Abstract
In mouse skin, sulfur mustard is a potent vesicant, damaging both the epidermis and the dermis. The extent of wounding is dependent on the dose of sulfur mustard and the duration of exposure. Initial responses include erythema, pruritus, edema, and xerosis; this is followed by an accumulation of inflammatory leukocytes in the tissue, activation of mast cells, and the release of mediators, including proinflammatory cytokines and bioactive lipids. These proinflammatory mediators contribute to damaging the epidermis, hair follicles, and sebaceous glands and to disruption of the epidermal basement membrane. This can lead to separation of the epidermis from the dermis, resulting in a blister, which ruptures, leading to the formation of an eschar. The eschar stimulates the formation of a neoepidermis and wound repair and may result in persistent epidermal hyperplasia. Epidermal damage and repair is associated with upregulation of enzymes generating proinflammatory and progrowth/pro–wound healing mediators, including cyclooxygenase-2 (COX-2), which generates prostanoids, inducible nitric oxide synthase (iNOS), which generates nitric oxide, fibroblast growth factor receptor 2 (FGFR2), and galectin-3. Characterization of the mediators regulating structural changes in the skin during sulfur mustard–induced tissue damage and wound healing will aid in the development of therapeutic modalities to mitigate toxicity and stimulate tissue repair processes.
Keywords: vesicants, skin, alkylating agents, DNA damage, sulfur mustard
Introduction
One of the more prominent skin-active chemical warfare agents is sulfur mustard (SM; bis(2-chloroethyl)sulfide), a highly reactive alkylating agent targeting many cellular components, including DNA, proteins, and lipids.1,2 Early responses of human skin to SM include erythema, pruritis, and inflammation, followed by degradation of the epidermis and dermis with the formation of fluid-filled blisters.3,4 Depending on the dose, the first signs of SM exposure usually develop after a latency of 2–24 h.5 Subsequently, fluid-filled blisters burst, resulting in the formation of necrotic lesions.6 Typically, mustard wounds resolve over several months; however, they often lead to changes in pigmentation, xerosis, and scarring.7,8 Understanding the cellular and molecular mechanisms leading to SM-induced skin injury and prolonged wound healing will be important in the development of effective medical countermeasures.
Experimental model systems in rabbits, pigs, and rodents have been developed to characterize the mechanisms of dermal injury induced by SM.9–14 The best characterized of these models involves the application of mustard as a dilute solution or in a vapor cup to mouse skin.10,15,16 In the vapor-cup model, 13-mm diameter vapor cups are mounted on the dorsal skin on both sides of the spine, producing an SM-exposed site and a contralateral control. SM vapor was produced by absorbing 10 μL of neat SM onto a filter disk within the vapor-cup assembly.11,17 Cutaneous responses to SM in this model include delayed onset of inflammation, microblister, and eschar formation at sites of exposure.18 This model offers the opportunity not only to understand the mechanisms of cutaneous injury, but also to screen for candidate therapeutics to mitigate SM-induced tissue injury.
