Abstract
Background
It is unknown whether aggressive medication strategies should be used for early COPD with or without lung hyperinflation. We aimed to explore the characteristics and bronchodilator responsiveness of early COPD patients (stages I and II) with/without lung hyperinflation.
Methods
Four hundred and six patients with COPD who performed both lung volume and bronchodilation tests were retrospectively analyzed. Residual volume to total lung capacity >120% of predicted values indicated lung hyperinflation. The characteristics and bronchodilator responsiveness were compared between the patients with and without lung hyperinflation across all stages of COPD.
Results
The percentages of patients with lung hyperinflation were 72.7% in the entire cohort, 19.4% in stage I, 68.5% in stage II, 95.3% in stage III, and 100.0% in stage IV. The patients with lung hyperinflation exhibited poorer lung function but better bronchodilator responsiveness of both forced expiratory volume in 1 second and forced vital capacity than those without lung hyperinflation during early COPD (t=2.21–5.70, P=0.000–0.029), especially in stage I, while age, body mass index, smoking status, smoking history, and disease duration were similar between the two subgroups in the same stages. From stages I to IV of subgroups with lung hyperinflation, stage I patients had the best bronchodilator responsiveness. Use of bronchodilator responsiveness of forced vital capacity to detect the presence of lung hyperinflation in COPD patients showed relatively high sensitivities (69.5%–75.3%) and specificities (70.3%–75.7%).
Conclusion
We demonstrated the novel finding that early COPD patients with lung hyperinflation are associated with poorer lung function but better bronchodilator responsiveness and established a simple method for detecting lung hyperinflation.
Keywords: GOLD I, GOLD II, RV/TLC, bronchodilation test
Introduction
COPD is a global disease with increasing morbidity, disability, and burden.1,2 A notable characteristic of COPD is the persistent airflow limitation, which usually develops progressively.1 The annual rates of decline in forced expiratory volume in 1 second (FEV1) in patients with early COPD (stages I and II) are more rapid than those in patients with stages III and IV COPD.3–5 For early stable COPD, the Global initiative for chronic Obstructive Lung Disease (GOLD) recommends regular and aggressive pharmacological treatment only for patients with high level of symptoms.1 However, drug interventions during early COPD that depend on subjective symptoms may not be sufficient, while most patients do not visit hospitals or clinics until they have developed more severe degree of COPD by the time the diagnosis is made.6 To achieve early COPD intervention, especially during GOLD stage I, objective “markers” for aggressive medication strategies should be taken into account.
In a previous study, the indices of lung hyperinflation increased exponentially across GOLD stages,7 resulting in increased dyspnea and limitation of exercise capacity for patients with COPD.8,9 Moreover, static hyperinflation is an independent predictor of frequent exacerbations10 and even mortality in patients with COPD.11,12 Patients with greater resting lung hyperinflation showed more bronchodilator-induced volume deflation effects.7 Given the earlier associations, considerable interest exists in evaluating the clinical utility of lung hyperinflation as objective “marker” in COPD. However, it is unknown whether aggressive medication strategies should be used during early COPD (particularly for GOLD stage I) with or without lung hyperinflation. In addition, when considering COPD management in primary care settings, simple methods that do not use advanced equipment and complex technology to detect lung hyperinflation should be established.
Therefore, the purposes of the present study were a) to explore the characteristics and bronchodilator responsiveness of early COPD patients with or without lung hyperinflation and b) to test the feasibility of using bronchodilator responsiveness to detect lung hyperinflation in patients with early COPD.
Methods
Patients
This retrospective study included 406 stable patients with COPD first diagnosed by pulmonary physicians using GOLD definition in the First Affiliated Hospital of Guangzhou Medical University from January 2011 to June 2015. COPD patients were aged ≥40 years, had been evaluated by using both lung volume and bronchodilation tests, and had postbronchodilator FEV1/forced vital capacity (FVC) values <0.70. Patients were excluded if they had experienced a respiratory infection or an exacerbation of COPD within the last 4 weeks, had a history of pulmonary resection or asthma, used supplemental oxygen for >12 hours per day, or had significant diseases other than COPD that might influence either the results of the study or the patient’s ability to perform tests. The stages of COPD conformed to GOLD guidelines: GOLD stage I, predicted FEV1 value (FEV1%pred) ≥80%; GOLD stage II, 50% ≤FEV1%pred <80%; GOLD stage III, 30% ≤FEV1%pred <50%; and GOLD stage IV, FEV1%pred <30%.1
Written informed consent of pulmonary function test (PFT) was obtained from all patients prior to the test, and the study protocol was approved by the First Affiliated Hospital of Guangzhou Medical University Ethics Committee. All patient information was kept confidential.
