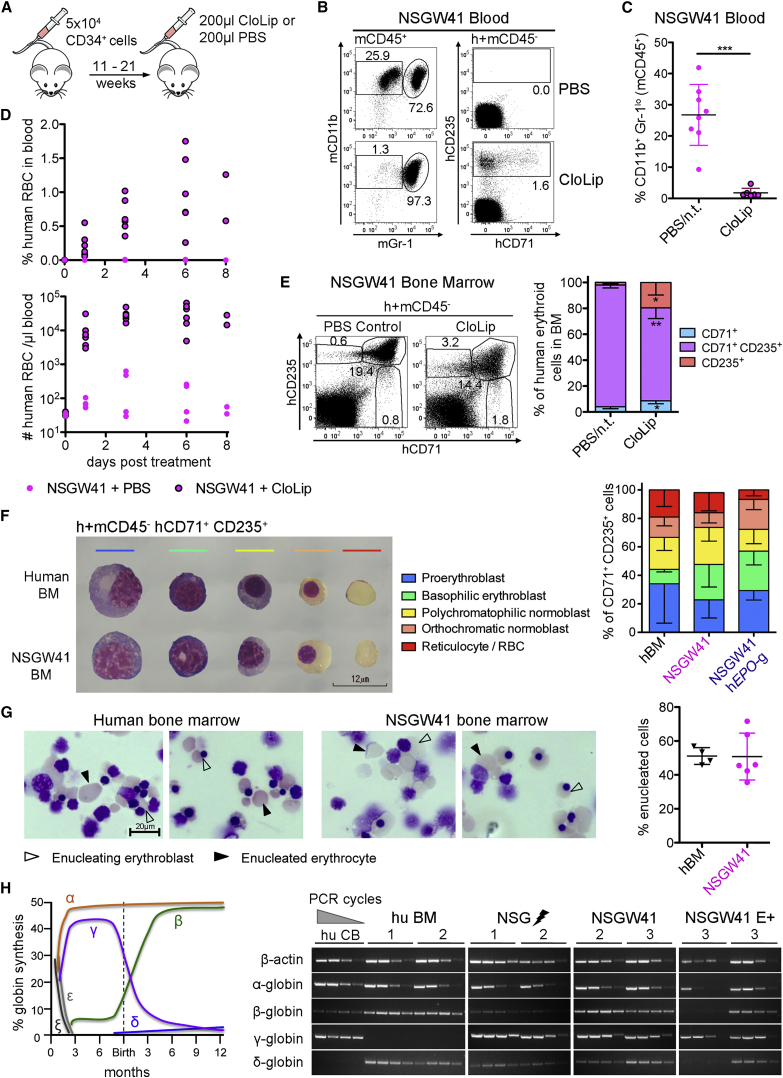
Depletion of Macrophages Partially Rescues Erythrocyte Maturation
Phagocytosis has been considered an alternative cause for lack of human RBCs in humanized mice (Hu et al., 2011). Endogenous murine macrophages are reduced after humanization (Figure S2A) but remain phagocytically active (Figure S2B). To deplete macrophages we injected clodronate-containing liposomes (CloLip) (Figure 3A), which cleared phagocytes from the blood ((Hu et al., 2011), Figures 3B and 3C) and resulted in the appearance of human RBCs in the circulation of humanized NSGW41 mice (Figures 3B and 3D). Correspondingly, the composition of human BM erythroid cells was altered and the frequency of Int-E/Late-E cells (CD71+ CD235+) was significantly reduced, whereas CD235+ Retic/RBCs increased (Figure 3E). We conclude that the depletion of macrophages improves human erythrocyte reconstitution in the blood of humanized mice but the shift toward more mature erythroid cells is incomplete, perhaps due to a block within Int-E/Late-E erythroblasts/normoblasts that contain terminal differentiation stages prior to enucleation, or insufficient enucleation.
Figure 3.
