Abstract
Leishmaniasis – human immunodeficiency virus (HIV) coinfection can manifest itself as tegumentary or visceral leishmaniasis. Almost 35 countries have reported autochthonous coinfections. Visceral leishmaniasis is more frequently described. However, usual and unusual manifestations of tegumentary leishmaniasis have been reported mainly in the Americas, but the real prevalence of Leishmania infection in HIV-infected patients is not clear. Regarding the clinical manifestations, there are some reports showing unusual manifestations in visceral leishmaniasis and tegumentary leishmaniasis in HIV-infected patients; yet, the usual manifestations are more frequent. Leishmaniasis diagnosis relies on clinical methods, but serological tests are used to diagnose visceral leishmaniasis despite them having a low sensitivity to tegumentary leishmaniasis. The search for the parasite is used to diagnose both visceral leishmaniasis and tegumentary leishmaniasis. Nevertheless, in HIV-infected patients, the sensitivity of serology is very low. Drugs available to treat leishmaniasis are more restricted and cause severe side effects. Furthermore, in HIV-infected patients, these side effects are more prominent and relapses and lethality are more recurrent. In this article, we discuss the current challenges of tegumentary leishmaniasis and visceral leishmaniasis–HIV infection, focusing mainly on the clinical manifestations, diagnosis, and treatment of leishmaniasis.
Keywords: leishmaniasis, HIV infection, coinfection, epidemiology, clinical manifestations, diagnosis, treatment
Introduction
The World Health Organization estimates that from about 900,000 to 1.3 million new cases of leishmaniasis are reported per year; of these, approximately 0.2–0.4 million are of visceral leishmaniasis (VL) and 0.7–1.2 million are of tegumentary leishmaniasis (TL).1,2 Leishmaniasis is endemic in over 98 countries and territories. It affects mainly some of the poorest people on earth, and is associated with malnutrition, population displacement, poor housing, a weak immune system, and lack of financial resources.1,3 The spread of the disease is linked to environmental changes such as deforestation, building of dams, irrigation schemes, and urbanization.1 More than 90% of global VL cases occur in six countries: Bangladesh, Brazil, Ethiopia, India, South Sudan, and Sudan. On the other hand, Afghanistan, Algeria, Brazil, Colombia, Costa Rica, Ethiopia, Iran, Peru, Sudan, and Syria, together account for 70%–75% of the estimated global incidence of TL.1 In Latin America, 96% of VL occurs in Brazil.1,2,4
Human immunodeficiency virus (HIV) infection is a major public health problem globally; there are about 36.9 million people living with HIV and 2.0 million new infections are reported per year.5 HIV is present in practically all countries and territories; however, the major burden of the disease is in sub-Saharan Africa, followed by Southeast Asia, the Americas, Europe, Western Pacific, and the Eastern Mediterranean area.6 Clearly, we can observe an overlap between the transmission areas of HIV and leishmaniasis. As a result, there have been an increasing number of cases of HIV–Leishmania coinfection, which has spread throughout the world. Since the end of the 1980s, Leishmania–HIV coinfection has been reported in 35 countries. Most reports on the coinfection are related to VL and HIV, and little is known about the actual situation of TL–HIV coinfection. In fact, HIV has affected the occurrence of leishmaniasis, mainly VL. Five to six percent of the total cases of VL–HIV around the world occur in the Mediterranean area.7 In some areas of Ethiopia, 35% of all leishmaniasis patients are coinfected with HIV,8 and the trend is spreading to neighboring countries such as Sudan. In India, the prevalence of VL–HIV has increased from 0.88% in 2000 to 2.18% in 2006.9 In Latin America, more precisely, in Brazil, the incidence of this coinfection has increased from 0.7% in 2001 to 8.5% in 2012.10
Some factors related to host and environment can influence the prevalence of VL–HIV coinfection. HIV and Leishmania share an immunopathological mechanism, compromising dendritic cells and macrophages. This fact contributes to replication of both the pathogens, accelerating the progression of VL and HIV.11,12 Notably, immunosuppression is an important factor for the development of VL in patients living with HIV in endemic areas for VL and also for VL reactivation, that is, when a patient infected by HIV presents a decrease in cell immunity, mainly related to CD4+ T-cell count. Factors related to the route of HIV infection also influence the transmission of leishmaniasis. In Spain, 70% of Leishmania–HIV coinfection occurs in intravenous drug users, which is characterized by an anthroponotic transmission cycle of Leishmania, in which Leishmania DNA is found in the shared needles of intravenous drug users.13,14 Another important factor for the occurrence of VL–HIV coinfection is associated with economic migration of people, that is, they become infected in urban areas and then return to rural areas where VL is prevalent. This fact could be observed in Ethiopia’s rural areas where HIV infection is a risk factor for the immigrants to develop VL.15
Demographic data related to Leishmania–HIV coinfection is now available, but abundant data is available for VL rather than TL. Young men are more prevalent in all the cohorts reported. In Spain, between 1997 and 2011, 99.4% of leishmaniasis–HIV coinfection occurred in males (86.4%) aged between 16 and 64 years.16 In other areas, different age groups are affected by VL without HIV. In Brazil, VL is more common in children; however, in the coinfection with HIV, the incidence is more among young males between 29 and 49 years of age, which is the same age group as HIV-infected patients.10 This point is crucial to detect an HIV infection in patients presenting with VL, because in 58% of them, VL is the first clinical manifestation of acquired immunodeficiency syndrome (AIDS).17 Therefore, offering them an HIV test is mandatory. Also, it is important to start the Highly Active Antiretroviral Therapy (HAART) and change the treatment schedule as the drug and the doses used in VL–HIV coinfection are different from those used in VL alone.10 Data related to TL and HIV are poor; there are some reports showing the prevalence in younger males,18,19 similar to that observed in patients not infected with HIV.
Especially in VL–HIV coinfection, a worrying situation is the increased relapse and lethality. Predictive factors of these outcomes are poorly known. Low CD4+ T-lymphocyte count, no increase in this cell count, and absence of secondary prophylaxis against VL in HIV-infected patients contribute to increased relapse and lethality.20 However, none of these factors are related to the parasite. It is necessary to know TCD4+ count to start HAART as soon as possible, preferably with the use of protease inhibitors.21 HAART can reduce not only relapse and lethality, but also the prevalence of VL–HIV coinfection. Clearly, a decrease of VL–HIV coinfection in Spain has been observed after the introduction of HAART.22–24 To date, there are no data about the impact of HAART on TL.
Different species of Leishmania can cause leishmaniasis, and they are involved in the development of TL (Table 1) or VL. The genus Leishmania is divided into the subgenera Leishmania and Viannia. Around the world, 21 species of Leishmania can cause tegumentary or visceral lesions; however, Leishmania from the subgenus Viannia causes mainly tegumentary lesions and is autochthonous in the Americas.25 Regarding the subgenus Leishmania, some species cause TL in Europe, Africa, and Asia, whereas some other species (Leishmania (Leishmania) infantum or Leishmania (Leishmania) infantum chagasi) cause VL in America and Europe and Leishmania (Leishmania) donovani causes VL in India and other Asian and African countries.26 The occurrence of HIV infection can disrupt this scenario because the species causing VL can also cause tegumentary lesions in HIV-infected patients and the species causing TL can affect the internal organs such as the spleen and liver.
