Mouse strain–dependent selective loss of apoptosis enzymes, with consequent decrease in susceptibility to apoptosis, occurs upon short-term hepatocyte culture and in vivo. These susceptibility differences likely reflect genetic modifiers that provide resistance or predisposition to hepatocyte death.
Abstract
Liver disease progression is modulated by genetic modifiers in mouse strains and across human races and ethnicities. We hypothesized that hepatocyte culture duration and genetic background regulate hepatocyte susceptibility to apoptosis. Hepatocytes were isolated from FVB/N, C57BL/6, and C3H/He mice and cultured or treated with Fas ligand or acetaminophen after different culture times. Protein and mRNA expressions of Fas receptor, caspases-3/7/8, and Bak/Bax/Bid proteins were determined. FVB/N hepatocytes manifested rapid decreases of caspases-3/7 but not caspase-8 as culture time increased, which paralleled decreased susceptibility to apoptosis. Some changes were also found in Fas-receptor and Bak, Bax, and Bid proteins; caspase mRNA decreases were also noted. Caspase protein degradation was partially reversed by lysosomal protease but not proteasome or autophagy inhibitors. C57BL/6 and FVB/N hepatocytes behaved similarly in their limited susceptibility to apoptosis, whereas C3H/He hepatocytes show limited alterations in caspases, with consequent increased susceptibility to apoptosis. Similarly, C3H/He mice were more susceptible than C57BL/6 and FVB/N mice to Fas-mediated liver injury. Therefore there are significant mouse strain–dependent differences in susceptibility to apoptosis and selective loss of caspases upon short-term hepatocyte culture, with consequent decrease in susceptibility to apoptosis. These differences likely reflect genetic modifiers that provide resistance or predisposition to hepatocyte death.
INTRODUCTION
Hepatocyte apoptosis is caused by multiple etiologies, including toxins and viruses, and is a hallmark of many acute and chronic liver diseases (Malhi et al., 2010). In addition, predisposition to liver disease is greatly influenced by genetic modifiers in humans and experimental mouse models (Hanada et al. 2008; Nguyen and Thuluvath, 2008; Weber et al., 2008; Anstee et al., 2011; Snider et al., 2011). Understanding the mechanisms of hepatocyte death and the involved genetic influences is likely to be beneficial for development of targeted treatments in a variety of liver diseases. Hepatocytes express several cell death receptors, including the Fas receptor (FasR, CD95; Smith et al., 1994). Administration of the anti-Fas antibody Jo2 to mice results in fulminant acute liver failure due to massive hepatocyte cell death (Galle et al., 1995). Fas-induced hepatocyte death in vivo occurs through the so-called type II pathway, which involves the Bcl-2 family protein Bid and results in the induction of the mitochondrial death signaling pathway (Walter et al., 2008). However, mouse primary hepatocytes cultured on a collagen I matrix undergo Fas-induced death via the type I signaling pathway, which does not require Bid (Walter et al., 2008). Apoptosis is commonly executed by caspases (Pop and Salvesen, 2009), and a subgroup of these proteases are classified as the initiator caspases (mainly 8–10), which trigger the apoptosis cascade (Pop and Salvesen, 2009; Malhi et al., 2010). The other subgroup, named executioner caspases, such as 3, 6, and 7, which are activated by active initiator caspases, degrade many intracellular proteins, leading to apoptotic cell death (Pop and Salvesen, 2009; Malhi et al., 2010).
Primary hepatocytes provide a robust and potentially physiologic ex vivo system in which to study a broad range of hepatocellular processes (Elaut et al., 2006; Hewitt et al., 2007). Isolation of primary hepatocytes from rodents involves a standard collagenase perfusion protocol (Berry and Friend, 1969; Klaunig et al., 1981a, b). The isolated primary hepatocytes resemble their in vivo counterparts, at least during the short-term culture period (Elaut et al., 2006; Hewitt et al., 2007). However, with long-term culture, hepatocytes dedifferentiate and undergo spontaneous cell death due to alterations in expression of many genes, limiting their utility for long-term studies (Vinken et al., 2011; Fraczek et al., 2013). During our studies of caspase digestion of the keratin cytoskeleton during apoptosis in the liver, we noted that mouse hepatocytes cultured ex vivo were relatively resistant to Fas-mediated apoptosis compared with the in vivo context (Weerasinghe et al., 2014), although the reason for this resistance was not investigated.
Given the importance of cell death and the genetic background in modulating liver injury, we hypothesized that short-term culture of normal hepatocytes and their genetic background both modulate hepatocyte susceptibility to Fas-mediated apoptosis. Our results show that primary hepatocytes, upon short-term culture, become resistant to apoptotic stimuli due to decreased levels of caspases 3 and 7 but not caspase 8. The change in caspase levels is highly dependent on the genetic background of the mice and correlates with lysosomal enzyme activation. Of importance, the findings using ex vivo cultures were also observed when mice were challenged with the Fas ligand (FasL) Jo2 antibody.
