Abstract
Purpose
Subsartorial saphenous nerve blockade (SSNB) is an effective analgesic alternative to femoral nerve blockade after anterior cruciate ligament (ACL) reconstruction with bone-tendon-bone (BTB) autograft. It was hypothesized that dexamethasone in a SSNB will prolong analgesia, improve pain and satisfaction, and reduce postoperative opioid requirements and side effects.
Methods
One hundred ninety-five patients undergoing ACL reconstruction with BTB autograft (ages 16–65) were enrolled. Subjects received SSNB with 13 mL of 0.5% bupivacaine (control group), 1 mg preservative-free dexamethasone+0.5% bupivacaine (treatment group I), or 4 mg preservative-free dexamethasone+0.5% bupivacaine (treatment group II). Subjects received identical perioperative management. On postoperative days 1 and 2, subjects reported perceived block duration, pain scores, satisfaction, opioid use, and side effects. Cox proportional hazards modeling was used to compare block duration, adjusting for body mass index, age, sex, tourniquet time, American Society of Anesthesiologists classification, and intravenous dexamethasone dose.
Results
Patient-perceived block duration was significantly increased in treatment group I (hazard ratio(95% confidence interval [CI]): 0.48(0.31–0.75); P=0.001) and treatment group II (hazard ratio(95% CI): 0.52(0.33–0.81); P=0.004) compared to control. The block was extended from a median(95% CI) of 33.1(28.4–37.3) to 41.2(32.4–50.9) and 46.5(35.8–48.9) hours, respectively. Additionally, patients in treatment group II reported increased time that block provided pain relief, higher patient satisfaction, lower pain scores at rest, and decreased drowsiness and confusion.
Conclusions
The addition of 1 mg and 4 mg of dexamethasone to the block injectate significantly increased SSNB duration by 8–13 hours compared to control.
Keywords: ACL reconstruction, patellar tendon autograft, subsartorial saphenous nerve block, postoperative pain
Introduction
A previous study [4] demonstrated that the subsartorial saphenous nerve block (SSNB) was as efficacious as the traditional femoral nerve block (FNB) [9,18] for postoperative analgesia following bone-tendon-bone (BTB)-anterior cruciate ligament (ACL) reconstruction. With the efficacy of SSNB having been established, this study investigates whether analgesia could be extended by the addition of preservative-free dexamethasone. Previous studies with perineural dexamethasone demonstrated prolonged brachial plexus blocks [5] as well as increased analgesic duration in sciatic nerve blocks [17]. The primary aim was to compare patient-derived SSNB duration for ACL patients who received either 0.5% bupivacaine or bupivacaine plus dexamethasone. The primary outcome, patient-perceived block duration, was assessed by the patient’s answer to the following question: “When did the nerve block entirely wear off?” It was hypothesized that the dexamethasone would prolong blockade from 6–10 hours [6,19], compared to a control nerve block containing only bupivacaine 0.5%. Secondary outcomes included reduced postoperative opioid consumption, numerical rating scale (NRS) pain scores on POD 1 and 2, patient satisfaction (as assessed using a 0–10 scale) and opioid side effects (as assessed by the opioid-related symptom distress scale, ORSDS [21]).
Materials and Methods
On the day of surgery, a co-investigator anesthesiologist approached the patient preoperatively. Eligible patients were all American Society of Anesthesiologists (ASA) Class I–III patients, ages 16 to 65, undergoing ambulatory surgery for ACL reconstruction with BTB autograft. Exclusion criteria were obesity (defined as body mass index [BMI] greater than 35 kg/m2), allergy to study medications, NRS pain scores greater than 3 with frequent opioid use prior to surgery (defined as daily for more than 3 weeks), lower extremity neurological dysfunction, diabetes, patients on steroids or requiring stress dose steroids, or contraindications to dexamethasone use.
Once written informed consent was obtained (patients under 18 signed an assent form, and consent was also obtained from their legal guardian), the investigator opened the sealed treatment envelope, which randomized the patient to the control group (SSNB: 0.5% bupivacaine plain), treatment group I (SSNB: 0.5% bupivacaine with 1 mg preservative-free dexamethasone), or treatment group II (SSNB: 0.5% bupivacaine with 4 mg preservative-free dexamethasone).
Randomizer™, an Excel-compatible randomizing program, was used to create a 1:1:1 random allocation sequence to 3 parallel treatment arms with a total target enrollment number of 195 patients. Non-study personnel prepared opaque sealed treatment envelopes.