Terminal Erythroid Differentiation Is Not Impaired in Erythroblasts Generated in Mice
(A) Scheme of macrophage depletion by CloLip 11 to 21 weeks after humanization.
(B) Representative pictures of macrophage depletion.
(C) Frequencies of murine CD11b+ Gr-1lo macrophages in the blood of humanized NSGW41 mice that have received CloLip 24 hr before. n.t., not treated. Two experiments, 2–4 mice per experiment.
(D) Frequencies (top) and numbers (bottom) of human CD235+ cells in the blood. Two experiments, 2–4 mice per experiment.
(E) Human BM erythroblasts/normoblasts 6–8 days after CloLip. One experiment, four mice.
(F) Giemsa-stained human RBC maturation stages within hCD71+ CD235+ cells. Composition of hCD71+ CD235+ cells in human BM (n = 3), BM from humanized NSGW41 mice (n = 5), and NSGW41 hEPO-tg mice (n = 3). Two experiments, ∼110 cells counted on each cytospin.
(G) In vitro enucleation assay. Pictures from hCD71+ CD235+ erythroid cells after 7 days of culture. Scale bar, 20 μm. Right: quantification of enucleated cells, 162–248 cells per cytospin counted. Three experiments, 1–2 replicates per experiment.
(H) Scheme of human globin expression during development. Semi-quantitative analysis of human globin mRNA expression in sorted hCD71+ CD235+ cells.
Data represent means ± SD. ∗p = 0.05–0.01, ∗∗p = 0.01–0.001, ∗∗∗p < 0.001. See also Figure S2.
Human Erythroblasts Generated in NSGW41 Mice Can Terminally Differentiate
The composition of CD71+ CD235+ progenitors that contain pro- and basophilic erythroblasts, polychromatophilic and orthochromatic normoblasts, and reticulocytes was comparable between humanized NSGW41, NSGW41 hEPO-tg, and human BM (Figure 3F), evidencing normal erythroid differentiation in our mouse strains. Furthermore, human CD71+ CD235+ Int-E/Late-E erythroblasts from human or NSGW41 BM enucleated at comparable frequencies (Figure 3G), suggesting that human erythroblasts generated in mice can efficiently enucleate. However, we cannot formally exclude that the enucleation frequency in vivo differs between humanized mice and human BM because the formation of erythroblastic islands may require factors that are incompatible between the species (Dzierzak and Philipsen, 2013). Adult globin chain gene expression was detected in CD71+ CD235+ cells from humanized mice, but the majority of transcripts encoded for fetal hemoglobin subunits (γ-globin) in all humanized mice (Figure 3H). However, humanized NSGW41 mice expressed increased amounts of transcripts encoding for adult-type β-globin. Furthermore, δ-globin, which is part of adult HbA2 hemoglobin, was not expressed in human CB but in all humanized mice, evidencing a globin switch in CB-derived erythroblasts. However, while silencing of γ-globin is not observed, β-globin activation seems to occur, suggesting that a globin switch is at least partially realized in humanized mice (Bauer and Orkin, 2011). The expression of fetal globin should not pose a problem to the final maturation and functionality of human erythrocytes in mice, as patients with a certain type of hereditary persistence of fetal hemoglobin who have 100% fetal hemoglobin display no anemia (Bauer and Orkin, 2011). We conclude that there is no block in human erythrocyte maturation in NSGW41 mice and assign paucity of human RBCs to either insufficient in vivo enucleation or SIRPalpha-independent phagocytosis.