Characteristics of cutaneous injury and wound healing following SM exposure
Studies in our laboratory have focused on exposure of the dorsal skin of SKH-1 hairless mice to SM using the vapor-cup model.10,11,19 The initial characteristic inflammatory responses in mouse skin after SM exposure include erythema and edema. This is followed by desquamation of the stratum corneum and disorganization of the basal layer, including changes in the cuboidal and columnar cells with karyolysis. Structural alterations are also observed in the rete ridges that appear flattened, decreased in numbers, and acellular with cell membrane remnants. Increased expression of keratin 10, an early marker of keratinocyte differentiation important in the formation of the stratum corneum, is also observed in suprabasal layers of the epidermis. SM treatment also results in collagen fibril compaction within the dermis. The papillary dermis becomes indistinguishable from the reticular dermis owing to thickened collagen fibrils and increased cellularity. Hemorrhage and inflammatory cells are also evident, most prominently between the dermal papillae. Hair follicles and sebaceous glands are also targets of SM.11 Following exposure, pilosebaceous units undergo degeneration and are not replaced during wound repair.11 In hair follicles, epidermal karyolysis within the hair root sheath, infundibulum, and isthmus are apparent. Simultaneously, decreases in numbers of sebocytes are apparent in sebaceous glands; the remaining cells express only low levels of fatty acid synthase, a key enzyme regulating the production of sebum. The remnant hair follicles and sebaceous glands undergo hyperplastic transformation and form engorged lamellated follicular cysts.10 Loss of the pilosebaceous unit has been suggested as a cause of xerosis in humans after exposure to SM.7,20
Loss of the viable epidermis following SM exposure is correlated with formation of a blister in humans and an eschar in mice. This is thought to be due to the breakdown of basement membrane proteins, including laminins, type IV collagen, and glycosaminoglycans.16 Degradation of the basement membrane is mediated by proteinases, including matrix metalloproteinases (MMP), particularly MMP-9.16 These enzymes also contribute to structural changes in the supporting papillary and reticular dermis.21,22 The eschar stimulates formation of a neoepidermis and the repair processes.
Upregulation of mediators of inflammation in the skin following SM exposure
One of the hallmarks of SM-induced skin injury is inflammation. This is associated with erythema and edema, as well as immune activation of stromal cells, including keratinocytes, Langerhans cells, and resident inflammatory cells, as well as an accumulation of infiltrating immune cells in the tissue.4,20 It is well established that these cells release large quantities of inflammatory mediators, including proteases, reactive oxygen and nitrogen species, eicosanoids, and cytokines, such as tumor necrosis factor α and interleukin (IL)-1β, which can contribute to tissue injury. These cells also release mediators, which contribute to tissue repair.23 In SM-exposed skin, we have identified mustard-induced modulation of mediators, including IL-1β, IL-6, tumor necrosis factor α, COX2, and vascular cell adhesion molecule (VCAM). These findings are consistent with a role for inflammatory mediators in tissue injury.24
In keratinocytes, we also found that cyclooxygenase-2 (COX-2), which generates prostaglandin E2, was upregulated after SM exposure, along with inducible nitric oxide synthase (iNOS), which generates nitric oxide, and galectin-3, a galactoside-binding protein whose expression and secretion are important in controlling proliferation, cell adhesion, and migration.25,26 This is important, as it is well recognized that inflammatory mediators and growth factors generated by keratinocytes can contribute to inflammation, the resolution of inflammation, and wound healing.27–29