Preoperative Procedures
Patients and the research assistants were blinded to the treatment groups. Preoperative assessments by blinded research assistants included a baseline sensory examination of the saphenous nerve distribution. A sterile alcohol swab was applied to the medial malleolus of the non-operative and operative legs, and any changes in cold or wet sensation were recorded. Basic demographic data were also collected by patient interview.
Intraoperative Procedures
Intravenous midazolam was administered in the operating room for anxiolysis and mild sedation. The SSNB was performed prior to the spinal anesthetic, under ultrasound guidance using a 6–13-MHz linear probe L25x by Sonosite®. At a distance of no more than 7 cm proximal to the medial condyle [13], a short-axis view of the sartorius and vastus medialis muscles was obtained with the saphenous nerve identified between the two muscles [4]. Using aseptic technique and under direct ultrasound visualization, a 22-gauge Chiba needle was advanced into the plane of the nerve, and the study injectate was administered. The 13-ml injectate was prepared by the anesthesiologist-investigator in the following fashion:
Control: Plain 0.5% bupivacaine
Treatment Group I: 0.5% bupivacaine mixed with 1 mg preservative-free dexamethasone (10 mg/ml)
Treatment Group II: 0.5% bupivacaine mixed with 4 mg preservative-free dexamethasone (10 mg/ml).
After nerve block administration, a spinal anesthetic of 60 mg (4 ml) of 1.5% mepivacaine was administered using a 27-gauge Whitacre needle. Intravenous propofol infusion was titrated at the anesthesiologist’s discretion. All patients received intravenous ondansetron (4 mg), dexamethasone (4 mg), and famotidine (20 mg) for postoperative nausea or vomiting prophylaxis. Intravenous ketorolac (30 mg) was administered at the end of the case. Tourniquet time and surgical duration were also documented.
Post-anesthesia care unit (PACU) Follow-ups
In the PACU, NRS pain scores were collected every 30 minutes, starting at 2.5 hours after block injection time, until discharge. Opioid medications were withheld until the patient reported NRS pain scores greater than or equal to 4. The discharge regimen consisted of an oral opioid (taken as needed), a non-steroidal anti-inflammatory drug (NSAID; taken on a regular schedule), and an anti-emetic (taken as needed). To determine the duration of the SSNB, patients were educated both at the time of consent and before discharge from the PACU regarding symptoms that might be expected as the nerve block resolved, including an increase in pain intensity and an experience of a full return of sensation in the operative leg. Patients were also educated regarding the saphenous nerve distribution, given a set of alcohol swabs, and shown how to test for sensation about the medial malleolus. The sensory exam was then administered prior to discharge.
Postoperative Phone Follow-ups
On POD 1 and POD 2, patients were contacted by telephone and asked to answer questions related to NRS pain (at rest, with movement), opioid consumption, and satisfaction with postoperative analgesia. The Optum Short Form (SF)-8 health survey was administered to assess mental and physical function [20]. The ORSDS [21] was administered to assess the severity of opioid-associated symptoms, including nausea, vomiting, constipation, and fatigue. Patients were asked to report durations of analgesia, as noted by times at which (1) they first felt pain in their knee, (2) the block stopped providing pain relief, and (3) the block entirely wore off. The duration from block injection time to the time of the patient-perceived block wear-off time was the primary outcome. Usage of anti-emetics and NSAIDs was also recorded. Blinding assessments [2] were performed on POD 2, and patients were asked to guess whether they did or did not receive dexamethasone in the block.
On POD 14, NRS pain scores at rest and with movement were collected via telephone follow-ups.
This double-blinded, randomized controlled trial was approved by the Institutional Review Board (IRB #2012-002) at the Hospital for Special Surgery. It was registered on clinicaltrials.gov on April 25, 2012.
