Abstract
The mechanisms underlying the spatiotemporal evolution of tumor ecosystems present a challenge in evaluating drug efficacy. In this Perspective, we address the use of three-dimensional in vitro culture models to delineate the dynamic interplay between the tumor and the host microenvironment in an effort to attain realistic platforms for assessing pharmaceutical efficacy in patients.
The past few decades have seen a greater understanding of the molecular and genetic underpinnings of tumor etiology. However, questions regarding indolent disease, metastatic colonization, dormancy, relapse, and the rapid evolution of drug resistance are inadequately addressed by the use of standard molecular and genetic characterization and standard monolayer cell culture models (1). The incidence of detected preinvasive neoplastic lesions—particularly for breast and prostate cancers—has increased because of improved diagnosis and access to routine screening (2). Clinicians often err on the side of caution and choose to aggressively treat these patients because it is difficult to predict which patients will progress to full-blown malignancy and which will continue to have stable disease. Regrettably, therapeutic intervention in itself may induce an emergent aggressive malignant state (3). Are specific phenotypic, genotypic, and cytogenetic signatures observed in patients with nonprogressive disease? Do they display distinctive morphogenetic programs that can be used in prospective studies for up-front identification at the time of presentation?
Another confounding fact is that patients rapidly acquire resistance to cancer therapeutics such as those that target DNA repair machinery, microtubules, and kinase signaling cascades (4), and this compromises the duration and quality of life. The mechanisms that drive these resistant phenotypes are poorly understood. Is this emergent state caused by the induction of resistance after drug treatment, development of de novo resistance mechanisms, selection for preexisting resistant subpopulations, or a combination of these? The interplay between intrinsic resistance mechanisms and physicochemical interactions of the tumor microenvironment contributes additional complexity to the determinants of contextual drug efficacy (5).
TAKING IT TO THREE DIMENSIONS
Recently, in efforts to “heal” the tissue, researchers have developed therapeutics that target the microenvironment, such as immune cell activation in melanoma or tumor vasculature in the breast (6). Because tumors are largely heterogeneous and aptly described as abnormal organs, understanding their individual components may be insufficient to predict the behavior of the whole (5). In the face of such complexity, integration of these concepts may seem daunting. In the field of condensed-matter physics, a paradigm shift occurred when simply knowing the laws and models for individual system components did not predict phenomena such as those observed in superconductivity, Bose-Einstein condensation, and superfluidity (7). Instead, new theoretical and computational models were developed to address the patterns and aggregate behavior that arose from complex interactions between large numbers of diverse particles. Cancer biologists are wrestling with comparable complexities. New tools combined with reductionist approaches are under way to successfully tackle the disease in all its complexity. One such tool uses three-dimensional (3D) culture models to reconstitute features of organs that enable in vitro recapitulation of in vivo function, including spatiotemporal gradients of chemicals and oxygen tension, mechanical cues, and heterotypic crosstalk (such as between the epithelium and the stroma). Drawing on techniques from fields such as regenerative medicine, these 3D biomimetic platforms spatially define multicellular assembly in an effort to study treatment-induced tissue responses. These models reconstitute some physiological complexities but allow for the dissection of problems into discrete units.
Although 2D growth on tissue culture plastic remains the de facto platform used for pharmaceutical studies, the cells grown in that setting often adopt physiologically irrelevant morphology and signaling patterns because they do not receive the external cues that allow them to “remember” and recapitulate their in vivo functions. In their place, 3D culture models are gaining traction in the field of drug discovery and development. In this Perspective, we focus on two critical gaps that are thought to be translational priorities for intervention (8): (i) preinvasive neoplasia and (ii) advanced metastatic disease.
DCIS IN A DISH
A growing number of patients are diagnosed with an intermediate state of malignancy, ductal carcinoma in situ (DCIS), or more generally, carcinoma in situ (CIS) (2). In the breast, loss of tissue architecture concomitant with a reactive stroma indicates that normal tissue homeostasis has been compromised but that these alterations are still restricted to the site of injury (2). These preinvasive lesions may remain quiescent for several decades and in some cases never progress to an invasive phenotype. Physicians are then faced with a dilemma: either recommend aggressive treatment or simply monitor the patient. Interrogation of 3D contextual models recapitulating these intermediate states may be able to explain the temporal regulation of the malignant transition and help inform clinical decisions. Thus, the first requirement is to create 3D models that recapitulate early-stage carcinogenesis.
