Abstract
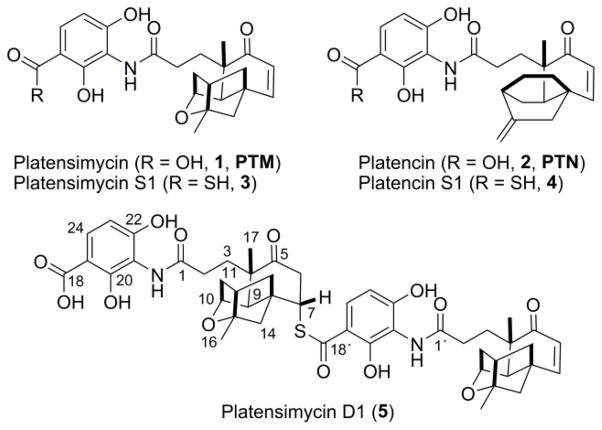
The platensimycin (PTM) and platencin (PTN) class of natural products are promising drug leads that target bacterial and mammalian fatty acid synthases. Natural congeners and synthetic analogues of PTM and PTN have been instrumental in determining their structure-activity relationships, with only a few analogues retaining the potencies of PTM and PTN. Here we describe the identification and isolation of two new sulfur-containing PTM congeners (3 and 5) from the engineered dual PTM-PTN overproducing Streptomyces platensis SB12029. Structure elucidation of platensimycin D1 (5), a sulfur-containing PTM pseudo-dimer, revealed the existence of its presumptive thioacid precursor (3). The unstable thioacid 3 was isolated and confirmed by structural characterization of its permethylated product (6). LC-MS analysis of crude extracts of SB12029 confirmed the presence of the thioacid analogue of PTN (4). The minimum inhibitory concentration (MIC) was determined for 5 revealing retention of the strong antibacterial activity of PTM.
Keywords: Platensimycin, Platencin, Natural products, Sulfur-containing, Antibacterial
Graphical abstract
1. Introduction
Platensimycin (PTM, 1) and platencin (PTN, 2) are a new class of promising natural product antibiotics. They are potent and selective inhibitors of bacterial fatty acid synthase (FASII) and are effective against a wide range of bacteria including methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant enterococci (VRE), and Mycobacterium tuberculosis.1–3 Although it is unclear whether FASII is a viable target for antibiotic development, as exogenous fatty acids in circulation may complement inhibited de novo fatty acid biosynthesis in the infecting bacteria,4,5 both PTM and PTN showed efficacy in mouse models while displaying no toxicity.1,2 They, however, currently require delivery via continuous infusion, ensuring adequate systemic exposure, for efficacy.1,2 The poor pharmacokinetic properties of PTM and PTN have dampened enthusiasm for these promising, yet undeveloped, antibiotic drug leads. PTM has also emerged as a drug lead for the treatment of diabetes as it is a potent and highly selective inhibitor of mammalian fatty acid synthase.6
PTM and PTN, originally isolated from Streptomyces platensis MA7327 and MA7339, respectively,1,2 are comprised of two distinct moieties, a polar 3-amino-2,4-dihydroxybenzoic acid (ADHBA) and a diterpene-derived lipophilic ketolide, linked by an amide bond (Fig. 1).7,8 We previously cloned and sequenced the ptm and ptn gene clusters from S. platensis MA7327 and MA7339, respectively, and discovered that MA7327 is a dual PTM-PTN producer.9 Inactivation of the negative transcriptional regulators ptmR1 and ptnR1, in the ptm and ptn gene clusters, respectively, yielded recombinant strains that dramatically (>100-fold, >300 mg L−1) increased production of PTM and PTN.10,11 These strains also produced impressive numbers of PTM and PTN congeners previously undetectable in the wild-type strains.11,12 Heterologous expression of the ptn cluster, with ptnR1 deleted, in Streptomyces lividans K4-114 also produced new PTN congeners, in addition to PTN.13
Figure 1.
Structures of PTM (1), PTN (2), and the congeners isolated from the recombinant strain S. platensis SB12029. Platencin S1 (4) was identified by LC-MS analysis.
We recently developed a high-throughput real-time PCR method for strain prioritization and natural products discovery.14 By screening the Actinomycetales collection at The Scripps Research Institute, we discovered six additional dual PTM-PTN producing S. platensis strains.14 Genome sequencing of two of the strains, CB00739 and CB00765, revealed that these producers possessed ptm gene clusters that were identical in genetic organization and highly homologous in sequence identity to that of S. platensis MA7327.14 To establish a model strain for the study of PTM and PTN, and bacterial diterpene biosynthesis in general, we constructed a genetically amenable, in-frame ΔptmR1 dual PTM-PTN overproducing strain, SB12029, from CB00739.15 As a demonstration of the utility of SB12029 for facile biosynthetic and bioengineering studies of the PTM and PTN class of natural products, a ΔptmR1 ΔptmO4 double mutant, SB12030, was created and fermented.15 Isolation of fourteen major PTM and PTN congeners from SB12030 shed new light on PTM and PTN biosynthesis.15
In this study, we report the identification of three new sulfur-containing PTM and PTN congeners from S. platensis SB12029 (Fig. 1). Preliminary analysis of 3 suggested it was a thioacid analogue of PTM. Isolation and structural elucidation of a sulfur-containing pseudo-dimer of PTM (5) supported the presence of its presumptive precursor (3). The unstable thioacid 3 was confirmed by permethylation and structural characterization of the resulting permethylated product (6). Furthermore, LC-MS analysis of SB12029 confirmed the presence of the thioacid analogue of PTN (4). Minimum inhibitory concentrations (MICs) were determined for 5 and 6 revealing that the isolated congener of PTM, 5, retained strong antibacterial activity.