Pulmonary function test
The PFT equipment (Jaeger Masterscreen Body, BD, Franklin Lakes, NJ, USA; Cosmed PFT Quark, COSMED, The Metabolic Company, Rome, Italy) met the criteria of the American Thoracic Society and the European Respiratory Society.13,14 Calibration checks were performed daily prior to all tests and validated that the devices were within the calibration limits. Patients were required to refrain from the use of short- and long-acting bronchodilators (for ≥6 and ≥24 hours, respectively), short- and long-acting theophylline (for ≥24 and ≥48 hours, respectively), antileukotrienes (for ≥48 hours), smoking, exercise, and tea/coffee (for ≥4 hours).
All lung volume and bronchodilation tests were conducted by skilled and certified doctors/technicians according to American Thoracic Society and the European Respiratory Society guidelines.13–15 For the lung volume test, body plethysmography or multiple-breath N2 washout was performed. Then, spirometry was performed before and 20–30 minutes after administration of 400 μg of salbutamol via metered dose inhaler. The change in FEV1 was expressed as 1) the percentage change relative to baseline (ΔFEV1%baseline), 2) the absolute change in the percentage of the predicted value (ΔFEV1%pred), and 3) the absolute change relative to baseline (ΔFEV1). The change in FVC was expressed in a similar manner. Predicted values of lung volume and spirometry were calculated from the reference equations published by Stocks et al16 and Zheng and Zhong,17 respectively. An abnormal ratio of residual volume to total lung capacity (RV/TLC) was considered pathological. RV/TLC values >120% of predicted values indicated lung hyperinflation.
Statistical analysis
Data were analyzed by using SPSS 18.0 software (SPSS Inc., Chicago, IL, USA). The characteristics of the study population are reported as mean ± standard deviation or as counts and percentages unless otherwise specified. Group differences between the presence and absence of lung hyperinflation within the entire cohort and each GOLD stage were explored using an unpaired t-test, a Mann–Whitney U-test, or a χ2-square test where appropriate. Comparisons across GOLD stages were analyzed using analysis of variance. Pearson’s correlation was used to evaluate the relationships between the RV/TLC%pred and bronchodilator responsiveness of the FVC and FEV1. Multivariate logistic regression analyses with age, sex, body mass index (BMI), technology of lung volume test, smoking status, self-reported smoking history, self-reported COPD duration, GOLD stage, ΔFVC, and ΔFEV1 were used to determine the association between these characteristics and the presence or absence of lung hyperinflation. Receiver operating characteristic curves were generated for selected cutoff values of bronchodilator responsiveness to detect lung hyperinflation. P-values <0.05 were considered significant in all analyses.
Results
Population characteristics
Of the 406 patients in the present study, 369 (90.9%) were males and 37 (9.1%) were females. The distribution of COPD stages was 16.5% in GOLD stage I, 39.9% in GOLD stage II, 31.3% in GOLD stage III, and 12.3% in GOLD stage IV (Table 1). The mean age, sex, and smoking status were similar within each GOLD stage subgroup, while greater self-reported smoking history (pack-year); longer self-reported COPD duration; higher RV%pred, TLC%pred, and RV/TLC%pred; and lower BMI, FVC%pred, FEV1%pred, and FEV1/FVC% were showed in the greater severity subgroup (F=2.89–972.11, P=0.000–0.035).
