Summary
A promising approach to understanding the mechanistic basis of speech is to study disorders that affect speech without compromising other cognitive or motor functions. Stuttering, also known as stammering, has been linked to mutations in the lysosomal enzyme-targeting pathway, but how this remarkably specific speech deficit arises from mutations in a family of general “cellular housekeeping” genes is unknown. To address this question, we asked whether a missense mutation associated with human stuttering causes vocal or other abnormalities in mice. We compared vocalizations from mice engineered to carry a mutation in the Gnptab (N-acetylglucosamine-1-phosphotransferase subunits alpha/beta) gene with wild type littermates. We found significant differences in the vocalizations of pups with the Gnptab stuttering mutation compared to littermate controls. Specifically, we found that mice with the mutation emitted fewer vocalizations per unit time, had longer pauses between vocalizations, and that the entropy of the temporal sequence was significantly reduced. Furthermore, Gnptab missense mice were similar to wild type mice on an extensive battery of non-vocal behaviors. We then used the same language-agnostic metrics for auditory signal analysis of human speech. We analyzed speech from people who stutter with mutations in this pathway and compared it to control speech, and found abnormalities similar to those found in the mouse vocalizations. These data show that mutations in the lysosomal enzyme targeting pathway produce highly specific effects in mouse pup vocalizations, and establish the mouse as an attractive model for studying this disorder.
Introduction
Speech disorders affect millions of people worldwide and are usually treated with behavioral therapy[1, 2]. A better understanding of human speech disorders may lead to a wider array of treatment options, and can provide insights into the genetic and neural underpinnings of human speech. To date, there are remarkably few speech disorders with a clearly identified genetic component [3-5]. Developmental stuttering is one of the most common speech disorders, with the persistent form of the disorder affecting 3 million adults in the United States. The disorder is characterized by frequent repetitions or prolongations of syllables or words, or speech that has frequent hesitations or pauses – known as “blocks” – that disrupt the smooth flow of speech [1]. As an inroad into mechanisms of speech production, stuttering is remarkable because affected individuals are, on average, normal by all other known measures of language, cognitive, and motor function [1]. Recently, it was found that persistent developmental stuttering unaccompanied by other deficits or symptoms can be linked to mutations in the lysosomal enzyme-targeting pathway (LETP) [6]. Mutations in the LETP pathway account for 9-16% of all cases of persistent nonsyndromic stuttering[7].
Lysosomes are degradative cellular organelles that contain acid hydrolases. These enzymes are targeted to lysosomes by tagging them with mannose 6-phosphate[8]. The mannose 6-phosphate is added to these acid hydrolases in a two-step process carried out by the products of three genes. Mutations in all three of these genes have been found in humans who stutter [6, 7]. However, the mechanisms by which mutations in an apparent “housekeeping gene” produce a deficit with the remarkable specificity of stuttering remain unclear.
While humans are irreplaceable for discovery of the genetic underpinnings of speech disorders, they have a number of disadvantages for analysis of the underlying mechanisms. An animal model would be an invaluable tool for investigations into the circuit and cellular mechanisms of a vocal disorder, despite the obvious difficulties in comparing human speech with any animal vocalization [3]. While no exact correspondence with human speech can be expected, it is possible that one or more “low-level” features—such as planning, initiation, timing, breath control, and/or temporal sequencing—may be shared both in phenotype and underlying neuronal circuitry across mammalian species.
Mouse vocalizations have been extensively studied and have been used successfully in the study of several disorders [9-13]. Mice produce ultrasonic vocalizations in a range of social situations. These vocalizations have repeated syllables and complex structure, and have been characterized as “songs” [14]. One type of innate mouse vocalization is the isolation call of pups which has also been extensively studied, especially in disease [3, 11, 15-20]. Mouse pups, when separated from their mother during the first two weeks of life, spontaneously vocalize [20]. The rate of vocalization peaks at around 5-7 days postnatal and decrease thereafter.
This approach is exemplified by studies of the speech disorder associated with mutations of the FOXP2 transcription factor. The Foxp2 gene is conserved across species [21]. In mice, disruption of the Foxp2 gene has been shown to cause changes in pup isolation calls [15-17, 22] (though not necessarily in adults [23]); in turn, such studies have been used to obtain numerous insights into the molecular and cellular functions of this gene and its role in human speech [3, 4, 11, 15-17, 24].
Here, we analyze pup isolation calls and other behaviors of mice with a knock-in mutation in the Gnptab gene, for which the equivalent mutation has been shown to cause stuttering in humans [6]. We show that such mice exhibit alterations in timing and sequencing of vocalization that are reminiscent of specific deficits observed in human stuttering.
Results
We engineered mice to carry a homozygous Glu1179Lys mutation in Gnptab (Gnptab mut/mut), homologous to the Glu1200Lys mutation in human GNPTAB well characterized for its role in stuttering [6, 25]. Mice were constructed on a pure BALB/c background (Figure 1A and S1), using a heterozygote × heterozygote breeding strategy to ensure the availability of littermate controls with matched pre- and post-natal environment. We recorded pup isolation calls on postnatal day 3, 5, and 8; data from all days are presented in figure supplements, with data from day 8 presented in the main figures below.
Figure 1. Experimental scheme.
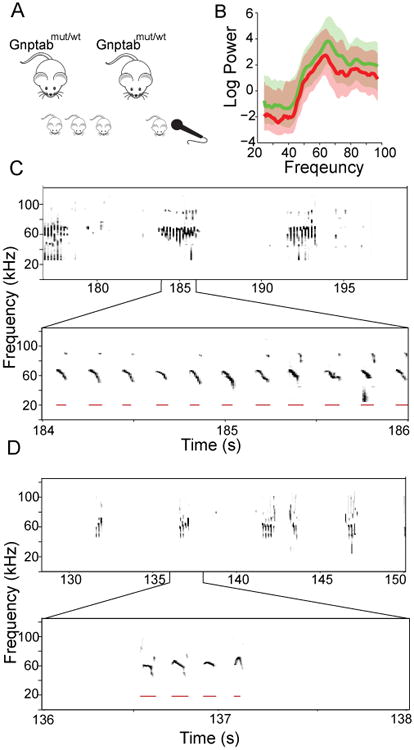
(A) Knock-in mice with a Glu1179Lys mutation in exon 19 of the Gnptab gene were constructed on a BALB/c background. Recordings were performed on mice pups separated from dam for recording on postnatal day 3, 5 and 8. (B) Frequency distribution of mouse pup vocalizations on day 8 in wild type littermates from the Gnptab wt/wt mice (green, n=20) and Gnptab mut/mut mice (red, n=19) (C) Sonogram of a wild type mouse pup vocalizing in isolation. Lower window is an expanded version of one portion, showing the repeated structure in the mouse vocalizations. Red bars indicate a detected vocalization. (D) Sonogram of a Gnptab mut/mut as in c. See also Figure S1.
Mouse vocalizations: rate, spectral characteristics, and timing
We first asked whether Gnptab mut/mut mice vocalized. Both wild-type littermates (Gnptab wt/wt), and Gnptab mut/mut mice produced vocalizations over the 3.5 minute recording session that appeared to have normal amplitude and spectral characteristics (Figure 1B-1D). We found that Gnptab mut/mut mice exhibited significantly fewer vocalizations compared to littermate controls (Gnptab mut/mut 64.2 ± 34.8 vocalizations; Gnptab wt/wt 184.5 ± 39.4 vocalizations; t-test p<0.034, Figure 2A) in the three and half minutes of a recording.