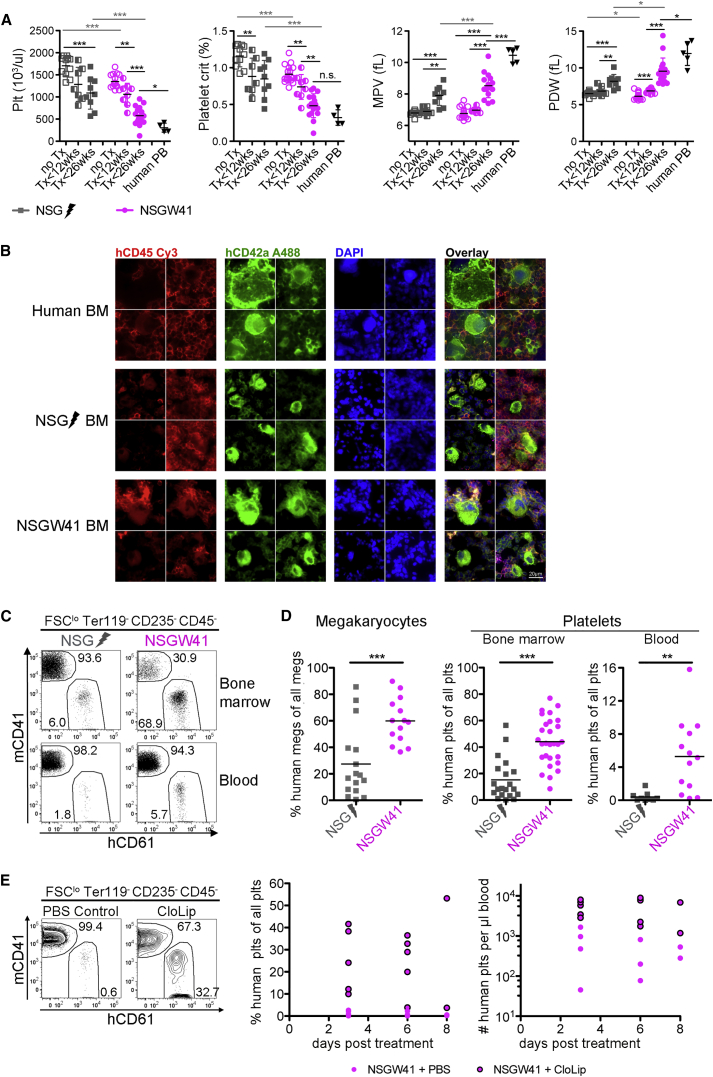
Human Platelet Formation Is Improved in NSGW41 Mice
The reconstitution of human thrombocytes is severely impaired in humanized mouse models (Hu and Yang, 2012, Rongvaux et al., 2011, Suzuki et al., 2007). Non-transplanted NSGW41 mice display thrombocytopenia (Figure 4A). After transplantation thrombocytes decreased in irradiated NSG and NSGW41 mice, but the decline was accelerated and more severe in NSGW41 mice. After humanization, mature human megakaryocytes were present in NSGW41 and NSG mice (Figure 4B), but the frequency was much improved in NSGW41 compared with NSG recipients (Figure 4D). Consistently, hCD61+ platelets were present in higher frequencies in BM and blood of humanized NSGW41 mice (Figures 4C and 4D). Despite a significant improvement of human thrombocyte reconstitution in NSGW41 mice, the frequency of circulating platelets was still low compared with overall human chimerism (Figure 1A). Macrophage depletion resulted in an increase of human platelets in the blood (Figure 4E). Quantification of thrombocytes in the BM showed no difference between macrophage-depleted and control mice (Figure S3), suggesting that megakaryocyte/thrombocyte lineage commitment and maturation were not affected by macrophage depletion but that survival in the periphery was enhanced. We conclude that maturation and survival of human thrombocytes are increased in NSGW41 mice compared with irradiated NSG mice.
Figure 4.
Human Thrombopoiesis in NSGW41 Mice
(A) Blood parameters of NSG and NSGW41 mice with and without humanization. Plt, platelet; MPV, mean platelet volume; PDW, platelet distribution width. No discrimination between human and mouse parameters. Five experiments, 2–8 mice per experiment.
(B) Immunofluorescence of human megakaryocytes in human BM cytospins and sections of bones from humanized NSGW41 and irradiated NSG mice. Scale bar, 20 μm.
