Abstract
BACKGROUND
High-fat diet (HFD)-induced obesity may promote age-related memory impairment via disturbances of ammonia-glutamine metabolism.
OBJECTIVE
We studied the effects of age and long-term HFD exposure on glutamine synthetase (GS) expression in the liver and hippocampus and recognition memory in mice.
METHODS
Adult (5-month-old) and aged (15-month-old) male C57BL/6 mice were exposed to control diet (CD, 14% calories from fat) or HFD (60% fat). Novel place recognition testing was conducted and tissue was collected after 4 and 5 months on HFD, respectively. Tissue GS expression levels were assessed using immunohistochemistry and image analysis.
RESULTS
The obese mice developed moderate/severe hepatic steatosis. GS immunoreactivity was observed in perivenous hepatocytes and in hippocampal astrocytes and neuropil. Hepatic GS immunoreactivity density was higher in aged mice on HFD (n = 8) than CD (n = 13, P = 0.004). In aged mice, hippocampal GS immunoreactivity density was higher with HFD than CD (P = 0.037). In the novel place recognition test, aged mice were classified into impaired (n = 7) and unimpaired (n = 12), relative to adult mice (n = 22). Hippocampal GS immunoreactivity density was higher in impaired than unimpaired aged mice (P < 0.05).
CONCLUSION
Long-term exposure of aged mice to HFD was associated with increased GS expression in the liver and hippocampus. Novel place recognition impairment in aged mice was associated with increased hippocampal GS expression. These findings suggest that excess ammonia is involved in the age-related effects of HFD exposure and in neurotoxicity.
Keywords: Aging, GLUL, glutamine synthetase, high-fat diet, hippocampus, liver
1. INTRODUCTION
The prevalence of adult obesity has increased greatly during the past three decades especially in America and Europe [1]. Long-term consumption of energy-dense diets high in fats and refined sugars appears to be a major contributor to the current epidemic of obesity [2]. In certain geographic regions like Republic of Palau (an island country in Micronesia), a recent survey [3] found that obesity and lifestyle-related diseases were prevalent in both women and men and appeared to be associated with high intake of calories from carbohydrate rather than fat. Redistribution of body fat storage from subcutaneous to visceral adipose tissue occurs progressively with age. Whereas subcutaneous fat responds to the equilibrium between energy demand and fuel supply, visceral fat is not coordinated with metabolic pathways regulating the state of fat deposition [4]. The intra-abdominal visceral deposition of adipose tissue, which characterizes abdominal (central) obesity (assessed by the waist circumference or waist / hip ratio), is a prime contributor to the development of metabolic syndrome (e.g. hypertension, elevated plasma insulin concentration and insulin resistance, hyperglycemia, and dyslipidemia) [2]. The association of obesity with increased risks of several chronic diseases (e.g. non-alcoholic fatty liver disease, type 2 diabetes mellitus, cardiovascular disease, and osteoarthritis) is well documented [1, 2]. The effect of obesity on brain function has been explored in both human epidemiologic studies and experimental animal models [5–7]. Older individuals in particular may be more susceptible to adverse effects of diet-induced obesity on cognitive functioning than young adults. For example, a systematic review showed that midlife obesity was associated with low cognitive abilities in late life [5].
Long-term exposure of C57BL/6 mice to high-fat diet (HFD) induces obesity accompanied by insulin resistance and hepatic steatosis (fatty liver), similar to the profile of diet-induced obesity in humans [8]. Evidence from animal models suggests that HFD exposure might affect ammonia metabolism. In the analysis of exhaled breath air of awake male Wistar rats based on proton transfer reaction time of flight mass spectrometry (PTR-ToF-MS), the ammonia concentration was higher in HFD-fed animals than in those fed with regular diet [9]. The ammonia concentration in fresh cecal digesta was higher in male New Zealand white rabbits exposed to HFD compared to regular diet [10]. Under physiological conditions, the small and large intestines are major sources of ammonia in the blood circulation via enzymatic breakdown of amino acids (mainly glutamine) and bacterial breakdown of urea and amino acids, respectively [11, 12]. The hepatic portal vein delivers ammonia to the liver, where ammonia is incorporated into urea via the (low affinity, high capacity) urea cycle within periportal hepatocytes or glutamine via the (high affinity, low capacity) glutamine synthetase (GS) reaction within perivenous hepatocytes [11, 12]. In urea cycle disorders or conditions leading to a decrease in hepatic urea synthesis capacity, GS-dependent glutamine synthesis has been suggested to be the most important alternative pathway for ammonia detoxification [11]. This notion is supported by the finding that hyperglutaminemia and hyperammonemia occurred in parallel in patients affected by urea cycle disorders [13, 14]. Nonetheless, the relationship between of long-term HFD exposure and hepatic GS-dependent glutamine synthesis remains unclear.
In the central nervous system, GS is expressed mainly in astrocytes. One of the primary functions of astrocytes is to protect neurons from ammonia toxicity and glutamate-mediated excitotoxicity by taking up ammonia readily crossing the blood-brain barrier and glutamate at synapses and converting them into glutamine in the GS-dependent reaction [15]. Astrocytic GS expression may be altered in response to the increased ammonia levels induced by long-term HFD consumption.
In the present study, we investigated the effects of age and long-term HFD exposure on GS expression in the liver and hippocampus of mice, which could indicate disturbances of ammonia-glutamine metabolism. Hippocampus-sensitive spatial memory deficits were consistently identified in cognitive decline related to both old age and long-term HFD exposure [7, 16]. Therefore, we investigated the association between hippocampal GS expression and novel place recognition performance (spatial memory shown to depend primarily on the hippocampal integrity [17]). We hypothesized that long-term HFD exposure would alter GS expression in the liver and hippocampus of aged mice. The alteration in hippocampal GS expression would correlate with performance in the novel place recognition test.