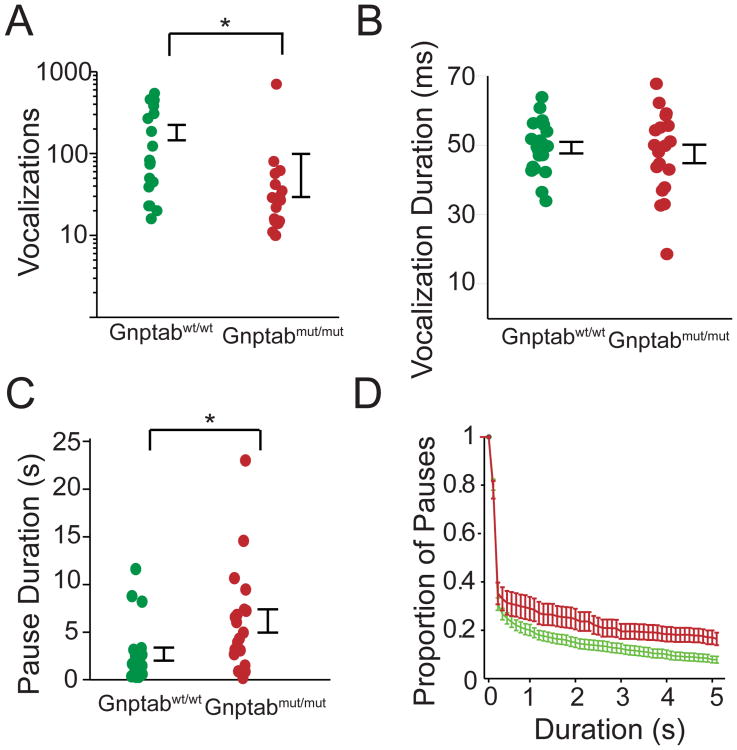
Figure 2. Differences in the vocalizations of mice with a mutation in the LETP compared to wild type littermates.
(A) Vocalizations per recording in mouse pup vocalizations in wild type littermates (Gnptab wt/wt, green, n=20), and mice homozygous for the Glu1179Lys mutation (Gnptab mut/mut, red, n=19). Note the significant decrease in the Gnptab mut/mut mice compared Gnptab wt/wt littermates (t-test, p < 0.034). (B) Duration of vocalization in Gnptab wt/wt and Gnptab mut/mut mice. No significant difference was found. (C) Pause duration between vocalizations, with a significant increase in the duration of pauses in the Gnptab wt/wt compared to the Gnptab mut/mut mice (t-test p<0.020). (D) Proportion of long pauses with a steadily increasing criterion for criterion for ‘long pauses’. Gnptab mut/mut mice had significantly more long pauses. Each dot represents the mean of one subject. Error bars represent standard error of the mean across individuals. Color scheme as in panels A-C. See also Figure S2 and S3.
The difference in rate of vocalizing in the homozygous mutants could be due to the length of vocalizations increasing, the length of pauses increasing, or a mixture of these. We found no significant difference in the duration of the vocalizations between the two groups of mice (t-test, p >0.5, Figure 2B). However, there was a significant difference in the mean pause length between vocalizations (Gnptab mut/mut 6.18 ± 1.23 s; Gnptab wt/wt 2.72 ± 0.69 s; t-test p<0.02, Figure 2C). This increase in the mean pause length in the Gnptab mut/mut could be due to increased duration of all pauses or an increase in a specific subset of pauses. Much like humans, mice exhibit “bouts” of vocalizations, separated by longer pauses (see Figure 1B and 1C). We found that the Gnptab mut/mut showed no difference in the length of intra-bout pauses, those short pauses between vocalizations inside a bout (Figure S2 and Experimental Procedures). Similarly, Gnptab mut/mut had a similar proportion of large pauses when the criterion for large pauses was not stringent (less than 0.8 seconds). However, using more stringent criteria for large pauses (setting the cutoff to 0.8 seconds or higher), the proportion of long pauses was increased in the Gnptab mut/mut mice (Figure S3). This result suggests that the key factor contributing to the reduced number of vocalizations in Gnptab mut/mut animals compared to their wild-type littermate controls was an increase in the duration of long pauses between bouts of vocalization.
We analyzed the same features of the vocalizations from heterozygous Gnptab mut/wt littermates, and found them to be more similar to the wildtype Gnptab wt/wt mouse vocalizations (Figure S3). We also found that the vocalization anomalies of the Gnptab mut/mut animals became more pronounced from day 3 to day 8 (Figure S3).
To obtain further insight into the changes in number of vocalizations, we also examined the number of bouts per recording and the number of vocalizations per bout in the Gnptab mut/mut mice. As expected from the increase in duration of long pauses, the number of bouts per recording was smaller in the Gnptab mut/mut mice (Gnptab mut/mut 14.74 ± 4.3 bouts; Gnptab wt/wt 45.35 ± 7.8 bouts; t-test p < .0023) (Figure 3A). There was no difference in the average number of vocalizations per bout (Gnptab mut/mut 3.0 ± .3 vocalizations; Gnptab wt/wt 3.6 ± .3 vocalizations; t-test p > .65, Figure 3B). In the Gnptab mut/mut mice, there was a small but significant increase in the percentage of bouts that contained only one vocalization compared to their wild type littermates (Gnptab mut/mut 45.5% ± .04%, Gnptab wt/wt 34.2% ± .03%, p <0.029). The percentage of bouts that contained two vocalizations was slightly smaller in the homozygous mutants mice (Gnptab mut/mut 13.0 % ± .02% Gnptab wt/wt 20.0% ± .03, p<0.05). There was no difference in the percentage of bouts that contained 3, 4, or 5 syllables (Figure 3C).
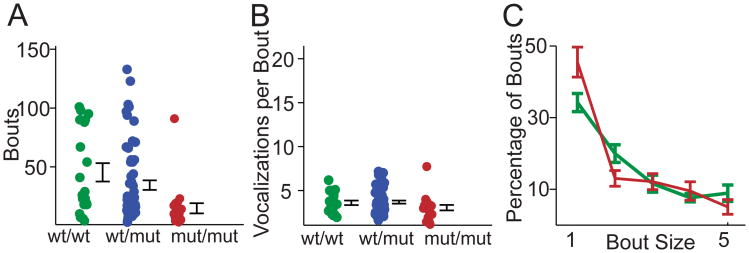
Figure 3. Number of bouts in Gnptab wt/mut mice vocalizations compared to Gnptab mut/mut and Gnptab wt/wt mice across days.
(A) Number of bouts per recording in mouse pup vocalizations in wild type littermates (Gnptab wt/wt, green), (Gnptab wt/mut, blue) and mice homozygous for the Glu1179Lys mutation (Gnptab mut/mut, red). Note there was a significant difference between Gnptab wt/wt and Gnptab mut/mut mice (t-test p < .0023). Error bars represent standard error of the mean across individuals. (B) No significant difference was found in the number of vocalizations per bout. (C) Percentage of bouts containing 1 (Gnptab mut/mut, red, 45.5% ± .04%, Gnptab wt/wt, green, 34.2% ± .03%, p < .029), 2 (Gnptab mut/mut 13.0 % ± .02% Gnptab wt/wt 20.0% ± .03, p<.05), 3, 4 or 5 vocalizations.