RESULTS AND DISCUSSION
Dramatic decrease of caspases 3 and 7 during hepatocyte culture parallels a decrease in susceptibility to apoptosis
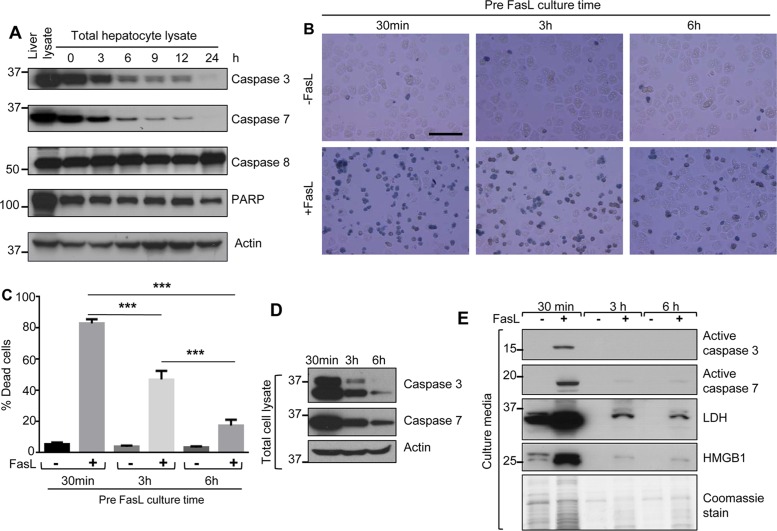
Isolated mouse primary hepatocytes (FVB/N mice) were cultured for 0–24 h, followed by analysis of caspases 3, 7, and 8 and poly(ADP-ribose) polymerase (PARP) levels as compared with protein expression in intact FVB/N liver. Caspase 3 and 7 levels decreased dramatically during hepatocyte culture duration starting from 3 h postseeding (Figure 1A). However, levels of caspase 8 and PARP were relatively constant throughout the culture duration (Figure 1A).
FIGURE 1:
Caspase 3 and 7 levels decrease rapidly with parallel relative resistance to apoptosis during short-term mouse hepatocyte culture. (A) Hepatocytes were isolated from FVB/N mice and cultured for the indicated times after plating onto collagen-coated plates. Total cell lysates were analyzed by blotting, using antibodies to the indicated proteins. A total liver lysate (lane 1) is included to show in situ whole-liver caspase levels, and an actin blot is included as a loading control. (B) Hepatocytes were isolated from FVB/N mice, followed by treatment with FasL (0.5 μg/ml, 6 h) in the presence of trypan blue (0.04%) after 30 min, 3 h, or 6 h of cell culture (see Supplemental Figure S1 for culture conditions). Scale bar, 200 μm. (C) Quantification of percentage of dead cells from the data in B, based on trypan blue uptake. A repeat experiment showed essentially identical findings. ***p < 0.001. (D) Total cell lysates were prepared from the hepatocytes used in B (just before the addition of FasL), followed by blotting for intact caspases 3 and 7. (E) Culture medium from a parallel experiment to that shown in B was concentrated and then analyzed by blotting to assess the release of the indicated proteins. A Coomassie stain of the concentrated culture medium is included and shows the limited amount of protein released when cells are cultured for 3 and 6 h before the addition of FasL (lanes 3 and 5).
We then tested the susceptibility of mouse hepatocytes to FasL-induced apoptosis after different times of culture (Supplemental Figure S1). We isolated hepatocytes from FVB/N mice and treated them with FasL or left them untreated after the indicated time of cell attachment. After 6 h in the presence of FasL, we assessed hepatocyte death by trypan blue staining and biochemically. When hepatocytes were treated with FasL after only 30 min of cell culture, the majority of cells underwent apoptosis (Figure 1B). In contrast, hepatocytes exposed to FasL after 3 or 6 h of culture had significantly reduced apoptotic cell death (Figure 1, B and C). In agreement with the reduced apoptosis after 3 or 6 h of culture, the expression of caspases 3 and 7 was markedly reduced compared with that at 30 min (Figure 1D). We validated the extent of hepatocyte death by analyzing the hepatocyte culture medium, which showed release of active caspases 3/7, lactate dehydrogenase (LDH), and high mobility group box 1 protein (HMGB1) primarily after 30 min but much less after 3 and 6 h of culture (Figure 1E). These results suggest that mouse primary hepatocytes become more resistant to apoptotic cell death even after short-term culture, likely due to reduced expression of caspases 3 and 7. Of note, the initiation of the apoptotic signaling cascade does not appear to be affected, since caspase 8 remains relatively constant throughout the culture duration (Figure 1A).
Mouse genetic background influences the susceptibility of hepatocytes to apoptosis
We tested the effect of mouse strain background on susceptibility of mouse hepatocytes to apoptosis upon culture as related to increasing culture duration. We compared hepatocytes isolated from C3H/He and C57BL/6 mice, which were selected because C57BL/6 are more susceptible than C3H/He mice to formation of Mallory–Denk body inclusions upon liver injury (Hanada et al., 2008) due to genetic modifiers that modulate the response to oxidative stress (Snider et al., 2011). Of note, formation of these inclusions in humans is a marker for poor liver disease outcome (Rakoski et al., 2011).
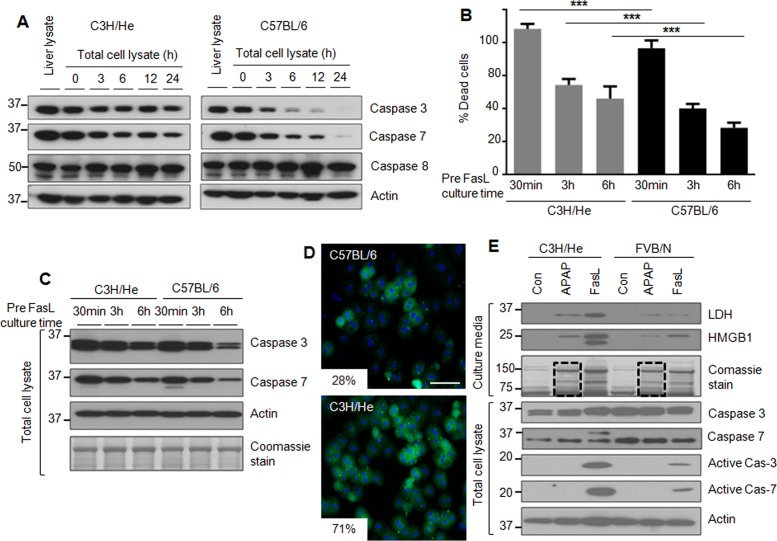
Isolated hepatocytes were cultured up to 24 h and then analyzed biochemically for the expression of caspases 3 and 7. C3H/He hepatocytes maintained relatively constant protein levels of caspases 3 and 7 as compared with C57BL/6 hepatocytes up to 24 h of culture (Figure 2A and Supplemental Figure S2). The dramatic changes in caspases 3 and 7 in C57BL/6 hepatocytes (Figure 2A) follow a similar pattern to that of FVB/N hepatocytes (Figure 1A). We then tested whether C3H/He hepatocytes are more susceptible to apoptosis due to the sustained expression of caspases 3 and 7 compared with C57BL/6 hepatocytes. C3H/He hepatocytes had significantly higher FasL-induced cell death at all three tested cell culture times (30 min, 3 h, and 6 h) compared with C57BL/6 hepatocytes (Figure 2B). In agreement with the reduced percentage of cell death observed in C57BL/6 hepatocytes during FasL treatment (Figure 2B), C57BL/6 hepatocytes express relatively lower levels of caspases 3 and 7 after 3 and 6 h of cell culture compared with CH3/He hepatocytes (Figure 2C). We also compared the FasL-induced cell death of C57BL/6 and C3H/He hepatocytes by immunostaining. C3H/He hepatocytes showed marked increase in annexin-V–positive staining compared with C57BL/6 hepatocytes (Figure 2D).