2. MATERIALS AND METHODS
2.1. Animals
We studied a male C57BL/6 mouse cohort consisting of 32 adult (5 months old) and 32 aged (15 months old, former breeders) mice (Taconic Biosciences, Taconic Farms, Hudson, NY, USA). The mice were single-housed for the duration of the study in a humidity- and temperature-controlled animal facility on a 12-h/12-h reverse light/dark cycle (lights off at 7:00 a.m.) with ad libitum access to food and water. Behavioral testing was conducted during the dark phase of the light/dark cycle. All of the experiments were performed in accordance with the guidelines of the American Association for the Accreditation of Laboratory Animal Care and National Research Council’s Guide for the Care and Use of Laboratory Animals, and approved by the University of California San Diego Institutional Animal Care and Use Committee.
Each of the two age groups was equally divided into two diet groups of mice (n = 16 per group) fed with control diet (CD; 32% calories from protein, 14% fat, and 54% carbohydrate; 8604 Teklad, Harlan Laboratories, Placentia, CA, USA) or HFD (20% calories from protein, 60% fat, and 20% carbohydrate; D12492, Research Diets, New Brunswick, NJ, USA). The degree and relative pattern of body weight gain among four age/diet groups of mice at 3 and 4.5 months after diet intervention were reported elsewhere [18].
2.2. Behavioral Testing
Mice were tested for anxiety-like behavior and locomotor activity, and in a battery of cognitive tasks, as reported elsewhere [18]. In the present study, to investigate the behavioral correlate of GS expression in the hippocampal formation, we selected the hippocampus-dependent novel place recognition test [17] to perform additional analyses of our previously published behavioral data [18]. We also analyzed locomotor activity data because skeletal muscle has a large potential capacity for ammonia metabolism [11, 12]. During testing in both of these behavioral tasks, adult and aged mice were 8.5−9 and 18.5−19 months old, respectively, and had been subject to HFD or CD for 3.5−4 months.
Briefly, locomotor activity was assessed in the dark for 60 min in four open field arenas equipped with infrared beams (Med Associates, St. Albans, VT, USA) for calculating total distances traveled [18]. Novel place recognition testing consisted of habituation, familiarization, and test phases [18]. In the habituation phase, mice were given access to all three compartments of a Perspex box and allowed to explore the arena for 10 min. In the familiarization phase, mice were confined to the middle compartment for 1 min before the dividers were removed and mice were given 10-min access to the entire arena and two identical objects placed (one per compartment) at one end of the side compartments. In the test phase, mice were again confined to the middle compartment for 1 min before they were given 10-min access to the entire arena where one of the objects was moved to the novel place (the opposite end of the side compartment). Data were expressed as the discrimination ratio of the duration of object interaction (= [Novel − Familiar] / [Novel + Familiar]) [18]. Positive discrimination ratios indicated greater interaction with the object at the novel place during the test phase. Higher discrimination ratios denoted better recognition memory performance.
2.3. Tissue Harvest
For the entire cohort, mouse ulcerative dermatitis [19] affected 3 of 16 (18.8%) adult and 4 of 16 (25%) aged mice on HFD, and none of 16 adult and 16 aged mice on CD (P = 0.032, Fisher's exact test). Tissue collection was performed when adult and aged mice were 10 and 20 months old, respectively, and in diet intervention for 5 months. Mice were divided into three batches for tissue collection over three weeks with order counterbalanced for age/diet groups. The liver and hemi-brain sections were immediately immersion fixed in 4% buffered paraformaldehyde for 72 h and then histologically processed for paraffin embedment. Five-µm-thick tissue sections were taken with Superfrost/Plus microscope slides. One liver slide and one brain slide from each mouse were stained with hematoxylin and eosin for standard histologic examination.
2.4. Immunohistochemistry
The tissue sections were deparaffinized with xylene and rehydrated through graded ethanol series and water. The sections were treated for 30 min with 0.3% hydrogen peroxide/phosphate-buffered saline (PBS) to quench endogenous peroxidase activity and then incubated for 30 min with 2.5% normal horse serum. Following 24-h incubation at 4°C with rabbit anti-GS primary antibody (#G2781, Sigma-Aldrich, St. Louis, MO, USA; 1:50,000 dilution for liver sections, 1:60,000 for brain sections), the sections were rinsed in 0.1% Tween 20/PBS and then incubated for 40 min at room temperature with horse anti-rabbit IgG peroxidase-polymer secondary antibody (ImmPRESS, Vector Laboratories, Burlingame, CA, USA). Following washing with 0.1% Tween 20/PBS, the signals were developed with diaminobenzidine (ImmPACT DAB peroxidase substrate, Vector Laboratories) for 5 min. The liver sections were counterstained with hematoxylin for 5 min. All the sections were dehydrated through graded ethanol series, cleared in xylene, and mounted. For the negative reagent control, the primary antibody was omitted. For each of the liver and brain slide sets, all the slides were processed in the same immunostaining batch.
