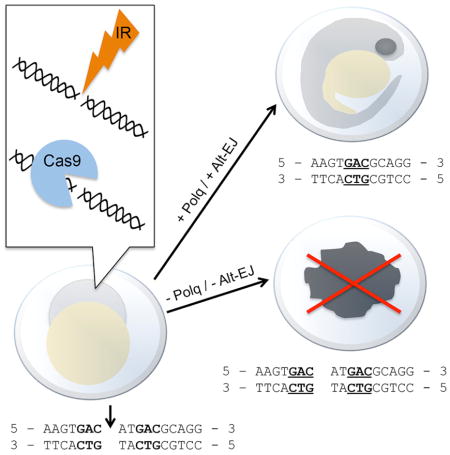
Summary
Error-prone repair of DNA double-strand breaks (DSBs) has been postulated to occur through classical non-homologous end joining (NHEJ) in systems ranging from nematode somatic tissues to zebrafish embryos. Contrary to this model, we show that zebrafish embryos mutant for DNA polymerase theta (Polq), a critical component of alternative end joining (alt-EJ), cannot repair DSBs induced by CRISPR/Cas9 or ionizing radiation. In the absence of DSBs, polq mutants are phenotypically normal, but they do not survive mutagenesis and display dramatic differences in the mutation profiles compared to wild type. These results show that alt-EJ repair is essential and dominant during the early development of a vertebrate.
Graphical abstract
Introduction
Repair of DNA double-strand breaks (DSBs) can occur through homology-directed repair (HDR) or through one of at least two distinct error-prone end joining mechanisms: classical non-homologous end joining (C-NHEJ) or alternative end joining (alt-EJ, sometimes called alt-NHEJ or microhomology-mediated end joining). In the absence of a template to guide HDR, end joining mechanisms with mutagenic outcomes are responsible for repair. C-NHEJ is well characterized and relies on Ku70/80 to dock onto the ends of DSBs, DNA-PKCS and Artemis to process ends, and DNA ligase 4 to join the processed ends (Weterings and Chen, 2008). This process generally relies on very little resection of DSB ends, and therefore typically results in accurate repair or small deletions and insertions (McVey and Lee, 2008; Yu and McVey, 2010). Alt-EJ is less well characterized, but this pathway is thought to rely on initiation of repair by Parp1, short resection by Mre11 and Ctip, DNA synthesis by Polq, and ligation by either DNA ligase 1 or DNA ligase 3 (Liang et al., 2008; Mateos-Gomez et al., 2015; Truong et al., 2013). Since alt-EJ depends on resection of DNA ends to find microhomologies, it typically results in larger deletions and templated insertions compared to C-NHEJ (McVey and Lee, 2008; Yu and McVey, 2010).
Alt-EJ is often considered to be a backup pathway that is active when C-NHEJ components are not available (Frit et al., 2014; Lin et al., 2013; Mladenov and Iliakis, 2011), but several lines of evidence indicate that alt-EJ repair occurs more frequently than previously thought, and can be the preferred method of repair in some situations (Ceccaldi et al., 2015; Gigi et al., 2014; Mateos-Gomez et al., 2015; Truong et al., 2013; van Schendel et al., 2015). For example, alt-EJ becomes the predominant repair pathway in mammalian cancer cells when HDR components are missing (Ceccaldi et al., 2015), and it can be induced by telomere de-protection (Mateos-Gomez et al., 2015) or the loss of C-NHEJ proteins (Bennardo et al., 2008; Secretan et al., 2004). Polymerase theta (Polq), a low-fidelity DNA polymerase (Hogg et al., 2012), is one of the crucial components of cancerous and induced alt-EJ in mammalian systems (Ceccaldi et al., 2015; Mateos-Gomez et al., 2015; Yousefzadeh et al., 2014), and is associated with human cancer (Ceccaldi et al., 2015; Lemee et al., 2010). In mammalian cells, it was found that Polq promotes the formation of chromosomal translocations and is essential for survival when HDR is impaired (Mateos-Gomez et al., 2015). Additionally, Polq-mediated alt-EJ has been observed in worms and flies. The Drosophila Polq ortholog Mus308 is responsible for alt-EJ (Chan et al., 2010; Yu and McVey, 2010), but its absence results in DNA repair through C-NHEJ, making alt-EJ dispensable for survival after DSBs (Chan et al., 2010). By contrast, in Caenorhabditis elegans, Polq and alt-EJ are essential for DSB repair in germ cells (van Schendel et al., 2015), while C-NHEJ is thought to be the dominant repair pathway in somatic tissues (Pontier and Tijsterman, 2009; Robert and Bessereau, 2007; van Schendel et al., 2015). Taken together, the studies in cancer cells and invertebrates indicate that under some circumstances Polq-mediated alt-EJ is more prevalent than the C-NHEJ (Sfeir and Symington, 2015).
There is no conclusive evidence that alt-EJ is an essential repair mechanism in normal vertebrate tissues, but the mutant alleles generated following Cas9-mediated DNA cleavage in mouse and zebrafish embryos suggest that repair during development might occur primarily through alt-EJ mechanisms (Gagnon et al., 2014; Hwang et al., 2013; Moreno-Mateos et al., 2015). For example, Cas9/gRNA-induced cleavage in zebrafish results in high rates of mutagenic end joining events, ranging in size from a single nucleotide to over fifty nucleotides in length (Gagnon et al., 2014; Moreno-Mateos et al., 2015). These types of alleles are similar to those observed for Cas9-induced DSB repair in mice (Yang et al., 2013; Yasue et al., 2014), rats (Shao et al., 2014), monkeys (Niu et al., 2014), and even humans (Liang et al., 2015), and are more consistent with alt-EJ repair than with C-NHEJ. Further, for some gRNAs, DNA repair generates a low number of mutagenic alleles, indicative of target site sequence features such as microhomologies that could be guiding the repair process (Gagnon et al., 2014).