Table 1.
Leishmania species related to clinical manifestation of tegumentary leishmaniasis.
| Localized cutaneous leishmaniasis | Leishmaniasis recidiva cutis | Disseminated leishmaniasis | Diffuse cutaneous leishmaniasis | Mucocutaneous leishmaniasis | |
|---|---|---|---|---|---|
| Tegumentary lesions | Round, well-delimited, painless ulcer with a central crust, sometimes hemorrhagic | Papule and vesicular lesions after clinical cure in or around the scar of an already healed lesion | Multiple pleomorphic lesions (10–300) often acneiform and papular, in two or more noncontiguous areas of the body surface | Nodular lesions that do not evolve into ulcerations, acting as an anergic pattern | Occurs years after the onset of TL. Destruction of oral–nasal and pharyngeal cavities that may evolve into disfiguring lesions. May start as a mild nasal inflammation and stuffiness, followed by ulcerations and perforations of the septum, extending to soft palate, pharynx, or larynx |
| Species | L. major, L. aethiopica, L. tropica, L. mexicana, L. amazonensis, L. (V.) braziliensis, L. (V.) guyanensis, L. (V.) shawi, L. (V.) lainsoni, and L. (V.) naiffi | L. aethiopica and L. (V.) braziliensis | L.(V.) braziliensis | L. mexicana and L. amazonensis in the Americas and L. aethiopica in Asia, Africa and Europe | L. (V.) braziliensis and L. (V.) guyanensis |
Abbreviation: TL, tegumentary leishmaniasis.
We assume that most cases of VL or TL occur in HIV-infected patients because some clinical manifestations could be mistaken as those of other opportunistic infections in immunosuppressed patients presenting with visceral or tegumentary lesions. In AIDS, TL can be mistaken with the cutaneous lesions caused by histoplasmosis, cryptococcosis or tuberculosis. Visceral involvement can be easily confused with diseases affecting the spleen, liver, or bone marrow, mainly disseminated mycobacteriosis or tuberculosis and histoplasmosis.
Clinical manifestations
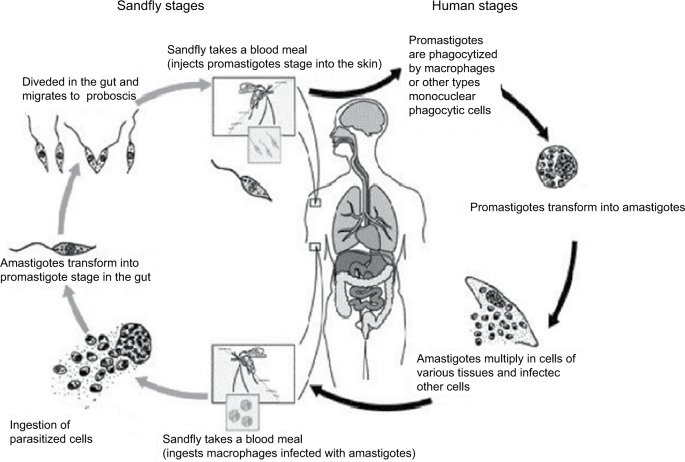
After transmission of Leishmania by sandfly or contaminated needles, the parasite enters into the macrophages or other types of cells. After multiplication of amastigotes, there is disruption of these cells and the parasites infect the other cells. Depending on the Leishmania species involved in the infection, TL or VL may be caused. After the sandfly takes a blood meal, ingesting the infected macrophages, transformation will occur into promastigotes within the digestive tract of the insect vector. When it takes a new blood meal, the infected females can transmit the promastigotes to the vertebrate host (Figure 1).
Figure 1.
Life cycle of Leishmania into the vertebrate and invertebrate host.
Note: Image modified from Centers for Disease Control and Prevention. Available from http://phil.cdc.gov/phil/home.asp.
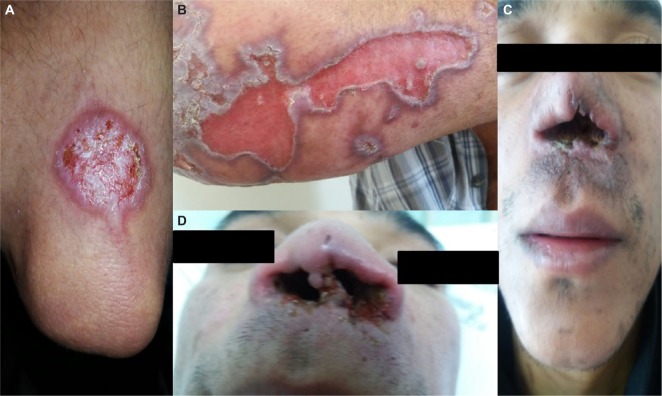
The wide spectrum of clinical presentations depends on the complex interplay between the infecting species, vector, immune and nutritional status of the host, age, genetic background of the host, inoculation site, and parasite load.27–29 Cutaneous, mucocutaneous, and visceral forms are the main clinical forms (Figures 2 and 3).30
Figure 2.
Lesions of tegumentary leishmaniasis.
Note: (A) Typical cutaneous lesion- localized cutaneous leishmaniasis, (B) Unusual manifestation of cutaneous leishmaniasis, characterized by large cutaneous ulceration, (C) and (D) Mucocutaneous leishmaniasis.
Figure 3.
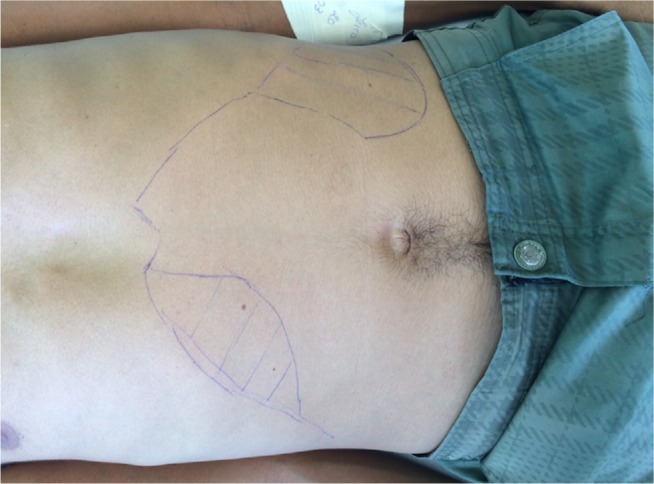
Clinical manifestation of visceral leishmaniasis in HIV-infected patient, showing hepatomegaly and splenomegaly.