FIGURE 2:
The genetic background alters hepatocyte susceptibility to apoptosis ex vivo. (A) Hepatocytes were isolated from C3H/He and C57BL/6 mice, followed by culture for the indicated times and then blotting of the lysates. A total liver lysate (lane 1 for each strain) is included to show in situ whole-liver protein levels. (B) Hepatocytes were isolated from C3H/He and C57BL/6 mice and then treated with FasL (0.5 μg/ml, 6 h) after the indicated times of culture. The histogram shows percentage of cell death. ***p < 0.001. (C) Hepatocyte lysates were prepared from duplicate cells to those in B, followed by blotting for caspases 3 and 7. An actin blot and Coomassie stain are included to show equal protein loading. (D) Hepatocytes were isolated from C3H/He and C57BL/6 mice and treated with FasL (0.1 μg/ml, 6 h) after 3 h of cell attachment. Apoptotic cells were visualized using annexin-V staining (green), with counterstaining with 4′,6-diamidino-2-phenylindole (DAPI; blue) to visualize nuclei. Percentage mean annexin-V+ cells is shown. p < 0.001; scale bar, 200 μm. (E) Isolated hepatocytes from C3H/He and FVB/N mice were treated with APAP (1 mM) or FasL (0.5 μg/ml, 6h) after 3 h of cell attachment. Concentrated culture medium was analyzed by blotting using antibodies to the indicated antigens. The Coomassie stain of the concentrated culture medium shows the increased release of cellular proteins in the presence of APAP (dashed lines in lanes 2 and 5) and FasL.
We then compared the expression of the FasR and the Bcl-2 family proteins Bid, Bax, and Bak in primary hepatocytes isolated from the mouse strains FVB/N, C57BL/6, and C3H/He. FasR protein showed gradual decreases during hepatocyte culture in all three strains, whereas the Bcl-2 family members (except Bid) did not manifest the same type of dramatic change noted for caspases 3 and 7 in the C57BL/6 and FVB/N strains (Supplemental Figure S3A). FasR mRNA isolated from hepatocytes of the three mouse strains showed a dramatic decrease beginning at 12 h (Supplemental Figure S3B).
We also compared the susceptibility to acetaminophen (APAP)-induced necrosis in C3H/He versus FVB/N hepatocytes. After 3 h of cell attachment, hepatocytes were treated with APAP or FasL or left untreated. Again, caspase activation was more prominent in C3H/He versus FVB/N hepatocytes upon FasL treatment, but cellular protein release into the medium upon APAP exposure in the absence of detectable caspase activation, which we take as a measure of necrosis, was similar in hepatocytes from both strains (Figure 2E, compare lanes 2 and 5 in the Coomassie-stained gel). Our results indicate that the sustained expression of caspases 3 and 7 in cultured C3H/He hepatocytes correlates with their observed increased susceptibility to apoptosis compared with C57BL/6 and FVB/N hepatocytes.
Ex vivo findings of C3H/He versus C57BL/6 and FVB/N hepatocyte susceptibility to Fas-mediated apoptosis also hold in vivo
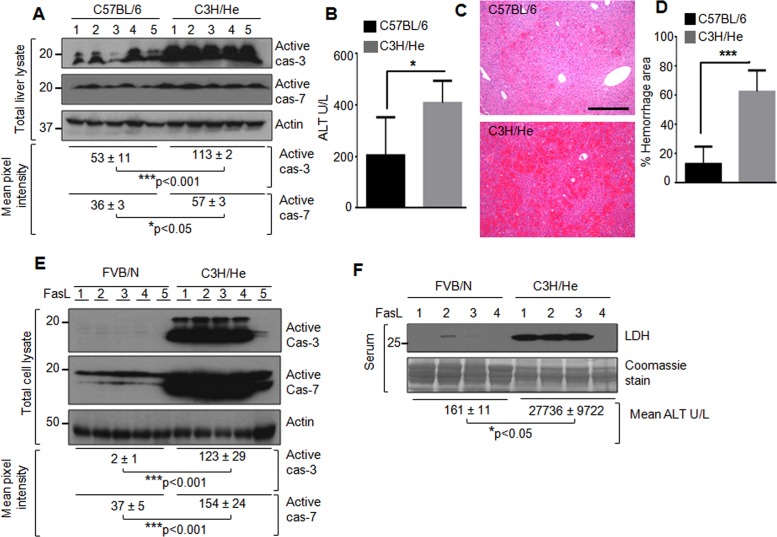
We tested C3H/He and C57BL/6 mice for their susceptibility to FasL-induced apoptosis to determine whether the ex vivo findings represent cell-autonomous effects. Mice were injected with FasL, followed by isolation of the livers for biochemical and histologic analysis, in addition to testing of serum alanine aminotransferase (ALT) levels. There was significant activation of caspases 3 and 7 in C3H/He as compared with C57BL/6 livers (Figure 3A). The activation of caspases 3 and 7 correlated with markedly increased ALT and liver damage in C3H/He mice (Figure 3, B–D). The results were reproducible using an independent biological replica experiment with five mice per strain (unpublished data). Comparison of C3H/He and FVB/N mice also showed parallel susceptibility to FasL injury to what was observed in the isolated hepatocytes: FVB/N mice had markedly less caspase activation and serum ALT and LDH levels compared with C3H/He mice (Figure 3, E and F). These findings, taken together with the hepatocyte ex vivo results, suggest a cell-autonomous effect and that genetic modifiers in the hepatocytes are likely to account for their dramatic difference in susceptibility to Fas-mediated apoptosis.