At the single-cell level, alterations in intracellular processes such as signal transduction, cell-cycle regulation, transcription, protein synthesis, and cell–extracellular matrix (ECM) crosstalk occur on the time scale of milliseconds to minutes and on length scales of nanometers, representative of individual enzymes, to micrometers, in measuring the diameter of a single cell (9). These processes work in concert to disrupt tissue architecture and alter local ECM boundaries spanning hundreds of micrometers (10). Newly formed aberrant organs generate new vasculature and evade immune surveillance by recruiting stromal cells to act as accomplices and to facilitate full-blown malignancy and invasion (9).
Human breast progression series of cell lines allow in vitro examination of the transition from normal to malignant phenotypes. When these are cultured as single cells in 3D biomimetic laminin-rich ECM, they form multicellular structures, recapitulating the progressive loss of tissue architecture and aberrant signaling associated with the transition from nonmalignant carcinoma in situ to full malignancy (11). The malignant transformation can be achieved by exogenous oncogenetic manipulation (MCF10a) or spontaneous evolution because of culture conditions (HMT3522). A similar architectural transformation has been observed by adjusting the mechanical forces of the microenvironment in 3D cell culture of non-malignant breast epithelial cells (5, 12). It was found that cells form normal breast acini units when cultured in the ECM, mimicking the physiological stiffness of the in vivo mammary gland, but the same cells undergo progressive loss of architecture concomitant with aberrant signaling when the stiffness of their microenvironment approaches that of the stroma surrounding a malignant tumor in vivo (12). Introduction of transformed stromal cells such as cancer-associated fibroblasts and macrophages in heterotypic cultures also facilitates neoplastic transformation of the epithelia (5). More recently, researchers used a microfluidics device that allowed the recreation of physiologically relevant DCIS architectures and incorporation of stromal constituents (13), thus providing an in vitro platform with which to probe possible mechanisms facilitating the transformation to an invasive phenotype (13). These results underlie the complexity of the determinants of contextual drug efficacy, in which both the physical and biochemical properties of the microenvironment must be considered. Nevertheless, these 3D surrogate organotypic culture models provide a tractable platform in which tissue architecture can be assessed for screening existing therapies as well as those in the preclinical pipeline. A future strategy may combine tissue biopsies with phenotype-driven drug testing of 3D culture of patient-derived tissue. Emergence of progressive loss or stable architecture after treatment may delineate those who will benefit from early intervention from those for whom treatment would be detrimental.
FORCING THE RESISTANCE
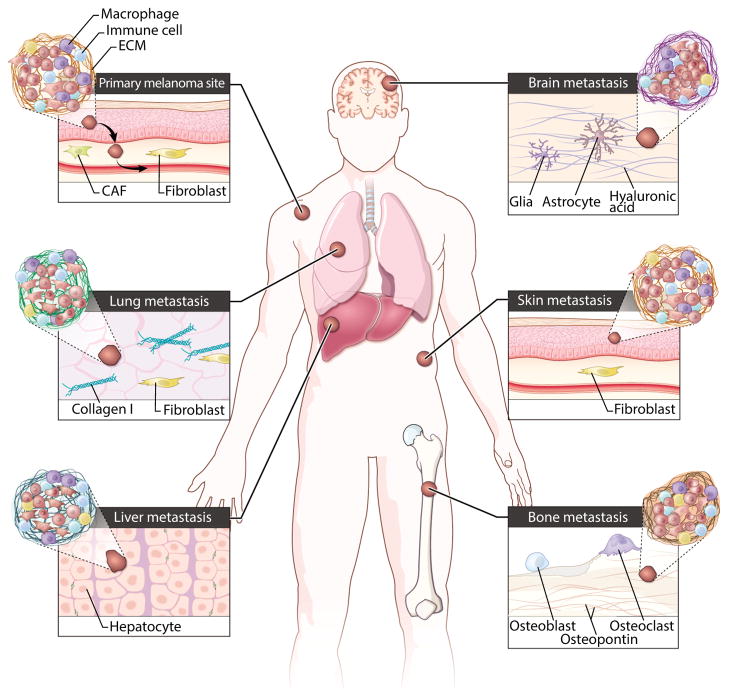
Unfortunately, by the time that many patients are diagnosed, disseminated tumor cells (DTCs) have already successfully evaded the constraints of the tissue adjacent to the primary tumor and entered into the lymphatic or vascular systems to initiate the metastatic cascade (14). In particular, patients diagnosed with cutaneous melanoma rapidly progress to full malignancy, ultimately succumbing if not diagnosed at very early stages of the disease (15). Metastatic disease also indicates that tumors are likely to be intrinsically resistant to treatment (16). DTCs en route to successful metastasis encounter distinct microenvironments