2.5. Quantification of Immunohistochemical Reactivity
The immunoreactivity signals were measured by means of two-dimensional computer-assisted image analysis. The immunostained tissue slides were scanned using a microscope slide scanner (Aperio ScanScope GL, Leica Biosystems, Buffalo Grove, IL, USA) equipped with a 20× objective lens (yielding the resolution of 0.5 µm per pixel). On the liver slide of each mouse, 3 different non-overlapping images (2,000 × 2,000 µm2 each) were extracted using the Aperio ImageScope software. For each liver image, the GS immunoreactivity signals were measured on the entire image using the Image-Pro Analyzer software (Version 6.3, Media Cybernetics, Bethesda, MA, USA). The same setting of histogram-based RGB color segmentation was applied to all liver images. The number of hepatocytes was estimated based on hematoxylin nuclear staining signals on the same image using the Image-Pro Analyzer software. To adjust for variability in the hepatocyte density among individual mice, the GS immunoreactivity level was normalized by the number of hepatocytes on each liver image. For each mouse, the average of three hepatic GS immunoreactivity density values was used for subsequent statistical analysis.
On the brain slide of each mouse, a 3,000 × 3,000 µm2 image covering the entire dorsal hippocampal formation was extracted from each of the 3 adjacent parasagittal brain sections on the same slide. On each hippocampus image, the outline of the entire hippocampal formation was digitally drawn with the Image-Pro Analyzer software, as previously described [20]. For each hippocampal formation, GS immunoreactivity signals were measured using the same color segmentation setting as that for the liver. The value of immunoreactivity normalized to the area measured (i.e. the immunoreactivity density) was calculated from three measurement values generated by the Image-Pro Analyzer software, as previously described [20]. For each mouse having three hippocampal GS immunoreactivity density values, one value that lay outside the mean ± one standard deviation was excluded and the average of the remaining two values was used for subsequent statistical analysis.
2.6. Statistical Analysis
Of 64 mice at the beginning of the study, nine (1 adult/HFD, 2 aged/CD, 6 aged/HFD) mice died before the scheduled tissue collection, and one aged/CD mouse was excluded from the study due to its extremely anxious behavior and severe body weight loss. The remaining 54 mice were subjected to histopathologic analyses.
The distribution of continuous data (original or transformed if necessary) in each mouse group was determined using the D' Agostino & Pearson omnibus normality test. Two-way analysis of variance (ANOVA) was employed with age and diet as between-subject factors for GS immunoreactivity density data and behavioral data. Post hoc comparisons were performed using the Holm-Sidak's multiple comparison test yielding adjusted P values. Other parametric statistical tests were used as indicated. The continuous data that did not pass the normality test were analyzed using non-parametric methods. Differences were considered statistically significant at two-tailed P < 0.05. The statistical analyses were performed on the GraphPad Prism 6 software (Version 6.0f, GraphPad Software, La Jolla, CA, USA) and (for Fisher's exact test) on http://www.physics.csbsju.edu/stats/exact.html.
3. RESULTS
3.1. Histopathology
Liver examination (n = 54) showed moderate/severe hepatic steatosis of mixed macro- and micro-vesicular type in all 15 adult and 9 of 10 aged mice on HFD but in none of 16 adult and 13 aged mice on CD (P < 0.0001, Fisher's exact test). One aged/HFD mouse having body weight loss did not show moderate/severe hepatic steatosis and thus was excluded from immunohistochemical analyses. All the obese mice had moderate/severe hepatic steatosis. In addition, there were background lesions (i.e. perivenous mononuclear inflammatory cell aggregates and multifocal neutrophil infiltrates/hepatocyte necrosis), which were distributed in all four age/diet groups without any significant difference in their frequencies (P = 0.28 and = 0.41, respectively, Fisher's exact test; data not shown).
Brain examination (n = 54) showed thalamic mineral deposits [19] in 3 of 13 aged/CD (23.1%) and 6 of 10 aged/HFD (60%) mice but not in any of 16 adult/CD or 15 adult/HFD mice (P < 0.0001, Fisher's exact test). In addition, one of aged/HFD mice had active leptomeningeal arteritis and was excluded from immunohistochemical analyses.
3.2. GS Immunoreactivity in the Liver
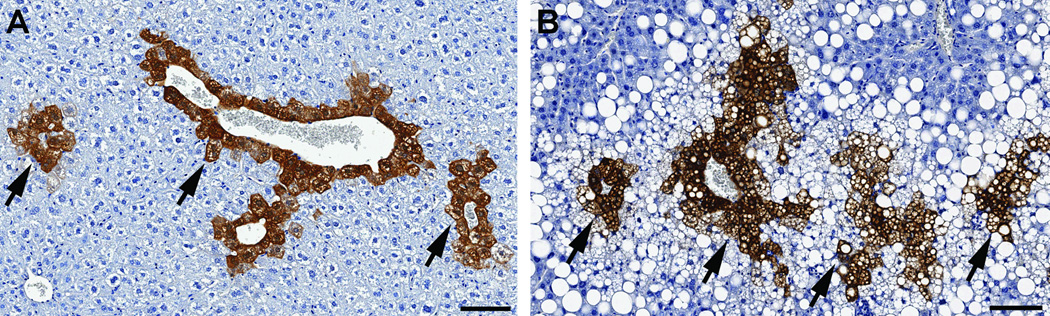
In the liver (n = 52), GS immunoreactivity was observed exclusively in perivenous hepatocytes (Fig. 1A and B). There was a main effect of diet on the hepatic GS immunoreactivity density (F(1,48) = 13.43, P = 0.0006; Fig. 2), as well as a trend for the main effect of age (F(1,48) = 3.76, P = 0.058). No significant age × diet interaction effect was present (F(1,48) = 2.10, P = 0.15). In aged mice the GS immunoreactivity density was higher with HFD than CD (adjusted P = 0.004); a similar trend was observed in adult mice (adjusted P = 0.083). On HFD feeding there was a trend for higher GS immunoreactivity density in aged mice compared to adult mice (adjusted P = 0.06), whereas on CD feeding no significant difference was found (adjusted P = 0.71).
Fig. (1).