FIGURE 3:
Effect of mouse genetic background on susceptibility to hepatocyte apoptosis in vivo. (A) C57BL/6 and C3H/He male mice (five mice/strain, 12 wk old) were injected with FasL. After 5 h, the livers were removed, followed by blotting of the liver lysates with antibodies to active caspase 3 (cas-3), caspase 7 (cas-7), and actin (loading control). Densitometry scanning was done to estimate the level of active caspases; mean and SEM, ***p < 0.001, *p < 0.05. Each lane represents an individual liver. (B) Serum ALT from the mice used in A. *p < 0.05. (C, D) Representative hematoxylin and eosin images (scale bar, 200 μm) and quantification of the hemorrhage. The percentage area of the hemorrhage, as compared with the total area of the liver, is shown. Two representative fields/liver per mouse were used for quantification. ***p < 0.001. (E) FVB/N and C3H/He (five male mice/strain, 10–11 wk old) were compared for their susceptibility to Fas-L–mediated liver injury. Liver lysates were blotted, followed by analysis as in A; ***p < 0.001. (F) Sera from mice used in E were analyzed (four mice/strain; blood could not be obtained from one mouse/strain) for release of LDH and ALT; *p < 0.05. Coomassie stain of the serum is included as a loading control.
Caspase inhibition rescued FasL-induced hepatocyte apoptosis
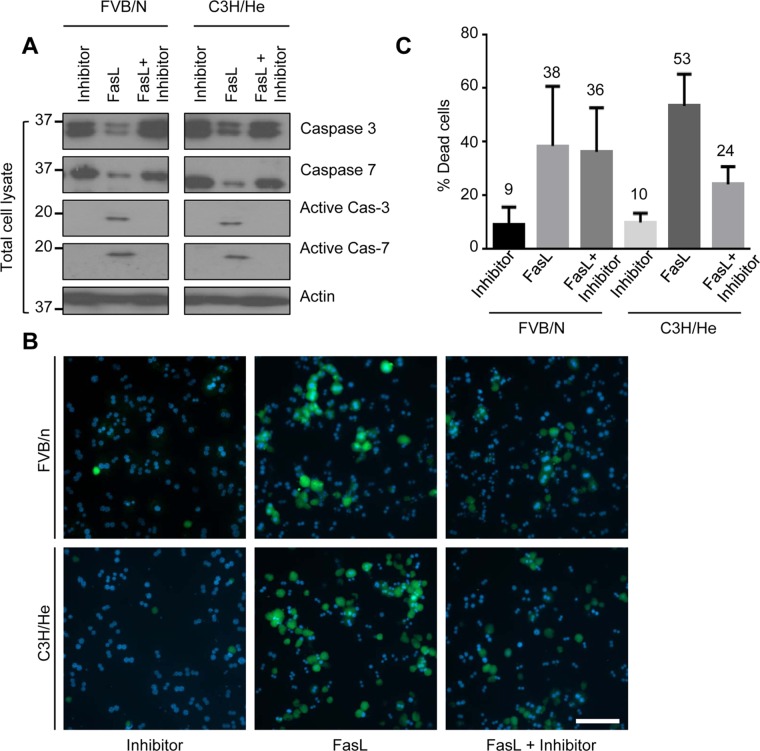
We tested whether the observed susceptibility of isolated hepatocytes to apoptosis during culture is caspase 3 and 7–dependent by assessing the effect of caspase inhibition. We treated hepatocytes from FVB/N and C3H/He mice, after 3 h of attachment, with FasL with or without the pancaspase inhibitor Z-VAD-FMK for 6 h. Both FVB/N and C3H/He hepatocytes showed no detectable caspase 3 and 7 activation when hepatocytes were challenged with FasL in the presence of the caspase inhibitor (Figure 4A). Apoptosis was also assessed by annexin-V staining, which showed marked reduction of annexin-V+ cells in the presence of the caspase inhibitor compared with cells treated with FasL alone (Figure 4, B and C). These results suggest that the resistance of cultured hepatocytes to FasL-mediated apoptosis is, at least in part, a caspase-dependent phenomenon.