Statistical Analysis
Median block duration was estimated using the Kaplan-Meier method to account for the presence of censored data (i.e., when patients had not experienced the event by the time of last interview). Block duration measures are reported as medians with 95% confidence intervals estimated via a log-log transformation. Cox proportional hazards modeling was used to compare block duration between groups, both with and without covariate adjustment, with results presented as hazard ratios with 95% confidence intervals. Normality of continuous variables was assessed via inspection of Q-Q plots and histograms. Normally-distributed continuous variables are summarized as means with standard deviations and non-normally-distributed continuous variables are summarized as medians with 1st and 3rd quartiles. Categorical variables are presented as counts and percentages. Categorical outcomes with a single value per patient were compared between groups using chi-square or Fisher’s exact test, as appropriate. Outcomes with two or more measurements per patient were analyzed using regression based on a generalized estimating equations (GEE) approach [16,22] with an identity link for continuous outcomes and a logit link for binary outcomes. An autoregressive [AR(1)] correlation structure was used for outcomes with three or more measurements per patient. The GEE method accounts for the correlation between repeated measurements on the same patient, where the AR(1) correlation structure assumes a greater degree of correlation among measurements recorded closer in time. A treatment by time interaction term was initially included in all GEE models to test for change in treatment effect over time. If no evidence of an interaction was found, the model was refit without an interaction term, and results were reported as overall difference in means between groups. All regression models were adjusted for age, sex, BMI, tourniquet time, ASA, and IV dexamethasone dose. Model covariates were chosen based on hypothesized association with the outcomes, and included in the final models regardless of statistical significance. Success of blinding was reported as Bang’s blinding indices with 95% confidence intervals for patients and investigators. Bang’s blinding index ranges from −1 to 1, where −1 indicates opposite guessing (i.e., all treatment assignment guesses are opposite of actual treatment), 0 indicates perfect blinding, and 1 indicates complete unblinding. All hypothesis tests were two-sided, with statistical significance defined as P<0.05. Statistical analyses were performed with SAS Version 9.3 (SAS Institute, Cary, NC).
The mean±standard deviation time until the block completely wore off, 18±16 hours, was based on Lundblad et al. [15]. A sample size of 59 patients in each of the three groups provided 80% power at a two-sided alpha of 0.05 to detect an 8 hour difference in block duration between each experimental group and the control group, assuming a common within-group standard deviation of 16 hours. A total of 195 patients were enrolled to account for a potential 10% dropout rate.
Results
Patient flow and demographic/intraoperative information
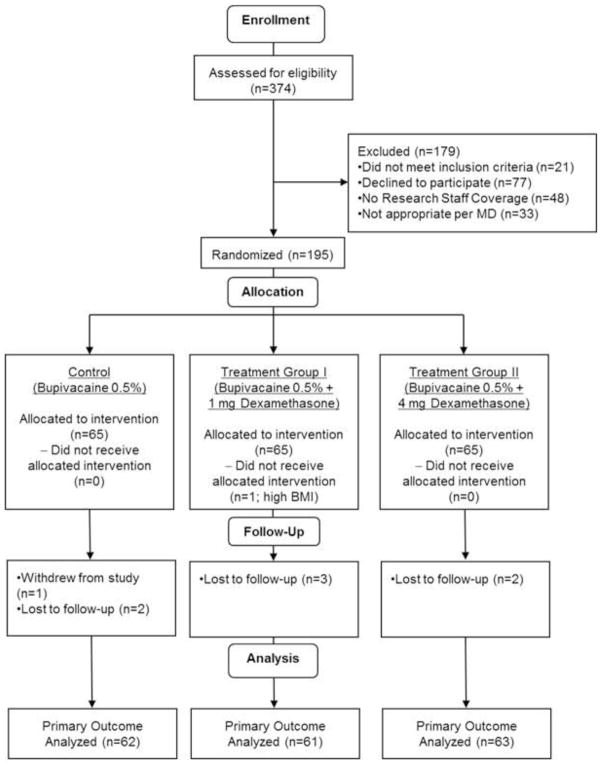
One patient dropped out of the study before data collection was complete, and 7 patients were lost to follow-up (Figure 1). One patient underwent an additional surgical procedure and one did not undergo ACL reconstruction. One patient was noted to have a high BMI in the operating room, did not receive the intervention, and was excluded from the study. Data from 186 patients were used for analysis. There were no significant differences in baseline characteristics among the three groups (Table 1). Most patients received 4 mg of intravenous dexamethasone. However, one patient in treatment group I and 3 patients in group II did not receive intravenous dexamethasone. In addition, one patient in treatment group 1 and one patient in treatment group II received 8 mg of intravenous dexamethasone.
Figure 1.
CONSORT Flow Diagram of Study Population. Numbers of assessed, approached, enrolled, randomized, and analyzed patients are presented.
Table 1.