In both early zebrafish and mouse embryos, all components of C-NHEJ are present and maternally available (Chew et al., 2013; Park et al., 2015). However, the important C-NHEJ components DNA-PKCS (prkdc) and artemis are transcribed at much lower levels than most other DNA repair proteins in both these organisms, raising the possibility that alt-EJ could be more dominant than C-NHEJ (Figure S1). Indeed, a recent morpholino-mediated knockdown experiment in zebrafish embryos indicated that Lig3, a ligase implicated in alt-EJ, might play a role in DNA repair. It remains unclear, however, if Lig3 is involved in endogenous genome repair, because repair was monitored using an injected plasmid with artificially designed microhomology repeats (He et al., 2015). To conclusively determine whether alt-EJ is required for DSB repair during vertebrate embryogenesis, we generated polq mutants and studied DNA repair after inducing DSBs. We found that Polq-mediated end joining is the dominant form of repair in early zebrafish embryos and is essential for survival following DSBs.
Results
To study the roles of Polq in vertebrate embryogenesis, we generated zebrafish polq mutants by injection of multiple gRNAs and Cas9 (Gagnon et al., 2014). We recovered an allele that eliminated 251 bases of polq, which is predicted to encode a frame-shifted protein truncated after 160 of 2,576 amino acids (Supplemental Methods). Similar to previously published mouse mutants (Masuda et al., 2005), adult homozygous fish were fertile, and homozygous maternal-zygotic (MZ) polq mutant embryos were viable with no morphological abnormalities (Figure 1a).
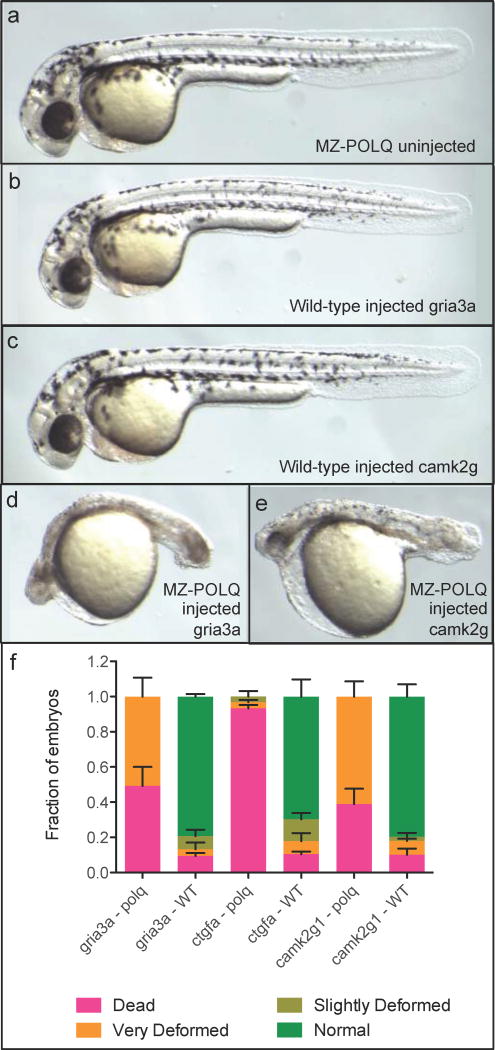
Figure 1. Polq is required for survival following Cas9-induced DNA DSBs.
All images are of approximately 36 hour post fertilization (hpf) embryos. Additional images are available in Figure S2. Sequences of the three gRNAs used are available in Table S1. a) Homozygous maternal-zygotic Polq (MZpolq) mutant. b) Wild-type (WT) embryo injected with Cas9 protein and gRNA for gria3a gene. c) WT embryo injected with Cas9 protein and gRNA for camk2g gene. d) MZpolq mutant injected with Cas9 protein and gRNA for gria3a gene. e) MZpolq mutant injected with Cas9 protein and gRNA for camk2g gene. f) Survival rates and fraction of deformed embryos observed for MZpolq and wild-type embryos injected with Cas9 protein and gRNAs targeting one of three genes. Embryos were injected at the 1-cell stage and all data was collected at approximately 36 hpf. Depictions of the observed deformities are available in Figure 1 and Figure S2a–c. The experiment was performed two times with n=15–47 for each injection. See also Figure S2 and Table S1.
To determine the importance of Polq in Cas9-induced DSB repair, we injected MZpolq and wild-type (WT) embryos at the one-cell stage with Cas9 and one of three individual gRNAs (Table S1). These gRNAs were previously found to generate high rates of mutagenesis but no or only minor phenotypic changes in wild-type embryos (Gagnon et al., 2014). In contrast, injection of any of the gRNAs with Cas9 into MZpolq embryos resulted in severely deformed or dead embryos (Figure 1b–e, Figure S2a, Figure S2b). For example, injection of Cas9 and a gRNA targeting ctgfa resulted in death of >90% of the MZpolq embryos, while fewer than 30% of wild-type embryos showed minor defects. MZpolq embryos injected with Cas9 or gRNA separately all survived, but interestingly, the majority of Cas9-injected mutants (13/15) displayed subtle abnormalities (Figure S2c). The dependence of DSB repair in Polq-deficient embryos was not limited to enzymatically-induced breaks: MZpolq embryos were significantly more sensitive to ionizing radiation than wild type (Figure 2a, Figure 2b, Figure S2d). In contrast, ultraviolet radiation did not result in reduced survival of MZpolq embryos compared to wild type (Figure 2c). These results indicate that Polq is essential for survival following DSBs.