2. Results and discussion
2.1. Identification of a sulfur-containing PTM congener (3) from S. platensis SB12029
Since our construction of the dual PTM-PTN overproducing S. platensis SB12029, we have utilized it as a model strain for the study of PTM and PTN, and bacterial diterpene biosynthesis in general.15 Given the enhanced productive nature of SB12029, as well as our previous experiences of identifying congeners of PTN in recombinant overproducing strains,11,12 we set out to examine SB12029 for novel natural congeners from a dual PTM-PTN overproducer.
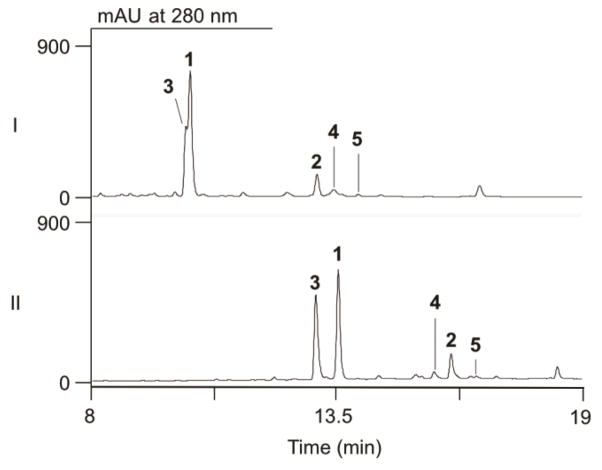
Following previously reported procedures for large-scale fermentation and extraction of PTM-PTN producing cultures,10,11,13–15 the crude extract of 6 L of fermentation broth was subjected to LC-MS analysis (Fig. 2). Along with the high titers of PTM and PTN, we noticed that the peak for PTM had a prominent polar shoulder (Fig. 2, panel I). Optimization of the solvent system used for LC-MS analysis, by replacing CH3CN with CH3OH, revealed this was in fact two separate compounds (Fig. 2, panel II). Mass spectroscopy analysis of this new peak gave an [M + H]+ ion at m/z 458, 16 Da higher than the [M + H]+ ion of PTM at m/z 442. We immediately suspected that this compound, 3, was a hydroxylated PTM analogue. Analogues of PTM, PTN, and biosynthetic intermediates containing superfluous hydroxyl groups have been previously isolated.11,15–17 Each of these analogues possessed an extra hydroxyl group on their diterpene-derived scaffold. This compound, however, appeared to have an unmodified ketolide as the electrospray ionization (ESI) MS spectrum showed a fragment ion at m/z 273, characteristic of the cleaved platensic acid, the ketolide of PTM (Fig. S1).18 Thus, we suspected this analogue had an extra hydroxyl group on the benzoic acid moiety.
Figure 2.
LC-MS analysis of S. platensis SB12029. The crude extract was analyzed using UV absorption at 280 nm (shown) and total ion current (TIC, not shown) using a solvent system of (I) CH3CN-H2O or (II) CH3OH-H2O. PTM (1), PTN (2), and congeners (3–5) isolated from fermentation of SB12029.
After fractionation of the crude extract, 3 was separated from PTM (1) and a number of other unknown peaks. However, 3 was unstable during and after purification, preventing structural characterization. High-resolution ESIMS (HRESIMS) of 3 afforded the [M + H]+ ion at m/z 458.1634 (Fig. S1). This m/z value was consistent with an analogue of PTM that had an oxygen atom replaced with a sulfur atom (calculated [M + H]+ ion for C24H28NO6S at m/z 458.1634) as opposed to a hydroxylated PTM analogue (calculated [M + H]+ ion for C24H28NO8 at m/z 458.1809).
Two sulfur-containing PTN congeners were previously isolated, although their biosynthetic origins are unknown. Platencin SL4, an N-acetylcysteamine (S-NAC) derivative of 7-hydroxy-ent-atiseren-19-oic acid was isolated from heterologous expression of the ptn gene cluster in S. lividans.13 Platencin A9, a hydroxylated PTN glucoside possessing a methyl thioester, was isolated from the PTN overproducing strain S. platensis SB12600.11 The [M + H]+ ion and predicted molecular formula of 3, along with its intrinsic instability, was indicative that 3 is a thioacid derivative of PTM. Thus, 3 was named platensimycin S1, with “S” designating this compound contains a sulfur atom.