(C) Expression of mCD41 and hCD61 on forward scatter low (FSClo), Ter119− CD235−, h + m CD45− BM (top), and blood (bottom) cells.
(D) Frequency of human megakaryocytes (FSChi hCD45− hCD61+) within all megakaryocytes (FSChi, Ter119− CD235−, h + m CD45−, mCD41+, or hCD61+) (five experiments, 2–4 mice per experiment) and frequency of human platelets within all BM (seven experiments, 1–4 mice per experiment) or blood platelets (four experiments, 1–4 mice per experiment).
(E) Human platelets in the blood of humanized NSGW41 mice 6 days after macrophage depletion (CloLip). Two experiments, 2–4 mice per experiment.
Data represent means ± SD. ∗p = 0.05–0.01, ∗∗p = 0.01–0.001, ∗∗∗p < 0.001; n.s., not significant. See also Figure S3.
Our results show that stable human HSC engraftment is sufficient to support the generation and maturation of human erythrocytes, thrombocytes, and other myeloid cells (Cosgun et al., 2014). The increased engraftment of human cells in NSGW41 mice occurs at the expense of murine erythroblasts and megakaryocytes, suggesting replacement of endogenous hematopoiesis. We conclude that NSGW41 mice are an improved tool for the study of human erythrocytes and RBC-associated diseases. Despite the presence of high numbers of human erythroblasts/normoblasts in the BM of NSGW41 mice, we could only detect human erythrocytes in the circulation after macrophage depletion. Significant differences between human and mouse erythropoiesis were reported (An et al., 2014, Pishesha et al., 2014) but direct comparisons are difficult, as murine erythroblasts are usually isolated from BM whereas human erythroblasts are derived from in vitro culture of CD34+ cells (Palis, 2014). We show here that there is no differentiation defect in the newly generated human erythroblasts, enucleation can take place, and even a partial globin chain switch occurs in mice, suggesting that growth factors important in RBC differentiation are cross-reactive between humans and mice. We reason that the murine NSGW41 environment enables human erythrocyte and thrombocyte differentiation without additional manipulation, allowing for the in vivo analysis of human erythropoiesis.
Experimental Procedures
Mice
NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ mice (NSG) were obtained from the Jackson Laboratory. NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ KitW41/W41 mice (NSGW41) were generated as previously described (Cosgun et al., 2014). To generate NSGW41 hEPO-tg mice, we backcrossed Tg6 mice (C57BL/6), which constitutively overexpress hEPO in a hypoxia-independent manner and show hematocrit values of up to 80% (Vogel and Gassmann, 2011), ten times onto NSG. Subsequently, NSG hEPO-tg mice were crossed with NSGW41 animals. All mice were bred and maintained under specific pathogen-free conditions at the animal facility of the TU Dresden. Animal experiments were performed in accordance with German animal welfare legislation and approved by the relevant authorities (Landesdirektion Dresden, Referat 24).
Human Donor Cells
Human umbilical CB samples were provided by the DKMS Cord Blood Bank, Dresden. Human BM samples were obtained from healthy BM donors in the Department of Hematology/Oncology of the University Hospital, Dresden. All human samples were used in accordance with the guidelines approved by the Ethics Committee of the Dresden University of Technology.
Transplantations
CD34+ progenitor cells were isolated from human CB as described previously (Cosgun et al., 2014). For humanization 5 × 104 CD34-enriched cells were injected intravenously in 150 μL of PBS/5% fetal calf serum into 4- to 12-week-old conditioned NSG (200 cGy; MaxiShot, Yxlon) or unconditioned NSGW41 or NSGW41 hEPO-tg mice. After transplantation, mice were given neomycin-containing drinking water for 3 weeks.
Blood Analysis
For complete blood counts, blood of mice was collected in EDTA-containing microtubes. Blood samples were diluted in a ratio of 1:5 with NaCl 0.9% and analyzed on a Sysmex XT2000iV or Sysmex XE-5000 blood analyzer.