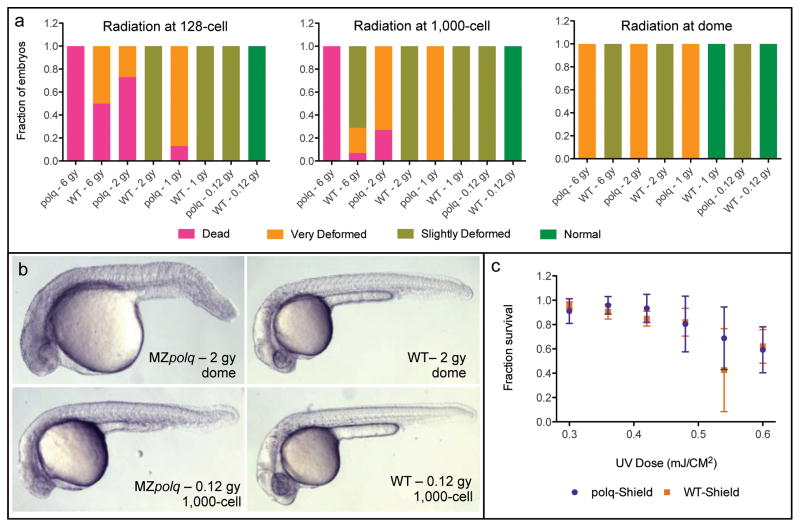
Figure 2. Polq is required for survival following DSBs induced by ionizing radiation.
a) Survival rates and fraction of deformed embryos observed for MZpolq and wild-type (WT) embryos exposed to varying levels of ionizing radiation at three stages of early development (n=14–32 for each condition). This data was collected at approximately 24 hpf. b) Examples of observed deformities in MZpolq embryos compared to wild-type embryos subjected to the same levels of radiation. Additional examples are available in Figure S2d. c) Survival rate observed for MZpolq and wild-type embryos exposed to varying levels of ultraviolet radiation at the shield stage of development. The experiment was performed two-four times per condition with n=14–54 each time. See also Figure S2.
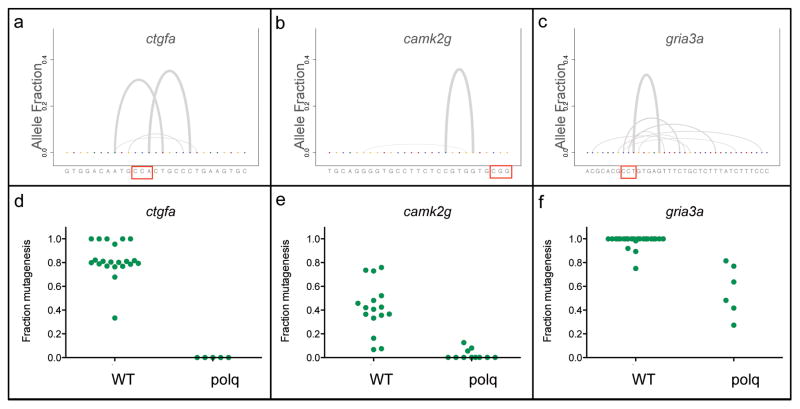
Some gRNAs only create a few predominant mutations, resulting in individual embryos that often have a small number of identical alleles (Gagnon et al., 2014). We hypothesized that short microhomologies might explain these observation by biasing the repair process to favor specific mutagenic alleles. To compare the nature and diversity of alleles following DSBs in wild type and MZpolq embryos, we individually injected three different gRNAs with Cas9 and analyzed mutations by deep sequencing. Two of the gRNAs we tested, targeting ctgfa and camk2g genes, produced highly stereotyped repair outcomes in wild-type embryos, with 1–2 predominant alleles (Figure 3a, Figure 3b, Figure S3a). Deficiency in the canonical NHEJ factor Lig4 did not alter repair outcomes (Figure S3b). Analyzing the sequences surrounding mutagenic repair alleles for these two gRNAs, as well as a number of other gRNAs, identified microhomologies of just three base-pairs in length (Figure S4). Cleavage with the third gRNA, targeting gria3a, produced a much more diverse repair profile compared to camk2g and ctgfa gRNAs and this target did not contain microhomologies of three or more base-pairs (Figure 3c). These results indicate that microhomologies play important roles in determining deletion size and allele diversity during DSB repair.
Figure 3. Distribution of mutagenic alleles generated by Cas9-induced DNA repair.
The types of alleles generated by Cas9 mutagenesis were determined by deep sequencing and are displayed with a gray arc connecting the bases flanking the deletion. The allele fraction is calculated by dividing the number of reads for the observed allele compared to the total reads for all mutated and wild-type (WT) alleles. The relative arc thickness also corresponds to this number. The PAM sequence for each gRNA is boxed in red. For each experiment 10–20 WT embryos were injected with a gRNA either targeting a) ctgfa, b) camk2g, or c) gria3a. Corresponding plots for single embryos are available in Figure S3a. Knockdown of Lig4 also does not influence the observed alleles (Figure S3b, Figure S3c). The observed mutations are likely guided by microhomologies for camk2g and ctgfa, as well as other sites, shown in Figure S4. Fraction mutagenesis, the number of sequencing reads containing an insertion or deletion induced by Cas9 cleavage and subsequent repair divided by the total number of sequence reads, for individual WT and surviving MZpolq embryos injected with gRNAs targeting d) ctgfa e) camk2g, and f) gria3a. In addition to reduced repair, oligonucleotide-mediated insertions are impaired in MZpolq embryos (Figure S5a, Figure S5b) and homologous recombination rates are not significantly higher (Figure S5c–e). See also Figure S3, S4, and S5.