2.2. Isolation and structural characterization of a sulfur-containing PTM pseudo-dimer (5)
During the isolation and purification of 3, we identified an additional compound of interest due to its UV absorption at 280 nm signifying the presence of an aromatic group, a fragmentation ion at m/z 273 implicating an intact ketolide of PTM, and a relatively large [M + H]+ ion at m/z 899. This compound was named “platensimycin D1” designating that this congener is a “dimeric” analogue of PTM.
Platensimycin D1 (5), isolated as a white solid, had a molecular formula of C48H55N2O13S based on HRESIMS analysis ([M + H]+ ion at m/z 899.3425; calculated [M + H]+ ion for C48H55N2O13S at m/z 899.3426). For comparison, a hydroxylated analogue with a molecular formula of C48H55N2O15 yields a calculated [M + H]+ ion at m/z 899.3597. As PTM is comprised of 24 carbons, we suspected that 5, with 48 carbons, was a dimer of PTM. The 1H and 13C NMR spectra (Table 1, Figs. S2 and S3) of 5 showed resonances that were very consistent with those of PTM (1).7 In addition, many of the peaks were duplicated with slight variations in the chemical shifts, suggestive of a dimeric compound. There were, however, two key differences between the monomers forming 5, as well as between 5 and 1, that revealed the structure of 5. While the enone functional group of one monomer was intact [C-5′ (δC 203.6), C-6′ (δC 127.6; δH 5.93, d, J = 9.8 Hz), C-7′ (δC 154.3; δH 6.37, d, J = 9.8 Hz)], the enone of the other monomer was lost [C-5 (δC 212.0), C-6 (δC 43.4; δH 3.28, dd, J = 14.7, 4.2 Hz and 2.74, m), C-7 (δC 50.1; δH 4.15, br t, J = 3.5 Hz)]. The loss of the α,β–unsaturated functionality suggested that dimer formation occurred through a Michael addition. The second major difference was from one of the carboxylic acid carbonyls of the ADHBA moiety. Compared with the chemical shift of the carboxylic acid carbonyl C-18 (δC 175.1), C-18′ (δC 194.1) was significantly shifted downfield, suggestive of a thiocarboxylic acid or ester. The sulfur of the thiocarboxylic acid is more nucleophilic than an oxygen of a carboxylic acid and was the likely culprit for dimer formation. The 13C chemical shift of C-7 (δC 50.1), along with an HMBC correlation (Figs. 3A and S6) between H-7 (δH 4.15) and C-18′ (δC 194.1) confirmed sulfur attachment at C-7. The stereochemistry at C-7 was determined by ROESY NMR analysis (Figs. 3B, S7, and S8). Correlations between H-7 (δH 4.15) and H-13 (δH 1.63) and H-14 (δH 1.57), along with a correlation between H-13 (δH 2.14) and CH3-17 (δH 1.20) established the sulfur was attached in the α orientation, corresponding to a configuration of 7S. The isolation of platensimycin D1 (5), a sulfur-containing pseudo-dimeric analogue of PTM, supported the presence and structure of 3.
Table 1.
Summary of 1H (700 MHz) and 13C (175 MHz) NMR data for 5 in pyridine-d5 (δ in ppm)a
| No. | δC | δH (J in Hz) | No. | δC | δH (J in Hz) |
|---|---|---|---|---|---|
| 1 | 174.9, s | 1′ | 174.5, s | ||
| 2 | 32.5, t | 3.05, 2H, m | 2′ | 32.1, t | 2.78, m 2.70, m |
| 3 | 34.1, t | 2.52, ddd (16.1, 9.8, 6.3) 2.20, mb |
3′ | 32.4, t | 2.69, mb 2.05, m |
| 4 | 50.0, s | 4′ | 47.0, s | ||
| 5 | 212.0, s | 5′ | 203.6, s | ||
| 6 | 43.4, t | 3.28, dd (14.7, 4.2) 2.74, mb |
6′ | 127.6, d | 5.93, d (9.8) |
| 7 | 50.1, d | 4.15, br t (3.5) | 7′ | 154.3, d | 6.37, d (9.8) |
| 8 | 48.6, s | 8′ | 46.5, s | ||
| 9 | 47.2, d | 2.46, br s | 9′ | 46.9, d | 2.43, br s |
| 10 | 77.3, d | 4.51, br s | 10′ | 76.8, d | 4.47, br s |
| 11 | 41.1, t | 1.98, br d (11.2) 1.94, m |
11′ | 41.1, t | 1.89, m 1.81, br d (11.2) |
| 12 | 46.0, d | 2.28, t (6.3) | 12′ | 45.3, d | 2.20, t (6.3) |
| 13 | 43.8, t | 2.14, dd (11.2, 2.1) 1.63, dd (11.2, 7.0) |
13′ | 43.3, t | 1.81, br d (11.2) 1.58, mb |
| 14 | 53.8, t | 1.99, br d (11.2) 1.57, br d (11.2) |
14′ | 55.3, t | 1.71, br d (10.5) 1.48, br d (10.5) |
| 15 | 86.2, s | 15′ | 87.1, s | ||
| 16 | 23.6, q | 1.40, s | 16′ | 23.6, q | 1.40, s |
| 17 | 26.2, q | 1.20, s | 17′ | 24.8, q | 1.14, s |
| 18 | 175.1, s | 18′ | 194.1, s | ||
| 19 | 107.4, s | 19′ | 114.0, s | ||
| 20 | 158.7, s | 20′ | 157.0, s | ||
| 21 | 115.7, s | 21′ | 115.5, s | ||
| 22 | 158.4, s | 22′ | 160.7, s | ||
| 23 | 110.3, d | 6.87, d (8.4) | 23′ | 110.8, d | 6.78, d (8.4) |
| 24 | 129.7, d | 8.10, d (8.4) | 24′ | 129.0, d | 7.63, d (8.4) |
| NH | 10.75, s | NH′ | 10.46, s |
Assignments were based on 1D and 2D NMR experiments.