Table 1.
Subject characteristics for each COPD stage
| Variables | All patients (n=406) | GOLD I (n=67) | GOLD II (n=162) | GOLD III (n=127) | GOLD IV (n=50) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
||||||||||||
| LH (72.7%) |
NLH (27.3%) |
Total | LH (19.4%) |
NLH (80.6%) |
Total | LH (68.5%) |
NLH (31.5%) |
Total | LH (95.3%) |
NLH (4.7%) |
Total | LH (100%) |
NLH (0%) |
Total | |
| Sex, male, % | 92.8 | 90.2 | 90.9 | 76.9 | 90.7 | 88.1 | 84.7 | 94.1 | 87.7 | 95.0 | 100.0 | 95.3 | 94.0 | – | 94.0 |
| Age, years | 64.8±7.7 | 66.3±8.8 | 65.2±8.0 | 67.2±6.6 | 66.2±8.1 | 66.4±7.7 | 65.9±8.3 | 65.8±9.8 | 65.9±8.7 | 64.8±6.8 | 71.2±5.0* | 65.1±6.8 | 63.9±7.9 | – | 63.9±7.9 |
| BMI, kg/m2 | 21.5±3.3 | 22.8±3.2*** | 21.9±3.3 | 22.9±3.0 | 23.1±3.1 | 23.0±3.1 | 22.8±3.4 | 22.8±3.3 | 22.8±3.3 | 21.0±2.9 | 20.0±1.7 | 20.9±2.9 | 19.8±3.0 | – | 19.8±3.0 |
| Smoking status (non-/former/current smoker), % | 12.9/54.2/32.9 | 10.8/46.8/42.4 | 12.3/52.2/35.5 | 15.4/53.8/30.8 | 11.1/40.7/48.2 | 11.9/43.3/44.8 | 14.4/54.1/31.5 | 11.8/51.0/37.2 | 13.6/53.1/33.3 | 11.6/55.4/33.0 | 16.7/50.0/33.3 | 11.8/55.1/33.1 | 12.0/56.0/32.0 | – | 12.0/56.0/32.0 |
| Smoking history, pack-year | 40.6±24.4 | 34.1±21.1* | 38.2±26.8 | 27.3±15.9 | 31.8±17.8 | 31.0±17.4 | 41.4±28.6 | 35.6±23.8 | 39.5±27.2 | 41.5±23.1 | 36.8±12.3 | 41.3±22.8 | 39.9±18.3 | – | 39.9±18.3 |
| Disease duration, years | 9.0±7.3 | 6.1±5.4*** | 8.2±6.9 | 5.4±3.5 | 4.7±4.2 | 4.8±4.0 | 8.3±7.3 | 7.4±6.2 | 8.0±7.0 | 9.2±7.1 | 8.3±6.3 | 9.1±7.0 | 11.0±7.8 | 11.0±7.8 | |
| FVC prebronchodilator, %pred | 73.5±16.7 | 102.1±16.6*** | 81.3±20.9 | 96.4±12.2 | 111.6±14.2*** | 108.7±15.0 | 81.9±13.5 | 94.2±12.7*** | 85.8±14.4 | 71.3±12.4 | 83.2±14.5* | 71.9±12.7 | 54.3±12.6 | – | 54.3±12.6 |
| FVC postbronchodilator, %pred | 82.2±17.2 | 104.8±15.6*** | 88.4±19.6 | 107.9±14.1 | 114.3±12.5 | 113.1±13.0 | 90.2±13.5 | 96.8±11.9** | 92.3±13.4 | 80.3±13.1 | 87.3±16.4 | 80.6±13.2 | 62.1±12.5 | – | 62.1±12.5 |
| FEV1 prebronchodilator, %pred | 41.9±15.3 | 75.2±18.1*** | 51.0±21.9 | 71.7±7.4 | 89.6±12.4*** | 86.2±13.6 | 54.3±9.3 | 63.9±7.7*** | 57.3±9.8 | 35.4±6.1 | 40.9±3.3* | 35.7±6.1 | 22.1±3.3 | – | 22.1±3.3 |
| FEV1 postbronchodilator, %pred | 47.6±16.9 | 80.3±18.0*** | 56.6±22.5 | 84.6±3.6 | 95.2±10.9*** | 93.1±10.8 | 61.4±8.7 | 68.9±7.0*** | 63.8±8.9 | 40.5±5.8 | 43.4±4.0 | 40.6±5.8 | 24.8±2.9 | – | 24.8±2.9 |
| FEV1/FVC prebronchodilator, % | 44.0±11.4 | 56.8±9.0*** | 47.5±12.2 | 58.4±7.4 | 62.3±4.8* | 61.6±5.5 | 52.4±9.1 | 53.1±8.0 | 52.6±8.7 | 39.4±7.7 | 38.3±7.0 | 39.3±7.7 | 33.0±6.7 | – | 33.0±6.7 |
| FEV1/FVC postbronchodilator, % | 44.8±11.7 | 59.1±8.9*** | 48.7±12.7 | 61.9±7.2 | 64.5±4.0 | 64.0±4.8 | 53.7±8.5 | 55.8±7.6 | 54.4±8.3 | 40.0±7.3 | 38.8±8.1 | 39.9±7.3 | 32.4±6.3 | – | 32.4±6.3 |
| RV, %pred | 160.0±39.5 | 100.6±17.9*** | 143.7±43.9 | 143.2±22.1 | 99.8±19.5*** | 108.2±26.3 | 146.5±30.9 | 101.0±16.7*** | 132.1±34.5 | 165.9±37.2 | 105.1±14.8*** | 163.0±38.7 | 179.8±52.4 | – | 179.8±52.4 |
| TLC, %pred | 105.2±16.9 | 96.3±11.1*** | 102.8±16.0 | 106.9±9.3 | 99.0±10.3* | 100.5±10.5 | 103.4±14.7 | 94.1±11.0*** | 100.5±14.3 | 106.2±16.7 | 91.4±14.6* | 105.5±16.8 | 106.1±20.9 | – | 106.1±20.9 |
| RV/TLC, %pred | 151.6±20.1 | 104.3±12.3*** | 138.7±27.9 | 133.1±9.5 | 100.1±13.4*** | 106.5±18.2 | 140.7±13.8 | 107.7±9.9*** | 130.3±20.0 | 154.0±16.1 | 113.5±6.7*** | 152.1±18.0 | 175.0±20.1 | – | 175.0±20.1 |
Notes: Data are presented as mean ± standard deviation or percentages.