Glutamine synthetase (GS) immunoreactivity in the liver of mice. Shown here are liver tissue sections from aged mice fed with control diet (A) or high-fat diet (HFD, B) for 5 months. GS immunoreactivity is present exclusively in perivenous hepatocytes (arrows). The HFD-fed mouse liver (B) is involved by moderate/severe mixed macro- and micro-vesicular hepatic steatosis (clear vacuoles). Diaminobenzidine immunohistochemical staining and hematoxylin counterstaining, scale bars = 100 µm.
Fig. (2).
Glutamine synthetase (GS) immunoreactivity density (arbitrary unit) in the liver among four age/diet groups of mice. In aged mice, the GS immunoreactivity density is higher with high-fat diet (HFD) than control diet (CD) feeding (**adjusted P = 0.004, Holm-Sidak's multiple comparison test following two-way analysis of variance). Data are expressed as mean and its 95% confidence interval.
3.3. GS Immunoreactivity in the Hippocampus
In the hippocampal formation (n = 52), GS immunoreactivity was observed in astrocytes and neuropil (Fig. 3A and B). There was an age × diet interaction effect on the hippocampal GS immunoreactivity density (F(1,48) = 5.73, P = 0.021; Fig. 4). In aged mice the GS immunoreactivity density was higher with HFD than CD (adjusted P = 0.037), whereas in adult mice no significant difference was observed (adjusted P = 0.43). On CD feeding there was a trend for lower GS immunoreactivity density in aged mice compared to adult mice (adjusted P = 0.059), whereas on HFD feeding the apparent difference was not statistically significant (adjusted P = 0.22).
Fig. (3).
Glutamine synthetase (GS) immunoreactivity in the dorsal hippocampal formation of mice. Shown here are brain tissue sections from aged mice fed with control diet (A) or high-fat diet (B) for 5 months. GS immunoreactivity is observed in astrocytes (B inset) and neuropil. Note that hippocampal pyramidal neurons and dentate gyrus granule cells are not immunoreactive for GS (clear layers). Diaminobenzidine immunohistochemical staining, scale bars = 200 µm.
Fig. (4).
Glutamine synthetase (GS) immunoreactivity density (arbitrary unit) in the dorsal hippocampal formation among four age/diet groups of mice. In aged mice, the GS immunoreactivity density is higher with high-fat diet (HFD) than control diet (CD) feeding (*adjusted P = 0.037, Holm-Sidak's multiple comparison test following two-way analysis of variance). Data are expressed as mean and its 95% confidence interval.
There was no significant correlation in GS immunoreactivity density between the hippocampus and liver in any of four age/diet groups (adult/CD: r = −0.14, P = 0.61, n = 16; adult/HFD: r = −0.14, P = 0.63, n = 15; aged/CD: r = −0.31, P = 0.31, n = 13; aged/HFD: r = 0.35, P = 0.40, n = 8, Pearson's correlation coefficient; data not shown).
3.4. Novel Place Recognition Memory and Hippocampal GS Immunoreactivity
Of 64 mice at the start of the study, 45 (12 adult/CD, 11 adult/HFD, 13 aged/CD, 9 aged/HFD) successfully underwent novel place recognition testing, whereas 15 failed to interact with any of the objects during familiarization phase, one showed extremely anxious behavior and severe body weight loss, and three died before behavioral testing, as previously reported [18]. In the test phase (n = 45), a trend for the main effect of age on discrimination ratios was found (F(1,41) = 3.73, P = 0.06), whereas there was no significant main effect of diet (F(1,41) = 0.44, P = 0.51) or age × diet interaction effect (F(1,41) = 1.25, P = 0.27; data not shown). Without regard to diet, there was a trend toward lower discrimination ratios in aged mice (n = 22, mean 0.11 [95% confidence interval (CI) −0.03, 0.25]) compared to adult mice (n = 23, mean 0.26 [95% CI 0.17, 0.35]; t(43) = 1.87, P = 0.07, unpaired t-test). Accordingly, aged mice with discrimination ratios below one standard deviation from the mean discrimination ratio of the 23 adult mice (i.e. discrimination ratios < 0.047) were classified as impaired aged (n = 8 [3 CD and 5 HFD]) and the remaining aged mice as unimpaired aged (n = 14 [10 CD and 4 HFD]). Similar statistics-based criteria have been used in the literature to classify the cognitive performance across aged rodents in relation to the young counterpart [21, 22]. No significant association between HFD exposure and novel place recognition memory impairment was observed in aged mice (P = 0.19, Fisher's exact test).
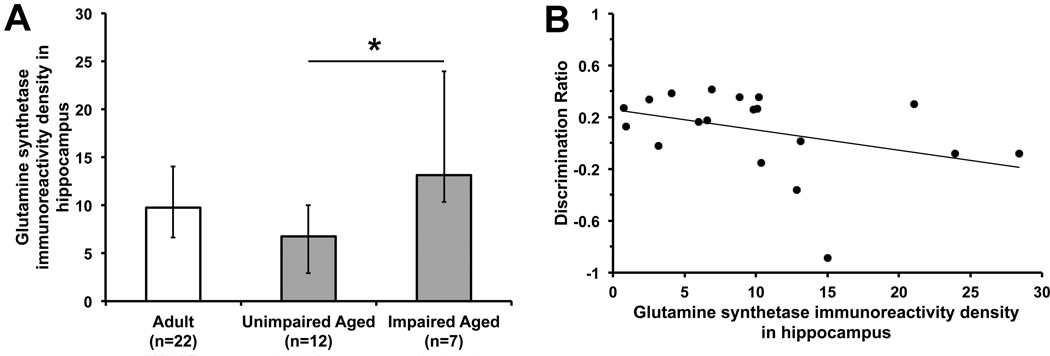
For behavioral-immunohistochemical analyses, there were 41 mice (12 adult/CD, 10 adult/HFD, 12 aged/CD, 7 aged/HFD) that had data on both behavioral performance and hippocampal GS immunoreactivity density. Differences in the hippocampal GS immunoreactivity density were observed among adult (n = 22), unimpaired aged (n = 12 [9 CD and 3 HFD]), and impaired aged (n = 7 [3 CD and 4 HFD]) mice (H(2) = 6.61, P = 0.037, Kruskal-Wallis test; Fig. 5A), in which the GS immunoreactivity density was higher in impaired compared to unimpaired aged mice (adjusted P < 0.05, Dunn's post hoc multiple comparison test). No significant difference in the GS immunoreactivity density was found between adult mice and unimpaired or impaired aged mice (adjusted P > 0.05). There was a trend toward inverse correlation between the hippocampal GS immunoreactivity density and discrimination ratio in aged mice (rs = −0.44, P = 0.057, n = 19, Spearman's rank correlation test; Fig. 5B), whereas no significant correlation was found in adult mice (rs = −0.14, P = 0.55, n = 22; data not shown).