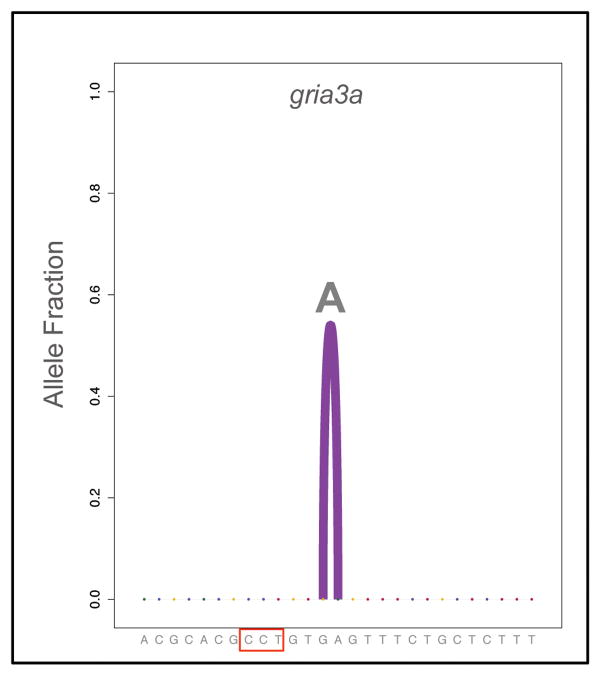
To determine the role of Polq in DNA repair, we analyzed allele diversity after Cas9/gRNA injection. We found that DNA repair in MZpolq embryos was severely impaired for all three gRNAs, irrespective of microhomology. Injection of Cas9 with ctgfa or camk2g gRNAs only led to the recovery of wild-type alleles (Figure 3d, Figure 3e). Together with the observed lethal phenotype in response to Cas9-induced cleavage (Figure 1) or ionizing radiation (Figure 2), this result indicates that DSB repair is severely impaired and that no other pathway can efficiently replace alt-EJ in these cases. Repair following injection of gRNA gria3a and Cas9 was substantially reduced and a single base-pair insertion dominated the repair process (Figure 4), dramatically reducing the allele diversity found in wild-type embryos (Figure 3e, Figure S3). These data reveal an essential role for Polq-dependent alt-EJ in DSB repair and allele diversification during early zebrafish development.
Figure 4. Types of mutagenic alleles observed in repair of Cas9-induced breaks in MZpolq mutant embryos.
Observed distribution of mutagenic alleles for MZpolq embryos injected with the gRNA targeting gria3a (n=6, fraction mutagenesis for each individual embryo is in Figure 3f). The purple arc indicates an insertion between the two ends of the arc (compared to the gray arcs representing deletions, Figure 3) and the inserted sequence is displayed at the top of the arc. The PAM sequence for the gria3a gRNA is boxed in red.
To test the role of Polq in homology-mediated processes, we measured the insertion rates of oligonucleotides and the homologous recombination rates of plasmids. Oligonucleotide-mediated genome engineering (Bedell et al., 2012) employs short homology arms to guide the insertion of a <100 bp DNA sequence following DSBs. Deep sequencing of MZpolq and wild-type embryos injected with Cas9, a gRNA targeting the camk2g site and an oligonucleotide containing a 6 base-pair insertion and 20 base-pair homology arms revealed no insertions in surviving MZpolq embryos, whereas wild-type embryos contained insertions (Figure S5a, Figure S5b). This result suggests that oligonucleotide insertion requires Polq-mediated alt-EJ. To determine whether loss of the Polq-mediated end joining pathway increases homologous recombination rates (Chu et al., 2015; Maruyama et al., 2015), we used a plasmid-based system to compare MZpolq and wild-type embryos (Figure S5c). Although both Cas9/guideRNA-injected wild-type and MZpolq embryos had significantly higher homologous recombination rates than control-injected embryos, loss of Polq did not significantly increase insertion rates. These results suggest that Polq-mediated alt-EJ might not be the major limitation to homologous recombination in zebrafish embryos.
Discussion
Our study reveals that Polq-mediated alt-EJ is required for the repair of Cas9-induced DSBs in zebrafish embryos. This result is surprising, as alt-EJ was considered to only be a backup form of repair and C-NHEJ was thought to be responsible for DSB repair in zebrafish (Auer et al., 2014; Bladen et al., 2005; Liu et al., 2012). More generally, these results suggest a broader role for alt-EJ in somatic tissues and in vertebrates than previously assumed (Frit et al., 2014; Lin et al., 2013; Mladenov and Iliakis, 2011).
Low levels of repair were observed in MZpolq embryos for only one of the three tested gRNAs (Figure 3f) and generated a single base-pair insertion (Figure 4), an allele more typical of C-NHEJ (McVey and Lee, 2008) and much shorter than alleles created in wild type (Figure 3c). This result indicates that some repair is possible in the absence of Polq, but the observed lethality of MZpolq embryos upon DSB induction and the minimal occurrence of repaired alleles indicate that no other pathway significantly contributes to DSB repair during early zebrafish embryogenesis.
Homologous recombination, which is inefficient in zebrafish embryos (Auer et al., 2014; Bedell et al., 2012; Hisano et al., 2015; Zu et al., 2013), was not significantly enhanced in the absence of Polq (Figure S5c–e). This result suggests that as in C. elegans (van Schendel et al., 2015) lack of Polq-mediated alt-EJ does not increase homologous recombination rates and that additional or other factors limit this process in zebrafish embryos. In contrast, our finding that DSB-induced oligonucleotide insertion is impaired in MZpolq embryos (Figure S5a, Figure S5b) indicates that alt-EJ is required for efficient oligonucleotide-mediated genome editing (Bedell et al., 2012).