P<0.05,
P<0.01,
P<0.001 compared to the lung hyperinflation group in the same COPD stage. – Represents no data.
Abbreviations: BMI, body mass index; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; GOLD, Global initiative for chronic Obstructive Lung Disease; %pred, percentage of predicted value; RV, residual volume; TLC, total lung capacity; LH, lung hyperinflation; NLH, not lung hyperinflation.
Characteristics and bronchodilator responsiveness of patients with or without lung hyperinflation
The percentages of patients with lung hyperinflation were 72.7% in the entire cohort, 19.4% in GOLD stage I, 68.5% in stage II, 95.3% in stage III, and 100.0% in stage IV (Table 1). Although patients with lung hyperinflation had lower BMIs and reported a greater smoking history and longer disease duration than those without lung hyperinflation in the entire cohort (|t|=2.02–4.30, P=0.000–0.044), no significant differences were found in terms of mean age, sex, BMI, smoking status, smoking history, and disease duration between patients with and without lung hyperinflation in GOLD stages I and II. Nevertheless, patients with lung hyperinflation had lower FVC%pred and FEV1%pred and higher RV%pred, TLC%pred, and RV/TLC%pred values than those without lung hyperinflation in GOLD stages I and II (|t|=2.54–17.28, P=0.000–0.013).
Regarding the bronchodilator responsiveness of the entire cohort (Table 2), patients with lung hyperinflation showed significant improvement in FVC compared to those without hyperinflation (0.27±0.21 L vs 0.09±0.15 L, t=9.88, P<0.001), but no significant improvement in FEV1 (0.14±0.12 L vs 0.13±0.10 L, t=1.28, P=0.201). However, the improvements of both FVC and FEV1 in patients with lung hyperinflation were significantly higher than those in patients without lung hyperinflation in GOLD stages I (0.32±0.20 L vs 0.08±0.15 L, t=4.83, P<0.001; 0.30±0.18 L vs 0.13±0.11 L, t=3.15, P=0.007, respectively) and II (0.26±0.22 L vs 0.09±0.15 L, t=5.70, P<0.001; 0.17±0.11 L vs 0.13±0.10 L, t=2.21, P=0.029, respectively). Among the patients with lung hyperinflation across each COPD stage, subgroup differences existed for FEV1 improvement (F=19.72, P<0.001), but not for FVC improvement (F=0.85, P=0.469). In addition, the present study showed no difference in the poor bronchodilator responsiveness between GOLD stages I and II without lung hyperinflation (|t|=0.08–1.56, P=0.121–0.940).
Table 2.
Bronchodilator responsiveness of FVC and FEV1 for each COPD stage
| Variables | All patients (n=406)
|
GOLD I (n=67)
|
GOLD II (n=162)
|
GOLD III (n=127)
|
GOLD IV (n=50)
|
P-value | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LH (72.7%) |
NLH (27.3%) |
Total | LH (19.4%) |
NLH (80.6%) |
Total | LH (68.5%) |
NLH (31.5%) |
Total | LH (95.3%) |
NLH (4.7%) |
Total | LH (100%) |
NLH (0%) |
Total | ||
| ΔFVC, L | 0.27±0.21 | 0.09±0.15*** | 0.22±0.22 | 0.32±0.20 | 0.08±0.15*** | 0.12±0.18 | 0.26±0.22 | 0.09±0.15*** | 0.20±0.22 | 0.29±0.23 | 0.14±0.15 | 0.28±0.23 | 0.25±0.16 | – | 0.25±0.16 | <0.001 |
| ΔFVC, Δ%baseline | 12.6±10.1 | 3.0±5.2*** | 10.0±10.0 | 12.2±7.8 | 2.8±5.0*** | 4.6±6.7 | 10.7±8.8 | 3.0±5.4*** | 8.3±8.6 | 13.2±10.2 | 4.9±4.5 | 12.8±10.2 | 15.7±12.2 | – | 15.7±12.2 | <0.001 |
| ΔFVC, Δ%pred | 8.6±6.5 | 2.7±4.8*** | 7.0±6.6 | 11.5±7.2 | 2.7±5.1*** | 4.4±6.5 | 8.3±6.6 | 2.6±4.7*** | 6.5±6.6 | 9.0±6.8 | 4.1±4.0 | 8.7±6.8 | 7.8±5.1 | – | 7.8±5.1 | <0.001 |