Fig. (5).
A. Glutamine synthetase (GS) immunoreactivity density (arbitrary unit) in the dorsal hippocampal formation among adult, unimpaired aged, and impaired aged mice with regard to novel place recognition memory. The hippocampal GS immunoreactivity density is higher in impaired than unimpaired aged mice (*adjusted P < 0.05, Dunn's multiple comparison test following non-parametric Kruskal-Wallis test). Data are expressed as median and interquartile range. B. Scattergraph with its trend line shows a trend toward inverse correlation between the hippocampal GS immunoreactivity density and discrimination ratio of the duration of object interaction (= [Novel − Familiar] / [Novel + Familiar]) in the test phase of the novel place recognition task in aged mice (rs = −0.44, P = 0.057, n = 19, Spearman's rank correlation test).
Regarding the 12 aged/CD mice, the hippocampal GS immunoreactivity density in impaired mice (n = 3, median 12.85 [interquartile range (IQR) 10.34, 15.03]) was higher than that in unimpaired mice (n = 9, median 5.97 [IQR 1.72, 7.88]; P = 0.009, Mann-Whitney U test). For the seven aged/HFD mice, the apparent difference in the hippocampal GS immunoreactivity density between impaired mice (n = 4, median 18.54 [IQR 5.68, 27.28]) and unimpaired mice (n = 3, median 10.21 [IQR 10.08, 21.07]) was not statistically significant (P = 0.57, Mann-Whitney U test).
3.5. Locomotor Activity
For behavioral-immunohistochemical analyses, the 41 mice were included. On the total distance traveled in the open field (log10-transformed data), there were main effects of both age (F(1,37) = 20.85, P < 0.0001) and diet (F(1,37) = 4.89, P = 0.033). No significant age × diet interaction effect was observed (F(1,37) = 1.29, P = 0.26). Aged mice had lower locomotor activity than adult mice on both CD and HFD feeding (adjusted P = 0.0001 and = 0.032, respectively). In adult mice, HFD-fed animals had lower locomotor activity than CD-fed animals (adjusted P = 0.034); however, in aged mice the difference was not statistically significant (adjusted P = 0.47; data not shown).
When the 41 mice were classified into subgroups based on their performance in the test phase of the novel place recognition task, there were differences in locomotor activity (log10-transformed data) among adult (n = 22, mean 3.81 [95% CI 3.74, 3.89]), unimpaired aged (n = 12, mean 3.61 [95% CI 3.52, 3.69]), and impaired aged (n = 7, mean 3.62 [95% CI 3.54, 3.71]) mice (F(2,38) = 9.52, P = 0.0004, one-way ANOVA). Whereas adult mice had higher locomotor activity than both unimpaired (adjusted P < 0.01, Tukey's post hoc multiple comparison test) and impaired (adjusted P < 0.05) aged mice, no significant difference was found between unimpaired and impaired aged mice (adjusted P > 0.05).
4. DISCUSSION
The present study showed higher GS immunoreactivity density in the liver of mice on HFD compared to CD feeding. The association between HFD exposure and increased hepatic GS immunoreactivity density was more pronounced in aged mice than in adult mice. It is possible that hepatic GS expression was increased in response to hyperammonemia induced by long-term HFD exposure. In support of this hypothesis, a study using male Wistar rats [9] showed that two-month HFD exposure was associated with higher ammonia concentration in exhaled breath air analyzed by PTR-ToF-MS, as compared to regular diet feeding. In male New Zealand white rabbits, four-week exposure to HFD was associated with higher ammonia concentration in fresh cecal digesta [10]. Since ammonia in the blood circulation derives largely from the intestines [11, 12], excess ammonia may result from changes in the gut microbiota composition [23, 24] in the way that promotes ammonia production [25]. A recent study revealed an increased relative abundance of Proteus mirabilis in rats exposed to HFD for 16 weeks, which correlated directly with metabolic parameters of obesity, as compared to regular diet feeding [25]. Proteus mirabilis is one of ammonia-producing bacteria using the enzyme urease [26]. The hepatic urea synthesis was probably suppressed in HFD-fed animals in our study, as previously reported in a rat model of HFD-induced steatohepatitis showing decreased expression of hepatic urea cycle enzymes and a reduction in the whole body urea-nitrogen synthesis [27]. Consequently, excess ammonia was diverted to hepatic GS-dependent glutamine synthesis, as proposed to be the case in patients with urea cycle disorders [11, 13, 14]. It seems reasonable to expect that hyperglutaminemia, in addition to hyperammonemia, may have occurred in HFD-fed mice in our cohort.