Asymptomatic infections
The role of asymptomatic infection remains poorly recognized. Its proportion is five to ten times higher among immunocompetent hosts than the number of apparent VL cases infected as shown by serological evidence of anti-Leishmania antibodies, by detection of parasite DNA in blood samples, or by a positive reaction to the leishmanin skin test.31–34 Epidemiology of this asymptomatic infection in the parasite cycle is still unknown. Regarding the HIV-infected patients, the number of asymptomatic carriers also seems to be larger than the clinically evident VL patients.35,36 Association of a higher parasite load has already been demonstrated in those with higher HIV viral loads and appears to be related to a higher risk to develop the clinical disease. It has been shown that HIV infection may increase the risk of developing VL by 100 up to 2,300 times in endemic areas.36–38
Visceral leishmaniasis
The clinical presentation of VL in HIV-infected patients is equivalent to that observed in non-coinfected individuals. Typical forms comprise initially intermittent fever followed by a continuous pattern; nontender hepatosplenomegaly due to involvement of the reticuloendothelial system; pancytopenia, mainly due to parasites directly invading the bone marrow, causing signs and symptoms related to each cytopenia and leading to anemia, hemorrhages, and concurrent infections. Weight loss and anorexia may be misdiagnosed with other opportunistic infections or AIDS wasting syndrome itself.31,39 Furthermore, atypical VL remains undiagnosed in clinical settings, or is diagnosed with substantial delay, when early recognition and treatment are utmostly necessary. Most of the unusual clinical manifestations occur in patients with HIV with very low CD4+ T-cell counts. These patients might also be affected with other opportunistic infections, making timely etiologic diagnosis challenging.40
In a trial regarding secondary prophylaxis for VL relapses, eight (15%) of 54 patients with VL coinfected with HIV, screened during 14 months, presented with atypical clinical presentation of leishmaniasis. Three of them had skin lesions (one patient with scattered nodular lesions and two with post–kala-azar dermal leishmaniasis-like lesions), two had oral lesions, two had lymph node involvement (one with intra-abdominal lymph node involvement), and one patient had rectal lesions.40
Nevertheless, the main clinical differences between HIV-infected and non-coinfected individuals seem to be a wide variety of atypical and overlapping clinical presentations, with Leishmania parasites isolated from unusual sites (gastrointestinal and oral mucosa, skin, pleura, pericardium, lymph nodes, Kaposi’s sarcoma lesions, and respiratory tract).41–53 Moreover, it is noteworthy that the lower response rate to the treatment and the higher rate of relapse may be reduced by HAART, but not avoided entirely.54
Tegumentary leishmaniasis
After the insect bite and an incubation period from 2 weeks to 3 months, a small erythematous, itchy papule or nodule appears, sometimes preceded or followed by draining lymph node enlargement. Its initial lesion may cure spontaneously or evolve into clinical disease after months.29
Regarding the clinical forms of TL, they can be divided as follows:55
Localized cutaneous leishmaniasis (LCL): It is the most frequent form that is commonly caused by dermotropic species. Often, lesions appear on an exposed area of the body surface (one to ten). The typical lesion is a round, well-delimited, painless ulcer with a central crust, which may be sometimes hemorrhagic. It may cure spontaneously, leading to a hypopigmented, smooth, thin scar.56 L. major, L. aethiopica, L. tropica, L. mexicana, L. amazonensis, L. (V.) braziliensis, L. (V.) guyanensis, L. (V.) shawi, L. (V.) lainsoni, and L. (V.) naiffi can cause localized cutaneous leishmaniasis.
Leishmaniasis recidiva cutis: It is more common in the Old World.57 It may produce papule and vesicular lesions after clinical cure in or around the scar of the already healed lesion, with a time frame that may vary from months to years. It is caused mainly by L. (L.) aethiopica and L. (V.) braziliensis.
Disseminated leishmaniasis: This causes multiple pleomorphic lesions (10–300), often acneiform and papular, in two or more noncontiguous areas of the body surface.58 In this form, L. (V.) braziliensis seems to be the only species encountered.59,60
Diffuse cutaneous leishmaniasis: It is a rare condition with nodular lesions that do not evolve into ulcerations, and thus act as an anergic pattern. Typically, the lesions are rich in parasites and the species involved are: L. (L.) mexicana and L. (L.) amazonensis in the Americas and L. (L.) aethiopica in the Old World.61
Mucocutaneous leishmaniasis: This occurs years after the onset of TL and is characterized by the destruction of oral–nasal and pharyngeal cavities that may evolve into disfiguring lesions. The clinical manifestations may start as a mild nasal inflammation and stuffiness, followed by ulcerations and perforations of the septum, extending to soft palate, pharynx, or larynx. It is caused mainly by L. (V.) braziliensis and L. (V.) guyanensis.
Regarding the HIV-infected patients, these manifestations seem to be similar to the non-coinfected ones, but they may be present in unusual forms. A wide variety of lesions have already been described in case series, such as papules, nodules, plaques, and diverse ulcerations forms. In addition, different forms of mucosal lesions have also been reported, such as widespread, diffuse infiltration of the mucosal surface of the palate and genital lesions, which were present in 27% of the patients in a series.18,62
IRIS and leishmaniasis
Generally, HAART has led to a significant decline in AIDS-associated morbidity and mortality due, in part, to the partial recovery of the immune system. Nevertheless, some individuals under HAART may experience clinical deterioration, despite concomitant increases in CD4+ T-lymphocyte counts and decreases in plasma HIV-1 viral loads. This fact is a result of an inflammatory response or “deregulation” of the immune system to both intact subclinical pathogens and residual antigens. As a result, clinical manifestations of this syndrome are diverse and depend on the infectious or noninfectious agents involved.63–66
In the case of Leishmania sp. infection, this restoration may be translated as a new disease or a progression of a latent one.55 Three clinical variations of leishmaniasis with dermatological involvement have been reported: diffuse mucocutaneous, post– or para–kala-azar dermal leishmaniasis, and sporotrichoid dermal and subcutaneous nodules.67 Few cases of TL as a manifestation of immune reconstitution inflammatory syndrome (IRIS) in patients with AIDS have been reported to date.68–70 In general, disseminated skin lesions (on the arms, lower limbs, and feet) and lesions in the nasal, oropharyngeal, as well as genital mucosa have been reported.68
Although VL seems to be rare in the context of IRIS, it must be considered as a cause of sudden fever of unknown origin following the initiation of antiretroviral treatment in HIV patients from (or with travel history to) endemic areas.71
Diagnosis of leishmaniasis in HIV-infected patient
Laboratory diagnosis of VL
Irrespective of the HIV status, parasitological diagnosis remains the confirmatory tool because of its high specificity.9,72 Lymph nodes, bone marrow, and spleen are the main tissues used for the demonstration of amastigote forms in preparations stained with Giemsa or Leishman stain.72 In HIV-infected as well as in non-HIV-infected individuals, samples for microscopy from the spleen tissues have the best sensitivity, followed by those from bone marrow and lymph nodes.73 Nevertheless, bone marrow aspiration is the most utilized exam due to its good sensitivity (67%–94%) and lower risk of complications compared to spleen aspiration.74,75 Culture can add sensitivity, but it needs a special medium (Novy–MacNeal–Nicolle medium), and it is usually not available in most endemic regions.26
Regarding serological diagnosis in HIV-infected patients, there is limited evidence of the performance of the tests with a large variety of studies.76 Serological tests are clearly less reliable in these patients,73 and there are doubts about which technique is superior to others in this context.10 For antibody demonstration, indirect immunofluorescence assay,32 direct agglutination test (DAT), enzyme immunoassays, and immunoblotting can be utilized with variable sensitivity.9,76 Cota et al conducted a review including 33 studies and 1,489 patients, and showed an overall limited sensitivity of serological tests and observed that DAT and immunoblotting had a better performance compared to enzyme-linked immunosorbent assay and IFA.76 In an original paper including 113 HIV-infected symptomatic patients, DAT exhibited good overall performance (positivity of 89%), with no statistical differences in comparison with molecular diagnosis. IFA and recombinant K39 antigen-based immunochromatographic test (rk39 dipstick test) presented the lowest sensitivity (45.6% and 60.9%, respectively).77
In theory, methods to detect antigens would work better on the diagnosis of active leishmaniasis in HIV-infected patients, because they could be related to the parasite burden.9 Despite this concept, a latex agglutination test kit (Katex) that detects antigen in urine samples showed satisfactory sensitivity and specificity in immunocompetent VL patients, but poorer sensitivity in HIV-coinfected individuals.9,78 In a study including a small sample of 13 individuals with HIV–Leishmania coinfection in Latin America, only five were Katex positive, while DAT positivity was 100%.79
Molecular diagnosis of VL using various Leishmania gene target sequences is becoming increasingly relevant both in non–HIV- and HIV-infected patients.9 Advantages include high sensitivity and specificity, possibility to use samples from peripheral blood or bone marrow, and possibility of laboratory follow-up in treated patients.10 Thus, besides the diagnosis, it would be possible to monitor the efficacy of therapy, avoiding invasive procedures.73 The positivity of polymerase chain reaction (PCR) varies considering the use of whole blood (less invasive) or bone marrow (83%–98% and 93%–100%, respectively).76 It is important to note that PCR is not a unique method and that it is very difficult to compare different studies due to the different PCR targets utilized. Probably, PCR assay based on amplification of kinetoplast is the most sensible method to detect DNA from Leishmania.77,80 Real-time qPCR is an alternative for diagnosis and follow-up of infection, with a positivity of 85.7% in HIV-infected patients in Latin America.77,81 Although this method is considered a useful tool for the diagnosis of coinfected patients, it is noteworthy that asymptomatic individuals could show positive results,77 which limits its use to the active disease diagnosis in areas of high transmission of VL.