Overlapping signals
Figure 3.
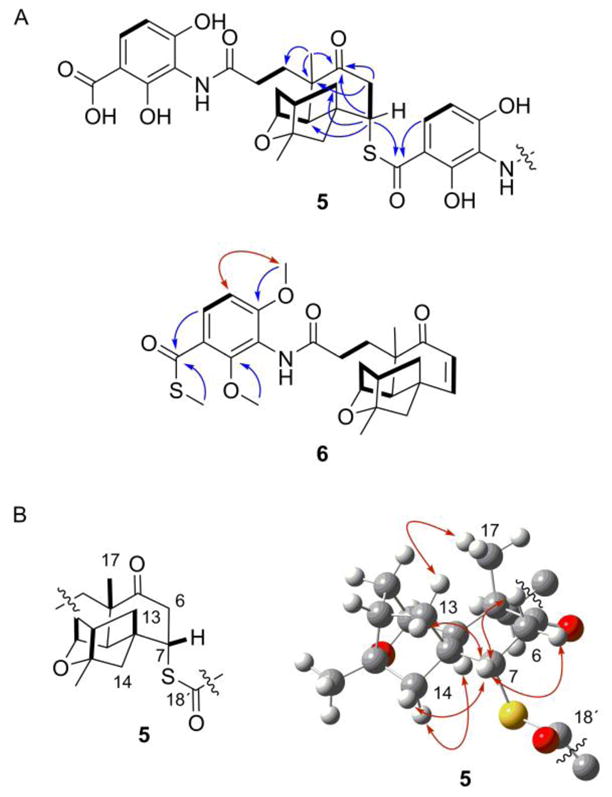
Key 2D NMR correlations supporting the structures of 3, 5, and 6. (A) Key COSY (bold lines), HMBC (blue arrows), and ROESY (red arrows) correlations. (B) Key ROESY correlations (red arrows) supporting the connection and stereochemistry of the thioester moiety at C-7.
2.3. Permethylation of 3 confirming the structure of platensimycin S1
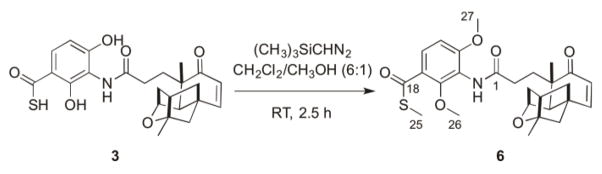
With the isolation of platensimycin D1 (5) supporting our preliminary hypothesis that 3 contains a free thioacid, we set out to confirm the structure of 3. Following a small scale (3 × 50 mL) fermentation of SB12029, 3 was partially purified from the resultant crude extract by preparative reverse-phase HPLC. The fraction containing 3 was quickly permethylated using trimethylsilyldiazomethane [(CH3)3SiCHN2] and subsequently purified to yield 6 (Scheme 1).19
Scheme 1.
Permethylation of platensimycin S1 (3) to afford 6 for structural confirmation.
As expected, the HRESIMS of 6 afforded an [M + H]+ ion at m/z 500.2105, consistent with a molecular formula of C27H34NO6S (calculated [M + H]+ ion at m/z 500.2107), exactly three methyl groups larger than the formula of 3. The 1H and 13C NMR spectra (Table 2, Figs. S9 and S10) of 6 clearly indicated, along with the characteristic signals for PTM, the presence of three additional methyl signals and a thioester carbonyl carbon instead of the usual carboxylic acid carbonyl carbon. The 1H and 13C chemical shifts of the three methyl groups represented one –SCH3 (C-25, δC 12.6; δH 2.39) and two –OCH3 (C-26 and C-27; δC 62.2, 56.6; δH 4.10, 3.75, respectively) functional groups. The 13C peak at δ 190.4 for C-18, along with HMBC correlations (Figs. 3A and S13) between the –SCH3 protons and C-18, indicated the presence of a methyl thioester in 6. Therefore, permethylation of 3, resulting in a methyl thioester analogue of PTM (6), confirms the structure of platensimycin S1 (3) as thioacid PTM.
Table 2.