Flow Cytometry and Cell Sorting
BM and blood samples were collected and prepared as described previously (Cosgun et al., 2014). Samples were acquired on an LSRII cytometer (BD Biosciences) or a MACS Quant Analyzer (Miltenyi) and analyzed using FlowJo software (TreeStar). Sorting of human erythrocyte populations was performed on a FACSAria II or III (BD Biosciences). For determining total cell numbers, a specific volume of the respective cell suspension was analyzed on a MACS Quant Analyzer. Details regarding antibodies can be found in Supplemental Experimental Procedures.
Colony Assay
Human CD45+ cells (5 × 104) were sorted from BM of humanized NSG and NSGW41 mice or fresh human BM samples and plated in duplicate into a 6-well plate with methylcellulose-containing medium supplemented with rhSCF, rhGM-CSF, rhG-CSF, rhIL-3, and rhEPO (MethoCult GF H84434; STEMCELL Technologies). After 14 days the number and types of colonies was analyzed using a STEMvision instrument (STEMCELL).
Enucleation Assay
Sorter-purified hCD71+ CD235+ BM cells (4.8 × 105 to 1×106) from humanized NSGW41 mice or fresh human BM samples were seeded in 1 mL of Iscove’s modified Dulbecco’s medium supplemented with 1% L-glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin, 330 μg/mL holo-human transferrin, 10 μg/mL recombinant human insulin, 2 IU/mL heparin, and 5% pooled AB serum (German Red Cross Blood Donation Service North-East), and 3 U/mL human erythropoietin into 24-well plates (Giarratana et al., 2011). Medium was renewed after 3 days. On day 7 cells were collected, and 2 × 105 to 5 × 105 cells were cytospun and analyzed.
Morphological Analysis
Sorted BM or cultured cells were cytospun onto polylysine-coated slides at 25–100 × g for 5 min and after ON drying stained with May-Gruenwald-Giemsa. Photographs were taken using Nikon Eclipse TS100 or Keyence BZ9000 microscopes.
Macrophage Depletion
Macrophages were depleted by intravenous injection of 200 μL of clodronate liposomes (ClodronateLiposomes.com). Controls received 200 μL of PBS. Macrophage depletion was confirmed 24 hr and red blood cell appearance 1–8 days after injection by flow cytometry.
EPO ELISA
Human or murine EPO levels were quantified from plasma or serum, respectively, using specific ELISAs (Quantikine; R&D Systems) according to the manufacturer's guidelines. For details on sample preparation see Supplemental Experimental Procedures.
Globin Chain Expression
For RNA isolation (RNeasy MicroKit, Qiagen) 4.4 × 104 to 8.3 × 105 hCD71+ CD235+ cells were sorted from BM of humanized NSG, NSGW41 and NSGW41 hEPO-tg mice, fresh human BM samples, or CB. cDNA was synthesized with the SuperScript First-Strand Synthesis System (Invitrogen). Semi-quantitative PCR was performed by titrating the number of amplification cycles. Primer sequences and number of amplification cycles are provided in Supplemental Experimental Procedures.
Immunohistochemistry
Fifteen weeks after humanization, cryosections from humeri were cut (3 μm) from paraformaldehyde-fixed, decalcified bones on a Cryotome CM1900 using the CryoJane Tape-Transfer System (Leica). Samples were analyzed by a fluorescence microscope (BZ-9000E; Keyence) and images were processed by BZ-Analyzer II software (Keyence) or Adobe Photoshop CS5 (Adobe). Details regarding antibodies can be found in Supplemental Experimental Procedures.
Statistical Analysis
Two-tailed Student's t tests were performed for all statistical analyses using Prism 5 for MacOSX software. In all graphs ∗p = 0.05–0.01, ∗∗p = 0.01–0.001, and ∗∗∗p < 0.001; data represent the mean ± SD.