Diagnosis of TL
Although there are case reports describing several clinical presentations of cutaneous leishmaniasis associated with HIV infection, there are no studies regarding laboratory diagnosis of cutaneous leishmaniasis exclusively in HIV-infected patients.57,82–84 Theoretically, HIV-infected patients should show greater positivity in direct exams and lower accuracy in serological methods because of the host cell-mediated immune deficiency. However, there are no studies comparing the positivity of diagnostic methods in HIV-infected patients; therefore, all information available comes from reports and case series.18
In VL, parasitological diagnosis remains the main test for diagnosis of cutaneous and mucocutaneous leishmaniasis due to its high specificity.58 Direct exam of biopsy specimens, scrapings, or impression smears for amastigote search can be performed by Giemsa staining. This is the most common diagnostic approach because of its availability and easy execution. The sensitivity of the direct examination is low, especially in the Americas chronic and mucosal cases, which could also compromise the diagnosis of HIV-infected patients.55,58 Nevertheless, in a case series including 15 individuals, positivity of direct examination was 80%, probably due to severe immunosuppression of the patients involved.18 Culture (Novy–MacNeal–Nicolle medium) of samples obtained through biopsy and aspirate samples can complement the diagnosis.55
Molecular diagnosis is a promising field that has seen major growth in the last decades. Studies with immunocompetent patients have shown PCR-based methods to be useful, especially in cases of expected low parasite load, like in mucosal forms. Other advantages include the possibility of using different biological materials and easy identification of Leishmania species, which is also possible by culture, but it is more time consuming and expensive. Specificity and sensitivity of PCR-based methods can reach values near 100%. Real-time PCR is another approach with promising results. This technique can also detect the Leishmania genus and species, and gives more rapid results and causes less contamination.55,58 Regarding the diagnosis based on immunological test, leishmanin skin test (Montenegro test) can be used, but it can refer to past or present infections. This method can be associated with other tests to improve diagnosis.25 Predominance of cellular immune response is related to positivity of leishmanin skin test. Therefore, it would be expected that patients with immunosuppression due to HIV infection could have negative Montenegro test as a result of predominance of T helper 2 (Th2) cell response in advanced disease. However, case reports showed high positivity of Montenegro test in HIV patients, opposing this concept and indicating that this test can help diagnosis, even in HIV-infected patients.19,84 Indirect immunofluorescence assay and enzyme-linked immunosorbent assay are the methods commonly used for the diagnosis of cutaneous leishmaniasis, but their sensitivity is considered low. This is a challenge in HIV-infected patients, because sometimes this is the only diagnostic tool available.25 In the Mediterranean region, as expected, serological tests showed low sensitivity in HIV–Leishmania-infected individuals. However, in Brazil, the sensitivity was higher, reaching 77% in a small case series.18 This discrepancy reveals the need for studies including more patients in order to establish the real importance of serological methods on leishmaniasis diagnosis in HIV-infected patients.
Treatment of Leishmania–HIV coinfection
As shown previously, leishmaniasis–HIV coinfection poses some difficulties for diagnosis, as well as in identifying the atypical clinical manifestations, either in visceral or tegumentary forms.85 Patients with severe immunosuppression, presenting with opportunistic diseases, and using the highly effective HAART may have drug interaction problems. Briefly, drug interaction is widespread during the treatment of opportunistic infections and AIDS. Additional toxicity in the treatment for Leishmania–HIV coinfection should be avoided, and minimal side effects have to be sought. Coinfection is associated with high initial failures, relapses, drug toxicity, and mortality.73
VL–HIV comorbidity has some particularities depending on the area studied, and drug combinations have been used. Liposomal amphotericin is the major drug used with better outcomes in coinfected individuals.85 However, dosing is still a great problem, as there is no consensus on which is the best choice for different populations worldwide. A study conducted in South Asia showed that a single dose of 10 mg/kg of liposomal amphotericin B (LAmB) is sufficient to treat VL–HIV coinfected individuals; on the other hand, higher doses are necessary for the treatment of the same VL–HIV coinfection in Ethiopia.86 In Brazil (where almost 90% of all VL cases from the Americas are reported), LAmB is recommended in a total dose of 20–40 mg/kg, although not based on clinical trials but only based on observational studies conducted in Latin America.10 Few studies about miltefosine are available worldwide, showing limited efficacy and acceptable toxicity.73 Although a combination therapy remains to be explored, a combination of LAmB (30 mg/kg) and miltefosine (100 mg/day for 14 days) has also been used for the treatment of coinfections in India with great results, as reported by Mahajan et al.87 Antimonials still continue to be used to treat VL patients in East Africa and Latin America; however, for VL–HIV coinfection, they pose some difficulties. They are not only unsafe because of life-threatening toxicity, but also have low effectiveness and should be avoided in this population.73,88 Nevertheless, secondary prophylaxis with LAmB (3–4 mg/kg) every 2–4 weeks is recommended for preventing relapses of VL in HIV-coinfected individuals, since they have a high rate of annual recurrence. Other alternatives include the use of pentamidine isethionate monthly at a dose of 4 mg/kg.89 HAART also has to be initiated to prevent VL relapses, as patients with lower CD4+ T-cell counts are at increased risk despite the use of secondary prophylaxis and effective initial treatment for VL.89 Also, HIV-1 protease inhibitors have shown some inhibitory effect on Leishmania in vitro, even though in doses that would be unacceptable for human beings in vivo. Anyway, HAART with protease inhibitors, as a backbone combination therapy, would be preferable in this coinfected population, as it acts as a primary prophylaxis in asymptomatic Leishmania infection, as an adjunctive therapy in those with clinical manifestations, or as a maintenance therapy in those who are treatment unresponsive.21
Studies regarding the treatment of TL–HIV coinfection are scarce and therapy must follow the guidelines established for immunocompetent individuals, based on epidemiology and the species of Leishmania involved. Instead of local therapy, systemic therapy is more frequently indicated for the treatment of TL–HIV coinfection. Moreover, the management of cutaneous or mucosal leishmaniasis can be challenging for the clinicians dealing with severe immunosuppression of HIV because there are higher risks of treatment failure and dissemination of Leishmania. Clinical healing of lesions and prevention of parasite dissemination by destroying them or improving the host’s immunity is the treatment goal of TL. As indicated by the Centers for Disease Control from the USA, antimonials (20 mg/kg Sb5+) intravenous/intramuscular for 28 days or LAmB as for VL (20–40 mg/kg) has shown great results in the treatment of cutaneous or mucocutaneous leishmaniasis in HIV-coinfected patients.25,55,73 However, as in VL treatment, use of antimonials for TL therapy implicates some side effects, mainly cardiotoxicity and renal failure. Another study conducted in Sao Paulo, Brazil showed a good response to LAmB when used for the treatment of mucosal leishmaniasis.90 Ultimately, no secondary prophylaxis is indicated for TL–HIV coinfection.25
An important point to be explored in leishmaniasis treatment, both visceral and tegumentary, is the search for new active drugs against all species of Leishmania, focusing on the mechanism of action to eliminate the parasite. We support this point because after clinical cure is obtained, parasites continue to be present in some organs or lesions, and if immunosuppression is still present, reactivations or relapses of leishmaniasis may occur.