Myeloid-derived mast cells are degranulated in mouse skin following exposure to SM.10,24 Mast cells release a variety of mediators that contribute not only to inflammation, but also to wound healing and angiogenesis, including serine proteases, histamine, eicosanoids, and various cytokines.30 As many of these mediators play roles in mustard-induced skin injury, inhibitors of mast cell degranulation have been proposed as potential countermeasures.10 In this regard, we have shown that an indomethacin prodrug that stabilizes mast cells suppresses mustard-induced injury in mouse skin.31
DNA damage in mouse skin following SM injury
SM is a bifunctional alkylating agent known to modify DNA, forming both monofunctional and bifunctional adducts.3,4,19 Intra- and interstrand biadducts are formed, as well as DNA–protein and DNA–glutathione adducts.32–34 Single- and double-strand DNA damage resulting from the formation of SM adducts can compromise DNA replication and repair.5,35,36 DNA damage also initiates single- and double-strand repair processes.37,38 Depending on the extent and type of DNA modifications, repair processes can restore the integrity and functioning of the DNA. However, the repair processes are also error prone; incomplete DNA repair or errors in repair can alter DNA replication, possibly leading to mutations that contribute to toxicity.39,40 Interestingly, topical application of SM has been reported to reduce both base-excision repair (BER) and excision/synthesis repair (ESR) activities in the skin.35 Limiting DNA repair following SM exposure increases the likelihood of persistent DNA damage, which can compromise wound healing, decreasing both keratinocyte proliferation and differentiation. This may in part explain the delayed wound healing reported in humans exposed to SM.1,20,41
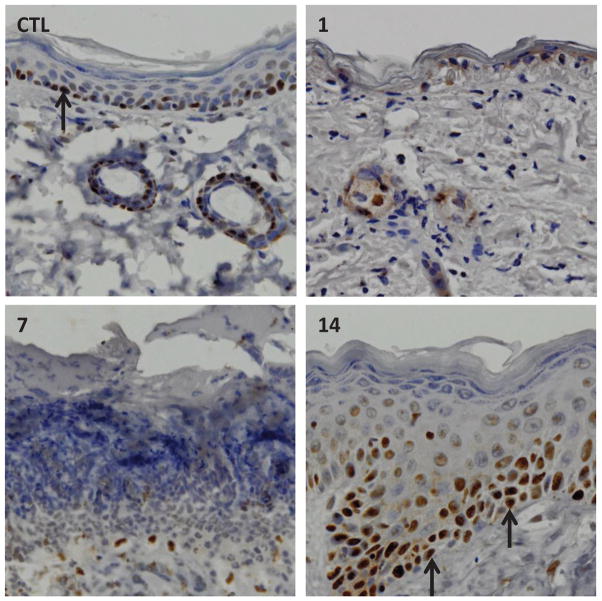
Previous studies by our laboratory have shown that SM induces double-strand DNA breaks.10,11 An early event in the repair process is phosphorylation of the histone H2A.X.40,42,43 This phosphorylated histone binds to double-strand DNA breaks, creating a recognition domain important in controlling DNA damage by recruiting DNA damage response proteins that initiate repair processes. We found that phospho-H2A.X appeared in the nuclei of basal cells of mouse epidermis within 1 day of exposure to SM (Fig. 1). Phospho-H2A.X persisted in the epidermis for at least 3 days, a time coordinate with degradation of the tissue and the formation of an eschar. Leukocytes infiltrating into the tissue beneath the eschar also expressed phospho-H2A.X (Fig. 1), a finding consistent with the fact that these cells are undergoing apoptosis.11,44 The basal cells in the neoepidermis formed during wound healing did not express phospho-H2A.X. These data indicate that SM induces double-strand DNA breaks in cells at early times postexposure. As these cells undergo apoptosis and necrosis, it is possible that double-strand DNA breaks contribute to these processes. The fact that the neoepidermis does not express phospho-H2A.X is in accord with the idea that proliferating cells in the neoepidermis formed during wound healing do not contain SM-induced double-strand DNA breaks.
Fig. 1.
Effects of SM exposure on phospho-H2A.X expression in mouse skin. Histological sections, prepared 1 day after exposure to control or 1, 7, and 14 days after sulfur mustard exposure, were stained with an antibody to phospho-H2A.X. Antibody binding was visualized using a Vectastain Elite ABC kit (original magnification, × 400). One representative section from six mice/treatment group is shown. Upper right panel: black arrows indicate phospho-H2A.X–expressing basal keratinocytes. Inflammatory cells beneath the eschar also express phospho-H2A.X. Data are Ref. 10.
It should also be noted that double-strand DNA breaks appear in basal and more differentiated cells of the dermal appendages of SM-treated skin, as evidenced by expression of phospho-H2A.X.10,11 Phospho-H2A.X was expressed in the outer root sheath surrounding hair follicles, in dermal papilla, sebocytes, and, notably, in the follicular bulge stem cell compartment. Expression of phospho-H2A.X was associated with upregulation of cleaved caspase-3, a marker of apoptosis. Mustard-induced double-strand DNA breaks can lead to inhibition of cellular migration and proliferation and even apoptotic cell death, ultimately resulting in defective wound healing hallmarked by the degradation and depletion of hair follicles and sebaceous glands in affected skin.