Demographics and Intraoperative Info
| Group | |||
|---|---|---|---|
| Control (n=62) | Treatment Group I (n=61) | Treatment Group II (n=63) | |
| Age; years; mean (SD) | 27 (10) | 26 (8) | 27 (10) |
| Weight; kg; mean (SD) | 76 (15) | 74 (15) | 76 (13) |
| BMI; kg/m2; mean (SD) | 24 (3) | 24 (4) | 25 (3) |
| Female; N (%) | 19 (31) | 27 (44) | 19 (30) |
| White Race; N (%) | 44 (71) | 45 (74) | 44 (70) |
| Hypertension; N (%) | 1 (2) | 4 (7) | 2 (3) |
| Hyperlipidemia; N (%) | 0 (0) | 1 (2) | 2 (3) |
| Smoker; N (%) | 1 (2) | 3 (5) | 3 (5) |
| Normal sensation in operative leg; N (%) | 61 (98) | 61 (100) | 63 (100) |
| NRS pain at rest; 0–10; median (Q1, Q3) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) |
| NRS pain with movement; 0–10; median (Q1, Q3) | 0 (0, 4) | 0 (0, 3.5) | 0 (0, 3) |
| ASA 1; N (%) | 53 (86) | 52 (85) | 53 (84) |
| Length of surgery; min; median (Q1, Q3) | 143 (133, 157) | 141 (130, 154) | 142 (131, 159) |
| Right Operative Leg; N (%) | 28 (45) | 34 (56) | 28 (44) |
| Tourniquet time; min; median (Q1, Q3) | 57.5 (22, 67) | 46 (18, 65) | 43 (16, 60) |
BMI = body mass index; NRS = numerical rating scale; ASA = American Society of Anesthesiologists Physical Status classification; SD = standard deviation; Q1 = 25th percentile; Q3 = 75th percentile
Block Duration
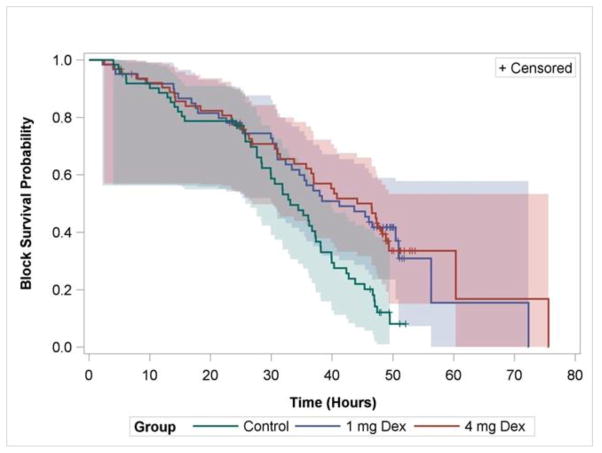
The primary outcome, patient-perceived block duration, was significantly increased in treatment group I (hazard ratio (95% CI): 0.48 (0.31–0.75)) and treatment group II ((hazard ratio (95% CI): 0.52 (0.33–0.81)) compared to control (Figure 2). This extended the block from a median (95% CI) of 33 (28.4–37.3) to 41 (32.4–50.9) (1 mg) and 46.5 (35.8–48.9) (4 mg) hours.
Figure 2.
Patient-Perceived Block Duration. Kaplan-Meier curve showing duration until the subsartorial saphenous nerve block completely wore off. Censored data are indicated as “+” and represent patients who had not experienced block resolution by the time of the postoperative day 2 follow-up or had partially known block resolution times. 4 mg Dex = Treatment Group I; 8 mg Dex = Treatment Group II.
Sensation of returning pain, as determined by the answer to the question of “When did you start having pain in your knee again?”, was similar in all groups. Termination of analgesic effect, as determined by answer to the question of “When did the nerve block stop providing pain relief?”, was also similar between the control group and treatment group I but demonstrated a statistically significant difference between the control group and treatment group II (Table 2).
Table 2.