Current contents
Leishmania–HIV coinfection has spread around the world due to several factors related to the environment, host, or parasite. Different species of Leishmania can cause visceral or tegumentary lesions. Two species can cause VL (L. (L.) donovani and L. (L.) infantum [or L. (L.) infantum chagasi in the Americas]). Other species from the Leishmania genus can cause TL in the New or Old World, and Leishmania from Viannia genus causes only TL in the Americas. In HIV-infected patients, there are anecdotal cases such as cutaneous lesions caused by L. (L.) infantum or visceral lesions caused by dermotropic species. Most patients with visceral or cutaneous leishmaniasis and HIV infection have clinical manifestations similar to those of patients not infected with HIV; but unusual manifestations of cutaneous leishmaniasis and VL and usual manifestations of these clinical forms, such as IRIS, are described in patients with HIV/AIDS. Regarding treatment response, we observed an increase in relapse and lethality in VL–HIV coinfected patients despite having the same clinical manifestations as those occurring in VL alone. Diagnosis based on serology or skin test has failed, with the sensitivity being under 50% for VL due to immunosuppression. Parasitological methods have presented high positivity both for TL and VL. Nowadays, it is of paramount importance that the physicians working in nonendemic areas for leishmaniasis watch out for the possible diagnosis of leishmaniasis in HIV-infected patients and the physicians working in endemic areas for leishmaniasis watch out for unusual manifestations of leishmaniasis or relapses, which could be associated with HIV infection.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Organization WH . Control of the Leishmaniases. Geneva: WHO; 2010. [Google Scholar]
- 2.Alvar J, Velez ID, Bern C, et al. Leishmaniasis worldwide and global estimates of its incidence. PloS One. 2012;7(5):e35671. doi: 10.1371/journal.pone.0035671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alvar J, Yactayo S, Bern C. Leishmaniasis and poverty. Trends Parasitol. 2006;22(12):552–557. doi: 10.1016/j.pt.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 4.Pan American Health Organization WHO . Leishmaniases: Epidemiological Report of the Americas. Pan American Health Organization, World Health Organization; Washington: 2013. [Google Scholar]
- 5.UNAIDS World AIDS Day Report 2012. [cited 2013 22 july 2013]; Available from: http://www.unaids.org/en/media/unaids/contentassets/documents/epidemiology/2012/gr2012/JC2434_WorldAIDSday_results_en.pdf.
- 6.UNAIDS . Global report: UNAIDS report on the global AIDS epidemic. Geneva: World Health Organization; 2012. [Google Scholar]
- 7.Monge-Maillo B, Norman FF, Cruz I, Alvar J, Lopez-Velez R. Visceral leishmaniasis and HIV coinfection in the Mediterranean region. PLoS Negl Trop Dis. 2014;8(8):e3021. doi: 10.1371/journal.pntd.0003021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diro E, Lynen L, Ritmeijer K, Boelaert M, Hailu A, van Griensven J. Visceral Leishmaniasis and HIV coinfection in East Africa. PLoS Negl Trop Dis. 2014;8(6):e2869. doi: 10.1371/journal.pntd.0002869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh S. Changing trends in the epidemiology, clinical presentation, and diagnosis of Leishmania-HIV co-infection in India. IJID. 2014;29:103–112. doi: 10.1016/j.ijid.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 10.Lindoso JA, Cota GF, da Cruz AM, et al. Visceral leishmaniasis and HIV coinfection in Latin America. PLoS Negl Trop Dis. 2014;8(9):e3136. doi: 10.1371/journal.pntd.0003136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garg R, Barat C, Ouellet M, Lodge R, Tremblay MJ. Leishmania infantum amastigotes enhance HIV-1 production in cocultures of human dendritic cells and CD4 T cells by inducing secretion of IL-6 and TNF-alpha. PLoS Negl Trop Dis. 2009;3(5):e441. doi: 10.1371/journal.pntd.0000441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bernier R, Turco SJ, Olivier M, Tremblay M. Activation of human immunodeficiency virus type 1 in monocytoid cells by the protozoan parasite Leishmania donovani. J Virol. 1995;69(11):7282–7285. doi: 10.1128/jvi.69.11.7282-7285.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alvar J, Canavate C, Gutierrez-Solar B, et al. Leishmania and human immunodeficiency virus coinfection: the first 10 years. Clin Microbiol Rev. 1997;10(2):298–319. doi: 10.1128/cmr.10.2.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Molina R, Gradoni L, Alvar J. HIV and the transmission of leishmania. Ann Trop Med Parasit. 2003;97(Suppl 1):29–45. doi: 10.1179/000349803225002516. [DOI] [PubMed] [Google Scholar]
- 15.Argaw D, Mulugeta A, Herrero M, et al. Risk factors for visceral Leishmaniasis among residents and migrants in Kafta-Humera, Ethiopia. PLoS Negl Trop Dis. 2013;7(11):e2543. doi: 10.1371/journal.pntd.0002543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herrador Z, Gherasim A, Jimenez BC, Granados M, San Martin JV, Aparicio P. Epidemiological changes in leishmaniasis in Spain according to hospitalization-based records, 1997–2011: raising awareness towards leishmaniasis in non-HIV patients. PLoS Negl Trop Dis. 2015;9(3):e0003594. doi: 10.1371/journal.pntd.0003594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cota GF, de Sousa MR, de Mendonca AL, Patrocinio A, Assunção LS, de Faria SR, Rabello A. Leishmania-HIV co-infection: clinical presentation and outcomes in an urban area in Brazil. PLoS Negl Trop Dis. 2014;8(4):e2816. doi: 10.1371/journal.pntd.0002816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lindoso JA, Barbosa RN, Posada-Vergara MP, Duarte MI, Oyafuso LK, Amato VS, Goto H. Unusual manifestations of tegumentary leishmaniasis in AIDS patients from the new world. Br J Dermatol. 2009;160(2):311–318. doi: 10.1111/j.1365-2133.2008.08908.x. [DOI] [PubMed] [Google Scholar]