The finding that alt-EJ is the dominant repair pathway for DSBs in zebrafish embryos supports and extends two recent studies. First, C. elegans Polq is required for the repair of DSBs in germ cells and of lesions caused by replication fork collapse in somatic cells (Koole et al., 2014; Roerink et al., 2014; van Schendel et al., 2015). Although C-NHEJ is thought to be the dominant DSB repair mechanism in somatic tissues in C. elegans (Pontier and Tijsterman, 2009; Robert and Bessereau, 2007; van Schendel et al., 2015), our study shows that alt-EJ is essential for somatic DNA repair in early zebrafish embryos. Second, morpholino-mediated inhibition in zebrafish of the alt-EJ ligase Lig3 reduced microhomology-mediated end joining repair of a GFP reporter plasmid (He et al., 2015). Our study shows that Polq-mediated alt-EJ is also essential for efficient repair of endogenous loci. While we find that microhomology is an important determinant of allele nature and diversity (Figure S4), repair that is not guided by obvious microhomologies, as in the case of the gria3a gRNA, is still dependent on Polq (Figure 3c). Previous studies indicate that microhomologies utilized in MMEJ are 5–25 base-pairs in length (McVey and Lee, 2008), while we find that even homologous sequences of three base-pairs can bias the repair process. Our results suggest that alt-EJ and MMEJ might comprise a single pathway that can utilize but does not depend on short microhomologies for repair.
Our study directly demonstrates that alt-EJ/MMEJ is essential for DSB repair in early vertebrate development, and observations in other systems suggest a similar role in mammalian embryos. Three studies of Cas9-injected mouse zygotes explicitly suggest that MMEJ is responsible for repair, rather than C-NHEJ (Li et al., 2015; Yang et al., 2013; Yasue et al., 2014), because the same alleles were repeatedly recovered in independently derived mice and repaired alleles appeared to utilize microhomologous repeat sequences. We also found that the alleles in Cas9-injected rats (Shao et al., 2014) and monkeys (Niu et al., 2014) have the sequence features expected from microhomology-mediated repair. There has only been a single publication on Cas9-induced DSB repair in injected human embryos, but our evaluation of the reported sequencing results suggests that Polq may also be responsible for repair in human embryos (Liang et al., 2015). For example, the frequent deletion of a GAG trinucleotide next to a second GAG trinucleotide is an expected outcome of microhomology-mediated repair. We therefore speculate that Polq-mediated alt-EJ/MMEJ is the major pathway for DSB repair in early vertebrate embryos.
Our results raise the question of why alt-EJ is the dominant and essential pathway for Cas9-mediated DSB repair in early zebrafish development. It is conceivable that the lower levels of some C-NHEJ components (DNA-PKCS (Prkdc), Artemis, Lig4) shift repair towards alt-EJ (Figure S1). In particular, DNA-PKCS has been shown to be an important determinant of C-NHEJ efficiency (Chan et al., 2010; Perrault et al., 2004). This explanation may extend to other organisms, such as mice and monkeys, in which similar mutagenic alleles are found after DSBs. As in zebrafish, the levels of both artemis and DNA-PKCS (prkdc) are low during early mouse development (Park et al., 2015) (Figure S1), while the mRNA levels of alt-EJ proteins such as Lig3, Parp1, and Polq increase during the first two cell cycles. It is possible that alt-EJ is better suited to repair DSBs in cells undergoing the rapid cycling found during early development. Short cell cycles might lead to increased replication stress, and Polq and alt-EJ have both been implicated in protecting the genome against stressors such as stalled replication forks (Roerink et al., 2014; Truong et al., 2013; Truong et al., 2014; Yousefzadeh and Wood, 2013). Our finding that injection of Cas9 protein alone induces phenotypic abnormalities in MZpolq embryos (Figure S2) might support this idea: Cas9 protein binds to DNA non-specifically (Sternberg et al., 2014) and could act as a genomic stressor, leading to DNA damage that requires repair by Polq-mediated alt-EJ. In this scenario, Polq-mediated alt-EJ is an important pathway to repair DNA damage generated during the rapid cleavage divisions of early embryogenesis
Experimental Procedures
Fish husbandry and microinjection
The polq mutant, ZFIN mutant line polqa153, was generated by simultaneous injection of three gRNAs, made as previously described (Gagnon et al., 2014), and approximately 0.5 nL of 50 μM Cas9 protein into TLAB embryos. The resulting mosaic adult with a germ line mutagenic deletion (Supplemental Methods) was crossed to TLAB and the offspring were mated to each other to generate MZpolq mutants. To assess DNA repair, zebrafish embryos, MZpolq mutant and TLAB wild type, were collected at the one-cell stage and injected with either 300 pg of Cas9 mRNA (Gagnon et al., 2014) or 0.5 nL of 50 μM Cas9 protein and excess (Gagnon et al., 2014; Hwang et al., 2013) (300–500 pg) gRNA. For oligonucleotide injections, approximately 0.5 nL of 2 μM oligonucleotide was co-injected. Lig4 deficiency was induced with a previously validated morpholino (He et al., 2015; Liu et al., 2012) obtained from Gene Tools (Oregon, USA), used at concentrations shown to be effective in these previous studies.