Postoperative Outcomes
| Outcome | Control (n=62) | Treatment Group I (n=61) | Treatment Group II (n=63) | Hazard Ratio or Difference in Means(95% CI) | P value | ||
|---|---|---|---|---|---|---|---|
| Treatment Group I vs. Control | Treatment Group II vs. Control | Treatment Group I vs. Control | Treatment Group II vs. Control | ||||
| Time until first pain; median[95% CI) | 15.3h [7.4–26.3) | 15.4h [5.9–23.1) | 18.9h [6.5–26.3) | 1.04 (0.67–1.62) | 0.99 (0.62–1.57) | n.s. | n.s. |
| Time until block stopped providing pain relief; median[95% CI) | 27.6h [19.9–31.0) | 30.9h [21.4–22.4) | 32.9h [24.3–37.0) | 0.76 (0.51–1.14) | 0.65 (0.43–1.00) | n.s. | 0.049* |
| Numerical rating scale pain at rest; 0–10; median(Q1, Q3) | |||||||
| Preoperative | 0(0, 0) | 0(0, 0) | 0(0, 0) | −0.09(−0.50–0.33) | −0.41(−0.79 – −0.03) | n.s. | 0.037* |
| 5h post-block administration | 3(2, 5) | 3(2, 5) | 3(1, 4) | ||||
| POD 1 | 3(2, 5) | 3(1, 4) | 2(1, 4) | ||||
| POD 2 | 3(2, 5) | 3(2, 5) | 3(2, 4) | ||||
| POD 14 | 1(0, 2) | 1(0, 2) | 0(0, 1) | ||||
| Patient satisfaction; 0–10; median(Q1, Q3) | |||||||
| POD 1 | 9(7.5, 9.5) | 9(7, 10) | 9(8, 10) | 0.13(−0.44–0.70) | 0.54(0.02–1.05) | n.s. | 0.042* |
| POD 2 | 8(6, 9) | 8(6, 9) | 9(8, 10) | ||||
| Opioid consumption; oral morphine equivalents; median(Q1, Q3) | |||||||
| PACU | 15(15, 22.5) | 15(7.5, 22.5) | 15(15, 22.5) | −3.12(−8.95–2.71) | −3.74(−9.66–2.18) | n.s. | n.s. |
| POD 1 | 30(16.5, 56.3) | 30(22.5, 45) | 31.9(15, 52.5) | ||||
| POD 2 | 45(26.3, 60) | 30(22.5, 60) | 30(15, 52.5) | ||||
| Antiemetic use; N (%) | |||||||
| PACU | 4 (7) | 6 (10) | 6 (10) | 0.97(0.41–2.28) | 0.62(0.24–1.60) | n.s. | n.s. |
| POD 1 | 9 (15) | 10 (16) | 7 (11) | ||||
| POD 2 | 10 (16) | 10 (16) | 7 (11) | ||||
| Nonsteroidal anti-inflammatory drug use; N (%) | |||||||
| POD 1 | 32 (52) | 40 (66) | 41 (65) | 1.66(0.85–3.22) | 1.66(0.86–3.21) | n.s. | n.s. |
| POD 2 | 34 (55) | 42 (69) | 42 (67) | ||||
| Pregabalin use; N (%) | |||||||
| PACU | 50 (81) | 55 (90) | 53 (84) | Chi-square test P value = 0.759 | |||
| Drowsiness | |||||||
| POD 1 | 1(0, 1.5) | 0(0, 1.4) | 0(0, 1.2) | −0.15(−0.4–0.1) | −0.25(−0.48 – −0.01) | n.s. | 0.038* |
| POD 2 | 1.1(0, 1.6) | 1(0, 1.7) | 0(0, 1.4) | ||||
| Confusion | |||||||
| POD 1 | 0(0, 0) | 0(0, 0) | 0(0, 0) | −0.04(−0.15–0.07) | −0.11(−0.18 – −0.03) | n.s. | 0.007* |
| POD 2 | 0(0, 0) | 0(0, 0) | 0(0, 0) | ||||
| Short Form-8 Scores; median(Q1, Q3) | |||||||
| POD 1 | PCS 34.2(29.2, 39.1); MCS 59.9(54.3, 62.3) | PCS 32.8(28.4, 39.6); MCS 58.4(51.9, 61.6) | PCS 34.2(28.8, 39.6); MCS 58.6(52.4, 63.2) | PCS 0.76 (−2.23–3.75); MCS −2.21 (−5.28–0.86) | PCS 2.30 (−0.63–5.23); MCS −0.94 (−4.17–2.29) | PCS n.s.; MCS n.s. | PCS n.s.; MCS n.s. |
| POD 2 | PCS 31.2(26.7, 39.6); MCS 59.1(56.4, 62.3) | PCS 35.3(28.4, 40.2); MCS 57.1(49.1, 61.3) | PCS 35.5(29.5, 41.2); MCS 58(54, 60.8) | ||||
P<0.05.
CI: confidence interval; Q1: 25th percentile; Q3: 75th percentile; h: hours; POD: postoperative day; PACU: postoperative anesthesia care unit; PCS: physical component score; MCS: mental component score
Pain scores, patient satisfaction, and medication usage
At rest, treatment group II had a lower overall pain score in comparison to control (Table 2). There was no difference in pain scores with movement among the three groups. Opioid consumption was also similar in all three groups. Additionally, there was no difference in the adjuvant use of pregabalin, antiemetics and NSAIDs (Table 2).
Patient satisfaction did not differ between treatment group I and the control group. However, it was increased in treatment group II compared to the control group, although the difference in means was relatively small and may not be clinically significant (Table 2). There were also significant improvements in the ORSDS components of confusion and drowsiness in treatment group II compared to the control group (Table 2).