- 19.Guerra JA, Coelho LI, Pereira FR, et al. American tegumentary leishmaniasis and HIV-AIDS association in a tertiary care center in the Brazilian Amazon. Am J Trop Med Hyg. 2011;85(3):524–527. doi: 10.4269/ajtmh.2011.11-0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cota GF, de Sousa MR, Rabello A. Predictors of visceral leishmaniasis relapse in HIV-infected patients: a systematic review. PLoS Negl Trop Dis. 2011;5(6):e1153. doi: 10.1371/journal.pntd.0001153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Griensven J, Diro E, Lopez-Velez R, et al. HIV-1 protease inhibitors for treatment of visceral leishmaniasis in HIV-co-infected individuals. Lancet Infect Dis. 2013;13(3):251–259. doi: 10.1016/S1473-3099(12)70348-1. [DOI] [PubMed] [Google Scholar]
- 22.Lopez-Velez R, Casado JL, Pintado V. Decline of a visceral leishmaniasis epidemic in HIV-infected patients after the introduction of highly active antiretroviral therapy (HAART) Clin Microbiol Infect. 2001;7(7):394–395. doi: 10.1046/j.1198-743x.2001.00270.x. [DOI] [PubMed] [Google Scholar]
- 23.Russo R, Nigro L, Panarello G, Montineri A. Clinical survey of Leishmania/HIV co-infection in Catania, Italy: the impact of highly active antiretroviral therapy (HAART) Ann Trop Med Parasitol. 2003;97(Suppl 1):149–155. doi: 10.1179/000349803225002624. [DOI] [PubMed] [Google Scholar]
- 24.del Giudice P, Mary-Krause M, Pradier C, et al. Impact of highly active antiretroviral therapy on the incidence of visceral leishmaniasis in a French cohort of patients infected with human immunodeficiency virus. J Infect Dis. 2002;186(9):1366–1370. doi: 10.1086/344325. [DOI] [PubMed] [Google Scholar]
- 25.Goto H, Lauletta Lindoso JA. Cutaneous and mucocutaneous leishmaniasis. Infect Dis Clin North Am. 2012;26(2):293–307. doi: 10.1016/j.idc.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 26.van Griensven J, Diro E. Visceral leishmaniasis. Infect Dis Clin North Am. 2012;26(2):309–322. doi: 10.1016/j.idc.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 27.Akilov OE, Khachemoune A, Hasan T. Clinical manifestations and classification of Old World cutaneous leishmaniasis. Int J Dermatol. 2007;46(2):132–142. doi: 10.1111/j.1365-4632.2007.03154.x. [DOI] [PubMed] [Google Scholar]
- 28.Schwartz E, Hatz C, Blum J. New world cutaneous leishmaniasis in travellers. Lancet Infect Dis. 2006;6(6):342–349. doi: 10.1016/S1473-3099(06)70492-3. [DOI] [PubMed] [Google Scholar]
- 29.Bogdan C. Leishmaniasis in rheumatology, haematology and oncology: epidemiological, immunological and clinical aspects and caveats. Ann Rheum Dis. 2012;71(Suppl 2):i60–i66. doi: 10.1136/annrheumdis-2011-200596. [DOI] [PubMed] [Google Scholar]
- 30.Saporito L, Giammanco GM, De Grazia S, Colomba C. Visceral leishmaniasis: host-parasite interactions and clinical presentation in the immunocompetent and in the immunocompromised host. IJID. 2013;17(8):e572–e576. doi: 10.1016/j.ijid.2012.12.024. [DOI] [PubMed] [Google Scholar]
- 31.Organization WH Control of the leishmaniases. World Health Organ Tech Rep Ser. 2010;(949):xii–xiii. 1–186. back cover. [PubMed] [Google Scholar]
- 32.Antinori S, Schifanella L, Corbellino M. Leishmaniasis: new insights from an old and neglected disease. Eur J Clin Microbiol Infect Dis. 2012;31(2):109–118. doi: 10.1007/s10096-011-1276-0. [DOI] [PubMed] [Google Scholar]
- 33.Desjeux P. Human leishmaniases: epidemiology and public health aspects. World Health Stat Q. 1992;45(2–3):267–275. [PubMed] [Google Scholar]
- 34.Sakthianandeswaren A, Foote SJ, Handman E. The role of host genetics in leishmaniasis. Trends Parasitol. 2009;25(8):383–391. doi: 10.1016/j.pt.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 35.Colomba C, Saporito L, Vitale F, et al. Cryptic Leishmania infantum infection in Italian HIV infected patients. BMC Infect Dis. 2009;9:199. doi: 10.1186/1471-2334-9-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ezra N, Ochoa MT, Craft N. Human immunodeficiency virus and leishmaniasis. J Glob Infect Dis. 2010;2(3):248–257. doi: 10.4103/0974-777X.68528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kallel K, Ammari L, Kaouech E, Belhadj S, Anane S, Kilani B, Chaker E. Asymptomatic bearing of Leishmania infantum among Tunisian HIV infected patients. Pathol Biol (Paris) 2007;55(10):521–524. doi: 10.1016/j.patbio.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 38.García-García JA, Martín-Sánchez J, Gállego M, et al. Use of noninvasive markers to detect leishmania infection in asymptomatic human immunodeficiency virus-infected patients. J Clin Microbiol. 2006;44(12):4455–4458. doi: 10.1128/JCM.00921-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pearson RD, Lareau SM, Jeronimo SM. Leishmaniasis at the end of the millennium. Curr Infect Dis Rep. 1999;1(5):448–452. doi: 10.1007/s11908-999-0057-8. [DOI] [PubMed] [Google Scholar]
- 40.Diro E, van Griensven J, Mohammed R, et al. Atypical manifestations of visceral leishmaniasis in patients with HIV in north Ethiopia: a gap in guidelines for the management of opportunistic infections in resource poor settings. Lancet Infect Dis. 2015;15(1):122–129. doi: 10.1016/S1473-3099(14)70833-3. [DOI] [PubMed] [Google Scholar]
- 41.Ejara ED, Lynen L, Boelaert M, Van Griensven J. Challenges in HIV and visceral Leishmania co-infection: future research directions. Trop Med Int Health. 2010;15(10):1266–1267. doi: 10.1111/j.1365-3156.2010.02612.x. [DOI] [PubMed] [Google Scholar]
- 42.Ara M, Maillo C, Peón G, Clavel A, Cuesta J, Grasa MP, Carapeto FJ. Visceral leishmaniasis with cutaneous lesions in a patient infected with human immunodeficiency virus. Br J Dermatol. 1998;139(1):114–117. doi: 10.1046/j.1365-2133.1998.02326.x. [DOI] [PubMed] [Google Scholar]
- 43.Bosch RJ, Rodrigo AB, Sánchez P, de Gálvez MV, Herrera E. Presence of Leishmania organisms in specific and non-specific skin lesions in HIV-infected individuals with visceral leishmaniasis. Int J Dermatol. 2002;41(10):670–675. doi: 10.1046/j.1365-4362.2002.01610.x. [DOI] [PubMed] [Google Scholar]