FIGURE 4:
Resistance to hepatocyte apoptosis during culture is caspase dependent. (A) Hepatocytes were isolated from FVB/N and C3H/He mice and treated with FasL (0.5 μg/ml, 6 h) ± the caspase inhibitor Z-VAD-FMK (10 μM) after 3 h of cell attachment. Total cell lysates were analyzed by blotting for the indicated antigens. (B) Apoptotic cells were visualized using annexin-V staining (green), with counterstaining using DAPI (blue) to visualize nuclei. Scale bar, 400 μm. (C) Quantification of percentage of annexin-V+ cells in B.
mRNA turnover and lysosomal protein degradation contribute to the reduced levels of caspases 3 and 7
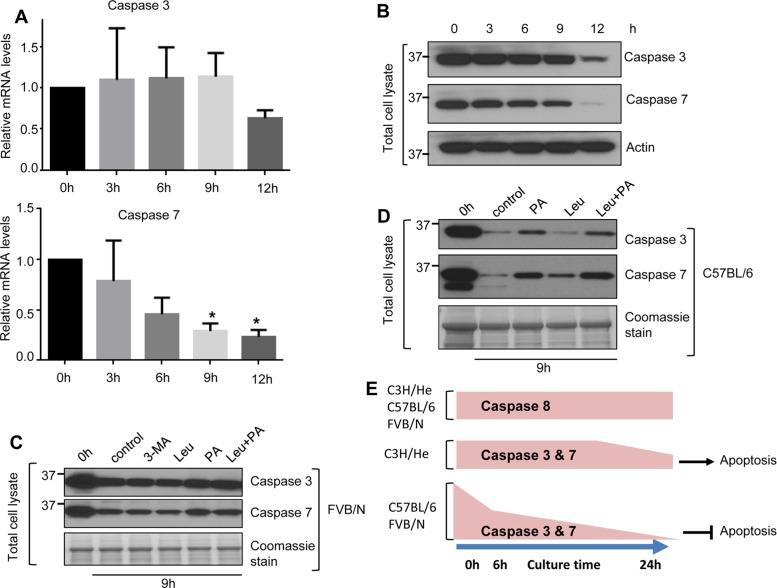
We tested the potential underpinnings for the observed changes in caspase 3 and 7 levels upon hepatocyte culture by measuring caspase mRNA levels and testing the effect of inhibiting several protein degradation pathways in hepatocytes isolated from FVB/N, C57BL/6, and C3H/He mice. By 12 h of culture, caspase 7 mRNA decreased more rapidly than that of caspase 3 in FVB/N hepatocytes (Figure 5A). Consistent with the decreased mRNA levels, caspase 3 and 7 protein levels from the same FVB/N hepatocytes also decreased markedly after 12 h of culture (Figure 5B). Similar patterns of caspase 3 and 7 mRNA degradation occurred in C3H/He and C57BL/6 hepatocytes by 12 h of culture (Supplemental Figure S4).
FIGURE 5:
mRNA and lysosomal protein degradation contribute to the depletion of caspases 3 and 7 in short-term cultured hepatocytes. Hepatocytes were isolated from FVB/N mice, and cells were collected at the indicated times and then processed to obtain total mRNA and total cell lysates. (A) Relative expression of caspase 3 and 7 mRNA compared with the 0-h baseline levels. *p < 0.05. (B) Cell lysates were analyzed by blotting. (C, D) Hepatocytes were isolated from FVB/N mice (C) and C57BL/6 mice (D) and then cultured with or without the autophagy inhibitor 3MA (5 mM, C) and the lysosomal protease inhibitor leupeptin (Leu; 40 μM) or pepstatin-A (PA; 10 μM; C and D). Hepatocytes were collected after 9 h of treatment, and total cell lysates were analyzed by blotting. (E) Schematic summarizing the overall findings. Short-term culture of mouse hepatocyte results in marked down-regulation of caspases 3 and 7 but not caspase 8. This down-regulation is accompanied by resistance to Fas-medicated apoptosis in a manner dependent on mouse genetic background.
We then examined whether caspase 3 and 7 protein degradation occurs via the proteasome by incubating cultured FVB/N hepatocytes with the proteasome inhibitors MG132 (Adams et al., 1998) and epoxomycin (Kim et al., 1999). As expected, both inhibitors enhanced protein ubiquitination after 24 h but did not rescue caspase 3 and 7 degradation (Supplemental Figure S4C, lanes 6 and7). Inhibition of autophagy using 3-methyladenine (3MA; Wu et al., 2010) also had no effect on caspase degradation (Figure 5C). In contrast, isolated cultured hepatocytes in the presence of the lysosomal protease inhibitor leupeptin (Hausott et al., 2012) or/and pepstatin-A (Marciniszyn et al., 1976) led to partial rescue of caspase 3 and 7 degradation in FVB/N (Figure 5C) and C57BL/6 hepatocytes (Figure 5D). These results indicate that RNA degradation and the lysosomal protein degradation pathway contribute to the depletion of caspases 3 and 7 in cultured mouse primary hepatocytes.
Discussion
Our findings provide a hitherto-unappreciated marked difference in mouse hepatocyte susceptibility to apoptosis upon short-term culture in a manner that depends on the mouse strain and provide an association of these differences with dramatic changes in the levels of some caspases due to protein and mRNA turnover. C3H/He mouse hepatocytes retain much of their in vivo properties in terms of susceptibility to FasL-mediated apoptosis, whereas FVB/N and C57BL/6 hepatocytes undergo dramatic degradation of caspase 3 and 7 but not caspase 8 protein. All three strains showed a prominent decrease in caspase 7 mRNA. The importance of caspase loss is supported by studies showing that administration of small interfering RNA (siRNA) to caspase 8 protected mice from FasL-induced liver injury (Zender et al., 2003) and of siRNA to caspase 3 or 8 protected mice from ischemia–reperfusion injury (Contreras et al., 2004). The consequences of caspase inhibition are likely to be compartment specific, given the observed different subcellular distribution of caspases 3 and 7 after Fas-induced apoptosis in mouse liver (Chandler et al., 1998). Our findings also show that caspase inhibition protected the cultured hepatocytes from undergoing apoptosis (Figure 4, B and C).
Changes in mRNA levels upon ex vivo culture of hepatocytes have been described, but, to our knowledge, not in the context of specific caspases and unique mouse strains. For example, short-term culture of C75BL/6 hepatocytes (up to 24 h) is accompanied by a dramatic decrease in major urinary protein mRNA within 6 h, whereas albumin mRNA remains relatively stable (Clayton and Darnell, 1983). However, longer-term cultures, beyond 24 h, result in a progressive decrease in albumin mRNA levels (Clayton and Darnell, 1983).