Administration of the SF-8 survey on POD 1 and POD2 assessed mental and physical function. No differences in mental component scores or physical component scores were observed among groups (Table 2).
Blinding assessment
Effective blinding was demonstrated [2]. Most patients, whether they received dexamethasone in the block or not, guessed that they had received the adjuvant.
Discussion
The most important finding of the present study was that low-dose perineural dexamethasone (1 mg) was as effective as a higher dose (4 mg) in providing significantly increased block duration by 8–13 hours. This concurs with the findings by Liu et al., who found that a dose of 1–2 mg or 4 mg dexamethasone as the perineural adjuvant prolonged the analgesic effect in supraclavicular blocks [14] and suggests that the optimal perineural dexamethasone dose can be quite small. Additional important findings in Group II vs. control were reduction in pain scores, decreased drowsiness and confusion, and increased patient satisfaction.
Review of the literature regarding perineural dexamethasone demonstrates conflicting results. Studies find increased block duration [6,10,19], with a recent meta-analysis identifying prolongation of analgesia with lower opioid consumption, specifically in brachial plexus blocks [7]. However, further studies have shown that prolonged analgesic duration can be obtained by the administration of either intravenous or perineural dexamethasone [1,8]. Rahangdale et al. found no difference in the quality of recovery from foot/ankle surgery following a sciatic nerve block with perineural or intravenous dexamethasone or saline, though duration of analgesia was prolonged in both additive groups compared to placebo [17]. These studies all administered doses of 8–10 mgs of dexamethasone. In contrast, Kawanishi et al., using 4 mg dexamethasone, found that the length of the interscalene sensory block was only extended with perineural administration [11]. Together, these findings are consistent with arguments that large doses (>0.1 mg/kg) of dexamethasone have a systemic mechanism of action that can obscure a locally mediated, perineural effect [3,7]. The optimal dose for perineural dexamethasone to exert a local effect has yet to be determined.
In this study, all patients received 4 mg dexamethasone intravenously as an anti-emetic. Total dexamethasone doses were 4 mg (control), 5 mg (treatment group I) and 8 mg (treatment group II). It is unlikely that increasing total dose from 4 mg (control) to 5 mg (treatment group I) caused the marked block prolongation. However, it is possible that improvements seen only in treatment group II (4 mg perineural dexamethasone; decreased NRS scores at rest, increased time until block stopped providing pain relief, increased patient satisfaction, and improved side effect profile) reflect a systemic effect from using a total of 8 mg dexamethasone.
Since patients received an anti-emetic dose of dexamethasone intraoperatively, one study limitation is that the dose of systemically administered dexamethasone was not rigorously compared to that given perineurally. A study comparing these two methods for the administration of low-dose dexamethasone is needed. Another study limitation is that longer block duration did not translate into reduced opioid consumption or lower pain scores in the 1 mg group compared to control. One explanation is that the SSNB for this study was performed 7–10 cm proximal to the medial condyle in order to minimize the possibility of anesthetizing the vastus component of the femoral nerve. Perhaps the resolution of the block, performed just above the knee itself, occurs in such a way that a significant difference between groups for onset of discomfort cannot be detected. Use of the more proximal midthigh adductor canal approach to the saphenous nerve may demonstrate a difference in analgesia between groups.
The clinical relevance of this work lies in the clear utility of the SSNB for pain control following ACL reconstruction with BTB autographs. This study demonstrated that addition of a very small dose of preservative-free dexamethasone (1–2 mg) to the local anesthetic used for the nerve block will increase this block’s duration. Clinically, it is best to use the lowest effective dose of medication so as to avoid potential side effects. The SSNB is particularly well suited for use with additives since it is a sensory block and quadriceps function is spared [12].
Conclusion
This study compared the outcomes of patients receiving a SSNB with plain bupivacaine, bupivacaine with 1 mg dexamethasone, or bupivacaine with 4 mg dexamethasone for postoperative analgesia following ACL reconstruction using BTB autograft. The addition of both 1 mg and 4 mg of dexamethasone to the block injectate significantly increased block duration by 8–13 hours. Additionally, patients in the 4 mg dexamethasone group reported lower NRS pain scores at rest, improved patient satisfaction and decreased drowsiness and confusion, which may reflect a systemic dexamethasone effect. There was no difference in measurements of NRS pain scores with movement. Medication (NSAIDs, pregabalin) usage and opioid consumption were similar in all three groups.