Homologous recombination assay
MZpolq mutant and TLAB wild-type embryos were collected at the one-cell stage and injected with 25 pg of each donor and acceptor plasmids (Figure S5c), 10 pg of Gal4 mRNA, 300 pg of Cas9 mRNA, and excess gRNA. The gRNA used was previously published to cleave the GFP DNA sequence with high efficiency and to not have any off-target cleavage sites in the zebrafish genome (Auer et al., 2014). The sequences of both plasmids are available in the supplemental methods. Control embryos were injected with all components of the reaction except the gRNA required to induce DSBs. Embryos were collected at approximately 30 hpf and RNA was recovered with TRIzol extraction (Ambion by Life Technologies). Approximately 20 embryos were pooled, with three groups of 20 assessed for each condition. Following RNA extraction, any remaining genomic and plasmid DNA was eliminated with a DNA-free DNA removal kit (ThermoFisher Scientific). cDNA was made from total RNA using BioRad iScript kit, and analyzed using SYBR® Green I (BioRad) and DNA Engine Opticon System (MJ Research).
Radiation experiments
Ionizing radiation was conducted on a slowly rotating platform at room temperature under a 137Cs source delivering 6 gy/minute. Ultraviolet radiation was conducted with a CL-1000 ultraviolet crosslinker, delivering a 254 nm wavelength. For each dose of ultraviolet radiation, the exposure was completed in thirds, swirling the dish between each partial dose to minimize differences in exposure due to embryo orientation.
Determination of somatic mutagenesis alleles
Sequences of repaired alleles were collected with MiSeq 150 base-pair paired-end sequencing (Illumina), as previously described (Gagnon et al., 2014). The forward and reverse reads were stitched using the fastq-join function in MacQIIME (Caporaso et al., 2010). Determination of the mutant alleles was done with a custom python script (supplemental file AlleleAnalysis.py). First, the fused reads were aligned to the genome reference sequence using the Needleman-Wunsch algorithm through EMBOSS suite with default parameters. Insertions and deletions were then determined from the alignments, using a BioPython module to import the alignments. Arc diagrams were plotted based on instructions from the website http://www.r-bloggers.com/arc-diagrams-in-r-les-miserables/. Prediction of potential alleles based on microhomologies was done with a custom python script (supplemental file, MicrohomologyFinder.py).
Supplementary Material
Acknowledgments
This research was supported by grants from the Damon Runyon Cancer Research Foundation (SBT) and NIH grants R01 GM056211, R01 HL109525, and R21 HD072733. The authors thank Dominic Mao for assistance with the ionizing radiation experiment, James Gagnon, Mehdi Goudarzi, Jeff Farrell, and Florian Merkle for helpful comments on the project, and Jeff Farrell, Mehdi Goudarzi, Megan Norris, Tessa Montague and Shristi Pandey for critical reading of the manuscript.
Footnotes
Author Contributions
SBT designed and conducted all experiments. SBT and AFS wrote the paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Auer TO, Duroure K, De Cian A, Concordet JP, Del Bene F. Highly efficient CRISPR/Cas9-mediated knock-in in zebrafish by homology-independent DNA repair. Genome Res. 2014;24:142–153. doi: 10.1101/gr.161638.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedell VM, Wang Y, Campbell JM, Poshusta TL, Starker CG, Krug RG, 2nd, Tan W, Penheiter SG, Ma AC, Leung AY, et al. In vivo genome editing using a high-efficiency TALEN system. Nature. 2012;491:114–118. doi: 10.1038/nature11537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennardo N, Cheng A, Huang N, Stark JM. Alternative-NHEJ is a mechanistically distinct pathway of mammalian chromosome break repair. PLoS genetics. 2008;4:e1000110. doi: 10.1371/journal.pgen.1000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bladen CL, Lam WK, Dynan WS, Kozlowski DJ. DNA damage response and Ku80 function in the vertebrate embryo. Nucleic acids research. 2005;33:3002–3010. doi: 10.1093/nar/gki613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Pena AG, Goodrich JK, Gordon JI, et al. QIIME allows analysis of high-throughput community sequencing data. Nature methods. 2010;7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceccaldi R, Liu JC, Amunugama R, Hajdu I, Primack B, Petalcorin MI, O’Connor KW, Konstantinopoulos PA, Elledge SJ, Boulton SJ, et al. Homologous-recombination-deficient tumours are dependent on Poltheta-mediated repair. Nature. 2015;518:258–262. doi: 10.1038/nature14184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan SH, Yu AM, McVey M. Dual roles for DNA polymerase theta in alternative end-joining repair of double-strand breaks in Drosophila. PLoS genetics. 2010;6:e1001005. doi: 10.1371/journal.pgen.1001005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew GL, Pauli A, Rinn JL, Regev A, Schier AF, Valen E. Ribosome profiling reveals resemblance between long non-coding RNAs and 5′ leaders of coding RNAs. Development. 2013;140:2828–2834. doi: 10.1242/dev.098343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu VT, Weber T, Wefers B, Wurst W, Sander S, Rajewsky K, Kuhn R. Increasing the efficiency of homology-directed repair for CRISPR-Cas9-induced precise gene editing in mammalian cells. Nature biotechnology. 2015;33:543–548. doi: 10.1038/nbt.3198. [DOI] [PubMed] [Google Scholar]