Author Contributions
S.R. designed and performed experiments, interpreted the data, and wrote the paper. R.K.W., N.K., and T.T. performed the enucleation assay and provided crucial help for morphological analysis, colony assay, and globin chain detection. J.F. performed immunohistology. A.N. analyzed erythroblast composition. M.B., R.O., and A.P. provided human cells. M.G. provided hEPO-tg (Tg6) mice and helped to write the manuscript. C.W. conceived the study, designed experiments, interpreted data, and wrote the paper.
Acknowledgments
We thank the laboratory staff of the DKMS Cord Blood Bank (Dresden) for providing cord blood samples. This work was supported by the German Research Foundation (DFG) through WA2837, FOR2033-A03, SFB655-B9, and TRR127-A5, and intramural seed grant funding by the DFG-Center for Regenerative Therapies to C.W.
Published: September 8, 2016
Footnotes
Supplemental Information includes Supplemental Experimental Procedures and three figures and can be found with this article online at http://dx.doi.org/10.1016/j.stemcr.2016.08.005.
Supplemental Information
References
- An X., Schulz V.P., Li J., Wu K., Liu J., Xue F., Hu J., Mohandas N., Gallagher P.G. Global transcriptome analyses of human and murine terminal erythroid differentiation. Blood. 2014;123:3466–3477. doi: 10.1182/blood-2014-01-548305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer D.E., Orkin S.H. Update on fetal hemoglobin gene regulation in hemoglobinopathies. Curr. Opin. Pediatr. 2011;23:1–8. doi: 10.1097/MOP.0b013e3283420fd0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q., Khoury M., Chen J. Expression of human cytokines dramatically improves reconstitution of specific human-blood lineage cells in humanized mice. Proc. Natl. Acad. Sci. USA. 2009;106:21783–21788. doi: 10.1073/pnas.0912274106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgun K.N., Rahmig S., Mende N., Reinke S., Hauber I., Schafer C., Petzold A., Weisbach H., Heidkamp G., Purbojo A. Kit regulates HSC engraftment across the human-mouse species barrier. Cell Stem Cell. 2014;15:227–238. doi: 10.1016/j.stem.2014.06.001. [DOI] [PubMed] [Google Scholar]
- Dzierzak E., Philipsen S. Erythropoiesis: development and differentiation. Cold Spring Harb. Perspect. Med. 2013;3:a011601. doi: 10.1101/cshperspect.a011601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geissler E.N., McFarland E.C., Russell E.S. Analysis of pleiotropism at the dominant white-spotting (W) locus of the house mouse: a description of ten new W alleles. Genetics. 1981;97:337–361. doi: 10.1093/genetics/97.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giarratana M.C., Rouard H., Dumont A., Kiger L., Safeukui I., Le Pennec P.Y., Francois S., Trugnan G., Peyrard T., Marie T. Proof of principle for transfusion of in vitro-generated red blood cells. Blood. 2011;118:5071–5079. doi: 10.1182/blood-2011-06-362038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Z., Yang Y.G. Full reconstitution of human platelets in humanized mice after macrophage depletion. Blood. 2012;120:1713–1716. doi: 10.1182/blood-2012-01-407890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Z., Van Rooijen N., Yang Y.G. Macrophages prevent human red blood cell reconstitution in immunodeficient mice. Blood. 2011;118:5938–5946. doi: 10.1182/blood-2010-11-321414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karagiannis P., Eto K. Manipulating megakaryocytes to manufacture platelets ex vivo. J. Thromb. Haemost. 2015;13(Suppl 1):S47–S53. doi: 10.1111/jth.12946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushansky K. Lineage-specific hematopoietic growth factors. N. Engl. J. Med. 2006;354:2034–2045. doi: 10.1056/NEJMra052706. [DOI] [PubMed] [Google Scholar]