Excess levels of both ammonia and glutamine in the systemic circulation readily cross the blood-brain barrier [15]. In the hippocampus, we found a strong trend toward lower GS immunoreactivity density in aged mice compared to adult mice on CD feeding. This finding is in agreement with that in an immunohistologic study by Rodriguez et al. [28] showing that the GS-immunoreactive astrocytic cell area was lower in the hippocampal cornu ammonis-1 and dentate gyrus of 24-month-old male SV129/C57BL6 mice compared to the 3-month-old counterpart. It is possible that a decrease in hippocampal GS expression with aging observed in our study was attributed to a reduction in ammonia production by skeletal muscle dependent on the intensity and duration of physical activity in aged mice [12], as we found that aged mice had lower locomotor activity than adult mice on CD feeding.
We found that hippocampal GS immunoreactivity density in HFD-fed aged mice was higher than that in CD-fed aged mice but not significantly higher than that in HFD-fed adult mice. As stated above, there was a strong trend toward lower GS immunoreactivity density in aged mice compared to adult mice on CD feeding, suggesting a decrease in hippocampal GS expression with aging. Therefore, the difference in hippocampal GS immunoreactivity density in aged mice between HFD and CD feeding seemed to result probably from both a decrease in GS expression in CD-fed animals and an increase in GS expression in HFD-fed animals. There was no significant difference in locomotor activity among aged mice with respect to diet (CD vs. HFD). It is possible that HFD-related excess ammonia up-regulated hippocampal GS expression and induced GS-dependent glutamine synthesis in the hippocampus of aged mice on HFD feeding [11, 12]. Changes in brain GS expression or activity with age or in response to metabolic disturbances appear to be region-specific. In studying the effects of age on astrocytic profiles in mice, Rodriguez et al. [28] observed an age-related decrease in GS-immunoreactive astrocytic cell area in the hippocampal formation, but not in the entorhinal cortex. Acosta et al. [29] found the GS enzyme activity to be increased in the hippocampus but decreased in the frontal cortex of male Wistar rats with surgically induced portal hypertension and hyperammonemia.
With regard to the novel place recognition task (a hippocampus-dependent spatial memory test [17]), we found that hippocampal GS immunoreactivity density was higher in impaired (n = 7) compared to unimpaired (n = 12) aged mice regardless of diet (12 CD and 7 HFD), whereas the hippocampal GS immunoreactivity density in unimpaired aged mice was not significantly different from that in adult mice (used as the normal memory reference). Specifically considering the subgroup of 12 aged mice on CD feeding, the hippocampal GS immunoreactivity density was also higher in impaired (n = 3) than in unimpaired (n = 9) animals. For the subgroup of seven aged mice on HFD feeding (4 impaired and 3 unimpaired), the apparently similar difference did not reach statistical significance, which was probably related to the small sample sizes. These findings suggest that relatively higher levels of hippocampal GS immunoreactivity density in impaired aged mice were abnormal, which occurred in response to excess levels of ammonia. Ammonia itself could induce cellular oxidative stress and energy deficit through disturbances of the nitric oxide pathway, inhibition of the Krebs cycle, and opening of the mitochondrial permeability transition [30]. Ammonia could also mediate the inhibition of glutamate uptake into astrocytes via the down-regulation of glutamate transporters, which might lead to glutamate-mediated neuronal excitotoxicity [30]. Glutamine (both entering the brain from the systemic circulation and formed in situ in astrocytes) could enter the mitochondria and be converted into glutamate and ammonia by the enzyme glutaminase, contributing to ammonia-induced mitochondrial dysfunction [30]. In agreement with this hypothesis, clinical studies of patients with urea cycle disorders showed that hyperglutaminemia and hyperammonemia occurred in parallel and both correlated with neurocognitive impairment [13, 14].
Although skeletal muscle has a large potential capacity for the production, uptake, and metabolism of ammonia, the skeletal musculature at rest may not be significantly involved in ammonia metabolism due to its low levels of GS activity and ammonia uptake [11, 12]. In our present study, there was no significant difference in locomotor activity among aged mice with regard to novel place recognition memory (unimpaired vs. impaired). Accordingly, it is unlikely that ammonia metabolism in the musculature had significantly accounted for specific GS immunoreactivity changes found in the hippocampus of aged mice.
We found no significant correlation in GS immunoreactivity density between the hippocampus and liver in any of four age/diet groups. In aged mice, a difference in GS immunoreactivity density between HFD and CD feeding was observed in both the hippocampus and liver. However, in the hippocampus the difference seemed to result probably from both a decrease in GS expression in CD-fed mice and an increase in GS expression in HFD-fed mice (Fig. 4), whereas in the liver the difference appeared to result only from an increase in GS expression in HFD-fed mice (Fig. 2). While there was evidence suggesting a decrease in GS expression with aging (i.e. on CD feeding) in the hippocampus, there was not such evidence in the liver. These findings may be related to the fact that the liver receives ammonia from the small and large intestines as major sources through the hepatic portal vein, as well as from the kidney and contracting skeletal muscle via the systemic circulation, whereas the central nervous system receives ammonia only through the systemic circulation [11, 12].
In addition to the relationship between diet-induced obesity and neurocognitive impairment, in cognitive aging research the potential neuroprotective effects of certain dietary practices [e.g. adherence to the Mediterranean diet (food rich in dietary fiber and mono- and poly-unsaturated fatty acids with a low ratio of omega-6 to omega-3 fatty acids [2]) and caloric restriction] and specific nutrient intake (e.g. fish oil, antioxidants, and vitamins) have received much attention [31]. Although observational studies have in general demonstrated neuroprotective effects of healthier dietary practices and dietary supplementation, randomized controlled trials of dietary intervention have yielded small benefits or equivocal findings [31].