conveying differential mechanical cues, and this exposure may induce epigenetic changes and enable de novo resistance. For example, exposure to shear forces induces cytoskeletal reorganization and cell adhesion, enabling the shape changes needed for efficient attachment to the vascular bed and organ infiltration (17). In vitro studies have shown that adhesion to specific substrata renders tumor cells more treatment-resistant. Furthermore, physicochemical properties such as the bulk stiffness, oxygen tension, and ECM composition of the local microenvironment of the infiltrated organ, along with tumor-directed tissue remodeling, might confer protection against therapeutic interventions (Fig. 1) (17). Consequently, drug treatment effective at the primary site may be rendered impotent for metastatic tumors (Fig. 1). Understanding these changes that occur along the way may help to prevent metastasis or, in the event of existing metastatic colonization, sensitize tumor cells to therapeutics.
Fig. 1. Designing in vitro platforms recapitulating diverse in vivo microenvironments.
Tumor cells may adopt different morphologies, patterns of ECM secretion, and modes of migration to successfully colonize distal organs. Clinically, cutaneous melanoma shows a broad tissue tropism and ability to metastasize to many organs. This illustration shows the architectural complexity at each of the diverse microenvironments in which both cell type and ECM composition might affect treatment efficacy. CAF, cancer-associated fibroblast.
CANCER ON A CHIP
Although commercially available substrates offer researchers the option of 3D culture, the question remains how to create an appropriate “biomimetic microenvironment” to ensure that one is not simply observing cell culture artifacts and can reliably reproduce findings. Bioengineered devices permit robust and precise manipulation of culture conditions (18). Microfabrication allows for defined scaffolds of different geometries to establish robust, spatially defined cocultures of different cell types. Microarray patterns in which precise combinations of ECM niches yield a defined morphology have provided insight into what constitutes “prometastatic” niches (19). The incorporation of microfluidics networks to transport soluble factors such as nutrients, hormones, growth factors, and oxygen would additionally facilitate tissue homeostasis via autocrine and paracrine signaling and recreate physiologically relevant mechanical cues that are absent from traditional cell culture (20). Recently, researchers achieved long-term differentiation of hepatocytes and osteoblasts, as evidenced by the finding that these cells exhibited normal physiological properties when cultured in bioreactors for up to 3 weeks (18). These bioreactors used complex microfluidic devices to both define and control the micro-environments by varying oxygen tension and fluid dynamics. Combining microfluidics and 3D microfabrication of ECM scaffolds makes possible the self-assembly of organotypic structures on experimental platforms, or “organs on a chip” (18). Complex organs such as the brain, liver, and gut have now been reconstructed by using in vitro systems (21–23). Of all human organs, tissue engineering of human skin has proven to be one of the most successful surrogates for modeling cancer growth (24). Commercially available cultured skin tissue can be tailored to incorporate human melanoma cell lines with characteristics of premetastatic stages: vertical growth phase (VGP) and radial growth phase (RGP) (25, 26). Thus, a future application of this technology may facilitate addressing organ-specific metastasis by incorporating tumor cells in these organotypic models.