Characteristics of wound healing following SM exposure
During wound healing in mouse skin following SM exposure, extensive hyperplasia is evident.10 This is reflected in the appearance of dystrophic squamous epithelial cells and the formation of pseudocolumnar epithelial cells along the basement membrane, persisting in the skin for at least 2 weeks. This is presumably due to the release of growth factors important in controlling proliferation and differentiation. In this regard, we found that fibroblast growth factor receptor 2 (FGFR2), which is critical for normal epidermal growth and hair follicle morphogenesis, is expressed within the hyperplastic stratum granulosum. These data suggest that fibroblast growth factors and related growth factors are associated with keratinocyte migration and re-epithelialization during wound repair.45
Of interest are our findings that, in normal mouse skin, proliferating cell nuclear antigen (PCNA), a DNA polymerase cofactor important in DNA replication and repair, is constitutively expressed in proliferating basal keratinocytes along the basement membrane (Fig. 2). However, during wound repair, the hyperplastic epidermis expresses PCNA not only in basal keratinocytes, but also in cells suprabasal to the basement membrane. These data suggest that both proliferating basal and suprabasal cells contribute to the dystrophic hyperplastic epidermis evident during SM wound repair.
Fig. 2.
Effects of SM exposure on PCNA expression in mouse skin. Histological sections, prepared 1 day after exposure to control or 1, 7, and 14 days after SM exposure, were stained with an antibody to PCNA. Antibody binding was visualized using a Vectastain Elite ABC kit (original magnification, × 400). One representative section from six mice/treatment group is shown. Black arrows indicate basal cells expressing PCNA. Data are from Ref. 10.
Conclusions
Critical in the development of effective countermeasures to SM poisoning is to define the precise sequence of events leading to inflammation, epidermal and dermal damage, and vesication, as well as subsequent wound healing. Our laboratory has been using a vapor-cup mouse skin model to characterize mechanisms leading to tissue injury. A number of questions arise as to how SM injury in the mouse model relates to human exposures. Most importantly, following SM exposure, what is the mechanism that causes blistering in human skin but only microblisters in mouse skin? One can speculate that this is due to differences in the composition and biochemistry of basement membrane proteins and/or enzymes activated by SM that degrade the extracellular matrix–stromal cell structure. Further studies are needed to better define SM-induced injury in mouse and human skin, as this will be useful in developing novel strategies to mitigate vesicant-induced cutaneous toxicity.
Acknowledgments
This work was supported was supported by the National Institutes of Health CounterACT program through the National Institutes of Arthritis and Musculoskeletal and Skin Diseases (Award U54AR055073) and by ES005022.