Mouse vocalizations: usage and temporal sequencing of syllables
In humans, some of the disfluencies in stuttering consist of silent blocks, but others—such as sound or syllable repetitions—are voiced. To ascertain whether Gnptab mut/mut mice showed such abnormalities in their vocalizations, we analyzed both the usage and temporal sequencing of syllable types as categorized by an established classification scheme based on presence and size of abrupt pitch jumps [10, 14] (Figure 4A). We chose this scheme because it was initially introduced based on quantitative observations of clustering, which makes it easy to automate and therefore unbiased. Several other syllable classification schemes have been used [11, 12, 18, 23, 26]; these alternative schemes differ primarily in the classification of syllables lacking pitch jumps, but they also exhibit many points of commonality. Wild type Gnptab wt/wt and knock-in Gnptab mut/mut mice were indistinguishable in the syllable types and their usage (see Experimental Procedures, p>.39, Figure S4). This indicates that these mutations do not affect the ability to produce different syllable types.
Figure 4. Mouse vocalizations from Gnptab wt/wt compared to vocalizations of Gnptab mut/mut.
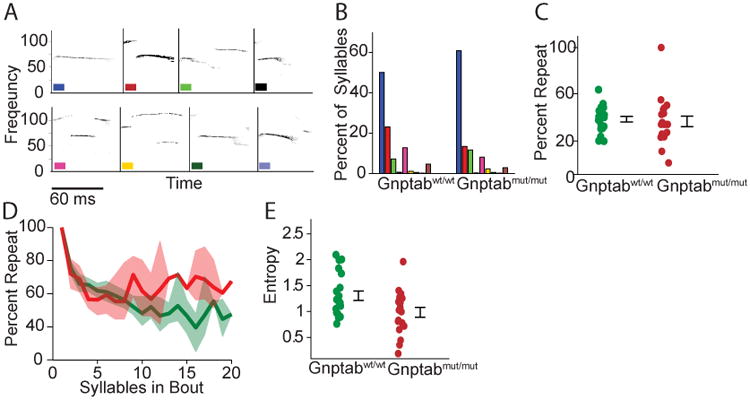
(A) Syllable identification scheme showing examples of each type of syllable. (B) Percentage of each type of syllable in wild type and knock-in mice. Differences were not statistically significant. Each color represents one syllable type. (C) Percentage of times that one syllable type was followed by the same syllable type. Differences were not statistically significant. (D) Percentage of syllables that were of the same type as the mode of each bout, for bout sizes ranging from 1 to 20. Data from Gnptab wt/wt and Gnptab mut/mut . Shaded areas represent 95% confidence intervals. (E) Diversity of vocalization sequences as quantified by the entropy of the corresponding first order Markov process (t-test, p<0.022). See also Figure S4 and S5.
We next looked at syllable repetition. Wild type mice normally exhibit a strong tendency towards syllable repetition, with doublets (defined as two of the same syllable type in a row, in bouts of two or more syllables) occurring 38% of the time. We found no significant increase in the presence of doublets (Figure 4C) in the vocalizations of the Gnptab mut/mut mice. However, within bouts, Gnptab mut/mut mice exhibited a higher percentage of syllables of a single type, suggesting that Gnptab mut/mut mice might be more stereotyped in their vocalizations (Figure 4D). We tested this possibility using a first order Markov process model to compare the entropy present in the temporal structure of these vocalizations, and found a tendency towards increased repetition in the vocalizations of the Gnptab mut/mut animals (t-test, p<0.022, Figure S5), indicating an overall reduction in temporal diversity. Consequently, Gnptab mut/mut mice use all syllable types in proportions similar to wild-type, but exhibit higher stereotypy in temporal sequencing.
Non-vocal phenotypes
We also asked whether phenotypic differences between wildtype Gnptab wt/wt and homozygous knock-in Gnptab mut/mut mice were specific to vocal behavior. Gnptab mut/mut pups exhibited normal weights (Figure S5).
We next examined adult wild type and homozygous knock-in mice using a wide variety of behavioral tests. Mice were evaluated first on a 1-h locomotor activity test. There was a significant decrease in ambulatory activity (total ambulations) on the part of the Gnptabmut/mut mice across the 60-min test session (Figure 5A, p=0.0498). In contrast, no differences were found between groups with regard to vertical rearing frequency, or time spent, entries made, or distance traveled in the central zone of the test field.
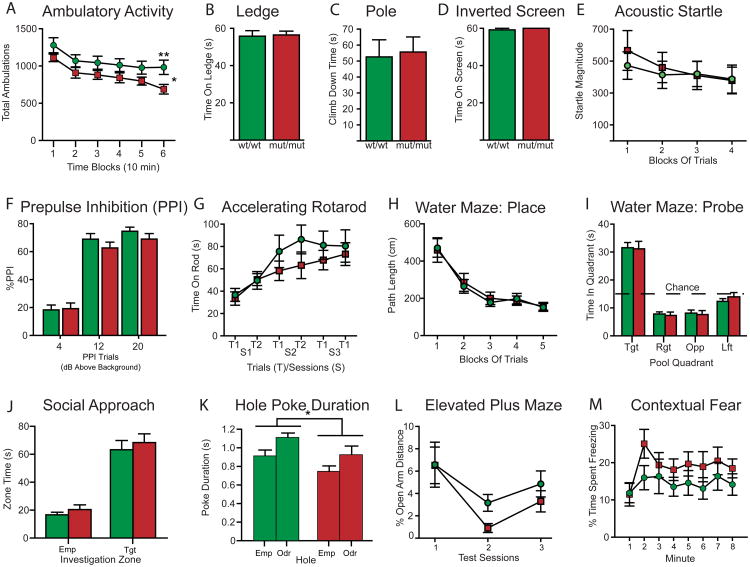
Figure 5. Non-vocal behavioral test results from Gnptab wt/wt and Gnptab mut/mut mice.
(A) Ambulatory activity was significantly decreased (*p<0.05) in mice homozygous for the Glu1179Lys mutation (Gnptab mut/mut, red, n=15) compared to wild type (Gnptab wt/wt, green, n=15) mice with differences being greatest during block 6 (**p=0.017). (B-D) The two groups of mice did not differ on several sensorimotor tests such as the time spent on an elevated ledge (B), the time to climb down a pole (C), or the time spent hanging upside down on an inverted screen before falling (D). (E-F) The Gnptab wt/wt and Gnptab mut/mut mice also exhibited similar magnitudes of the acoustic startle response (arbitrary units) (E) and PPI (F). (G) Time spent on accelerating rotorod before falling. No significant differences were found. (H) Path length on Morris Water maze for place trials. (I-J) The performance of the Gnptab mut/mut mice was not impaired during the spatial learning (place) trials (I) nor in terms of retention performance during a probe trial where each group showed spatial bias for the target quadrant by spending significantly more time in it versus the times spent in each of the other quadrants (p<0.00005). (J) The Gnptab wt/wt and Gnptab mut/mut mice showed comparable levels of sociability in that each group spent significantly more time in an investigation zone surrounding a stimulus mouse that remained inside a withholding cage compared to levels exhibited toward an empty cage (p<0.00005), although there were no differences between the two groups for either of the two conditions. (K) For each group, the duration of hole pokes was significantly greater for the odorant-containing versus the empty holes (p<0.024), although the Gnptab wt/wt mice had significantly higher poke durations on average across the two hole types (*p=0.028). (L) The two groups did not differ in levels of anxiety-related behaviors in the elevated plus maze as exemplified by the lack of significant effects involving genotype on the percentage of distance traveled in the open arms out of the total distances traveled in all of the arms. (M) The absence of any significant effects involving genotype suggested that there were no differences in contextual fear conditioning between the groups.