Our present study was limited by the absence of data on ammonia and glutamine levels in blood and hippocampal tissue to support the proposed mechanistic scenario based on alterations in the GS immunoreactivity density in both the liver and brain. Data on the GS enzyme activity were not available in either the liver or hippocampus. Blood test results of liver function tests were not available. More aged mice than adult mice, particularly those aged mice exposed to HFD, died prior to tissue collection. Therefore, a selection bias might be introduced because the tissue analyses were performed only on samples from mice that survived. High attrition rates are common in age-related studies using C57BL/6 mice since spontaneous cancers, hematopoietic and vascular neoplasms in particular, are prevalent in this inbred strain [19].
CONCLUSION
Long-term exposure of aged mice to HFD was associated with increased GS expression in the liver and hippocampus. These findings suggest that excess ammonia is involved in the age-related effect of HFD exposure. The finding that impaired novel place recognition memory in aged mice was associated with increased hippocampal GS expression suggests that excess ammonia and glutamine may be a factor in neurotoxicity [30]. Further studies are warranted to identify alterations in the gut microbiota composition, to measure ammonia and glutamine levels in blood and brain tissue, and to measure the GS enzyme activity in the liver and brain in animal models of HFD-induced obesity.
Acknowledgments
This work was supported by Sam and Rose Stein Institute for Research on Aging and U.S. National Institutes of Health grants: R01 MH094151 and T32 MH019934 (D.V. Jeste), P50 DA026306 (S. Semenova, C.L. Achim, V. Soontornniyomkij), and R25 MH081482 (J.P. Kesby).
LIST OF ABBREVIATIONS
- ANOVA
analysis of variance
- CD
control diet
- CI
confidence interval
- GS
glutamine synthetase
- HFD
high-fat diet
- IQR
interquartile range
- PBS
phosphate-buffered saline
- PTR-ToF-MS
proton transfer reaction time of flight mass spectrometry
Footnotes
CONFLICT OF INTEREST
All of the authors declare that they have no conflicts of interest.
D.V. Jeste conceived, designed and supervised the study, and obtained funding. V. Soontornniyomkij reviewed the literature, designed the study, optimized immunohistochemistry protocols, examined histopathology, performed image analysis, analyzed the data, interpreted the results, wrote the first article draft, and completed the final article. J.P. Kesby designed and performed animal behavioral testing, collected the data, and wrote the behavioral testing part. S. Semenova designed and supervised animal behavioral testing, and revised the behavior-related parts. B. Soontornniyomkij collected and processed tissue specimens, performed immunohistochemical experiments, and scanned tissue slides. J.J. Kim designed the study and performed tissue harvest. T. Kisseleva and C.L. Achim designed and supervised the study. All the authors approved the final article.
REFERENCES
- 1.Haslam DW, James WP. Obesity. Lancet. 2005;366(9492):1197–1209. doi: 10.1016/S0140-6736(05)67483-1. [DOI] [PubMed] [Google Scholar]
- 2.Kopelman P. Health risks associated with overweight and obesity. Obes Rev. 2007;8(Suppl 1):13–17. doi: 10.1111/j.1467-789X.2007.00311.x. [DOI] [PubMed] [Google Scholar]
- 3.Komatsu F, Kagawa Y, Kawabata T, Kaneko Y, Ishiguro K. Relationship of dietary habits and obesity to oxidative stress in Palauan people: compared with Japanese and Mongolian people. Curr Aging Sci. 2009;2(3):214–222. doi: 10.2174/1874609810902030214. [DOI] [PubMed] [Google Scholar]
- 4.Zafon C. Fat and aging: A tale of two tissues. Curr Aging Sci. 2009;2(2):83–94. doi: 10.2174/1874609810902020083. [DOI] [PubMed] [Google Scholar]
- 5.Dahl AK, Hassing LB. Obesity and cognitive aging. Epidemiol Rev. 2013;35(1):22–32. doi: 10.1093/epirev/mxs002. [DOI] [PubMed] [Google Scholar]
- 6.Freeman LR, Haley-Zitlin V, Rosenberger DS, Granholm AC. Damaging effects of a high-fat diet to the brain and cognition: a review of proposed mechanisms. Nutr Neurosci. 2014;17(6):241–251. doi: 10.1179/1476830513Y.0000000092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Winocur G, Greenwood CE. Studies of the effects of high fat diets on cognitive function in a rat model. Neurobiol Aging. 2005;26(Suppl 1):46–49. doi: 10.1016/j.neurobiolaging.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 8.Collins S, Martin TL, Surwit RS, Robidoux J. Genetic vulnerability to diet-induced obesity in the C57BL/6J mouse: physiological and molecular characteristics. Physiol Behav. 2004;81(2):243–248. doi: 10.1016/j.physbeh.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 9.Aprea E, Morisco F, Biasioli F, et al. Analysis of breath by proton transfer reaction time of flight mass spectrometry in rats with steatohepatitis induced by high-fat diet. J Mass Spectrom. 2012;47(9):1098–1103. doi: 10.1002/jms.3009. [DOI] [PubMed] [Google Scholar]