| ΔFEV1, L | 0.14±0.11 | 0.13±0.10 | 0.14±0.11 | 0.3±0.18 | 0.13±0.11*** | 0.16±0.14 | 0.17±0.11 | 0.13±0.10* | 0.16±0.11 | 0.13±0.10 | 0.06±0.06 | 0.13±0.10 | 0.07±0.05 | – | 0.07±0.05 | <0.001 |
| ΔFEV1, %baseline | 14.7±11.2 | 7.3±6.2*** | 12.7±10.6 | 19.0±10.7 | 6.5±5.6*** | 8.9±8.4 | 13.9±9.7 | 8.4±6.8*** | 12.2±9.2 | 15.5±12.9 | 6.0±6.1 | 15.1±12.8 | 13.6±10.2 | – | 13.6±10.2 | <0.001 |
| ΔFEV1, %pred | 5.8±4.4 | 5.1±3.9 | 5.6±4.3 | 13.0±6.7 | 5.4±4.2*** | 6.9±5.6 | 7.0±4.3 | 5.1±3.7** | 6.4±4.2 | 5.1±3.7 | 2.4±2.5 | 5.0±3.7 | 2.8±2.0 | – | 2.8±2.0 | <0.001 |
| ΔFEV1/FVC, % | 0.8±3.5 | 2.3±2.8*** | 1.2±3.4 | 3.5±3.3 | 2.2±2.8 | 2.4±2.9 | 1.3±3.6 | 2.6±2.9* | 1.7±3.5 | 0.6±3.4 | 0.5±1.7 | 0.6±3.3 | −0.6±2.8 | – | −0.6±2.8 | <0.001 |
Notes: Data are presented as mean ± standard deviation or percentages.
P<0.05,
P<0.01,
P<0.001 compared to the lung hyperinflation group in the same COPD stage. – Represents no data. P-value, compared among the total groups of GOLD I to IV.
Abbreviations: FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; GOLD, Global initiative for chronic Obstructive Lung Disease; Δ, absolute change relative to baseline; Δ%baseline, percentage change relative to baseline; Δ%pred, absolute change in percentage of the predicted value; LH, lung hyperinflation; NLH, not lung hyperinflation.
Logistic regression for patients with lung hyperinflation
The variables from the multivariate logistic regression model are listed in Table 3. The odds for meeting lung hyperinflation criteria tended to be higher for females than males. The model also showed that higher odds of lung hyperinflation were associated with increased COPD severity. The ΔFVC was the most significant factor, and patients with greater ΔFVC tended to exhibit higher odds of lung hyperinflation. However, age, BMI, technology of lung volume test, smoking status, smoking history, disease duration, and ΔFEV1 were not significant in the logistic regression analysis.
Table 3.
Multivariate logistic regression analysis of patients with lung hyperinflation
| Variables | OR (95% CI) | P-value |
|---|---|---|
| Sex | 0.207 (0.067–0.636) | 0.006 |
| Age | 0.996 (0.955–1.038) | 0.844 |
| BMI | 0.958 (0.865–1.061) | 0.411 |
| Technology of lung volume test | 1.206 (0.581–2.503) | 0.616 |
| Smoking status | 0.957 (0.553–1.655) | 0.874 |
| Smoking history | 1.006 (0.992–1.019) | 0.409 |
| Disease duration | 1.052 (0.999–1.106) | 0.052 |
| GOLD stage | 9.852 (5.368–18.082) | <0.001 |
| ΔFVC | 144.836 (17.755–1,181.484) | <0.001 |
| ΔFEV1 | 7.840 (0.227–270.436) | 0.254 |
Abbreviations: BMI, body mass index; CI, confidence interval; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; GOLD, Global initiative for chronic Obstructive Lung Disease; OR, odds ratio; Δ, absolute change relative to baseline.
Cutoff values of bronchodilator responsiveness used to detect lung hyperinflation
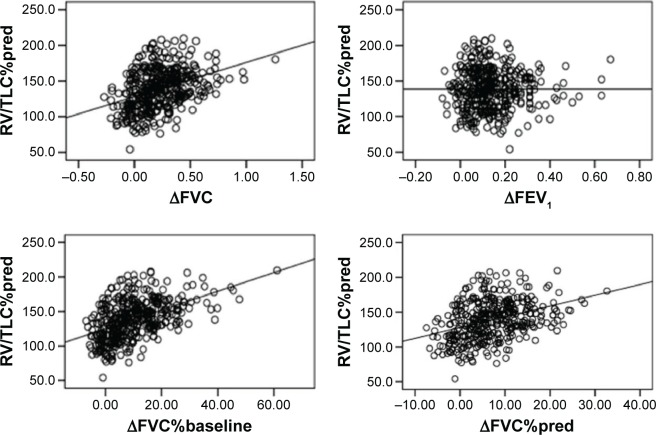
Linear relationships were observed between RV/TLC%pred and ΔFVC (r=0.386, P<0.001), ΔFVC%baseline (r=0.495, P<0.001), and ΔFVC%pred (r=0.378, P<0.001) (Figure 1). The relationships between RV/TLC%pred and ΔFEV1 (r=−0.002, P=0.975) and ΔFEV1%pred (r=−0.012, P=806) were poor, except for RV/TLC%pred and ΔFEV1%baseline (r=0.362, P<0.001).