The mice were then tested on a battery of sensorimotor measures to assess possible functional deficits. No performance differences were observed between groups on the ledge (Figure 5B), pole (Figure 5C), inverted screen tests (Figure 5D), or on the walking initiation, platform, or inclined screens tests. These results suggest that balance, strength, co-ordination, and initiation of movement were not grossly disturbed in the Gnptabmut/mut mice. These animals and their wild type littermates also performed equivalently on tests of acoustic startle (Figure 5E), prepulse inhibition of startle (PPI) (Figure 5F), and rotarod tests (Figure 5G).
Spatial learning and memory capabilities of the Gnptabmut/mut mice were assessed using the Morris water maze task with a computerized tracking system as previously described [27]. Cued (visible platform, variable location), and place (submerged, hidden platform, constant location) trials were conducted. No significant effects involving genotype were found (Figure 5H and 5I).
We next examined the sociability of the mice as assessed by measuring the time investigating a conspecific stimulus mouse that was loosely sequestered in a small withholding cage compared to the investigation times observed for an empty cage and found no significant differences between genotypes [28] (Figure 5J).
Mice were next evaluated for differences in exploratory behaviors and olfactory preference using a previously published procedure [29], where hole poking with the nose served as the main behavioral response. The apparatus contained 4 corner and 4 side holes in the floor. One corner hole contained a familiar odorant and the diagonally opposite corner hole contained a novel odorant. Gnptabmut/mut and control mice performed similarly on several test variables including the frequency of nose pokes made into the side or corner holes, and with regard to pokes made into odorant-containing versus empty corner holes, and for pokes made into the corner holes containing a novel versus a familiar odorant. However, wild type mice showed a significant preference for the hole containing the familiar odorant (p=0.006) while the Gnptabmut/mut mice did not show a significant preference for the familiar odorant, though there was a trend in that direction (p=0.11). In addition, the Gnptabmut/mut mice had decreased poke durations on average across empty and odorant-containing holes as indicated by a significant Genotype effect (Figure 5K, p=0.028). The Gnptabmut/mut and Wild type groups each had significantly longer poke durations for the odorant-containing holes versus the empty ones, (p=0.024 and 0.012, respectively).
People who stutter (PWS) have been shown to have a higher incidence of anxiety disorders [30]. Therefore we assessed anxiety-like behaviors using the elevated plus maze (EPM) and fear conditioning. Analysis of the classic open arm variables in the EPM including time spent, entries made, and distance traveled in the open arms did not yield any significant effects involving genotype (Figure 5L). We also evaluated the performance of Gnptabmut/mut mice on a fear-associated Pavlovian conditioning test and found no genotype differences (Figure 5M).
Human Vocalizations
Given that the deficits of mice were primarily vocal in nature, we compared these vocal deficits with those of PWS. Stuttered and normal speech have been compared using a wide variety of language-based and acoustic analyses [1, 31-37]. To ensure optimal comparability, we applied our mouse-centric analyses to the vocalizations of PWS. Moreover, we were able to analyze vocalizations from normally fluent human controls (CT), PWS, and PWS and carry a well-characterized mutation in one of the three genes encoding the LETP (PWS-L) (Table S1). We thus tested whether the vocal abnormalities found in the Gnptab mut/mut mice could be seen in the more complex vocalizations of humans. We obtained recorded speech samples using a standard 500-word stuttering diagnostic reading passage containing balanced representation of all speech sound classes [38]. To ensure comparability with mouse data, no language-specific criteria were used to determine vocalization segments. Computer-assigned vocalization segments could therefore include more than one syllable or word.
We compared the rate of vocalizations in PWS versus controls (CT). Consistent with previous studies [1, 39-41], stuttering case subjects had significantly fewer vocalizations per minute (PWS, 90.2 ± 3.0 vocalizations; CT, 124.9 ± 3.1; mean ± s.e.m.; t-test, p< 10-11, Figure 6B). Like the Gnptab mut/mut mice, the PWS-L group also displayed significantly fewer vocalizations per minute than controls (PWS-L, 81.1 ± 4 vocalizations per minute; t-test, p< 10-11, Figure 6B). These differences were comparable to the difference found between the Gnptab mut/mut mice and Gnptab wt/wt mice.
Figure 6. Vocalizations of human controls, PWS, and PWS with a known mutation in the LETP, as measured by a language agnostic analysis.
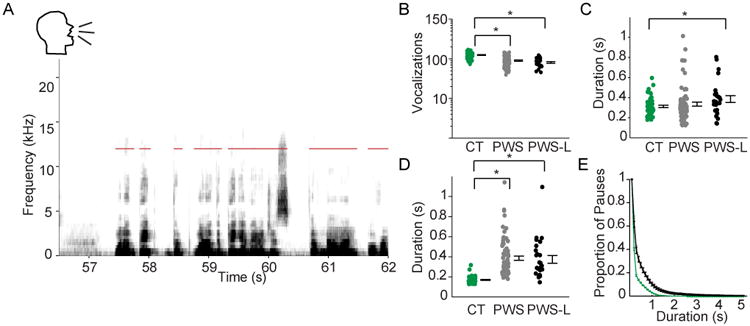
(A) Sonogram of a person saying, “(Breath) One of big…the…one of the big advantages of nylon over” while reading from a standard diagnostic script. Red bars indicate detected vocalization intervals. (B) Vocalizations per minute in the control subjects reading the script (CT; green, n=51), PWS (PWS; gray, n=74), and PWS with a known mutation in the LETP reading the same script (PWS-L; black, n=26). Each dot represents the mean of one subject. Error bars represent standard error of the mean across individuals. Brackets and stars indicate significance. There was a significant decrease in the number of vocalizations in PWS group (t-test, p<10-11), and in the PWS-L (t-test, p<10-11) group compared to the CT group. Data on log scale. (C) Duration of vocalizations. Note there was no significant difference in the duration of vocalizations between the CT and PWS groups while there was a significant difference between the CT and PWS-L groups (t-test, p<0.02). (D) Mean pause duration between vocalizations, with a significant increase in the PWS group (t-test, p< 10-12) as well as in the PWS-L group (t-test, p< 10-9) compared to the CT group. (E) Proportion of long pauses, with a steadily increasing criterion for ‘long pauses’, in the recordings of the subjects reading the script using the same color scheme as in panels B-D showing that over a range of criteria, PWS in both categories had more ‘long’ pauses. Bars indicate standard error of mean. See also Table S1.
An analysis of the durations of vocalizations revealed no significant difference between the general stuttering cases and controls (PWS mean 0.335 ± 0.020 sec; CT mean 0.313 ± 0.012 sec; t-test, p>0.3). However, there was a significant increase in the duration of the vocalizations in stuttering cases carrying an identified mutation in the LETP (PWS-L mean 0.385 ± 0.033 sec, t-test, p < 0.02, Figure 6C).