Acknowledgments
Funding: Department of Anesthesiology, Hospital for Special Surgery (study) and National Institutes of Health: Clinical and Translational Science Center Grant UL-1RR024996 (REDCAP; data collection)
This study was funded by the Research and Education Fund of the Department of Anesthesiology at the Hospital for Special Surgery. The use of REDCap was funded by the National Institutes of Health (#UL1TR000457).
Footnotes
IRB: This study was approved by the IRB at the Hospital for Special Surgery.
Clinicaltrials.gov Registration Number: NCT #01586806
Level of Evidence: Therapeutic Level I
Conflicts of Interest
M.F.C., J.C., K.G.F., D.B.M., G.A.L., M.A.G., V.M.Z., and J.T.Y. declare that they have no conflicts of interest. R.G.M. declares the following conflicts of interest: employment on the Editorial Board of Journal of Bone and Joint Surgery and two books (“Revision ACL Reconstruction: Indications and Technique” [Springer, 2013] and “The ACL Solution” [Desmos Health, 2012]).
References
- 1.Abdallah FW, Johnson J, Chan V, Murgatroyd H, Ghafari M, Ami N, Jin R, Brull R. Intravenous dexamethasone and perineural dexamethasone similarly prolong the duration of analgesia after supraclavicular brachial plexus block: a randomized, triple-arm, double-blind, placebo-controlled trial. Reg Anesth Pain Med. 2015;40(2):125–132. doi: 10.1097/AAP.0000000000000210. [DOI] [PubMed] [Google Scholar]
- 2.Bang H, Flaherty SP, Kolahi J, Park J. Blinding assessment in clinical trials: A review of statistical methods and a proposal of blinding assessment protocol. Clin Res Regul Aff. 2010;27(2):42–51. [Google Scholar]
- 3.Bolin ED, Wilson S. Perineural Versus Systemic Dexamethasone: Questions Remain Unanswered. Reg Anesth Pain Med. 2015;40(4):393–394. doi: 10.1097/AAP.0000000000000262. [DOI] [PubMed] [Google Scholar]
- 4.Chisholm MF, Bang H, Maalouf DB, Marcello D, Lotano MA, Marx RG, Liguori GA, Zayas VM, Gordon MA, Jacobs J, YaDeau JT. Postoperative Analgesia with Saphenous Block Appears Equivalent to Femoral Nerve Block in ACL Reconstruction. HSS J. 2014;10(3):245–251. doi: 10.1007/s11420-014-9392-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choi S, Rodseth R, McCartney CJ. Effects of dexamethasone as a local anaesthetic adjuvant for brachial plexus block: a systematic review and meta-analysis of randomized trials. Br J Anaesth. 2014;112(3):427–439. doi: 10.1093/bja/aet417. [DOI] [PubMed] [Google Scholar]
- 6.Cummings KC, 3rd, Napierkowski DE, Parra-Sanchez I, Kurz A, Dalton JE, Brems JJ, Sessler DI. Effect of dexamethasone on the duration of interscalene nerve blocks with ropivacaine or bupivacaine. Br J Anaesth. 2011;107(3):446–453. doi: 10.1093/bja/aer159. [DOI] [PubMed] [Google Scholar]
- 7.De Oliveira GS, Jr, Almeida MD, Benzon HT, McCarthy RJ. Perioperative single dose systemic dexamethasone for postoperative pain: a meta-analysis of randomized controlled trials. Anesthesiology. 2011;115(3):575–588. doi: 10.1097/ALN.0b013e31822a24c2. [DOI] [PubMed] [Google Scholar]
- 8.Desmet M, Braems H, Reynvoet M, Plasschaert S, Van Cauwelaert J, Pottel H, Carlier S, Missant C, Van de Velde M. I.V. and perineural dexamethasone are equivalent in increasing the analgesic duration of a single-shot interscalene block with ropivacaine for shoulder surgery: a prospective, randomized, placebo-controlled study. Br J Anaesth. 2013;111(3):445–452. doi: 10.1093/bja/aet109. [DOI] [PubMed] [Google Scholar]
- 9.Ekmekci P, Bengisun ZK, Akan B, Kazbek BK, Ozkan KS, Suer AH. The effect of magnesium added to levobupivacaine for femoral nerve block on postoperative analgesia in patients undergoing ACL reconstruction. Knee Surg Sports Traumatol Arthrosc. 2013;21(5):1119–1124. doi: 10.1007/s00167-012-2093-4. [DOI] [PubMed] [Google Scholar]