- Lambert M.P., Sullivan S.K., Fuentes R., French D.L., Poncz M. Challenges and promises for the development of donor-independent platelet transfusions. Blood. 2013;121:3319–3324. doi: 10.1182/blood-2012-09-455428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massberg S., Brand K., Gruner S., Page S., Muller E., Muller I., Bergmeier W., Richter T., Lorenz M., Konrad I. A critical role of platelet adhesion in the initiation of atherosclerotic lesion formation. J. Exp. Med. 2002;196:887–896. doi: 10.1084/jem.20012044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mende N., Kuchen E.E., Lesche M., Grinenko T., Kokkaliaris K.D., Hanenberg H., Lindemann D., Dahl A., Platz A., Hofer T. CCND1-CDK4-mediated cell cycle progression provides a competitive advantage for human hematopoietic stem cells in vivo. J. Exp. Med. 2015;212:1171–1183. doi: 10.1084/jem.20150308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mende N., Rahmig S., Waskow C. Multilineage readout after HSC expansion—erythrocytes matter. Cell Cycle. 2016;15:1032–1033. doi: 10.1080/15384101.2016.1156904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palis J. Of mice and men. Blood. 2014;123:3367–3368. doi: 10.1182/blood-2014-04-565457. [DOI] [PubMed] [Google Scholar]
- Pishesha N., Thiru P., Shi J., Eng J.C., Sankaran V.G., Lodish H.F. Transcriptional divergence and conservation of human and mouse erythropoiesis. Proc. Natl. Acad. Sci. USA. 2014;111:4103–4108. doi: 10.1073/pnas.1401598111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmig S., Bornstein S.R., Chavakis T., Jaeckel E., Waskow C. Humanized mouse models for type 1 diabetes including pancreatic islet transplantation. Horm. Metab. Res. 2015;47:43–47. doi: 10.1055/s-0034-1390446. [DOI] [PubMed] [Google Scholar]
- Rongvaux A., Willinger T., Takizawa H., Rathinam C., Auerbach W., Murphy A.J., Valenzuela D.M., Yancopoulos G.D., Eynon E.E., Stevens S. Human thrombopoietin knockin mice efficiently support human hematopoiesis in vivo. Proc. Natl. Acad. Sci. USA. 2011;108:2378–2383. doi: 10.1073/pnas.1019524108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rongvaux A., Willinger T., Martinek J., Strowig T., Gearty S.V., Teichmann L.L., Saito Y., Marches F., Halene S., Palucka A.K. Development and function of human innate immune cells in a humanized mouse model. Nat. Biotechnol. 2014;32:364–372. doi: 10.1038/nbt.2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma Y., Astle C.M., Harrison D.E. Heterozygous kit mutants with little or no apparent anemia exhibit large defects in overall hematopoietic stem cell function. Exp. Hematol. 2007;35:214–220. doi: 10.1016/j.exphem.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K., Hiramatsu H., Fukushima-Shintani M., Heike T., Nakahata T. Efficient assay for evaluating human thrombopoiesis using NOD/SCID mice transplanted with cord blood CD34+ cells. Eur. J. Haematol. 2007;78:123–130. doi: 10.1111/j.1600-0609.2006.00783.x. [DOI] [PubMed] [Google Scholar]
- Vogel J., Gassmann M. Erythropoietic and non-erythropoietic functions of erythropoietin in mouse models. J. Physiol. 2011;589:1259–1264. doi: 10.1113/jphysiol.2010.196147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waskow C., Paul S., Haller C., Gassmann M., Rodewald H. Viable c-Kit(W/W) mutants reveal pivotal role for c-kit in the maintenance of lymphopoiesis. Immunity. 2002;17:277–288. doi: 10.1016/s1074-7613(02)00386-2. [DOI] [PubMed] [Google Scholar]
- Waskow C., Terszowski G., Costa C., Gassmann M., Rodewald H.R. Rescue of lethal c-KitW/W mice by erythropoietin. Blood. 2004;104:1688–1695. doi: 10.1182/blood-2004-04-1247. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.