A primary feature of stuttering is involuntary pauses known as blocks, and such pauses have been found to be important in assessing fluency of speech [11, 34]. Like the differences in the pause lengths in the Gnptab mut/mut mice and Gnptab wt/wt mouse vocalizations, the speech of PWS had significantly longer pauses between vocalizations compared to that of control subjects (PWS 0.385 ± 0.021 sec; CT, 0.170 ± 0.005 sec; t-test, p < 10-12, Figure 6D). This was also true for case subjects carrying an LETP mutation (PWS-L, 0.375 ± .038 sec; t-test, p <10-9). Moreover, we found a significantly greater proportion of long pauses in both case groups compared to the control subjects over a wide range of cutoff values for defining “long” pauses (Figure 6E). These data indicate that we can detect a statistically significant difference in specific features of vocalization in PWS using the same methods used to analyze the mouse data. Such measures are “language agnostic” because they do not depend on detailed features (formant structure, vowels and consonants, etc.) of human speech. Using just two such features, number of vocalizations per minute and length of pauses, a k-nearest neighbor classifier could distinguish a PWS from a control subject 79% of the time, significantly better than chance (shuffled data, p < 10-5).
To determine whether these same phenomena apply to stuttering during extemporaneous speech, we also examined the length and number of speech intervals in 80 three-minute samples obtained from publicly-available podcasts, for both controls and PWS. Consistent with the results from people reading a script, we found that in extemporaneous speech, PWS had fewer vocalizations per unit time compared to controls. This difference was significant (PWS 274.7 ± 8.4 vocalizations; CT 434.8 ± 6.5 vocalizations; t-test p < 10-12, Figure 7A). The average length of vocalization also differed significantly between groups (PWS 415 ± 17 ms; CT 258 ± 6; t-test p < 10-10; Figure 7B). We also found that the mean length of pauses between speech intervals was significantly longer in PWS compared to controls (PWS 255 ± 12 ms; CT 160 ± 3; t-test p < 10-8, Figure 7C).
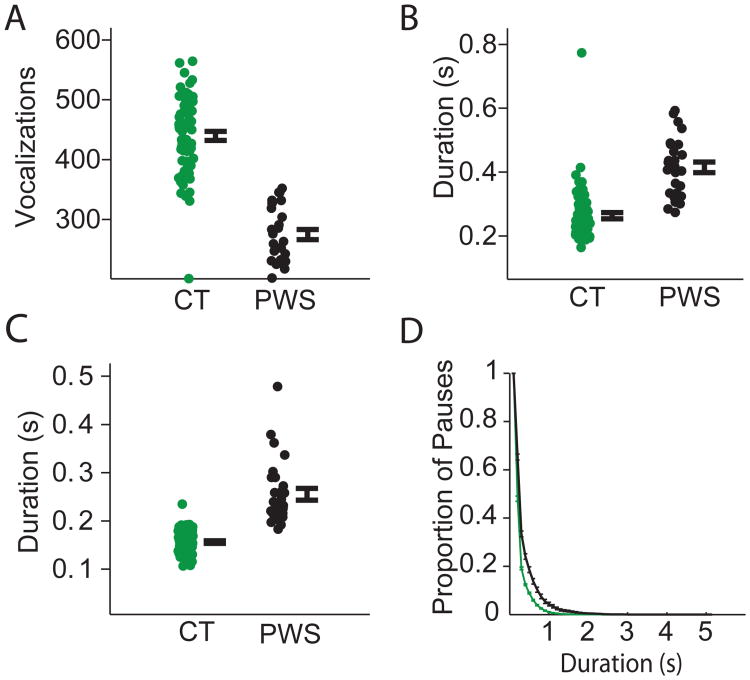
Figure 7. Extemporaneous vocalizations of human controls and PWS.
(A) Vocalizations per minute in podcast segments from controls (CT; green, n=68), and PWS (PWS; black, n=28, t-test; p<10-21). Each dot represents the mean of one subject. Error bars represent standard error of the mean across individuals. (B) Duration of vocalization periods. Note the significant increase in PWS group (t-test, p< p<10-12) compared to the CT group. (C) Pause duration between vocalizations, with a significant increase in the duration of pauses in the PWS group (t-test, p<10-17) compared to the CT group. (D) Proportion of long pauses with a steadily increasing criterion for ‘long pauses’, in the recordings of the controls subjects reading the script (CT; green), and PWS (ST; black). Bars indicate standard error of the mean. These results are similar to that of reading a script Figure 6.
Discussion
Vocal alterations: mice
Identification of some of the genetic underpinnings of human stuttering has created an opportunity to study whether homologous mutations could affect vocalization in a genetically-tractable animal model. We found that the vocalizations of Gnptab mut/mut mice were significantly different than those of their Gnptab wt/wt littermates. Gnptab mut/mut mice had fewer vocalizations per unit time and showed an increased incidence of long pauses. Gnptab mut/mut mice produced all the same syllables as their wildtype littermates, and used them in similar proportions. We note that these conclusions are specific to the syllable classification scheme adopted here, and it remains possible that other classification schemes would come to a different conclusion. From manual inspection of the sonograms, at present we have not seen evidence leading us to suspect any kind of difference in syllable repertoire.
However, by two measures mice with an LETP mutation showed more temporal stereotypy in their vocalizations. First, during bouts of vocalization, a higher percentage of syllables were of a single type. Second, the entropy of the first order Markov process was reduced, indicating an overall reduction in temporal diversity.
Vocal alterations: human
The techniques used to analyze mouse vocalization were validated by applying them to human speech from PWS, PWS with a mutation in the LETP, and controls. Stuttered speech has been extensively analyzed using a variety of measures including words per minute, voice onset time, formant frequencies of consecutive glottal periods, and other language-based acoustic techniques [31, 33, 34, 40]. Previous studies have demonstrated that stuttering is strongly correlated with fewer vocalizations per unit time and an increase in pause length [39, 40]. We confirmed and extended these results using language agnostic measures designed to be generalizable to mice. In addition, we found that using just three such language-agnostic features (number of vocalizations per minute, length of syllables and length of pauses), we could classify a recording as coming from a PWS or control.
Stuttering is heterogeneous disorder, and there have been many proposed subtypes of stuttering [42]. Here we have analyzed the speech of a subset of PWS with a genetically identified etiology and compared it to the speech of a general group of PWS. Our results show that using our language-agnostic analyses, PWS with a known mutation in the LETP showed similar abnormalities as those displayed by PWS of an unknown etiology.
Vocal alterations: comparison between mouse and human
Human speech is produced by air flow induced vocal fold vibrations [43]. In contrast, mouse vocalizations are thought to be a form of whistle [44]. Many types of comparisons between human speech and mouse vocalization are unlikely to be informative due to anatomical differences and the complex and communicative nature of human speech. In the case of stuttering, it is important to note that while stuttering may manifest as deficiencies in the control of vocal tract movements, there is no evidence that these are the proximal cause of the disorder. In fact, there is evidence that persons who are deaf or hearing impaired stutter when using sign language suggesting the cause of stuttering resides elsewhere [45]. In addition, mouse vocalizations require control of airflow, subglottal pressure, and glottal adduction and these share some common neural pathways with human vocal production [3, 10, 44]. Moreover, there is evidence linking these more cognitive pathways to stuttering [46, 47].
GNPTAB mutations exhibit several common features in both organisms. PWS, and specifically subjects carrying an LETP mutation (PWS-L), showed fewer vocalizations per unit time and as fluency blocks, which acoustically correspond to large pauses. Also like the mouse, the tendency towards syllable repetition is a defining characteristic of human stuttering. That some of the prominent features of human stuttering are recapitulated by the mouse model suggests that such a model may be useful in discovering the circuit and neurochemical underpinnings of at least some aspects of stuttering. One interpretation of these results is that a primary deficit in stuttered speech is the inability to initiate vocalization sequences, and that this specific aspect of the behavior is shared between organisms and can be disrupted by similar genetic means.