- 10.Fritsch G, Danninger T, Allerberger K, Tsodikov A, Felder TK, Kapeller M, Gerner P, Brummett CM. Dexmedetomidine added to ropivacaine extends the duration of interscalene brachial plexus blocks for elective shoulder surgery when compared with ropivacaine alone: a single-center, prospective, triple-blind, randomized controlled trial. Reg Anesth Pain Med. 2014;39(1):37–47. doi: 10.1097/AAP.0000000000000033. [DOI] [PubMed] [Google Scholar]
- 11.Kawanishi R, Yamamoto K, Tobetto Y, Nomura K, Kato M, Go R, Tsutsumi YM, Tanaka K, Takeda Y. Perineural but not systemic low-dose dexamethasone prolongs the duration of interscalene block with ropivacaine: a prospective randomized trial. Local Reg Anesth. 2014;7:5–9. doi: 10.2147/LRA.S59158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim DH, Lin Y, Goytizolo EA, Kahn RL, Maalouf DB, Manohar A, Patt ML, Goon AK, Lee YY, Ma Y, Yadeau JT. Adductor canal block versus femoral nerve block for total knee arthroplasty: a prospective, randomized, controlled trial. Anesthesiology. 2014;120(3):540–550. doi: 10.1097/ALN.0000000000000119. [DOI] [PubMed] [Google Scholar]
- 13.Krombach J, Gray AT. Sonography for saphenous nerve block near the adductor canal. Reg Anesth Pain Med. 2007;32(4):369–370. doi: 10.1016/j.rapm.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 14.Liu J, Richman KA, Grodofsky SR, Bhatt S, Huffman GR, Kelly JDt, Glaser DL, Elkassabany N. Is there a dose response of dexamethasone as adjuvant for supraclavicular brachial plexus nerve block? A prospective randomized double-blinded clinical study. J Clin Anesth. 2015;27(3):237–242. doi: 10.1016/j.jclinane.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 15.Lundblad M, Kapral S, Marhofer P, Lonnqvist PA. Ultrasound-guided infrapatellar nerve block in human volunteers: description of a novel technique. Br J Anaesth. 2006;97(5):710–714. doi: 10.1093/bja/ael241. [DOI] [PubMed] [Google Scholar]
- 16.Ma Y, Mazumdar M, Memtsoudis SG. Beyond repeated-measures analysis of variance: advanced statistical methods for the analysis of longitudinal data in anesthesia research. Reg Anesth Pain Med. 2012;37(1):99–105. doi: 10.1097/AAP.0b013e31823ebc74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rahangdale R, Kendall MC, McCarthy RJ, Tureanu L, Doty R, Jr, Weingart A, De Oliveira GS., Jr The effects of perineural versus intravenous dexamethasone on sciatic nerve blockade outcomes: a randomized, double-blind, placebo-controlled study. Anesth Analg. 2014;118(5):1113–1119. doi: 10.1213/ANE.0000000000000137. [DOI] [PubMed] [Google Scholar]
- 18.Svediene S, Andrijauskas A, Ivaskevicius J, Saikus A. The efficacy comparison of on-demand boluses with and without basal infusion of 0.1 % bupivacaine via perineural femoral catheter after arthroscopic ACL reconstruction. Knee Surg Sports Traumatol Arthrosc. 2013;21(3):641–645. doi: 10.1007/s00167-012-1971-0. [DOI] [PubMed] [Google Scholar]
- 19.Vieira PA, Pulai I, Tsao GC, Manikantan P, Keller B, Connelly NR. Dexamethasone with bupivacaine increases duration of analgesia in ultrasound-guided interscalene brachial plexus blockade. Eur J Anaesthesiol. 2010;27(3):285–288. doi: 10.1097/EJA.0b013e3283350c38. [DOI] [PubMed] [Google Scholar]
- 20.Ware JE, Kosinski M, Dewey J, Gandek B. How to score and interpret single-item health status measures: a manual for users of the SF-8 health survey. QualityMetric, Inc; Lincoln, RI: 2001. [Google Scholar]
- 21.Yadeau JT, Liu SS, Rade MC, Marcello D, Liguori GA. Performance characteristics and validation of the Opioid-Related Symptom Distress Scale for evaluation of analgesic side effects after orthopedic surgery. Anesth Analg. 2011;113(2):369–377. doi: 10.1213/ANE.0b013e31821ae3f7. [DOI] [PubMed] [Google Scholar]
- 22.Zeger SL, Liang KY, Albert PS. Models for longitudinal data: a generalized estimating equation approach. Biometrics. 1988;44(4):1049–1060. [PubMed] [Google Scholar]