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Ghabili K, Agutter PS, Ghanei M, et al. Sulfur mustard toxicity: history, chemistry, pharmacokinetics, and pharmacodynamics. Crit Rev Toxicol. 2011;41:384–403. doi: 10.3109/10408444.2010.541224. [DOI] [PubMed] [Google Scholar]
- 2.McAdams AJ., Jr A study of mustard vesication. J Invest Dermatol. 1956;26:317–26. doi: 10.1038/jid.1956.42. [DOI] [PubMed] [Google Scholar]
- 3.Vogt RF, Jr, Dannenberg AM, Jr, Schofield BH, et al. Pathogenesis of skin lesions caused by sulfur mustard. Fundam Appl Toxicol. 1984;4:S71–83. doi: 10.1016/0272-0590(84)90139-8. [DOI] [PubMed] [Google Scholar]
- 4.Shakarjian MP, Heck DE, Gray JP, et al. Mechanisms mediating the vesicant actions of sulfur mustard after cutaneous exposure. Toxicol Sci. 2010;114:5–19. doi: 10.1093/toxsci/kfp253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kehe K, Thiermann H, Balszuweit F, et al. Acute effects of sulfur mustard injury--Munich experiences. Toxicology. 2009;263:3–8. doi: 10.1016/j.tox.2009.04.060. [DOI] [PubMed] [Google Scholar]
- 6.Jenner J, Graham SJ. Treatment of sulphur mustard skin injury. Chem Biol Interact. 2013;206:491–5. doi: 10.1016/j.cbi.2013.10.015. [DOI] [PubMed] [Google Scholar]
- 7.Firooz A, Sadr B, Davoudi SM, et al. Long-term skin damage due to chemical weapon exposure. Cutan Ocul Toxicol. 2011;30:64–8. doi: 10.3109/15569527.2010.529547. [DOI] [PubMed] [Google Scholar]
- 8.Ghanei M, Poursaleh Z, Harandi AA, et al. Acute and chronic effects of sulfur mustard on the skin: a comprehensive review. Cutan Ocul Toxicol. 2010;29:269–77. doi: 10.3109/15569527.2010.511367. [DOI] [PubMed] [Google Scholar]
- 9.Casillas RP, Kiser RC, Truxall JA, et al. Therapeutic approaches to dermatotoxicity by sulfur mustard. I. Modulaton of sulfur mustard-induced cutaneous injury in the mouse ear vesicant model. J Appl Toxicol. 2000;20(Suppl 1):S145–51. doi: 10.1002/1099-1263(200012)20:1+<::aid-jat665>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 10.Joseph LB, Gerecke DR, Heck DE, et al. Structural changes in the skin of hairless mice following exposure to sulfur mustard correlate with inflammation and DNA damage. Experimental and Molecular Pathology. 2011;91:515–527. doi: 10.1016/j.yexmp.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Joseph LB, Heck DE, Cervelli JA, et al. Structural changes in hair follicles and sebaceous glands of hairless mice following exposure to sulfur mustard. Exp Mol Pathol. 2014;96:316–27. doi: 10.1016/j.yexmp.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Isidore MA, Castagna MP, Steele KE, et al. A dorsal model for cutaneous vesicant injury by 2-chloroethyl ethyl sulfide using C57BL/6 mice. Cutan Ocul Toxicol. 2007;26:265–76. doi: 10.1080/15569520701521914. [DOI] [PubMed] [Google Scholar]
- 13.Monteiro-Riviere NA, Inman AO. Indirect immunohistochemistry and immunoelectron microscopy distribution of eight epidermal-dermal junction epitopes in the pig and in isolated perfused skin treated with bis (2-chloroethyl) sulfide. Toxicol Pathol. 1995;23:313–25. doi: 10.1177/019262339502300308. [DOI] [PubMed] [Google Scholar]
- 14.Tewari-Singh N, Rana S, Gu M, et al. Inflammatory biomarkers of sulfur mustard analog 2-chloroethyl ethyl sulfide-induced skin injury in SKH-1 hairless mice. Toxicol Sci. 2009;108:194–206. doi: 10.1093/toxsci/kfn261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang YC, Wang JD, Svoboda KK, et al. Sulfur mustard induces an endoplasmic reticulum stress response in the mouse ear vesicant model. Toxicol Appl Pharmacol. 2013;268:178–87. doi: 10.1016/j.taap.2013.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shakarjian MP, Bhatt P, Gordon MK, et al. Preferential expression of matrix metalloproteinase-9 in mouse skin after sulfur mustard exposure. J Appl Toxicol. 2006;26:239–46. doi: 10.1002/jat.1134. [DOI] [PubMed] [Google Scholar]