It is worth noting that mice are deaf during the postnatal period until at least P10 depending on the frequency [48]. Auditory feedback in humans has been alternatively shown to reduce or increase stuttering [49]. However with humans being able to hear is not necessary for stuttering behavior. It has been found that people that are hard of hearing and people who are deaf, stutter, both in oral speech and sign language [45, 50].
We also noted some differences between human stuttering and the mouse model. There have been several cases of PWS who are heterozygous for a mutation in the LETP; in contrast, we found that the vocalizations of heterozygous mice were more similar to those of wild type controls. In humans, repetition of a single syllable is unusual and strongly indicative of stuttered speech; in mice, syllable repetition is commonplace, with the consequence that only longer sequences of repetition exhibited significant differences. Finally, an important outstanding question is whether the Gnptab mut/mut mice were attempting to vocalize during the abnormal pauses or simply remaining quiet.
Non-vocal behaviors
We examined sensory-motor function and learning in a series of non-vocal behavioral paradigms. We ran an exhaustive behavioral screening on these animals including 50 statistical comparisons. We found that Gnptab mut/mut mice were normal over the large majority of these measurements. Two measures which did exhibit differences were general ambulatory activity (p = 0.007 (corrected for 6 bins, p=.042) during the final time bin, or p = 0.049 over the entire time interval) and exploratory behaviors related to hole pokes (p = 0.01-0.03). This is not significantly better than chance (one would expect 2.5 tests to pass this criterion by chance). Nor was any single p-value sufficiently strong to survive Bonferroni correction for multiple comparisons. With such an extensive battery of tests we therefore conclude that our finding appears to be a specific abnormality in vocalization. An alternative interpretation of these findings is that Gnptab mut/mut mice may show reduced rate of certain spontaneous behaviors.
Summary
Our data show that mutations in the lysosomal enzyme targeting pathway affect mouse pup isolation calls and human speech in similar ways, suggesting that there is a commonly disrupted vocalization function in both. We suggest that this animal model could allow the large array of genetic and neurobiological tools that exist in mice to be applied to the study of the cellular and molecular features of stuttering and perhaps normal speech.
Experimental Procedures
Data Acquisition
Studies were conducted under institutionally reviewed and approved animal study protocols. (NIDCD/NIH #1318-13, D.D. Principal Investigator and Washington University protocol 20130179, T.H. Principal Investigator, and 20120110, D.W., Principal Investigator). Knock-in mice were constructed on a BALB/c background using the strategy shown in Figure S1. To generate test subjects, heterozygous mice were mated. Females were separated from all other mice before they gave birth. Dams were checked for pups daily and the first day that a litter was discovered was considered as postnatal day zero (P0). Mice came from a total of 18 litters. We recorded from mice on postnatal three (P3), five (P5), and eight (P8). Mouse data were collected blind to genotype.
Analysis of Vocalizations
All analyses were done using in-house MATLAB software, available online at http://holylab.wustl.edu/. Analysis code implemented a fully-automated algorithm and were therefore blind to genotype. For mice, waveforms were pre-processed, band-pass filtered (25–110 kHz), and vocalizations identified using mean frequency, “spectral purity” (fraction of total power concentrated into a single frequency bin), and the “spectral discontinuity” (the change in the allocation of power across frequencies between two adjacent time bins) [14]. Stored acoustical waveforms were processed using MATLAB to compute the sonogram (512 samples/block, half-overlap, resulting in a time resolution of 1.02 ms and a frequency resolution of 0.98 kHz). We also examined broad band ‘clicks’ in the mouse vocalizations that were defined as non-contiguous milliseconds where less than 200 of the 512 sampled half frequencies were empty. No difference in the number of clicks was detected between groups. Stored acoustical waveforms were processed in MATLAB to compute the sonogram (512 samples/block, half-overlap, resulting in a time resolution of 1.02 ms and a frequency resolution of 0.98 kHz).
For humans, vocalizations were identified in terms of supra-threshold power in either of two frequency bands (0.6 -1.6 kHz and 5.6-9.6 kHz). Vocalization periods separated by gaps shorter than 20 ms were merged. Comparison with manual scoring demonstrated that breathing and other non-speech noises were excluded (Figure 5A). We examined the length and number of speech intervals and non-speech intervals (pauses between speech) in the speech samples. These intervals did not necessarily have only one word; many times words were run together without an appreciable non-speech interval.
To calculate the number of syllables and the duration of syllables and pauses, each vocalization or pause contributed to the mean for each animal or subject; each individual's mean was then averaged to obtain the group average. A t-test was then performed to compare groups with each individual's mean. An alternative analysis, where each syllable contributes to the overall mean was also performed and produced comparable results, not shown.
For the syllable analysis, the definitions set forth in Arriaga et al. were used, with the exception of classes “i, j, k” these syllable types were grouped into the category ‘other’ [10, 14]. This classification was done using a fully-automated algorithm. To determine whether the usage frequency differed significantly between genotypes, we first picked a random pair of recordings from the Gnptab mut/mut mice. We performed a bootstrap analysis of the differences in syllable usage between two individual mice, measuring the difference by
where s is the syllable type index, and ãs and b̃s are the normalized numbers of observed or predicted syllables of type s. The normalization was performed in terms of A = Σas and B = Σbs (where variables without ∼ denote raw counts), scaling and . As a consequence, the recording with more syllables was scaled to a number equal to that of the recording with fewer syllables, and the one with fewer was unmodified. The bootstrap was calculating the mean difference D among randomly-selected pairs of Gnptab mut/mut mice, and then comparing this mean against the distribution of mean D obtained from an identical number of wildtype/mut pairs, with wildtypes resampled to match the number of syllables produced by mut animals.
We used a t-test to compare the number of doublets and found no difference between experimental groups. Bout-level analyses defined bouts based on histograms of pause lengths for all groups of mice in each day we recorded. Histograms were constructed with a range of bin sizes (50 ms to 300 ms). The middle of minimum bin in the range of 0.15 to 0.33 seconds was averaged across all bin sizes to determine the criteria for an inter-bout pause. The resulting minimum intra-bout/inter-bout cutoff was determined to be 0.33 s in day 3 recordings, 0.28 s in day 5 and 0.22 s in day 8 recordings. To analyze bout-level repetition, the syllables in each bout were analyzed to determine how many of the syllables in each bout were the same type as the mode of that bout. In Figure 4D, the confidence intervals were calculated by bootstrapping bouts; bout-sizes with fewer than 3 exemplars are depicted only in terms of their mean. Entropy of syllable usage was calculated from the proportion of different syllable types; entropy for the temporal sequence (modeled as a first-order Markov process) was given by H2 = −Σp(X)Σp(X|Y) log2p (X|Y) with X and Y being each syllable type [19].
For the classification analysis, the number of vocalizations per minute, the average duration of each vocalization and the average duration of each pause were normalized to a range of 0-1 and plotted in 3 dimensional space. We then categorized each recording as being either from a control or person who stutters based on nearest neighbors. Choices of 1, 3, and 5 closest neighbors all yielded very similar results. We analyzed three cases: 1) all recordings from controls and PWS, 2) controls and PWS with an unknown cause, and 3) controls and PWS with a known mutation in the LETP. Data in all cases were significant compared to empirically calculated p value of .05 (data labels (e.g. controls, person who stutters) shuffled 10,000 times, 95% confidence intervals).
Supplementary Material
Highlights.