- Frit P, Barboule N, Yuan Y, Gomez D, Calsou P. Alternative end-joining pathway(s): bricolage at DNA breaks. DNA repair. 2014;17:81–97. doi: 10.1016/j.dnarep.2014.02.007. [DOI] [PubMed] [Google Scholar]
- Gagnon JA, Valen E, Thyme SB, Huang P, Ahkmetova L, Pauli A, Montague TG, Zimmerman S, Richter C, Schier AF. Efficient mutagenesis by Cas9 protein-mediated oligonucleotide insertion and large-scale assessment of single-guide RNAs. PloS one. 2014;9:e98186. doi: 10.1371/journal.pone.0098186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gigi V, Lewis S, Shestova O, Mijuskovic M, Deriano L, Meng W, Luning Prak ET, Roth DB. RAG2 mutants alter DSB repair pathway choice in vivo and illuminate the nature of ‘alternative NHEJ’. Nucleic acids research. 2014;42:6352–6364. doi: 10.1093/nar/gku295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He MD, Zhang FH, Wang HL, Wang HP, Zhu ZY, Sun YH. Efficient ligase 3-dependent microhomology-mediated end joining repair of DNA double-strand breaks in zebrafish embryos. Mutat Res. 2015;780:86–96. doi: 10.1016/j.mrfmmm.2015.08.004. [DOI] [PubMed] [Google Scholar]
- Hisano Y, Sakuma T, Nakade S, Ohga R, Ota S, Okamoto H, Yamamoto T, Kawahara A. Precise in-frame integration of exogenous DNA mediated by CRISPR/Cas9 system in zebrafish. Scientific reports. 2015;5:8841. doi: 10.1038/srep08841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogg M, Sauer-Eriksson AE, Johansson E. Promiscuous DNA synthesis by human DNA polymerase theta. Nucleic acids research. 2012;40:2611–2622. doi: 10.1093/nar/gkr1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang WY, Fu Y, Reyon D, Maeder ML, Tsai SQ, Sander JD, Peterson RT, Yeh JR, Joung JK. Efficient genome editing in zebrafish using a CRISPR-Cas system. Nature biotechnology. 2013;31:227–229. doi: 10.1038/nbt.2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koole W, van Schendel R, Karambelas AE, van Heteren JT, Okihara KL, Tijsterman M. A Polymerase Theta-dependent repair pathway suppresses extensive genomic instability at endogenous G4 DNA sites. Nature communications. 2014;5:3216. doi: 10.1038/ncomms4216. [DOI] [PubMed] [Google Scholar]
- Lemee F, Bergoglio V, Fernandez-Vidal A, Machado-Silva A, Pillaire MJ, Bieth A, Gentil C, Baker L, Martin AL, Leduc C, et al. DNA polymerase theta up-regulation is associated with poor survival in breast cancer, perturbs DNA replication, and promotes genetic instability. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:13390–13395. doi: 10.1073/pnas.0910759107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Shou J, Guo Y, Tang Y, Wu Y, Jia Z, Zhai Y, Chen Z, Xu Q, Wu Q. Efficient inversions and duplications of mammalian regulatory DNA elements and gene clusters by CRISPR/Cas9. Journal of molecular cell biology. 2015;7:284–298. doi: 10.1093/jmcb/mjv016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang L, Deng L, Nguyen SC, Zhao X, Maulion CD, Shao C, Tischfield JA. Human DNA ligases I and III, but not ligase IV, are required for microhomology-mediated end joining of DNA double-strand breaks. Nucleic acids research. 2008;36:3297–3310. doi: 10.1093/nar/gkn184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang P, Xu Y, Zhang X, Ding C, Huang R, Zhang Z, Lv J, Xie X, Chen Y, Li Y, et al. CRISPR/Cas9-mediated gene editing in human tripronuclear zygotes. Protein & cell. 2015;6:363–372. doi: 10.1007/s13238-015-0153-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin WY, Wilson JH, Lin Y. Repair of chromosomal double-strand breaks by precise ligation in human cells. DNA repair. 2013;12:480–487. doi: 10.1016/j.dnarep.2013.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Gong L, Chang C, Liu C, Peng J, Chen J. Development of novel visual-plus quantitative analysis systems for studying DNA double-strand break repairs in zebrafish. J Genet Genomics. 2012;39:489–502. doi: 10.1016/j.jgg.2012.07.009. [DOI] [PubMed] [Google Scholar]
- Maruyama T, Dougan SK, Truttmann MC, Bilate AM, Ingram JR, Ploegh HL. Increasing the efficiency of precise genome editing with CRISPR-Cas9 by inhibition of nonhomologous end joining. Nature biotechnology. 2015;33:538–542. doi: 10.1038/nbt.3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda K, Ouchida R, Takeuchi A, Saito T, Koseki H, Kawamura K, Tagawa M, Tokuhisa T, Azuma T, JOW DNA polymerase theta contributes to the generation of C/G mutations during somatic hypermutation of Ig genes. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:13986–13991. doi: 10.1073/pnas.0505636102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateos-Gomez PA, Gong F, Nair N, Miller KM, Lazzerini-Denchi E, Sfeir A. Mammalian polymerase theta promotes alternative NHEJ and suppresses recombination. Nature. 2015;518:254–257. doi: 10.1038/nature14157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVey M, Lee SE. MMEJ repair of double-strand breaks (director’s cut): deleted sequences and alternative endings. Trends in genetics: TIG. 2008;24:529–538. doi: 10.1016/j.tig.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mladenov E, Iliakis G. Induction and repair of DNA double strand breaks: the increasing spectrum of non-homologous end joining pathways. Mutation research. 2011;711:61–72. doi: 10.1016/j.mrfmmm.2011.02.005. [DOI] [PubMed] [Google Scholar]