Figure 1.
Scatter diagrams for RV/TLC%pred versus ΔFVC, ΔFVC%baseline, ΔFVC%pred, and ΔFEV1.
Abbreviations: FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; RV, residual volume; TLC, total lung capacity; %pred, percentage of predicted value; Δ, absolute change relative to baseline; Δ%baseline, percentage change relative to baseline.
The area under the receiver operating characteristic curve (AUC) for the entire cohort for detecting lung hyperinflation with ΔFVC%baseline was highest (0.811), followed by ΔFVC and ΔFVC%pred, with AUCs of 0.766 and 0.767, respectively (Figure 2). In addition, the AUCs of the GOLD stages I–III were 0.838, 0.774, and 0.756, respectively, when using ΔFVC%baseline to predict lung hyperinflation, indicating that earlier stages exhibited larger AUCs. The cutoff values with the best sensitivity and specificity for each functional variable were selected according to the receiver operating characteristic analysis: 0.15 L of ΔFVC with a sensitivity of 70.2% and a specificity of 71.2%, 6.0% of ΔFVC%baseline with a sensitivity of 75.3% and a specificity of 75.7%, and 5.0% of ΔFVC%pred with a sensitivity of 69.5% and a specificity of 70.3%.
Figure 2.
Receiver operating characteristic curves for detecting lung hyperinflation in patients with COPD.
Notes: (A) The entire cohort, (B) COPD stage I, (C) COPD stage II, and (D) COPD stage III.
Abbreviations: FVC, forced vital capacity; Δ, absolute change relative to baseline; Δ%baseline, percentage change relative to baseline; Δ%pred, absolute change in percentage of the predicted value.
Discussion
The main findings of the present study were as follows: 1) early COPD patients with lung hyperinflation showed poorer lung function but better bronchodilator responsiveness than those without lung hyperinflation, especially in GOLD stage I, while age, BMI, smoking status, smoking history, and disease duration were similar between the two subgroups for the same COPD stages; 2) the bronchodilator responsiveness of FVC was similar in patients with lung hyperinflation across COPD stages, while greater bronchodilator responsiveness of FEV1 was detected in earlier COPD patients with lung hyperinflation; and 3) use of bronchodilator responsiveness of FVC to detect the presence of lung hyperinflation in early COPD is a simple method with relatively high sensitivity and specificity.
Up to 72.7% of patients exhibited lung hyperinflation in the entire study cohort, while the rate increased with the stage of COPD. Unlike previous studies that focused on the bronchodilator responsiveness and treatment effect in each stage of COPD as a whole,7,18,19 the present study provided a novel analysis of the two subgroups within each COPD stage (patients with/without lung hyperinflation) in terms of characteristics, lung function, and bronchodilator responsiveness. The study of Ortega et al20 reported that better bronchodilator responsiveness was associated with poorer quality of life and exercise capacity in patients with severe COPD. Our study found that early COPD patients with lung hyperinflation were associated with poorer lung function but better bronchodilator responsiveness (Tables 1 and 2). Hence, we hypothesized that poorer lung function but better bronchodilator responsiveness might associate with poorer quality of life and exercise capacity. In addition, the present study showed no difference in the poor bronchodilator responsiveness between GOLD stages I and II without lung hyperinflation. Therefore, the presence of lung hyperinflation may serve as an objective “marker” for aggressive pharmacological intervention for early COPD, especially for GOLD stage I, which complements subjective assessments.
Within the patient population, some characteristics and bronchodilator responsiveness appeared to be associated with the presence of lung hyperinflation in the multivariate logistic regression. Females appeared to exhibit lung hyperinflation more often than males, but this observation should be viewed with caution given the limited sample size of female patients. Patients without lung hyperinflation generally exhibited milder COPD, and the indices that were not associated with lung hyperinflation, such as BMI, smoking history, and disease duration, could be explained based on the COPD stage. Moreover, the ΔFVC was the most significant factor associated with lung hyperinflation. In addition, we also found a positive correlation between FVC improvement and the RV/TLC%pred. Therefore, it is possible to use the bronchodilator responsiveness of FVC to detect high RV/TLC%pred (lung hyperinflation).