An immediate application currently under way, however, is the addressing of drug toxicity effects. The metabolic activity of the liver and the gut largely dictates the toxic effects of many drugs. Pharmacokinetic studies are traditionally performed in rodents or in 2D polarized intestinal cells, but several compounds have still proven toxic to humans after such testing (21). Toxicology screening was recently performed in a system comprising interconnected channels and chambers representative of distinct tissue types (27). Liver, lung, and fat cells were cultured in interconnected compartments, allowing interrogation of physiologically relevant features, such as the timing of circulation and interchange of metabolites (27). These units can be adapted for high-throughput technologies, in which rapid screens of patient samples can be performed to identify suitable drug targets, and emergence of resistant phenotypes may help to determine what treatment should be administered. A more extensive review on this topic has been published previously (28).
LET’S TAKE A LOOK
Imaging of tissue dynamics has refined our understanding of the basic principles of cell migration. Just as phenotype reflects malignancy, distinct morphogenetic programs may reflect temporal dynamics of transitory states.
Earlier work showed the ability of tumor cells to exhibit distinct modes of motility, switching between “mesenchymal” and “amoeboid” migration to enable single-cell dissemination in three dimensions (29, 30). These motilities have been confirmed as strategies used by tumor cells in vivo by using intravital imaging in mouse studies (31, 32). However, the coupling of 3D organotypic models with the appropriate imaging modalities to dissect the morphodynamics of these multicellular structures has been less explored. Direct visualization of noncancerous epithelial tissue undergoing acinar and branching morphogenesis revealed geometrically specific motilities that facilitate the establishment of multicellular architecture (29, 33–35). Using these in vitro platforms and genetic manipulation (largely informed by patient data), a resulting phenotype can be assessed for normal or tumorigenic architecture. For example, induction of extracellular signal–regulated kinase (ERK) in 3D culture of normal human mammary epithelial cells caused the cells to become randomly motile within the acini, mimicking preinvasive breast neoplastic lesions (36). Similarly, collective invasion—a common strategy for carcinoma dissemination—was shown to be specific to tumor cells with genotypes similar to basal epithelial cells in 3D organotypic breast cancer studies and in mice (37).
These data suggest that a distinct type of cell motility occurs in preinvasive stages of epithelial tumor growth and hint at an underlying morphogenetic program that facilitates full-blown malignancy. Using intravital imaging after therapy-induced cell death revealed that distinct components of the microenvironment regulated drug efficacy. Specifically, increased vascular leakage increased drug efficacy, whereas the treatment-induced recruitment of immune cells regulated the durability of treatment responses (32). Additionally, many metastatic lesions and treatment-resistant tumors are associated with overabundance of ECM proteins (10). The ECM proteins may act as a physical barrier against drug transport and as signaling cues to induce resistance in cancer cells. The establishment of this protective cocoon of ECM and its relation to cell motility are currently underexplored. Further studies may yet uncover the motility determinants of colonization and organ specificity in tumor establishment and metastasis.
REALITY CHECK
The merging of tissue engineering and cancer biology will allow for robust interrogation of contextual drug efficacy; however, the ultimate goal remains informed patient care. Additionally, it is difficult to reconcile the time scale of days to weeks for a typical assay with the dynamics of clinical cancers that take many years to grow. A few points must be considered in order to ensure that these technologies will generate meaningful data. Namely, all ECMs are not created equal. Natural systems such as collagen extracts and laminin-rich gels such as Matrigel (BD Biosciences, San Jose, CA) are conveniently available but vary from lot to lot in consistency of protein concentration, gel stiffness, and concentrations of growth factors, which must be monitored. Synthetic materials incorporating defined cell ligands and proteolytic enzymes are attractive alternatives, but fabrication of these materials requires specific expertise and larger expense. Furthermore, cell lines that may be easy to manipulate and thus commonly used may no longer show physiological relevance to patient samples (1). Last, cells are not static but actively remodel their local environment, changing the gradients of metabolites and mechanical forces, thus disrupting the “predefined” environment. We may have to sacrifice a priori understanding of cellular mechanisms and focus on phenotypic outcomes at the tissue level of organization as we get closer to deciphering the laws of cancer.