- Moreno-Mateos MA, Vejnar CE, Beaudoin JD, Fernandez JP, Mis EK, Khokha MK, Giraldez AJ. CRISPRscan: designing highly efficient sgRNAs for CRISPR-Cas9 targeting in vivo. Nature methods. 2015 doi: 10.1038/nmeth.3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu Y, Shen B, Cui Y, Chen Y, Wang J, Wang L, Kang Y, Zhao X, Si W, Li W, et al. Generation of gene-modified cynomolgus monkey via Cas9/RNA-mediated gene targeting in one-cell embryos. Cell. 2014;156:836–843. doi: 10.1016/j.cell.2014.01.027. [DOI] [PubMed] [Google Scholar]
- Park SJ, Shirahige K, Ohsugi M, Nakai K. DBTMEE: a database of transcriptome in mouse early embryos. Nucleic acids research. 2015;43:D771–776. doi: 10.1093/nar/gku1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrault R, Wang H, Wang M, Rosidi B, Iliakis G. Backup pathways of NHEJ are suppressed by DNA-PK. Journal of cellular biochemistry. 2004;92:781–794. doi: 10.1002/jcb.20104. [DOI] [PubMed] [Google Scholar]
- Pontier DB, Tijsterman M. A robust network of double-strand break repair pathways governs genome integrity during C. elegans development. Curr Biol. 2009;19:1384–1388. doi: 10.1016/j.cub.2009.06.045. [DOI] [PubMed] [Google Scholar]
- Robert V, Bessereau JL. Targeted engineering of the Caenorhabditis elegans genome following Mos1-triggered chromosomal breaks. The EMBO journal. 2007;26:170–183. doi: 10.1038/sj.emboj.7601463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roerink SF, van Schendel R, Tijsterman M. Polymerase theta-mediated end joining of replication-associated DNA breaks in C. elegans. Genome research. 2014;24:954–962. doi: 10.1101/gr.170431.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Secretan MB, Scuric Z, Oshima J, Bishop AJ, Howlett NG, Yau D, Schiestl RH. Effect of Ku86 and DNA-PKcs deficiency on non-homologous end-joining and homologous recombination using a transient transfection assay. Mutation research. 2004;554:351–364. doi: 10.1016/j.mrfmmm.2004.05.016. [DOI] [PubMed] [Google Scholar]
- Sfeir A, Symington LS. Microhomology-Mediated End Joining: A Back-up Survival Mechanism or Dedicated Pathway? Trends in biochemical sciences. 2015;40:701–714. doi: 10.1016/j.tibs.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Y, Guan Y, Wang L, Qiu Z, Liu M, Chen Y, Wu L, Li Y, Ma X, Liu M, et al. CRISPR/Cas-mediated genome editing in the rat via direct injection of one-cell embryos. Nature protocols. 2014;9:2493–2512. doi: 10.1038/nprot.2014.171. [DOI] [PubMed] [Google Scholar]
- Sternberg SH, Redding S, Jinek M, Greene EC, Doudna JA. DNA interrogation by the CRISPR RNA-guided endonuclease Cas9. Nature. 2014;507:62–67. doi: 10.1038/nature13011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truong LN, Li Y, Shi LZ, Hwang PY, He J, Wang H, Razavian N, Berns MW, Wu X. Microhomology-mediated End Joining and Homologous Recombination share the initial end resection step to repair DNA double-strand breaks in mammalian cells. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:7720–7725. doi: 10.1073/pnas.1213431110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truong LN, Li Y, Sun E, Ang K, Hwang PY, Wu X. Homologous recombination is a primary pathway to repair DNA double-strand breaks generated during DNA rereplication. The Journal of biological chemistry. 2014;289:28910–28923. doi: 10.1074/jbc.M114.576488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Schendel R, Roerink SF, Portegijs V, van den Heuvel S, Tijsterman M. Polymerase Theta is a key driver of genome evolution and of CRISPR/Cas9-mediated mutagenesis. Nat Commun. 2015;6:7394. doi: 10.1038/ncomms8394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weterings E, Chen DJ. The endless tale of non-homologous end-joining. Cell research. 2008;18:114–124. doi: 10.1038/cr.2008.3. [DOI] [PubMed] [Google Scholar]
- Yang H, Wang H, Shivalila CS, Cheng AW, Shi L, Jaenisch R. One-step generation of mice carrying reporter and conditional alleles by CRISPR/Cas-mediated genome engineering. Cell. 2013;154:1370–1379. doi: 10.1016/j.cell.2013.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasue A, Mitsui SN, Watanabe T, Sakuma T, Oyadomari S, Yamamoto T, Noji S, Mito T, Tanaka E. Highly efficient targeted mutagenesis in one-cell mouse embryos mediated by the TALEN and CRISPR/Cas systems. Scientific reports. 2014;4:5705. doi: 10.1038/srep05705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousefzadeh MJ, Wood RD. DNA polymerase POLQ and cellular defense against DNA damage. DNA repair. 2013;12:1–9. doi: 10.1016/j.dnarep.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousefzadeh MJ, Wyatt DW, Takata K, Mu Y, Hensley SC, Tomida J, Bylund GO, Doublie S, Johansson E, Ramsden DA, et al. Mechanism of suppression of chromosomal instability by DNA polymerase POLQ. PLoS genetics. 2014;10:e1004654. doi: 10.1371/journal.pgen.1004654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu AM, McVey M. Synthesis-dependent microhomology-mediated end joining accounts for multiple types of repair junctions. Nucleic acids research. 2010;38:5706–5717. doi: 10.1093/nar/gkq379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zu Y, Tong X, Wang Z, Liu D, Pan R, Li Z, Hu Y, Luo Z, Huang P, Wu Q, et al. TALEN-mediated precise genome modification by homologous recombination in zebrafish. Nature methods. 2013;10:329–331. doi: 10.1038/nmeth.2374. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.