Summary of 1H (700 MHz) and 13C (175 MHz) NMR data for 6 in pyridine-d5 (δ in ppm)a
| No. | δC | δH (J in Hz) | No. | δC | δH (J in Hz) |
|---|---|---|---|---|---|
| 1 | 172.8, s | 15 | 87.1, s | ||
| 2 | 32.2, t | 2.81, mb 2.75, mb |
16 | 23.6, q | 1.40, s |
| 3 | 32.7, t | 2.81, mb 2.20, mb |
17 | 24.9, q | 1.19, s |
| 4 | 47.1, s | 18 | 190.4, s | ||
| 5 | 203.7, s | 19 | 122.3, s | ||
| 6 | 127.6, d | 5.96, d (9.8) | 20 | 157.9, s | |
| 7 | 154.3, d | 6.39, d (9.8) | 21 | 125.1, s | |
| 8 | 46.5, s | 22 | 161.1, s | ||
| 9 | 46.9, d | 2.51, br s | 23 | 107.7, d | 6.81, d (9.1) |
| 10 | 76.8, d | 4.57, br t (2.8) | 24 | 129.3, d | 7.96, d (9.1) |
| 11 | 41.1, t | 1.90, m 1.83, br d (11.2) |
25 | 12.6, q | 2.39, s |
| 12 | 45.4, d | 2.21, t (7.0) | 26 | 62.2, q | 4.10, s |
| 13 | 43.4, t | 1.83, dd (11.2, 3.5) 1.58, dd (11.2, 7.0) |
27 | 56.6, q | 3.75, s |
| 14 | 55.3, t | 1.73, dd (11.2, 3.5) 1.50, br d (11.2) |
NH | 10.36, s |
Assignments were based on 1D and 2D NMR experiments.
Overlapping signals.
2.4. SB12029 also produces platencin S1 (4)
With the presence and structure of platensimycin S1 (3) confirmed, we were interested if SB12029 also produced the thioacid PTN derivative. Retrospective analysis of the crude extract by LC-MS revealed a minor compound (4) that migrated faster than PTN (using the CH3OH–H2O solvent system, Fig 2, panel II) and gave an [M + H]+ ion at m/z 16 Da higher than the [M + H]+ ion of PTN at m/z 426 (Fig. 2). Similar to 3, this compound also appeared to have an unmodified ketolide as the ESIMS spectrum showed a fragment ion at m/z 257, characteristic of cleaved platencinic acid, the ketolide of PTN (Fig. S1). HRESIMS of this peak afforded an [M + H]+ ion at m/z 442.1681, consistent with a sulfur-containing PTN analogue (calculated [M + H]+ ion for C24H28NO5S at 442.1683) as opposed to hydroxylated PTN (calculated [M + H]+ ion for C24H28NO7 at 442.1860). Consequently, 4 was concluded to be the thioacid analogue PTN (4) and named platencin S1.
2.5. Biological evaluation of 5 and 6 revealing that platensimycin D1 (5) retains strong antibacterial activity
Both 5 and 6 were tested for antibacterial activity in comparison with PTM (1) against Staphylococcus aureus ATCC 25923 and Micrococcus luteus ATCC 9431. MICs were determined using the broth dilution method (Table 3).20 PTM (1), used as a positive control, had MICs of 0.5 μg mL−1 against both strains, matching previously reported MIC values.1 The sulfur-containing PTM pseudo-dimer (5) retained the strong antibacterial activity of PTM against both strains, with an equivalent MIC (0.5 μg mL−1) value against S. aureus. Due to the differences in molecular weights, the molar MICs of 5 against S. aureus and M. luteus were 0.56 and 0.28 μM, respectively, a 2–4-fold increase in potency compared to 1.13 μM for PTM (1). Unsurprisingly, 6 was inactive (MICs 64 or >64 μg mL−1), as several key functional groups required for FabF binding were methylated.21
Table 3.
Antibacterial activities of 5 and 6 in comparison to PTM (1) as measured by MICs (μg mL−1)a
| Strain | 1 | 5 | 6 |
|---|---|---|---|
| Staphylococcus aureus ATCC 25923b | 0.5c | 0.5c | >64 |
| Micrococcus luteus ATCC 9431 | 0.5 | 0.25 | 64 |
Each MIC was performed in duplicate or triplicate.
Methicillin sensitive.
A concentration of 0.5 μg mL−1 equals 1.13 μM for 1 and 0.56 μM for 5.
3. Conclusions
Study of the PTM and PTN family of natural products continues to reveal new and unexpected phenomena. The dual PTM-PTN and PTN-only overproducing recombinant strains have provided a means to explore and elucidate the biosynthesis of PTM and PTN by producing high titers of countless congeners.10,11,15 Typically, natural congeners can be separated into three different categories, biosynthetic intermediates, shunt metabolites, or post-biosynthetic modifications from adventitious enzymes. In the case of PTM and PTN, these three categories generally do not translate into the retention of biological activity. Correspondingly, very few analogues of PTM and PTN, of either natural or synthetic origin, retain the potency of PTM and PTN’s natural antibacterial activity.22,23 Therefore, it is intriguing that 5 retains antibacterial activity, being 2–4-fold more potent (in molar concentration) than PTM. It is currently unknown if 5 (i) hydrolyzes in vivo to form PTM or other bioactive analogues, (ii) is accommodated into the active site of FabF, or (iii) is active through a distinct mechanism. In addition, it will be fascinating to investigate whether 5 acquired improved pharmacokinetic properties, compared to PTM, while retaining in vivo efficacy.