Mutations in the lysosomal enzyme-targeting pathway cause stuttering in humans
Mice with a mutation in this pathway also show abnormalities in vocalizations
Other behaviors in the knock-in mice appear largely intact
People who stutter with this type of mutation have similar features in their speech
Acknowledgments
We thank the Hollins Communication Research Institute (HCRI) for assistance with subject recruitment and stuttering diagnosis. We thank Peter Rietzes of StutterTalk for help with speech samples from PWS. We thank Grace Liao and Cassandra Roberts for help with vocal recordings of mice, Wayne Barnes and Thanh Loan Nguyen for their help with mouse genotyping, and Sara Conyers for her work involving the non-vocalization behavioral testing. This work was supported by the NIH Director's Pioneer Award (DP1 OD006437), Intellectual and Developmental Disabilities Research Center grant (P30 HD062171), and NIDCD Intramural grant Z1A-000046-15
Footnotes
Author Contributions: T.D.B. designed experiments, gathered mouse vocalization data, analyzed human and mouse vocalization data, and wrote the paper. D.F.W. directed the non-vocalization behavioral studies, which involved statistical and graphical analyses of the results and wrote the paper. T.U.H. provided comments on the manuscript and made Figure S1. D.D., had the original idea for the paper, recruited human subjects, designed knock-in mice, supervised experiments, and wrote the paper. J.G. recruited subjects and obtained speech recordings from scripts. T.E.H supervised mouse experiments, analyzed data, and wrote the paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Terra D. Barnes, Email: terra.barnes@gmail.com.
Dennis Drayna, Email: drayna@nidcd.nih.gov.
Timothy E. Holy, Email: holy@wustl.edu.
References
- 1.Bloodstein O, Bernstein Ratner N, editors. A Handbook on Stuttering. 6th 2008. [Google Scholar]
- 2.Conture EG. Stuttering: Its Nature, Dignosis, and Treatment. Allyn and Bacon; 2001. [Google Scholar]
- 3.Fisher SE, Lai CSL, Monaco AP. Deciphering the Genetic Basis of Speech and Language Disorders. Annual Review of Neuroscience. 2003;26:57–80. doi: 10.1146/annurev.neuro.26.041002.131144. [DOI] [PubMed] [Google Scholar]
- 4.MacDermot KD, Bonora E, Sykes N, Coupe AM, Lai CSL, Vernes SC, Vargha-Khadem F, McKenzie F, Smith RL, Monaco AP, et al. Identification of FOXP2 Truncation as a Novel Cause of Developmental Speech and Language Deficits. The American Journal of Human Genetics. 2005;76:1074–1080. doi: 10.1086/430841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fedorenko E, Morgan A, Murray E, Cardinaux A, Mei C, Tager-Flusberg H, Fisher SE, Kanwisher N. A highly penetrant form of childhood apraxia of speech due to deletion of 16p11. 2. European Journal of Human Genetics. 2015 doi: 10.1038/ejhg.2015.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kang C, Riazuddin S, Mundorff J, Krasnewich D, Friedman P, Mullikin JC, Drayna D. Mutations in the Lysosomal Enzyme–Targeting Pathway and Persistent Stuttering. New England Journal of Medicine. 2010;362:677–685. doi: 10.1056/NEJMoa0902630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raza MH, Domingues CEF, Webster R, Sainz E, Paris E, Rahn R, Gutierrez J, Chow HM, Mundorff J, Kang Cs, et al. Mucolipidosis types II and III and non-syndromic stuttering are associated with different variants in the same genes. Eur J Hum Genet. 2015 doi: 10.1038/ejhg.2015.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kornfeld S. Trafficking of lysosomal enzymes. The FASEB Journal. 1987;1:462–468. doi: 10.1096/fasebj.1.6.3315809. [DOI] [PubMed] [Google Scholar]
- 9.Fischer J, Hammerschmidt K. Ultrasonic vocalizations in mouse models for speech and socio-cognitive disorders: insights into the evolution of vocal communication. Genes, Brain and Behavior. 2010:no–no. doi: 10.1111/j.1601-183X.2010.00610.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arriaga G, Zhou EP, Jarvis ED. Of Mice, Birds, and Men: The Mouse Ultrasonic Song System Has Some Features Similar to Humans and Song-Learning Birds. PLoS ONE. 2012;7:e46610. doi: 10.1371/journal.pone.0046610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Enard W, Gehre S, Hammerschmidt K, Hölter SM, Blass T, Somel M, Brückner MK, Schreiweis C, Winter C, Sohr R, et al. A Humanized Version of Foxp2 Affects Cortico-Basal Ganglia Circuits in Mice. Cell. 2009;137:961–971. doi: 10.1016/j.cell.2009.03.041. [DOI] [PubMed] [Google Scholar]
- 12.Scattoni ML, Ricceri L, Crawley JN. Unusual repertoire of vocalizations in adult BTBR T+ tf/J mice during three types of social encounters. Genes, Brain and Behavior. 2011;10:44–56. doi: 10.1111/j.1601-183X.2010.00623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anne-Marie EENT, Sourd L, Boeckers CSLTM, Thomas PF. The Autism ProSAP1/Shank2 Mouse Model Displays Quantitative and Structural Abnormalities in Ultrasonic Vocalisations. 2013 doi: 10.1016/j.bbr.2013.08.031. [DOI] [PubMed] [Google Scholar]
- 14.Holy TE, Guo Z. Ultrasonic Songs of Male Mice. PLoS Biol. 2005;3:e386. doi: 10.1371/journal.pbio.0030386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fujita E, Tanabe Y, Shiota A, Ueda M, Suwa K, Momoi MY, Momoi T. Ultrasonic vocalization impairment of Foxp2 (R552H) knockin mice related to speech-language disorder and abnormality of Purkinje cells. Proceedings of the National Academy of Sciences. 2008;105:3117–3122. doi: 10.1073/pnas.0712298105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gaub S, Groszer M, Fisher SE, Ehret G. The structure of innate vocalizations in Foxp2-deficient mouse pups. Genes, Brain and Behavior. 2010;9:390–401. doi: 10.1111/j.1601-183X.2010.00570.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shu W, Cho JY, Jiang Y, Zhang M, Weisz D, Elder GA, Schmeidler J, De Gasperi R, Sosa MAG, Rabidou D, et al. Altered ultrasonic vocalization in mice with a disruption in the Foxp2 gene. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:9643–9648. doi: 10.1073/pnas.0503739102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grimsley JMS, Monaghan JJM, Wenstrup JJ. Development of Social Vocalizations in Mice. PLoS ONE. 2011;6:e17460. doi: 10.1371/journal.pone.0017460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ey E, Torquet N, Le Sourd AM, Leblond CS, Boeckers TM, Faure P, Bourgeron T. The Autism ProSAP1/Shank2 mouse model displays quantitative and structural abnormalities in ultrasonic vocalisations. Behavioural Brain Research. 2013;256:677–689. doi: 10.1016/j.bbr.2013.08.031. [DOI] [PubMed] [Google Scholar]
- 20.Ehret G. Categorical perception of mouse-pup ultrasounds in the temporal domain. Animal Behaviour. 1992;43:409–416. [Google Scholar]
- 21.Lai CSL, Fisher SE, Hurst JA, Vargha-Khadem F, Monaco AP. A forkhead-domain gene is mutated in a severe speech and language disorder. Nature. 2001;413:519–523. doi: 10.1038/35097076. [DOI] [PubMed] [Google Scholar]