Acknowledgments
The authors apologize to researchers whose work was not reflected in the references owing to space constraints. We thank A. Hoofring of the National Institutes of Health (NIH) Medical Arts for assistance with figure illustration. This effort was supported by the Intramural Research Program of NIH, the National Cancer Institute.
REFERENCES AND NOTES
- 1.Gillet JP, Calcagno AM, Varma S, Marino M, Green LJ, Vora MI, Patel C, Orina JN, Eliseeva TA, Singal V, Padmanabhan R, Davidson B, Ganapathi R, Sood AK, Rueda BR, Ambudkar SV, Gottesman MM. Redefining the relevance of established cancer cell lines to the study of mechanisms of clinical anti-cancer drug resistance. Proc Natl Acad Sci USA. 2011;108:18708–18713. doi: 10.1073/pnas.1111840108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bissell MJ, Hines WC. Why don’t we get more cancer? A proposed role of the microenvironment in restraining cancer progression. Nat Med. 2011;17:320–329. doi: 10.1038/nm.2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roesch A, Vultur A, Bogeski I, Wang H, Zimmermann KM, Speicher D, Körbel C, Laschke MW, Gimotty PA, Philipp SE, Krause E, Pätzold S, Villanueva J, Krepler C, Fukunaga-Kalabis M, Hoth M, Bastian BC, Vogt T, Herlyn M. Overcoming intrinsic multidrug resistance in melanoma by blocking the mitochondrial respiratory chain of slow-cycling JARID1B(high) cells. Cancer Cell. 2013;23:811–825. doi: 10.1016/j.ccr.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holohan C, Van Schaeybroeck S, Longley DB, Johnston PG. Cancer drug resistance: An evolving paradigm. Nat Rev Cancer. 2013;13:714–726. doi: 10.1038/nrc3599. [DOI] [PubMed] [Google Scholar]
- 5.Swartz MA, Iida N, Roberts EW, Sangaletti S, Wong MH, Yull FE, Coussens LM, DeClerck YA. Tumor microenvironment complexity: Emerging roles in cancer therapy. Cancer Res. 2012;72:2473–2480. doi: 10.1158/0008-5472.CAN-12-0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ackerman A, Klein O, McDermott DF, Wang W, Ibrahim N, Lawrence DP, Gunturi A, Flaherty KT, Hodi FS, Kefford R, Menzies AM, Atkins MB, Long GV, Sullivan RJ. Outcomes of patients with metastatic melanoma treated with immunotherapy prior to or after BRAF inhibitors. Cancer. 2014;120:1695–1701. doi: 10.1002/cncr.28620. [DOI] [PubMed] [Google Scholar]
- 7.Anderson PW. More is different. Science. 1972;177:393–396. doi: 10.1126/science.177.4047.393. [DOI] [PubMed] [Google Scholar]
- 8.Eccles SA, Aboagye EO, Ali S, Anderson AS, Armes J, Berditchevski F, Blaydes JP, Brennan K, Brown NJ, Bryant HE, Bundred NJ, Burchell JM, Campbell AM, Carroll JS, Clarke RB, Coles CE, Cook GJ, Cox A, Curtin NJ, Dekker LV, Silva IS, Duffy SW, Easton DF, Eccles DM, Edwards DR, Edwards J, Evans D, Fenlon DF, Flanagan JM, Foster C, Gallagher WM, Garcia-Closas M, Gee JM, Gescher AJ, Goh V, Groves AM, Harvey AJ, Harvie M, Hennessy BT, Hiscox S, Holen I, Howell SJ, Howell A, Hubbard G, Hulbert-Williams N, Hunter MS, Jasani B, Jones LJ, Key TJ, Kirwan CC, Kong A, Kunkler IH, Langdon SP, Leach MO, Mann DJ, Marshall JF, Martin L, Martin SG, Macdougall JE, Miles DW, Miller WR, Morris JR, Moss SM, Mullan P, Natrajan R, O’Connor JP, O’Connor R, Palmieri C, Pharoah PD, Rakha EA, Reed E, Robinson SP, Sahai E, Saxton JM, Schmid P, Smalley MJ, Speirs V, Stein R, Stingl J, Streuli CH, Tutt AN, Velikova G, Walker RA, Watson CJ, Williams KJ, Young LS, Thompson AM. Critical research gaps and translational priorities for the successful prevention and treatment of breast cancer. Breast Cancer Res. 2013;15:R92. doi: 10.1186/bcr3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19:1423–1437. doi: 