Platensimycin D1 (5) is the first “dimeric” analogue of PTM or PTN. While 5 most likely is the result of a nonenzymatic reaction, due to increased nucleophilicity of the sulfur atom attacking the enone via a heteroatom Michael addition, we are confident that 3 and 4 are indeed true natural products. This begs the question of what role the thioacid functionality of 3 and 4 have in either the biosynthesis or modification of PTM and PTN, respectively. The production of 3 and 4, in relatively high titers, raises a provocative question: Are PTM and PTN not the final biosynthetic products of their respective gene clusters, only hydrolyzed products of 3 and 4, respectively? One such example is the Pseudomonas siderophore quinolobactin (QB). The true final biosynthetic product of the qb gene cluster was discovered to be thioquinolobactin (TQB), an unstable thioacid analogue that spontaneously hydrolyzes into QB in aqueous solutions.24,25 Interestingly, TQB inhibits growth of the oomycete Pythium while QB does not.25 Subsequent biosynthetic investigations on TQB revealed a variation of the sulfur transfer chemistry used in molybdopterin and thiamine biosynthesis.26 We are now investigating the biological and biosynthetic significance of the thioacid analogues of PTM and PTN.
Overall, PTM and PTN, through the use of engineered overproducers, are revealing their treasure-trove of undiscovered biosynthetic information. Continued study of the production, SAR, and biosynthesis of the PTM and PTN class of natural products promises to be extremely rewarding.
4. Experimental section
4.1. Chemistry
4.1.1. General experimental procedures
All chemicals and medium components were purchased from commercial sources. All 1H, 13C, and 2D NMR (1H-1H COSY, 1H-13C HSQC, 1H-13C HMBC, 1H-1H ROESY) spectra were collected using a Bruker Avance III Ultrashield 700 at 700 MHz for 1H and 175 MHz for 13C nuclei. MPLC separation was carried out on a Biotage Isolera One equipped with a Biotage SNAP Cartridge HP-Sil column (60 g). Semi-preparative HPLC was performed on a Varian HPLC system equipped with a Prostar 330 detector and an Agilent Zorbax SB-C13 column (250 mm × 9.4 mm, 5 μm). Preparative HPLC was performed on an Agilent 1260 Infinity LC equipped with an Agilent Eclipse XDB-C18 column (250 mm × 21.2 mm, 7 μm). LC-MS was conducted on an Agilent 1260 Infinity LC coupled to a 6230 TOF (HRESI) equipped with an Agilent Poroshell 120 EC-C18 column (50 mm × 4.6 mm, 2.7 μm). UV and IR spectra were obtained with NanoDrop 2000C (Thermo Scientific) and Spectrum One FT-IR (PerkinElmer) spectrophotometers, respectively. Optical rotations were measured using an AUTOPOL IV automatic polarimeter (Rudolph Research Analytical).
4.1.2. Bacterials trains and culture conditions
Fermentation of S. platensis SB12029 was performed using previously reported protocols.10,14,15 Briefly, spores of S. platensis were inoculated into Bacto™ tryptic soy broth (BD) and incubated at 28 °C and 250 rpm for 2 d. PTM fermentation medium supplemented with 3% (w/v) Amberlite XAD-16 resin (Sigma-Aldrich) was inoculated with 4% (v/v) of the resultant seed culture. After shaking at 250 rpm for 7 d at 28 °C, the fermented broth was harvested and centrifuged to pellet the resin.
4.1.3. Extraction and isolation
Extraction from resin used in small-scale fermentations of S. platensis SB12029 followed previously reported procedures.15 After harvesting the resin from the fermentation broth, the resin was subsequently washed three times with H2O and extracted three times with CH3OH. The CH3OH extract was directly used for LC-MS analysis. Liquid chromatography for LC-MS analysis was conducted using an 18 min solvent gradient (0.4 mL min−1) of 5–100% of either CH3CN or CH3OH containing 0.1% formic acid in H2O containing 0.1% formic acid.
For large-scale fermentation (6 L) of SB12029, 12 × 2 L baffled flasks containing 500 mL of PTM medium and supplemented with 15 g of Amberlite XAD-16 resin were inoculated with 16 mL of seed culture. After fermentation, the resin was harvested by centrifugation, washed three times with H2O, and extracted three times with ca. 600 mL of CH3OH. Methanol was removed in vacuo and the resulting oil was adsorbed onto C18 reverse-phase resin (Biotage) and fractionated by MPLC with an elution system of CH3OH-H2O (5–100%) to get four fractions (Fr01–Fr04). The major compounds of fractions Fr01 and Fr03 were PTM (1) and 3, and PTN (2) and 4, respectively. LC-MS analysis of fraction Fr04 revealed a dimeric compound with m/z of 899. Further preparative reverse-phase HPLC using a gradient elution system of 30–100% CH3CN in H2O containing 0.1% formic acid yielded 5 (50 mg).