It remains to be determined whether the strain-dependent differences in isolated hepatocyte susceptibility to apoptosis, which were also noted in intact C3H/He, C57BL/6, and FVB/N mice, are also present in hepatocytes from humans of different races or ethnic backgrounds. Our findings suggest the effects we observed are cell autonomous, in that they were found in both hepatocytes and intact livers. Cell interactions with extracellular matrix components are critical for isolated cells and intact liver (Maher and Bissell, 1993). Furthermore, the type of matrix (e.g., dried, stiff collagen, as is the case in coated plates vs. gelled collagen) is critical for susceptibility of cultured mouse hepatocytes to apoptosis, as noted for transforming growth factor-β–induced apoptosis (Godoy et al., 2010). Most studies that involve isolation of mouse hepatocytes involve a standard two-step perfusion of the liver, followed by culturing for short-term or longer-term experiments (Li et al., 2010). An important caveat, based on the findings here, is that cultured mouse hepatocytes undergo dramatic loss of a subset of caspases at the protein and mRNA levels in a caspase-selective manner, which explains the shift toward marked resistance of the cultured hepatocytes to undergoing apoptosis. We did not carry out an exhaustive analysis of changes in all apoptosis-related proteins, but testing of FasR and the Bcl-2 family proteins Bax, Bid, and Bak showed variable alterations that were not as dramatic as observed in caspases 3 and 7 in primary hepatocytes or intact livers. The mouse strain C3H/He is relatively refractory to changes in the caspases and therefore offers an alternative, more robust mouse system in which to study hepatocyte apoptosis ex vivo. The C3H/He strain has an additional unique property in terms of injury models, namely resistance to endotoxin due to a mutation in the Toll-like receptor 4 (Hoshino et al., 1999). The mechanism of how the genetic background in the mouse lines we tested influences Fas-mediated apoptosis in vivo remains to be investigated.
MATERIALS AND METHODS
Isolation and treatment of primary mouse hepatocytes
Hepatocytes were isolated from age-matched male (FVB/N, C3H/He, or C57BL/6; Jackson Laboratory, Bar Harbor, ME) mice for primary culture as described (Weerasinghe et al., 2014), with the following modifications. The liver was perfused with 1–2 ml of perfusion medium through the portal vein with a flow rate of 3 ml/min, followed by perfusion with 10–15 ml of digestion medium containing 150 U/ml collagenase-II (Worthington Biochemical Corporation, Lakewood, NJ) at the same flow rate. After the first wash, the cell pellet was suspended in 6 ml of ice-cold wash medium and separated on a Percoll gradient (Sigma-Aldrich, St. Louis, MO; 15% in phosphate-buffered saline, pH 7.4, 4°C, 500 rpm, 10 min) to remove dead cells. The cell pellet was washed with ice-cold wash medium and suspended in culture medium (William’s medium E supplemented with 10% heat-inactivated fetal bovine serum and 1% penicillin-streptomycin, 37°C) and plated (2 × 105 cells/ml on collagen-I–coated six-well plates; BD BioCoat, San Jose, CA) for subsequent analysis of the released proteins and the biochemical experiments. After 30 min (37°C, 5% CO2) to allow attachment, the cell culture medium was replaced (to remove any debris or unattached cells) with fresh medium and cultured for indicated times (37°C, 5% CO2) or treated with Fas ligand (FasL; Jo2 antibody, 0.5 μg/ml; BD Biosciences PharMingen; Supplemental Figure S1) or with APAP (1 mM; Sigma-Aldrich). To calculate the percentage cell death after FasL, the number of round (apoptotic) cells and the total number of cells were counted under bright field (40× magnification). The percentage dead cell count was calculated with respect to total number of cells, using up to 15 fields for each time point.
Preparation of liver and hepatocyte lysates and biochemical analysis
Total hepatocyte and liver tissue lysates were prepared by homogenizing the cells or liver tissues, respectively, using 2× Tris-glycine SDS-containing sample buffer (under reducing conditions that include 5% β-mercaptoethanol). Proteins were separated using SDS–PAGE and then stained with Coomassie blue or transferred to polyvinylidene fluoride membranes, followed by blotting with antibodies to intact caspases 3 and 7, active caspases 3 and 7, PARP (Cell Signaling Technology, Boston, MA), HMGB1 (Abcam, Cambridge, MA), actin (Lab Vision), Fas receptor, Bax (Santa Cruz Biotechnology, Fremont, CA), Bid (R&D Systems, Minneapolis, MN), Bak (NeoMarkers, Fremont, CA), annexin-V (Clontech, Mountain View, CA), and LDH (LifeSpan BioSciences, Seattle, WA). Protein degradation pathways were assessed using 3MA, leupeptin, and pepstatin-A (Sigma-Aldrich), epoxomycin (BostonBiochem, Cambridge, MA), and MG132 (Cayman Chemical Company, Ann Arbor, MI). Caspase inhibition was performed using Z-VAD-FMK, a cell-permeable and irreversible pancaspase inhibitor (R&D Systems). Annexin-V staining was performed using ApoAlert Annexin V-FITC Apoptosis Kit (Clontech).
Analysis of the medium of cultured cells
After FasL treatment, culture plates were centrifuged (1200 rpm, 5 min), followed by collection of the culture medium without disturbing the cells. The collected medium was recentrifuged (1200 rpm, 10 min), and the supernatant was concentrated using Centricon YM-10 filters (Millipore) and then mixed with 4× SDS–PAGE sample buffer for subsequent immunoblotting.