10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tanner K. Regulation of the basement membrane by epithelia generated forces. Phys Biol. 2012;9:065003. doi: 10.1088/1478-3975/9/6/065003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rizki A, Weaver VM, Lee SY, Rozenberg GI, Chin K, Myers CA, Bascom JL, Mott JD, Semeiks JR, Grate LR, Mian IS, Borowsky AD, Jensen RA, Idowu MO, Chen F, Chen DJ, Petersen OW, Gray JW, Bissell MJ. A human breast cell model of preinvasive to invasive transition. Cancer Res. 2008;68:1378–1387. doi: 10.1158/0008-5472.CAN-07-2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, Reinhart-King CA, Margulies SS, Dembo M, Boettiger D, Hammer DA, Weaver VM. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8:241–254. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 13.Bischel LL, Beebe DJ, Sung KE. Microfluidic model of ductal carcinoma in situ with 3D, organotypic structure. BMC Cancer. 2015;15:12. doi: 10.1186/s12885-015-1007-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nguyen DX, Bos PD, Massagué J. Metastasis: From dissemination to organ-specific colonization. Nat Rev Cancer. 2009;9:274–284. doi: 10.1038/nrc2622. [DOI] [PubMed] [Google Scholar]
- 15.Chin L. The genetics of malignant melanoma: Lessons from mouse and man. Nat Rev Cancer. 2003;3:559–570. doi: 10.1038/nrc1145. [DOI] [PubMed] [Google Scholar]
- 16.Villanueva J, Herlyn M. Melanoma and the tumor micro-environment. Curr Oncol Rep. 2008;10:439–446. doi: 10.1007/s11912-008-0067-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kumar S, Weaver VM. Mechanics, malignancy, and metastasis: The force journey of a tumor cell. Cancer Metastasis Rev. 2009;28:113–127. doi: 10.1007/s10555-008-9173-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huh D, Matthews BD, Mammoto A, Montoya-Zavala M, Hsin HY, Ingber DE. Reconstituting organ-level lung functions on a chip. Science. 2010;328:1662–1668. doi: 10.1126/science.1188302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reticker-Flynn NE, Malta DF, Winslow MM, Lamar JM, Xu MJ, Underhill GH, Hynes RO, Jacks TE, Bhatia SN. A combinatorial extracellular matrix platform identifies cell-extracellular matrix interactions that correlate with metastasis. Nat Commun. 2012;3:1122. doi: 10.1038/ncomms2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jaeger AA, Das CK, Morgan NY, Pursley RH, McQueen PG, Hall MD, Pohida TJ, Gottesman MM. Microfabricated polymeric vessel mimetics for 3-D cancer cell culture. Biomaterials. 2013;34:8301–8313. doi: 10.1016/j.biomaterials.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ebrahimkhani MR, Neiman JA, Raredon MS, Hughes DJ, Griffith LG. Bioreactor technologies to support liver function in vitro. Adv Drug Deliv Rev. 2014;69–70:132–157. doi: 10.1016/j.addr.2014.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lancaster MA, Renner M, Martin CA, Wenzel D, Bicknell LS, Hurles ME, Homfray T, Penninger JM, Jackson AP, Knoblich JA. Cerebral organoids model human brain development and microcephaly. Nature. 2013;501:373–379. doi: 10.1038/nature12517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sato T, Clevers H. Growing self-organizing mini-guts from a single intestinal stem cell: Mechanism and applications. Science. 2013;340:1190–1194. doi: 10.1126/science.1234852. [DOI] [PubMed] [Google Scholar]