Platensimycin S1 (3)
HRESIMS affording the [M + H]+ ion at m/z 458.1634 (calcd [M + H]+ for C24H28NO6S at 458.1634).
Platencin S1 (4)
HRESIMS affording the [M + H]+ ion at m/z 442.1681 (calcd [M + H]+ for C24H28NO5S at 442.1683).
Platensimycin D1 (5)
White solid; −194.3 (c 0.46, CH3OH); UV (DMSO) λmax (log ε) 304 (4.28), 259 (4.34) nm; IR (film) νmax 2963, 1655, 1607, 1537, 1446, 1380, 1302, 1235, 1084, 830 cm−1; 1H and 13C NMR data, see Table 1; HRESIMS affording the [M + H]+ ion at m/z 899.3425 (calcd [M + H]+ for C48H55N2O13S at 899.3419).
4.1.4. Permethylation of 3 yielding 6
A 150 mL (3 × 50 mL in 250 mL baffled flasks) fermentation of SB12029 was harvested as described above. The crude extract was subjected to preparative reverse-phase HPLC using a gradient elution system of 10–100% CH3OH in H2O containing 0.1% formic acid to afford a partially pure fraction (14.1 mg), the major compound of which was 3. The fraction was dissolved in a mixture of CH2Cl2/CH3OH (6:1, 2.8 mL) and 192 μL of (trimethylsilyl)diazomethane19 (2 M in diethyl ether, Sigma-Aldrich) was added and stirred at room temperature under argon. After stirring for 2.5 h, the LC-MS profile showed that 3 had completely disappeared. The solution was directly concentrated in vacuo and purified by semi-preparative reverse-phase HPLC using a gradient elution system of 20–75% CH3CN in H2O containing 0.1% formic acid to yield 6 (1.0 mg) as a white solid. −523.8 (c 0.08, CH3OH); UV (DMSO) λmax (log ε) 282 (3.96), 252 (3.95) nm; IR (film) 3270, 2965, 1670, 1591, 1464, 1413, 1294, 1257, 1102, 1002, 832 cm−1; 1H and 13C NMR data, see Table 2. HRESIMS affording the [M + H]+ ion at m/z 500.2105 (calcd [M + H]+ for C27H34NO6S at 500.2107).
4.2. Antibacterial assay
The antibacterial activities of compounds 5 and 6 were tested against S. aureus ATCC 25923 and M. luteus ATCC 9431. MIC values were determined using a 96-well plate format with Müller-Hinton (MH) broth.20 Each strain was grown in MH broth to an OD600 = 0.5, diluted 100× with MH broth, and 98 μL of the resultant cell culture was pipetted into each well. Two microliters of each compound, serially diluted in DMSO, were added to each well, resulting in final concentrations of 0.125–64 μg mL−1. DMSO and PTM (1) were used as negative and positive controls, respectively. The MIC values were determined after incubation at 37 °C for 18 h. Each MIC determination was performed in duplicate or triplicate.
Supplementary Material
Acknowledgments
This work is supported in part by the National Institutes of Health Grant GM114353.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.Wang J, Soisson SM, Young K, Shoop W, Kodali S, Galgoci A, Painter R, Parthasarathy G, Tang YS, Cummings R, Ha S, Dorso K, Motyl M, Jayasuriya H, Ondeyka J, Herath K, Zhang C, Hernandez L, Allocco J, Basilio A, Tormo JR, Genilloud O, Vicente F, Pelaez F, Colwell L, Lee SH, Michael B, Felcetto T, Gill C, Silver LL, Hermes JD, Bartizal K, Barrett J, Schmatz D, Becker JW, Cully D, Singh SB. Nature. 2006;441:358. doi: 10.1038/nature04784. [DOI] [PubMed] [Google Scholar]
- 2.Wang J, Kodali S, Lee SH, Galgoci A, Painter R, Dorso K, Racine F, Motyl M, Hernandez L, Tinney E, Colletti SL, Herath K, Cummings R, Salazar O, Gonzalez I, Basilio A, Vicente F, Genilloud O, Pelaez F, Jayasuriya H, Young K, Cully DF, Singh SB. Proc Natl Acad Sci U S A. 2007;104:7612. doi: 10.1073/pnas.0700746104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown AK, Taylor RC, Bhatt A, Futterer K, Besra GS. PLoS One. 2009;4:e6306. doi: 10.1371/journal.pone.0006306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brinster S, Lamberet G, Staels B, Trieu-Cuot P, Gruss A, Poyart C. Nature. 2009;458:83. doi: 10.1038/nature07772. [DOI] [PubMed] [Google Scholar]