- 17.Ricketts KM, Santai CT, France JA, et al. Inflammatory cytokine response in sulfur mustard-exposed mouse skin. J Appl Toxicol. 2000;20(Suppl 1):S73–6. doi: 10.1002/1099-1263(200012)20:1+<::aid-jat685>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 18.Jain AK, Tewari-Singh N, Inturi S, et al. Histopathological and immunohistochemical evaluation of nitrogen mustard-induced cutaneous effects in SKH-1 hairless and C57BL/6 mice. Exp Toxicol Pathol. 2014;66:129–38. doi: 10.1016/j.etp.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chang YC, Wang JD, Hahn RA, et al. Therapeutic potential of a non-steroidal bifunctional anti-inflammatory and anti-cholinergic agent against skin injury induced by sulfur mustard. Toxicol Appl Pharmacol. 2014;280:236–44. doi: 10.1016/j.taap.2014.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Graham JS, Schoneboom BA. Historical perspective on effects and treatment of sulfur mustard injuries. Chem Biol Interact. 2013;206:512–22. doi: 10.1016/j.cbi.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 21.Michopoulou A, Rousselle P. How do epidermal matrix metalloproteinases support re-epithelialization during skin healing? Eur J Dermatol. 2015;25(Suppl 1):33–42. doi: 10.1684/ejd.2015.2553. [DOI] [PubMed] [Google Scholar]
- 22.Steffensen B, Hakkinen L, Larjava H. Proteolytic events of wound-healing--coordinated interactions among matrix metalloproteinases (MMPs), integrins, and extracellular matrix molecules. Crit Rev Oral Biol Med. 2001;12:373–98. doi: 10.1177/10454411010120050201. [DOI] [PubMed] [Google Scholar]
- 23.He C, Carter AB. The Metabolic Prospective and Redox Regulation of Macrophage Polarization. J Clin Cell Immunol. 2015;6 doi: 10.4172/2155-9899.1000371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mouret S, Wartelle J, Batal M, et al. Time course of skin features and inflammatory biomarkers after liquid sulfur mustard exposure in SKH-1 hairless mice. Toxicol Lett. 2014;232:68–78. doi: 10.1016/j.toxlet.2014.09.022. [DOI] [PubMed] [Google Scholar]
- 25.Cao Z, Said N, Amin S, et al. Galectins-3 and -7, but not galectin-1, play a role in re-epithelialization of wounds. J Biol Chem. 2002;277:42299–305. doi: 10.1074/jbc.M200981200. [DOI] [PubMed] [Google Scholar]
- 26.Wollina U, Lange D, Paus R, et al. Expression of galectin-1 and -3 and of accessible binding sites during murine hair cycle. Histol Histopathol. 2000;15:85–94. doi: 10.14670/HH-15.85. [DOI] [PubMed] [Google Scholar]
- 27.Liu W, Hsu DK, Chen HY, et al. Galectin-3 regulates intracellular trafficking of EGFR through Alix and promotes keratinocyte migration. J Invest Dermatol. 2012;132:2828–37. doi: 10.1038/jid.2012.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feldmeyer L, Werner S, French LE, et al. Interleukin-1, inflammasomes and the skin. Eur J Cell Biol. 2010;89:638–44. doi: 10.1016/j.ejcb.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 29.Nestle FO, Di Meglio P, Qin JZ, et al. Skin immune sentinels in health and disease. Nat Rev Immunol. 2009;9:679–91. doi: 10.1038/nri2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shiota N, Nishikori Y, Kakizoe E, et al. Pathophysiological role of skin mast cells in wound healing after scald injury: study with mast cell-deficient W/W(V) mice. Int Arch Allergy Immunol. 2010;151:80–8. doi: 10.1159/000232573. [DOI] [PubMed] [Google Scholar]