- 44.González-Beato MJ, Moyano B, Sánchez C, González-Beato MT, Pérez-Molina JA, Miralles P, Lázaro P. Kaposi’s sarcoma-like lesions and other nodules as cutaneous involvement in AIDS-related visceral leishmaniasis. Br J Dermatol. 2000;143(6):1316–1318. doi: 10.1046/j.1365-2133.2000.03909.x. [DOI] [PubMed] [Google Scholar]
- 45.Dereure J, Duong Thanh H, Lavabre-Bertrand T, et al. Visceral leishmaniasis. Persistence of parasites in lymph nodes after clinical cure. J Infect. 2003;47(1):77–81. doi: 10.1016/s0163-4453(03)00002-1. [DOI] [PubMed] [Google Scholar]
- 46.Aliaga L, Cobo F, Mediavilla JD, et al. Localized mucosal leishmaniasis due to Leishmania (Leishmania) infantum: clinical and microbiologic findings in 31 patients. Medicine (Baltimore) 2003;82(3):147–158. doi: 10.1097/01.md.0000076009.64510.b8. [DOI] [PubMed] [Google Scholar]
- 47.Albrecht H, Stellbrink HJ, Gross G, Berg B, Helmchen U, Mensing H. Treatment of atypical leishmaniasis with interferon gamma resulting in progression of Kaposi’s sarcoma in an AIDS patient. Clin Investig. 1994;72(12):1041–1047. doi: 10.1007/BF00577752. [DOI] [PubMed] [Google Scholar]
- 48.Alonso MJ, Muñoz E, Picazo A, et al. Duodenal leishmaniasis diagnosed by biopsy in two HIV-positive patients. Pathol Res Pract. 1997;193(1):43–47. doi: 10.1016/s0344-0338(97)80092-1. discussion 49–50. [DOI] [PubMed] [Google Scholar]
- 49.Angarano G, Maggi P, Rollo MA, Larocca AM, Quarto M, Scalone A, Gradoni L. Diffuse necrotic hepatic lesions due to visceral leishmaniasis in AIDS. J Infect. 1998;36(2):167–169. doi: 10.1016/s0163-4453(98)80007-8. [DOI] [PubMed] [Google Scholar]
- 50.Balkhair A, Ben Abid F. Gastric and cutaneous dissemination of visceral leishmaniasis in a patient with advanced HIV. IJID. 2008;12(1):111–113. doi: 10.1016/j.ijid.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 51.Betz P, Elsing C, Purrmann J, Frenzel H. Leishmaniasis of the upper gastrointestinal tract in an HIV positive patient. Pathologe. 1990;11(2):97–100. German. [PubMed] [Google Scholar]
- 52.Cánovas DL, Carbonell J, Torres J, Altés J, Buades J. Laryngeal leishmaniasis as initial opportunistic disease in HIV infection. J Laryngol Otol. 1994;108(12):1089–1092. doi: 10.1017/s002221510012897x. [DOI] [PubMed] [Google Scholar]
- 53.Heudier P, Taillan B, Garnier G, Marty P, Fuzibet JG, Dujardin P. Pulmonary site of visceral leishmaniasis in HIV infection. Presse Med. 1993;22(22):1060. [PubMed] [Google Scholar]
- 54.López-Vélez R. The impact of highly active antiretroviral therapy (HAART) on visceral leishmaniasis in Spanish patients who are co-infected with HIV. Ann Trop Med Parasitol. 2003;97(Suppl 1):143–147. doi: 10.1179/000349803225002615. [DOI] [PubMed] [Google Scholar]
- 55.Goto H, Lindoso JA. Current diagnosis and treatment of cutaneous and mucocutaneous leishmaniasis. Expert Rev Anti Infect Ther. 2010;8(4):419–433. doi: 10.1586/eri.10.19. [DOI] [PubMed] [Google Scholar]
- 56.Scarisbrick JJ, Chiodini PL, Watson J, et al. Clinical features and diagnosis of 42 travellers with cutaneous leishmaniasis. Travel Med Infect Dis. 2006;4(1):14–21. doi: 10.1016/j.tmaid.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 57.Machado ES, Braga Mda P, Da Cruz AM, et al. Disseminated American muco-cutaneous leishmaniasis caused by Leishmania braziliensis braziliensis in a patient with AIDS: a case report. Mem Inst Oswaldo Cruz. 1992;87(4):487–492. doi: 10.1590/s0074-02761992000400005. [DOI] [PubMed] [Google Scholar]
- 58.Reithinger R, Dujardin JC, Louzir H, Pirmez C, Alexander B, Brooker S. Cutaneous leishmaniasis. Lancet Infect Dis. 2007;7(9):581–596. doi: 10.1016/S1473-3099(07)70209-8. [DOI] [PubMed] [Google Scholar]
- 59.Turetz ML, Machado PR, Ko AI, et al. Disseminated leishmaniasis: a new and emerging form of leishmaniasis observed in northeastern Brazil. J Infect Dis. 2002;186(12):1829–1834. doi: 10.1086/345772. [DOI] [PubMed] [Google Scholar]
- 60.Costa JM, Marsden PD, Llanos-Cuentas EA, et al. Disseminated cutaneous leishmaniasis in a field clinic in Bahia, Brazil: a report of eight cases. J Trop Med Hyg. 1986;89(6):319–323. [PubMed] [Google Scholar]
- 61.Barral A, Costa JM, Bittencourt AL, Barral-Netto M, Carvalho EM. Polar and subpolar diffuse cutaneous leishmaniasis in Brazil: clinical and immunopathologic aspects. Int J Dermatol. 1995;34(7):474–479. doi: 10.1111/j.1365-4362.1995.tb00613.x. [DOI] [PubMed] [Google Scholar]
- 62.Puig L, Pradinaud R. Leishmania and HIV co-infection: dermatological manifestations. Ann Trop Med Parasitol. 2003;97(Suppl 1):107–114. doi: 10.1179/000349803225002589. [DOI] [PubMed] [Google Scholar]
- 63.Narita M, Ashkin D, Hollender ES, Pitchenik AE. Paradoxical worsening of tuberculosis following antiretroviral therapy in patients with AIDS. Am J Respir Crit Care Med. 1998;158(1):157–161. doi: 10.1164/ajrccm.158.1.9712001. [DOI] [PubMed] [Google Scholar]
- 64.Gea-Banacloche JC, Clifford Lane H. Immune reconstitution in HIV infection. AIDS (London, England) 1999;13(Suppl A):S25–S38. [PubMed] [Google Scholar]
- 65.Stone SF, Price P, Keane NM, Murray RJ, French MA. Levels of IL-6 and soluble IL-6 receptor are increased in HIV patients with a history of immune restoration disease after HAART. HIV Med. 2002;3(1):21–27. doi: 10.1046/j.1464-2662.2001.00096.x. [DOI] [PubMed] [Google Scholar]
- 66.Koval CE, Gigliotti F, Nevins D, Demeter LM. Immune reconstitution syndrome after successful treatment of Pneumocystis carinii pneumonia in a man with human immunodeficiency virus type 1 infection. Clin Infect Dis. 2002;35(4):491–493. doi: 10.1086/341974. [DOI] [PubMed] [Google Scholar]
- 67.Amerson EH, Maurer TA. Immune reconstitution inflammatory syndrome and tropical dermatoses. Dermatol Clin. 2011;29(1):39–43. doi: 10.1016/j.det.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 68.Posada-Vergara MP, Lindoso JA, Tolezano JE, Pereira-Chioccola VL, Silva MV, Goto H. Tegumentary leishmaniasis as a manifestation of immune reconstitution inflammatory syndrome in 2 patients with AIDS. J Infect Dis. 2005;192(10):1819–1822. doi: 10.1086/497338. [DOI] [PubMed] [Google Scholar]