- 22.Groszer M, Keays DA, Deacon RMJ, de Bono JP, Prasad-Mulcare S, Gaub S, Baum MG, French CA, Nicod J, Coventry JA, et al. Impaired Synaptic Plasticity and Motor Learning in Mice with a Point Mutation Implicated in Human Speech Deficits. Current biology : CB. 2008;18:354–362. doi: 10.1016/j.cub.2008.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hammerschmidt K, Schreiweis C, Minge C, Pääbo S, Fischer J, Enard W. A humanized version of Foxp2 does not affect ultrasonic vocalization in adult mice. Genes, Brain and Behavior. 2015;14:583–590. doi: 10.1111/gbb.12237. [DOI] [PubMed] [Google Scholar]
- 24.Groszer M, Keays DA, Deacon RMJ, de Bono JP, Prasad-Mulcare S, Gaub S, Baum MG, French CA, Nicod J, Coventry JA, et al. Impaired Synaptic Plasticity and Motor Learning in Mice with a Point Mutation Implicated in Human Speech Deficits. Current Biology. 2008;18:354–362. doi: 10.1016/j.cub.2008.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fedyna A, Drayna D, Kang C. Characterization of a mutation commonly associated with persistent stuttering: evidence for a founder mutation. J Hum Genet. 2011;56:80–82. doi: 10.1038/jhg.2010.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grimsley JM, Gadziola MA, Wenstrup JJ. Automated classification of mouse pup isolation syllables: from cluster analysis to an Excel-based “mouse pup syllable classification calculator”. Frontiers in Behavioral Neuroscience. 2013;6:89. doi: 10.3389/fnbeh.2012.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wozniak DF, Hartman RE, Boyle MP, Vogt SK, Brooks AR, Tenkova T, Young C, Olney JW, Muglia LJ. Apoptotic neurodegeneration induced by ethanol in neonatal mice is associated with profound learning/memory deficits in juveniles followed by progressive functional recovery in adults. Neurobiology of disease. 2004;17:403–414. doi: 10.1016/j.nbd.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 28.Dougherty JD, Maloney SE, Wozniak DF, Rieger MA, Sonnenblick L, Coppola G, Mahieu NG, Zhang J, Cai J, Patti GJ. The disruption of Celf6, a gene identified by translational profiling of serotonergic neurons, results in autism-related behaviors. The Journal of Neuroscience. 2013;33:2732–2753. doi: 10.1523/JNEUROSCI.4762-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wozniak DF, Diggs-Andrews KA, Conyers S, Yuede CM, Dearborn JT, Brown JA, Tokuda K, Izumi Y, Zorumski CF, Gutmann DH. Motivational disturbances and effects of L-dopa administration in neurofibromatosis-1 model mice. PLoS ONE. 2013;8:e66024. doi: 10.1371/journal.pone.0066024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Menzies RG, Onslow M, Packman A. Anxiety and stuttering: Exploring a complex relationship. American Journal of Speech-Language Pathology. 1999;8:3. doi: 10.1044/1058-0360(2011/10-0091). [DOI] [PubMed] [Google Scholar]
- 31.Czyzewski A, Kaczmarek A, Kostek B. Intelligent Processing of Stuttered Speech. Journal of Intelligent Information Systems. 2003;21:143–171. [Google Scholar]
- 32.Healey EC, Ramig PR. Acoustic Measures of Stutterers' and Nonstutterers' Fluency in Two Speech Contexts. Journal of Speech, Language, and Hearing Research. 1986;29:325–331. doi: 10.1044/jshr.2903.325. [DOI] [PubMed] [Google Scholar]
- 33.Howell P, Sackin S, Glenn K. Development of a Two-Stage Procedure for the Automatic Recognition of Dysfluencies in the Speech of Children Who Stutter: II. ANN Recognition of Repetitions and Prolongations With Supplied Word Segment Markers. Journal of speech, language, and hearing research : JSLHR. 1997;40:1085–1096. doi: 10.1044/jslhr.4005.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lustyk T, Bergl P, Cmejla R. Evaluation of disfluent speech by means of automatic acoustic measurements. The Journal of the Acoustical Society of America. 2014;135:1457–1468. doi: 10.1121/1.4863646. [DOI] [PubMed] [Google Scholar]
- 35.Riper Cv. The nature of stuttering. Englewood Cliffs, nj: Prentice–Hall; 1982. [Google Scholar]
- 36.Robb M, Blomgren M, Chen Y. Formant frequency fluctuation in stuttering and nonstuttering adults. Journal of Fluency Disorders. 1998;23:73–84. [Google Scholar]
- 37.Seery CH, Watkins RV, Mangelsdorf SC, Shigeto A. Subtyping stuttering II: Contributions from language and temperament. Journal of Fluency Disorders. 2007;32:197–217. doi: 10.1016/j.jfludis.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Webster RL. Empirical considerations regarding stuttering therapy. Controversies about stuttering therapy. 1979:209–240. [Google Scholar]
- 39.Andrade CRFd, Cervone LM, Sassi FC. Relationship between the stuttering severity index and speech rate. Sao Paulo Medical Journal. 2003;121:81–84. doi: 10.1590/S1516-31802003000200010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Johnson W. Measurements of oral reading and speaking rate and disfluency of adult male and female stutterers and nonstutterers. The Journal of speech and hearing disorders. 1961:1. [PubMed] [Google Scholar]
- 41.Ryan BP. Articulation, Language, Rate, and Fluency Characteristics of Stuttering and Nonstuttering Preschool Children. Journal of Speech, Language, and Hearing Research. 1992;35:333–342. doi: 10.1044/jshr.3502.333. [DOI] [PubMed] [Google Scholar]
- 42.Yairi E. Subtyping stuttering I: A review. Journal of Fluency Disorders. 2007;32:165–196. doi: 10.1016/j.jfludis.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 43.Honda M. Human speech production mechanisms. NTT Technical Review. 2003;1:24–29. [Google Scholar]
- 44.Johnson AM, Ciucci MR, Russell JA, Hammer MJ, Connor NP. Ultrasonic output from the excised rat larynx. The Journal of the Acoustical Society of America. 2010;128:EL75–EL79. doi: 10.1121/1.3462234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Montgomery BM, Fitch JL. The prevalence of stuttering in the hearing-impaired school age population. Journal of Speech and Hearing Disorders. 1988;53:131–135. doi: 10.1044/jshd.5302.131. [DOI] [PubMed] [Google Scholar]
- 46.Fox PT, Ingham RJ, Ingham JC, Zamarripa F, Xiong JH, Lancaster JL. Brain correlates of stuttering and syllable production. Brain. 2000;123:1985–2004. doi: 10.1093/brain/123.10.1985. [DOI] [PubMed] [Google Scholar]
- 47.Rosenbek J, Messert B, Collins M, Wertz RT. Stuttering following brain damage. Brain and language. 1978;6:82–96. doi: 10.1016/0093-934x(78)90046-9. [DOI] [PubMed] [Google Scholar]
- 48.Ehret G. Development of absolute auditory thresholds in the house mouse (Mus musculus) Ear and Hearing. 1976;1:179–184. [PubMed] [Google Scholar]
- 49.Kalinowski J, Armson J, Stuart A, Gracco VL. Effects of alterations in auditory feedback and speech rate on stuttering frequency. Language and Speech. 1993;36:1–16. doi: 10.1177/002383099303600101. [DOI] [PubMed] [Google Scholar]
- 50.Snyder G. Unpublished manuscript. University of Mississippi, University; 2006. The existence of stuttering in sign language and other forms of expressive communication: Sufficient cause for the emergence of a new stuttering paradigm. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.