- 10.Jurgonski A, Juskiewicz J, Zdunczyk Z, Matusevicius P, Kolodziejczyk K. Polyphenol-rich extract from blackcurrant pomace attenuates the intestinal tract and serum lipid changes induced by a high-fat diet in rabbits. Eur J Nutr. 2014;53(8):1603–1613. doi: 10.1007/s00394-014-0665-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olde Damink SWM, Jalan R, Dejong CHC. Interorgan ammonia trafficking in liver disease. Metab Brain Dis. 2009;24(1):169–181. doi: 10.1007/s11011-008-9122-5. [DOI] [PubMed] [Google Scholar]
- 12.Wilkinson DJ, Smeeton NJ, Watt PW. Ammonia metabolism, the brain and fatigue; revisiting the link. Prog Neurobiol. 2010;91(3):200–219. doi: 10.1016/j.pneurobio.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 13.Serrano M, Ormazábal A, Vilaseca MA, et al. Assessment of plasma ammonia and glutamine concentrations in urea cycle disorders. Clin Biochem. 2011;44(8–9):742–744. doi: 10.1016/j.clinbiochem.2011.03.136. [DOI] [PubMed] [Google Scholar]
- 14.Batshaw ML, Tuchman M, Summar M, Seminara J Members of the Urea Cycle Disorders. A longitudinal study of urea cycle disorders. Mol Genet Metab. 2014;113(1–2):127–130. doi: 10.1016/j.ymgme.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suárez I, Bodega G, Fernández B. Glutamine synthetase in brain: effect of ammonia. Neurochem Int. 2002;41(2–3):123–142. doi: 10.1016/s0197-0186(02)00033-5. [DOI] [PubMed] [Google Scholar]
- 16.Klencklen G, Després O, Dufour A. What do we know about aging and spatial cognition? Reviews and perspectives. Ageing Res Rev. 2012;11(1):123–135. doi: 10.1016/j.arr.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 17.Barker GR, Warburton EC. When is the hippocampus involved in recognition memory? J Neurosci. 2011;31(29):10721–10731. doi: 10.1523/JNEUROSCI.6413-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kesby JP, Kim JJ, Scadeng M, et al. Spatial cognition in adult and aged mice exposed to high-fat diet. PLoS One. 2015;10(10):e0140034. doi: 10.1371/journal.pone.0140034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brayton CF, Treuting PM, Ward JM. Pathobiology of aging mice and GEM: background strains and experimental design. Vet Pathol. 2012;49(1):85–105. doi: 10.1177/0300985811430696. [DOI] [PubMed] [Google Scholar]
- 20.Soontornniyomkij V, Risbrough VB, Young JW, et al. Short-term recognition memory impairment is associated with decreased expression of FK506 binding protein 51 in the aged mouse brain. Age (Dordr) 2010;32(3):309–322. doi: 10.1007/s11357-010-9145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pawlowski TL, Bellush LL, Wright AW, Walker JP, Colvin RA, Huentelman MJ. Hippocampal gene expression changes during age-related cognitive decline. Brain Res. 2009;1256:101–110. doi: 10.1016/j.brainres.2008.12.039. [DOI] [PubMed] [Google Scholar]
- 22.Rowe WB, Blalock EM, Chen KC, et al. Hippocampal expression analyses reveal selective association of immediate-early, neuroenergetic, and myelinogenic pathways with cognitive impairment in aged rats. J Neurosci. 2007;27(12):3098–3110. doi: 10.1523/JNEUROSCI.4163-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mujico JR, Baccan GC, Gheorghe A, Díaz LE, Marcos A. Changes in gut microbiota due to supplemented fatty acids in diet-induced obese mice. Br J Nutr. 2013;110(4):711–720. doi: 10.1017/S0007114512005612. [DOI] [PubMed] [Google Scholar]
- 24.Hildebrandt MA, Hoffmann C, Sherrill-Mix SA, et al. High-fat diet determines the composition of the murine gut microbiome independently of obesity. Gastroenterology. 2009;137(5):716–724. e1–e2. doi: 10.1053/j.gastro.2009.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lecomte V, Kaakoush NO, Maloney CA, et al. Changes in gut microbiota in rats fed a high fat diet correlate with obesity-associated metabolic parameters. PLoS One. 2015;10(5):e0126931. doi: 10.1371/journal.pone.0126931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mobley HL, Island MD, Hausinger RP. Molecular biology of microbial ureases. Microbiol Rev. 1995;59(3):451–480. doi: 10.1128/mr.59.3.451-480.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thomsen KL, Grønbæk H, Glavind E, et al. Experimental nonalcoholic steatohepatitis compromises ureagenesis, an essential hepatic metabolic function. Am J Physiol Gastrointest Liver Physiol. 2014;307(3):G295–G2301. doi: 10.1152/ajpgi.00036.2014. [DOI] [PubMed] [Google Scholar]
- 28.Rodríguez JJ, Yeh CY, Terzieva S, Olabarria M, Kulijewicz-Nawrot M, Verkhratsky A. Complex and region-specific changes in astroglial markers in the aging brain. Neurobiol Aging. 2014;35(1):15–23. doi: 10.1016/j.neurobiolaging.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 29.Acosta GB, Fernández MA, Roselló DM, Tomaro ML, Balestrasse K, Lemberg A. Glutamine synthetase activity and glutamate uptake in hippocampus and frontal cortex in portal hypertensive rats. World J Gastroenterol. 2009;15(23):2893–2899. doi: 10.3748/wjg.15.2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Norenberg MD, Rama Rao KV, Jayakumar AR. Signaling factors in the mechanism of ammonia neurotoxicity. Metab Brain Dis. 2009;24(1):103–117. doi: 10.1007/s11011-008-9113-6. [DOI] [PubMed] [Google Scholar]
- 31.Smith PJ, Blumenthal JA. Diet and neurocognition: Review of evidence and methodological considerations. Curr Aging Sci. 2010;3(1):57–66. doi: 10.2174/1874609811003010057. [DOI] [PMC free article] [PubMed] [Google Scholar]