Except for lung volume tests, recent evidence has supported using radiography methods, such as computed tomography, to evaluate lung hyperinflation severity in patients with COPD.21–23 These assessments are intuitive and accurate, but the radiographic method is not a repeatable or acceptable choice due to its high cost and level of radiation exposure. Considering the lack of advanced equipment and complex technology in primary care settings, especially in developing countries, the present study provided a simple method to detect lung hyperinflation, in which the cutoff values of ΔFVC and its derivatives exhibited relatively high sensitivity and specificity, especially for GOLD stage I patients. Although the shortcoming of the cutoff value of FVC change is somewhat similar to that of the reversibility of FEV1,24 this simple method still could use for screening lung hyperinflation in a COPD population. In addition, the same meaning as FVC, increase of inspiratory capacity after bronchodilator inhalation suggests a reduction of dynamic hyperinflation. However, FVC could be obtained simultaneously when spirometry maneuver was performed; by comparison, inspiratory capacity has to be tested separately. Moreover, the reliability and quality control of inspiratory capacity are poor in some instances. Therefore, FVC has the advantage of easier obtainment in the clinical practice.
Limitations
Some limitations were present in our study. As a retrospective study, we could not record additional clinical data from the COPD patients, such as modified medical research council scores, COPD assessment test scores, and the number of exacerbation events during the previous year. The multiple-breath N2 washout was performed in 78.6% of patients, while the lung volume measured by body plethysmography differs with that measured by multiple-breath N2 washout in COPD, the more, the higher grade of obstruction.14 However, the present study focused on early COPD that with lower grade of obstruction, and the technology of lung volume test did not show any significance in the logistic regression analysis for detecting lung hyperinflation. In addition, the number of patients with GOLD stage I and female patients was relatively small, although they did reflect the reality of COPD management in the People’s Republic of China.25 Nevertheless, we found evidence that lung hyperinflation in patients with early COPD is associated with poorer lung function but better bronchodilator responsiveness. These limitations have been considered in our upcoming study, which prospectively focuses on the long-term treatment effects and clinical outcomes of patients with or without lung hyperinflation in early COPD, especially in GOLD stage I.
Conclusion
This study provided novel findings regarding the presence of lung hyperinflation in COPD patients, which may serve as an objective “marker” for aggressive pharmacological intervention for early COPD. In addition, the present study established a simple method using the bronchodilator responsiveness of FVC to detect lung hyperinflation.
Acknowledgments
This work was supported by chronic respiratory diseases funding, Chinese Medical Association research projects, National Key Technology R&D Program, and Natural Science Foundation for Young of China (2012BAI05B01, 2013BAI09B09, 2014Y2-00540, 2015BAI12B10, 81300017).
Footnotes
Author contributions
JZ is the guarantor of the paper, had full access to all the data in the study, and takes responsibility for the integrity of the data and the accuracy of the data analysis. WJ contributed to study design, quality control, acquisition of the data, statistical analysis, and revising of the manuscript. CC contributed to quality control, acquisition of the data, statistical analysis, and drafting of the manuscript. All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work. All the authors have read and approved the final manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Vestbo J, Hurd SS, Agustí AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: gold executive summary. Am J Respir Crit Care Med. 2013;187(4):347–365. doi: 10.1164/rccm.201204-0596PP. [DOI] [PubMed] [Google Scholar]
- 2.Buist AS, Vollmer WM, McBurnie MA. Worldwide burden of COPD in high- and low-income countries. Part I. The burden of obstructive lung disease (BOLD) initiative. Int J Tuberc Lung Dis. 2008;12(7):703–708. [PubMed] [Google Scholar]
- 3.The Lung Health Study Research Group Effect of inhaled triamcinolone on the decline in pulmonary function in chronic obstructive pulmonary disease. N Engl J Med. 2000;343(26):1902–1909. doi: 10.1056/NEJM200012283432601. [DOI] [PubMed] [Google Scholar]
- 4.Tashkin DP, Celli B, Senn S, et al. UPLIFT Study Investigators A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med. 2008;359(15):1543–1554. doi: 10.1056/NEJMoa0805800. [DOI] [PubMed] [Google Scholar]