- 69.Sinha S, Fernández G, Kapila R, Lambert WC, Schwartz RA. Diffuse cutaneous leishmaniasis associated with the immune reconstitution inflammatory syndrome. Int J Dermatol. 2008;47(12):1263–1270. doi: 10.1111/j.1365-4632.2008.03804.x. [DOI] [PubMed] [Google Scholar]
- 70.Chrusciak-Talhari A, Ribeiro-Rodrigues R, Talhari C, et al. Tegumentary leishmaniasis as the cause of immune reconstitution inflammatory syndrome in a patient co-infected with human immunodeficiency virus and Leishmania guyanensis. Ann Trop Med Parasitol. 2009;81(4):559–564. doi: 10.4269/ajtmh.2009.09-0077. [DOI] [PubMed] [Google Scholar]
- 71.Schleenvoigt BT, Ignatius R, Baier M, et al. Development of visceral leishmaniasis in an HIV(+) patient upon immune reconstitution following the initiation of antiretroviral therapy. Infection. 2016;44(1):115–119. doi: 10.1007/s15010-015-0813-7. [DOI] [PubMed] [Google Scholar]
- 72.Srivastava P, Dayama A, Mehrotra S, Sundar S. Diagnosis of visceral leishmaniasis. Trans R Soc Trop Med Hyg. 2011;105(1):1–6. doi: 10.1016/j.trstmh.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.van Griensven J, Carrillo E, Lopez-Velez R, Lynen L, Moreno J. Leishmaniasis in immunosuppressed individuals. Clin Microbiol Infect. 2014;20(4):286–299. doi: 10.1111/1469-0691.12556. [DOI] [PubMed] [Google Scholar]
- 74.Alvar J, Aparicio P, Aseffa A, et al. The relationship between leishmaniasis and AIDS: the second 10 years. Clin Microb Rev. 2008;21(2):334–359. doi: 10.1128/CMR.00061-07. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lima IP, Muller MC, Holanda TA, Harhay M, Costa CH, Costa DL. Human immunodeficiency virus/Leishmania infantum in the first foci of urban American visceral leishmaniasis: clinical presentation from 1994 to 2010. Rev Soc Bras Med Trop. 2013;46(2):156–160. doi: 10.1590/0037-8682-0033-2012. [DOI] [PubMed] [Google Scholar]
- 76.Cota GF, de Sousa MR, Demarqui FN, Rabello A. The diagnostic accuracy of serologic and molecular methods for detecting visceral leishmaniasis in HIV infected patients: meta-analysis. PLoS Negl Trop Dis. 2012;6(5):e1665. doi: 10.1371/journal.pntd.0001665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cota GF, de Sousa MR, de Freitas Nogueira BM, et al. Comparison of parasitological, serological, and molecular tests for visceral leishmaniasis in HIV-infected patients: a cross-sectional delayed-type study. Am J Trop Med Hyg. 2013;89(3):570–577. doi: 10.4269/ajtmh.13-0239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Attar ZJ, Chance ML, el-Safi S, et al. Latex agglutination test for the detection of urinary antigens in visceral leishmaniasis. Acta Trop. 2001;78(1):11–16. doi: 10.1016/s0001-706x(00)00155-8. [DOI] [PubMed] [Google Scholar]
- 79.Barbosa Junior WL, Ramos de Araujo PS, Dias de Andrade L, et al. Rapid tests and the diagnosis of visceral leishmaniasis and human immunodeficiency virus/acquired immunodeficiency syndrome coinfection. Am J Trop Med Hyg. 2015;93(5):967–969. doi: 10.4269/ajtmh.14-0798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nuzum E, White F, 3rd, Thakur C, Dietze R, Wages J, Grogl M, Berman J. Diagnosis of symptomatic visceral leishmaniasis by use of the polymerase chain reaction on patient blood. J Infect Dis. 1995;171(3):751–754. doi: 10.1093/infdis/171.3.751. [DOI] [PubMed] [Google Scholar]
- 81.Bossolasco S, Gaiera G, Olchini D, et al. Real-time PCR assay for clinical management of human immunodeficiency virus-infected patients with visceral leishmaniasis. J Clin Microbiol. 2003;41(11):5080–5084. doi: 10.1128/JCM.41.11.5080-5084.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hozannah A, Santos M, Chrusciak-Talhari A, Talhari C. Leishmaniasis and AIDS coinfection. An Bras Dermatol. 2013;88(6):992–993. doi: 10.1590/abd1806-4841.20132191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sampaio RN, Salaro CP, Resende P, de Paula CD. American cutaneous leishmaniasis associated with HIV/AIDS: report of four clinical cases. Rev Soc Bras Med Trop. 2002;35(6):651–654. doi: 10.1590/s0037-86822002000600017. Portuguese. [DOI] [PubMed] [Google Scholar]
- 84.Mattos M, Caiza A, Fernandes O, Gonçalves AJ, Pirmez C, Souza CS, Oliveira-Neto MP. American cutaneous leishmaniasis associated with HIV infection: report of four cases. J Eur Acad Dermatol Venereol. 1998;10(3):218–225. [PubMed] [Google Scholar]
- 85.Zijlstra EE. Visceral leishmaniasis: a forgotten epidemic. Arch Dis Child. 2016;101(6):561–567. doi: 10.1136/archdischild-2015-309302. [DOI] [PubMed] [Google Scholar]
- 86.Balasegaram M, Ritmeijer K, Lima MA, et al. Liposomal amphotericin B as a treatment for human leishmaniasis. Expert Opin Emerg Drugs. 2012;17(4):493–510. doi: 10.1517/14728214.2012.748036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mahajan R, Das P, Isaakidis P, et al. Combination treatment for visceral leishmaniasis patients coinfected with human immunodeficiency virus in India. Clin Infect Dis. 2015;61(8):1255–1262. doi: 10.1093/cid/civ530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Diro E, Lynen L, Mohammed R, Boelaert M, Hailu A, van Griensven J. High parasitological failure rate of visceral leishmaniasis to sodium stibogluconate among HIV co-infected adults in Ethiopia. PLoS Negl Trop Dis. 2014;8(5):e2875. doi: 10.1371/journal.pntd.0002875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Diro E, Ritmeijer K, Boelaert M, et al. Use of pentamidine as secondary prophylaxis to prevent visceral leishmaniasis relapse in HIV infected patients, the first twelve months of a prospective cohort study. PLoS Negl Trop Dis. 2015;9(10):e0004087. doi: 10.1371/journal.pntd.0004087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cunha MA, Leao AC, de Cassia Soler R, Lindoso JA. Efficacy and safety of liposomal amphotericin B for the treatment of mucosal leishmaniasis from the new world: a retrospective study. Am J Trop Med Hyg. 2015;93(6):1214–1218. doi: 10.4269/ajtmh.15-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]