- 5.Balemans W, Lounis N, Gilissen R, Guillemont J, Simmen K, Andries K, Koul A. Nature. 2010;463:E3. doi: 10.1038/nature08667. [DOI] [PubMed] [Google Scholar]
- 6.Wu M, Singh SB, Wang J, Chung CC, Salituro G, Karanam BV, Lee SH, Powles M, Ellsworth KP, Lassman ME, Miller C, Myers RW, Tota MR, Zhang BB, Li C. Proc Natl Acad Sci U S A. 2011;108:5378. doi: 10.1073/pnas.1002588108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singh SB, Jayasuriya H, Ondeyka JG, Herath KB, Zhang C, Zink DL, Tsou NN, Ball RG, Basilio A, Genilloud O, Diez MT, Vicente F, Pelaez F, Young K, Wang J. J Am Chem Soc. 2006;128:11916. doi: 10.1021/ja062232p. [DOI] [PubMed] [Google Scholar]
- 8.Jayasuriya H, Herath KB, Zhang C, Zink DL, Basilio A, Genilloud O, Diez MT, Vicente F, Gonzalez I, Salazar O, Pelaez F, Cummings R, Ha S, Wang J, Singh SB. Angew Chem, Int Ed. 2007;46:4684. doi: 10.1002/anie.200701058. [DOI] [PubMed] [Google Scholar]
- 9.Smanski MJ, Yu Z, Casper J, Lin S, Peterson RM, Chen Y, Wendt-Pienkowski E, Rajski SR, Shen B. Proc Natl Acad Sci U S A. 2011;108:13498. doi: 10.1073/pnas.1106919108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smanski MJ, Peterson RM, Rajski SR, Shen B. Antimicrob Agents Chemother. 2009;53:1299. doi: 10.1128/AAC.01358-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu Z, Smanski MJ, Peterson RM, Marchillo K, Andes D, Rajski SR, Shen B. Org Lett. 2010;12:1744. doi: 10.1021/ol100342m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu Z, Rateb ME, Smanski MJ, Peterson RM, Shen B. J Antibiot. 2013;66:291. doi: 10.1038/ja.2013.1. [DOI] [PubMed] [Google Scholar]
- 13.Smanski MJ, Casper J, Peterson RM, Yu Z, Rajski SR, Shen B. J Nat Prod. 2012;75:2158. doi: 10.1021/np3005985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hindra, Huang T, Yang D, Rudolf JD, Xie P, Xie G, Teng Q, Lohman JR, Zhu X, Huang Y, Zhao L-X, Jiang Y, Duan Y, Shen B. J Nat Prod. 2014;77:2296. doi: 10.1021/np5006168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rudolf JD, Dong LB, Huang T, Shen B. Mol BioSyst. 2015;11:2717. doi: 10.1039/c5mb00303b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singh SB, Jayasuriya H, Herath KB, Zhang C, Ondeyka JG, Zink DL, Ha S, Parthasarathy G, Becker JW, Wang J, Soisson SM. Tetrahedron Lett. 2009;50:5182. [Google Scholar]
- 17.Zhang C, Ondeyka J, Dietrich L, Gailliot FP, Hesse M, Lester M, Dorso K, Motyl M, Ha SN, Wang J, Singh SB. Bioorg Med Chem. 2010;18:2602. doi: 10.1016/j.bmc.2010.02.030. [DOI] [PubMed] [Google Scholar]
- 18.Zhang C, Ondeyka J, Guan Z, Dietrich L, Burgess B, Wang J, Singh SB. J Antibiot. 2009;62:699. doi: 10.1038/ja.2009.106. [DOI] [PubMed] [Google Scholar]
- 19.Hashimoto N, Aoyama T, Shioiri T. Chem Pharm Bull. 1981;29:1475. [Google Scholar]
- 20.Wiegand I, Hilpert K, Hancock REW. Nat Protoc. 2008;3:163. doi: 10.1038/nprot.2007.521. [DOI] [PubMed] [Google Scholar]
- 21.Singh SB, Herath KB, Wang J, Tsou N, Ball RG. Tetrahedron Lett. 2007;48:5429. [Google Scholar]
- 22.Saleem M, Hussain H, Ahmed I, van Ree T, Krohn K. Nat Prod Rep. 2011;28:1534. doi: 10.1039/c1np00010a. [DOI] [PubMed] [Google Scholar]
- 23.Martens E, Demain AL. J Antibiot. 2011;64:705. doi: 10.1038/ja.2011.80. [DOI] [PubMed] [Google Scholar]
- 24.Matthijs S, Baysse C, Koedam N, Tehrani KA, Verheyden L, Budzikiewicz H, Schaefer M, Hoorelbeke B, Meyer JM, De Greve H, Cornelis P. Mol Microbiol. 2004;52:371. doi: 10.1111/j.1365-2958.2004.03999.x. [DOI] [PubMed] [Google Scholar]
- 25.Matthijs S, Tehrani KA, Laus G, Jackson RW, Cooper RM, Cornelis P. Environ Microbiol. 2007;9:425. doi: 10.1111/j.1462-2920.2006.01154.x. [DOI] [PubMed] [Google Scholar]
- 26.Godert AM, Jin M, McLafferty FW, Begley TP. J Bacteriol. 2007;189:2941. doi: 10.1128/JB.01200-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.