- 24.Netzlaff F, Lehr CM, Wertz PW, Schaefer UF. The human epidermis models EpiSkin, SkinEthic and EpiDerm: An evaluation of morphology and their suitability for testing phototoxicity, irritancy, corrosivity, and substance transport. Eur J Pharm Biopharm. 2005;60:167–178. doi: 10.1016/j.ejpb.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 25.Li L, Shukla S, Lee A, Garfield SH, Maloney DJ, Ambudkar SV, Yuspa SH. The skin cancer chemotherapeutic agent ingenol-3-angelate (PEP005) is a substrate for the epidermal multidrug transporter (ABCB1) and targets tumor vasculature. Cancer Res. 2010;70:4509–4519. doi: 10.1158/0008-5472.CAN-09-4303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li L, Fukunaga-Kalabis M, Herlyn M. The three-dimensional human skin reconstruct model: A tool to study normal skin and melanoma progression. J Vis Exp. 2011;54:2937. doi: 10.3791/2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Esch MB, King TL, Shuler ML. The role of body-on-a-chip devices in drug and toxicity studies. Annu Rev Biomed Eng. 2011;13:55–72. doi: 10.1146/annurev-bioeng-071910-124629. [DOI] [PubMed] [Google Scholar]
- 28.Astashkina A, Grainger DW. Critical analysis of 3-D organoid in vitro cell culture models for high-throughput drug candidate toxicity assessments. Adv Drug Deliv Rev. 2014;69–70:1–18. doi: 10.1016/j.addr.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 29.Wolf K, Mazo I, Leung H, Engelke K, von Andrian UH, Deryugina EI, Strongin AY, Bröcker EB, Friedl P. Compensation mechanism in tumor cell migration: Mesenchymal-amoeboid transition after blocking of pericellular proteolysis. J Cell Biol. 2003;160:267–277. doi: 10.1083/jcb.200209006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sanz-Moreno V, Gadea G, Ahn J, Paterson H, Marra P, Pinner S, Sahai E, Marshall CJ. Rac activation and inactivation control plasticity of tumor cell movement. Cell. 2008;135:510–523. doi: 10.1016/j.cell.2008.09.043. [DOI] [PubMed] [Google Scholar]
- 31.Condeelis J, Segall JE. Intravital imaging of cell movement in tumours. Nat Rev Cancer. 2003;3:921–930. doi: 10.1038/nrc1231. [DOI] [PubMed] [Google Scholar]
- 32.Nakasone ES, Askautrud HA, Kees T, Park JH, Plaks V, Ewald AJ, Fein M, Rasch MG, Tan YX, Qiu J, Park J, Sinha P, Bissell MJ, Frengen E, Werb Z, Egeblad M. Imaging tumor-stroma interactions during chemotherapy reveals contributions of the microenvironment to resistance. Cancer Cell. 2012;21:488–503. doi: 10.1016/j.ccr.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tanner K, Mori H, Mroue R, Bruni-Cardoso A, Bissell MJ. Coherent angular motion in the establishment of multicellular architecture of glandular tissues. Proc Natl Acad Sci USA. 2012;109:1973–1978. doi: 10.1073/pnas.1119578109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ewald AJ, Brenot A, Duong M, Chan BS, Werb Z. Collective epithelial migration and cell rearrangements drive mammary branching morphogenesis. Dev Cell. 2008;14:570–581. doi: 10.1016/j.devcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang H, Lacoche S, Huang L, Xue B, Muthuswamy SK. Rotational motion during three-dimensional morphogenesis of mammary epithelial acini relates to laminin matrix assembly. Proc Natl Acad Sci USA. 2013;110:163–168. doi: 10.1073/pnas.1201141110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pearson GW, Hunter T. Real-time imaging reveals that noninvasive mammary epithelial acini can contain motile cells. J Cell Biol. 2007;179:1555–1567. doi: 10.1083/jcb.200706099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cheung KJ, Gabrielson E, Werb Z, Ewald AJ. Collective invasion in breast cancer requires a conserved basal epithelial program. Cell. 2013;155:1639–1651. doi: 10.1016/j.cell.2013.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]