Isolation of RNA and quantitative PCR analysis
Total RNA was isolated from cultured hepatocytes using an RNeasy kit (Qiagen). RNA was translated into cDNA using the TaqMan reverse transcription kit (Applied Biosystems). cDNA was amplified by Brilliant SYBR Green Master Mix using the mouse-specific caspase 3 primers 5′-TGGGCCTGAAATACCAAGTC-3′ and 5′-TCCCATAAATGACCCCTTCA-3′, the caspase 7 primers 5′-ACTTCGACAAAGCGACAGGT-3′ and 5′-GGTCCTCCTCAGAGGCTTTT-3′, and the Fas receptor primers 5′-AGCTGAGGAGGCGGGTTCGTG-3′ and 5′- CATGGGGCGCAGGTTGGTGTA-3′, using quantitative real-time PCR (MyiQ real-time PCR detection system; Bio-Rad Laboratories). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal control. The relative mRNAs of caspases 3 and 7 and Fas receptor for each culture time point relative to GAPDH were normalized to that of the 0-h isolation (which was set to be 1.0) to calculate the mRNA fold change.
In vivo FasL administration and liver damage analysis
C57BL/6, FVB/N, and C3H/He mice (Taconic Biosciences, Rensselaer, NY; 10- to 12-wk-old males) were fasted overnight (with water provided ad libitum) and then injected intraperitoneally with FasL (0.5 μg/g body weight). Mice (five mice/strain) were killed after 4 h, and liver damage was assessed biochemically, serologically, and histologically. Serum ALT levels were measured using a commercial kit (Pointe Scientific) per manufacturer’s instructions. Hemorrhage quantification was done using ImageJ software. Repeat independent experiments were done using five mice/strain.
Statistical analysis
Statistical analysis was performed using analysis of variance or t test (for cell count experiments and RNA comparison) using GraphPad Prism 6 statistical software.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health (NIH) Grant DK52951 and the Department of Veterans Affairs (M.B.O.), NIH Grant DK088752 Summer Undergraduate Research Fellowship to D.A.P., institutional NIH Grant DK034933 to the University of Michigan, and a University of Michigan Postdoctoral Translational Scholars Program Award (M.J.P.).
Abbreviations used:
- ALT
alanine aminotransferase
- APAP
acetaminophen
- FasL
fas ligand
- FasR
fas receptor
- LDH
lactate dehydrogenase.
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E15-06-0423) on August 17, 2016.
REFERENCES
- Adams J, Behnke M, Chen S, Cruickshank AA, Dick LR, Grenier L, Klunder JM, Ma YT, Plamondon L, Stein RL. Potent and selective inhibitors of the proteasome: dipeptidyl boronic acids. Bioorg Med Chem Lett. 1998;8:333–338. doi: 10.1016/s0960-894x(98)00029-8. [DOI] [PubMed] [Google Scholar]
- Anstee QM, Daly A K, Day CP. Genetics of alcoholic and nonalcoholic fatty liver disease. Semin Liver Dis. 2011;31:128–146. doi: 10.1055/s-0031-1276643. [DOI] [PubMed] [Google Scholar]
- Berry MN, Friend DS. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol. 1969;43:506–520. doi: 10.1083/jcb.43.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler JM, Cohen GM, MacFarlane M. Different subcellular distribution of caspase-3 and caspase-7 following Fas-induced apoptosis in mouse liver. J Biol Chem. 1998;273:10815–10818. doi: 10.1074/jbc.273.18.10815. [DOI] [PubMed] [Google Scholar]
- Clayton DF, Darnell JE. Changes in liver-specific compared to common gene transcription during primary culture of mouse hepatocytes. Mol Cell Biol. 1983;3:1552–1561. doi: 10.1128/mcb.3.9.1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras JL, Vilatoba M, Eckstein C, Bilbao G, Anthony Thompson J, Eckhoff DE. Caspase-8 and caspase-3 small interfering RNA decreases ischemia/reperfusion injury to the liver in mice. Surgery. 2004;136:390–400. doi: 10.1016/j.surg.2004.05.015. [DOI] [PubMed] [Google Scholar]
- Elaut G, Henkens T, Papeleu P, Snykers S, Vinken M, Vanhaecke T, Rogiers V. Molecular mechanisms underlying the dedifferentiation process of isolated hepatocytes and their cultures. Curr Drug Metab. 2006;7:629–660. doi: 10.2174/138920006778017759. [DOI] [PubMed] [Google Scholar]
- Fraczek J, Bolleyn J, Vanhaecke T, Rogiers V, Vinken M. Primary hepatocyte cultures for pharmaco-toxicological studies: at the busy crossroad of various anti-dedifferentiation strategies. Arch Toxicol. 2013;87:577–610. doi: 10.1007/s00204-012-0983-3. [DOI] [PubMed] [Google Scholar]
- Galle PR, Hofmann WJ, Walczak H, Schaller H, Otto G, Stremmel W, Krammer P H, Runkel L. Involvement of the CD95 (APO-1/Fas) receptor and ligand in liver damage. J Exp Med. 1995;182:1223–1230. doi: 10.1084/jem.182.5.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godoy P, Schug M, Bauer A, Hengstler JG. Reversible manipulation of apoptosis sensitivity in cultured hepatocytes by matrix-mediated manipulation of signaling activities. Methods Mol Biol. 2010;640:139–155. doi: 10.1007/978-1-60761-688-7_7. [DOI] [PubMed] [Google Scholar]
- Hanada S, Strnad P, Brunt EM, Omary MB. The genetic background modulates susceptibility to mouse liver Mallory-Denk body formation and liver injury. Hepatology. 2008;48:943–952. doi: 10.1002/hep.22436. [DOI] [PubMed] [Google Scholar]
- Hausott B, Vallant N, Hochfilzer M, Mangger S, Irschick R, Haugsten EM, Klimaschewski L. Leupeptin enhances cell surface localization of fibroblast growth factor receptor 1 in adult sensory neurons by increased recycling. Eur J Cell Biol. 2012;91:29–38. doi: 10.1016/j.ejcb.2011.09.009. [DOI] [PubMed] [Google Scholar]