- 5.Decramer M, Celli B, Kesten S, Mehra S, Tashkin DP, UPLIFT investigators Effect of tiotropium on outcomes in patients with moderate chronic obstructive pulmonary disease (UPLIFT): a pre-specified subgroup analysis of a randomized controlled trial. Lancet. 2009;374(9696):1171–1178. doi: 10.1016/S0140-6736(09)61298-8. [DOI] [PubMed] [Google Scholar]
- 6.Price D, Freeman D, Cleland J, Kaplan A, Cerasoli F. Earlier diagnosis and earlier treatment of COPD in primary care. Prim Care Respir J. 2011;20(1):15–22. doi: 10.4104/pcrj.2010.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deesomchok A, Webb KA, Forkert L, et al. Lung hyperinflation and its reversibility in patients with airway obstruction of varying severity. COPD. 2010;7(6):428–437. doi: 10.3109/15412555.2010.528087. [DOI] [PubMed] [Google Scholar]
- 8.O’Donnell DE, Laveneziana P. Dyspnea and activity limitation in COPD: mechanical factors. COPD. 2007;4(3):225–236. doi: 10.1080/15412550701480455. [DOI] [PubMed] [Google Scholar]
- 9.O’Donnell DE, Revill SM, Webb KA. Dynamic hyperinflation and exercise intolerance in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164(5):770–777. doi: 10.1164/ajrccm.164.5.2012122. [DOI] [PubMed] [Google Scholar]
- 10.Oh YM, Sheen SS, Park JH, et al. Emphysematous phenotype is an independent predictor for frequent exacerbation of COPD. Int J Tuberc Lung Dis. 2014;18(12):1407–1414. doi: 10.5588/ijtld.14.0205. [DOI] [PubMed] [Google Scholar]
- 11.Casanova C, Cote C, de Torres JP, et al. Inspiratory-to-total lung capacity ratio predicts mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;171(6):591–597. doi: 10.1164/rccm.200407-867OC. [DOI] [PubMed] [Google Scholar]
- 12.Tantucci C, Donati P, Nicosia F, et al. Inspiratory capacity predicts mortality in patients with chronic obstructive pulmonary disease. Respir Med. 2008;102(4):613–619. doi: 10.1016/j.rmed.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 13.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 14.Wanger J, Clausen JL, Coates A, et al. Standardisation of the measurement of lung volumes. Eur Respir J. 2005;26(3):511–522. doi: 10.1183/09031936.05.00035005. [DOI] [PubMed] [Google Scholar]
- 15.Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26(5):948–968. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 16.Stocks J, Quanjer PH. Reference values for residual volume, functional residual capacity and total lung capacity. Eur Respir J. 1995;8(3):492–506. doi: 10.1183/09031936.95.08030492. [DOI] [PubMed] [Google Scholar]
- 17.Zheng J, Zhong N. Normative values of pulmonary function testing in Chinese adults. Chin Med J (Engl) 2002;115(1):50–54. [PubMed] [Google Scholar]
- 18.Zhang FQ, Zheng JP, Wang JH, et al. Comparison of lung volume response with airflow response to bronchodilator in patients with chronic obstructive pulmonary disease. Zhonghua Jie He He Hu Xi Za Zhi. 2010;33(2):109–113. [PubMed] [Google Scholar]
- 19.Jian W, Zheng J, Hu Y, Li Y, Gao Y, An J. What is the difference between FEV1 change in percentage predicted value and change over baseline in the assessment of bronchodilator responsiveness in patients with COPD? J Thorac Dis. 2013;5(4):393–399. doi: 10.3978/j.issn.2072-1439.2013.08.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ortega F, Márquez-Martín E, Valencia B, et al. Impact of bronchodilator responsiveness on quality of life and exercise capacity in patients with COPD. Respir Care. 2014;59(1):81–89. doi: 10.4187/respcare.02399. [DOI] [PubMed] [Google Scholar]
- 21.Alves GR, Marchiori E, Irion KL, et al. The effects of dynamic hyperinflation on CT emphysema measurements in patients with COPD. Eur J Radiol. 2014;83(12):2255–2259. doi: 10.1016/j.ejrad.2014.08.014. [DOI] [PubMed] [Google Scholar]
- 22.Stoel BC, Putter H, Bakker ME, et al. Volume correction in computed tomography densitometry for follow-up studies on pulmonary emphysema. Proc Am Thorac Soc. 2008;5(9):919–924. doi: 10.1513/pats.200804-040QC. [DOI] [PubMed] [Google Scholar]
- 23.Sun XW, Gu SY, Li QY, et al. Pulmonary function parameters in high-resolution computed tomography phenotypes of chronic obstructive pulmonary disease. Am J Med Sci. 2015;349(3):228–233. doi: 10.1097/MAJ.0000000000000395. [DOI] [PubMed] [Google Scholar]
- 24.Albert P, Agusti A, Edwards L, et al. Bronchodilator responsiveness as a phenotypic characteristic of established chronic obstructive pulmonary disease. Thorax. 2012;67(8):701–708. doi: 10.1136/thoraxjnl-2011-201458. [DOI] [PubMed] [Google Scholar]
- 25.Zhong N, Wang C, Yao W, et al. Prevalence of chronic obstructive pulmonary disease in China: a large, population-based survey. Am J Respir Crit Care Med. 2007;176(8):753–760. doi: 10.1164/rccm.200612-1749OC. [DOI] [PubMed] [Google Scholar]