- 31.Composto GM, Laskin JD, Laskin DL, et al. Mitigation of Nitrogen Mustard Mediated Skin Injury by a Novel Bifunctional Pro-drug. Experimental and Molecular Pathology. 2016 doi: 10.1016/j.yexmp.2016.05.008. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yue L, Wei Y, Chen J, et al. Abundance of four sulfur mustard-DNA adducts ex vivo and in vivo revealed by simultaneous quantification in stable isotope dilution-ultrahigh performance liquid chromatography-tandem mass spectrometry. Chem Res Toxicol. 2014;27:490–500. doi: 10.1021/tx4003403. [DOI] [PubMed] [Google Scholar]
- 33.Batal M, Rebelo-Moreira S, Hamon N, et al. A guanine-ethylthioethyl-glutathione adduct as a major DNA lesion in the skin and in organs of mice exposed to sulfur mustard. Toxicol Lett. 2015;233:1–7. doi: 10.1016/j.toxlet.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 34.Smith KJ, Hurst CG, Moeller RB, et al. Sulfur mustard: its continuing threat as a chemical warfare agent, the cutaneous lesions induced, progress in understanding its mechanism of action, its long-term health effects, and new developments for protection and therapy. J Am Acad Dermatol. 1995;32:765–76. doi: 10.1016/0190-9622(95)91457-9. [DOI] [PubMed] [Google Scholar]
- 35.Sauvaigo S, Sarrazy F, Batal M, et al. Impact of topical application of sulfur mustard on mice skin and distant organs DNA repair enzyme signature. Toxicol Lett. 2016;241:71–81. doi: 10.1016/j.toxlet.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 36.Moser J, Meier HL. Comparison of cell size in sulfur mustard-induced death of keratinocytes and lymphocytes. J Appl Toxicol. 2000;20(Suppl 1):S23–30. doi: 10.1002/1099-1263(200012)20:1+<::aid-jat693>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 37.Ceccaldi R, Rondinelli B, D’Andrea AD. Repair Pathway Choices and Consequences at the Double-Strand Break. Trends Cell Biol. 2016;26:52–64. doi: 10.1016/j.tcb.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Black AT, Hayden PJ, Casillas RP, et al. Expression of proliferative and inflammatory markers in a full-thickness human skin equivalent following exposure to the model sulfur mustard vesicant, 2-chloroethyl ethyl sulfide. Toxicol Appl Pharmacol. 2010;249:178–87. doi: 10.1016/j.taap.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rodgers K, McVey M. Error-Prone Repair of DNA Double-Strand Breaks. J Cell Physiol. 2016;231:15–24. doi: 10.1002/jcp.25053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mah LJ, El-Osta A, Karagiannis TC. gammaH2AX: a sensitive molecular marker of DNA damage and repair. Leukemia. 2010;24:679–86. doi: 10.1038/leu.2010.6. [DOI] [PubMed] [Google Scholar]
- 41.Rice P. Sulphur mustard injuries of the skin. Pathophysiology and management. Toxicol Rev. 2003;22:111–8. doi: 10.2165/00139709-200322020-00006. [DOI] [PubMed] [Google Scholar]
- 42.Clingen PH, Wu JY, Miller J, et al. Histone H2AX phosphorylation as a molecular pharmacological marker for DNA interstrand crosslink cancer chemotherapy. Biochem Pharmacol. 2008;76:19–27. doi: 10.1016/j.bcp.2008.03.025. [DOI] [PubMed] [Google Scholar]
- 43.Jowsey PA, Williams FM, Blain PG. The role of homologous recombination in the cellular response to sulphur mustard. Toxicol Lett. 2010;197:12–8. doi: 10.1016/j.toxlet.2010.04.020. [DOI] [PubMed] [Google Scholar]
- 44.Chow SH, Deo P, Naderer T. Macrophage cell death in microbial infections. Cell Microbiol. 2016;18:466–74. doi: 10.1111/cmi.12573. [DOI] [PubMed] [Google Scholar]
- 45.Lomash V, Jadhav SE, Ahmed F, et al. Evaluation of wound-healing formulation against sulphur mustard-induced skin injury in mice. Hum Exp Toxicol. 2012;31:588–605. doi: 10.1177/0960327111429139. [DOI] [PubMed] [Google Scholar]