- Hewitt NJ, Lechon MJ, Houston JB, Hallifax D, Brown HS, Maurel P, Kenna JG, Gustavsson L, Lohmann C, Skonberg C, et al. Primary hepatocytes: current understanding of the regulation of metabolic enzymes and transporter proteins, and pharmaceutical practice for the use of hepatocytes in metabolism, enzyme induction, transporter, clearance, and hepatotoxicity studies. Drug Metab Rev. 2007;39:159–234. doi: 10.1080/03602530601093489. [DOI] [PubMed] [Google Scholar]
- Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, Takeda K, Akira S. Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J Immunol. 1999;162:3749–3752. [PubMed] [Google Scholar]
- Kim KB, Myung J, Sin N, Crews CM. Proteasome inhibition by the natural products epoxomicin and dihydroeponemycin: insights into specificity and potency. Bioorg Med Chem Lett. 1999;9:3335–3340. doi: 10.1016/s0960-894x(99)00612-5. [DOI] [PubMed] [Google Scholar]
- Klaunig JE, Goldblatt PJ, Hinton DE, Lipsky MM, Chacko J, Trump BF. Mouse liver cell culture. I. Hepatocyte isolation. In Vitro. 1981a;17:913–925. doi: 10.1007/BF02618288. [DOI] [PubMed] [Google Scholar]
- Klaunig JE, Goldblatt PJ, Hinton DE, Lipsky MM, Trump BF. Mouse liver cell culture. II. Primary culture. In Vitro. 1981b;17:926–934. doi: 10.1007/BF02618289. [DOI] [PubMed] [Google Scholar]
- Li WC, Ralphs KL, Tosh D. Isolation and culture of adult mouse hepatocytes. Methods Mol Biol. 2010;640:139–155. doi: 10.1007/978-1-59745-019-5_13. [DOI] [PubMed] [Google Scholar]
- Maher JJ, Bissell DM. Cell-matrix interactions in liver. Semin Cell Biol. 1993;4:189–201. doi: 10.1006/scel.1993.1023. [DOI] [PubMed] [Google Scholar]
- Malhi H, Guicciardi ME, Gores GJ. Hepatocyte death: a clear and present danger. Physiol Rev. 2010;90:1165–1194. doi: 10.1152/physrev.00061.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marciniszyn J, Hartsuch JA, Tang J. Mode of inhibition of acid proteases by pepstatin. J Biol Chem. 1976;251:7088–7094. [PubMed] [Google Scholar]
- Nguyen GC, Thuluvath PJ. Racial disparity in liver disease: biological, cultural, or socioeconomic factors. Hepatology. 2008;47:1058–1066. doi: 10.1002/hep.22223. [DOI] [PubMed] [Google Scholar]
- Pop C, Salvesen GS. Human caspases: activation, specificity, and regulation. J Biol Chem. 2009;284:21777–21781. doi: 10.1074/jbc.R800084200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakoski MO, Brown MB, Fontana RJ, Bonkovsky HL, Brunt EM, Goodman ZD, Lok AS, Omary MB. For the HALT-C Trial Group. Mallory-Denk bodies are associated with outcomes and histological features in patients with chronic hepatitis C. Clin Gastroenterol Hepatol. 2011;9:902–909. doi: 10.1016/j.cgh.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CA, Farrah T, Goodwin RG. The TNF receptor superfamily of cellular and viral proteins: activation, costimulation, and death. Cell. 1994;76:959–962. doi: 10.1016/0092-8674(94)90372-7. [DOI] [PubMed] [Google Scholar]
- Snider NT, Weerasinghe SV, Singla A, Leonard JM, Hanada S, Andrews PC, Lok AS, Omary MB. Energy determinants GAPDH and NDPK act as genetic modifiers for hepatocyte inclusion formation. J Cell Biol. 2011;195:217–229. doi: 10.1083/jcb.201102142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinken M, Decrock E, Doktorova T, Ramboer E, De Vuyst E, Vanhaecke T, Leybaert L, Rogiers V. Characterization of spontaneous cell death in monolayer cultures of primary hepatocytes. Arch Toxicol. 2011;85:1589–1596. doi: 10.1007/s00204-011-0703-4. [DOI] [PubMed] [Google Scholar]
- Walter D, Schmich K, Vogel S, Pick R, Kaufmann T, Hochmuth FC, Haber A, Neubert K, McNelly S, von Weizsäcker F, et al. Switch from type II to I Fas/CD95 death signaling on in vitro culturing of primary hepatocytes. Hepatology. 2008;48:1942–1953. doi: 10.1002/hep.22541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber S, Gressner OA, Hall R, Grunhage F, Lammert F. Genetic determinants in hepatic fibrosis: from experimental models to fibrogenic gene signatures in humans. Clin Liver Dis. 2008;12:747–757. doi: 10.1016/j.cld.2008.07.012. [DOI] [PubMed] [Google Scholar]
- Weerasinghe SV, Ku N-O, Altshuler PJ, Kwan R, Omary MB. Mutation of caspase-digestion sites in keratin 18 interferes with filament reorganization, and predisposes to hepatocyte necrosis and loss of membrane integrity. J Cell Sci. 2014;127:1464–1475. doi: 10.1242/jcs.138479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu YT, Tan HL, Shui G, Bauvy C, Huang Q, Wenk MR, Ong CN, Codogno P, Shen HM. Dual role of 3-methyladenine in modulation of autophagy via different temporal patterns of inhibition on class I and III phosphoinositide 3-kinase. J Biol Chem. 2010;285:10850–10861. doi: 10.1074/jbc.M109.080796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zender L, Hutker S, Liedtke C, Tillmann HL, Zender S, Mundt B, Waltemathe M, Gosling T, Flemming P, Malek NP, et al. Caspase 8 small interfering RNA prevents acute liver failure in mice. Proc Natl Acad Sci USA. 2003;100:7797–7802. doi: 10.1073/pnas.1330920100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.