Abstract
Cholinergic status epilepticus (CSE) quickly becomes self-sustaining, independent of its initial trigger, and resistant to benzodiazepines and other antiepileptic drugs. We review a few of the many physiological changes associated with CSE, with an emphasis on receptor trafficking. Time-dependent internalization of synaptic GABAA receptors explains, in part, the loss of inhibition and the loss of response to benzodiazepines in the early stages of CSE. The increase in NMDA receptors may contribute to the runaway excitation and excitotoxicity of CSE. These changes have therapeutic implications. The time-dependent increase in maladaptive changes points to the importance of early treatment. The involvement of both inhibitory and excitatory systems challenges current therapeutic guidelines, which recommend treating only one system, and questions the rationale for monotherapy. It suggests that polytherapy may be needed, especially when treatment is delayed, so that drugs can only reach a much reduced number of GABAA receptors. Finally, it raises the possibility that the current practice of waiting for one treatment to fail before starting the next drug may need to be re-evaluated.
Keywords: refractory status epilepticus, cholinergic seizures, pharmacoresistance, polytherapy, nerve agent
Introduction
While we have made considerable progress in treating epilepsy, status epilepticus (SE) remains a therapeutic challenge that still carries a 27% mortality and a high morbidity.1 Despite treatment, many SE sufferers end up with permanent brain damage, especially in the limbic system, resulting in memory loss, cognitive dysfunction, epilepsy, and other neurological conditions.1–4 Treatment of cholinergic SE (CSE) induced by organophosphates and other poisons is even more problematic. Once seizures have started, they quickly become self-sustaining, independent of their initial cholinergic trigger, and refractory to standard antiseizure drugs.5 In experimental animals, they cause severe brain damage and chronic epilepsy,6 and they may well have similar effects in humans.7,8
The tendency of SE to become self-sustaining and pharmacoresistant, and the fact that it is more than a series of severe seizures, were recognized as early as the 19th century.9 In a few models of SE, usually done under anesthesia, seizure response is tightly coupled with the epileptogenic stimulus.10 However, in awake, free-running animals, SE tends to become self-sustaining and to continue for hours after the epileptogenic stimulus is withdrawn. This is true of seizures induced by chemical10–12 and electrical stimulation.13–21 Understanding the conditions that are critical for the transition from stimulus-bound seizures to self-sustaining SE (SSSE) may help us to understand at what point SE becomes intractable and brain damaging and how to prevent these consequences.
Time-dependent pharmacoresistance is a major therapeutic problem in SE and CSE. As seizures continue, pharmacoresistance develops progressively. The antiseizure potency of benzodiazepines can decrease 20-fold in 30 min of seizures.22 Phenytoin and barbiturates also lose potency, but more slowly.17 By contrast, N-methyl-d-aspartate receptor (NMDAR) blockers remain potent, even in late SE, in some animal models.20 In human SE, evidence for pharmacoresistance is abundant but indirect. Early treatment is much more effective than late treatment: in the VA Cooperative Study, four treatments were randomly rotated.23 The first treatment was successful in 53% of patients. The third treatment given was successful in 2% of patients, and the development of pharmacoresistance during the interval between the first and third treatments is the most likely of several possible explanations for these results. Many clinical studies show that delays in initiating treatment, delays between treatments, and ineffective dosing24 are associated with poor outcomes. In the Ramparts study, a 4-min difference in the timing of treatment was associated with a significant difference in outcome.25 Like most human studies, this is compatible with the hypothesis of a rapid development of pharmacoresistance, but does not prove it.
Pharmacological responsiveness differentiates the initiation phase of SE and CSE from their maintenance phase. A large number of toxins and pharmacological agents are able to induce SSSE (Table 1), suggesting that the circuit that maintains self-sustaining seizures has many potential points of entry. However, pharmacological responsiveness during initiation of SSSE and during established SSSE are strikingly different. Minute amounts of many agents that enhance inhibitory transmission or reduce excitatory transmission easily block the development of SSSE (Table 1), suggesting that brain circuits are biased against it, and that “all systems must be go” in order for the phenomenon to develop. This is hardly surprising, since SSSE is a rare, life-threatening event. However, once seizures are self-sustaining, few agents are effective in terminating them, and they usually work only in large concentration. Some of the most effective agents are blockers of NMDA synapses or presynaptic inhibitors of glutamate release (Table 1).
Table 1. Initiators and blockers of self-sustaining status epilepticus.
| Initiators | Blockers of initation phase | Blockers of maintenance phase |
|---|---|---|
|
|
|
Mechanisms of the transition from single seizures to SE: GABAAR internalization
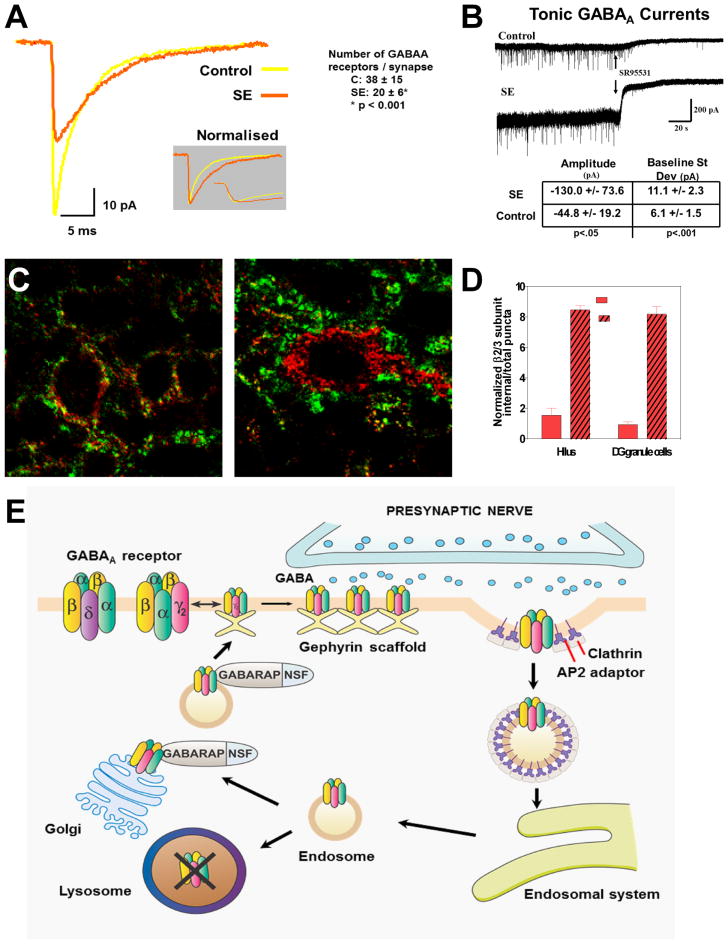
Since GABAergic agents lose therapeutic effectiveness as SE proceeds, and many studies show a diminished inhibitory tone of hippocampal circuits,25,26 (as indicated by loss of paired-pulse inhibition in vivo), we examined the effects of SE on GABAA synapses using whole-cell patch-clamp recordings of miniature inhibitory postsynaptic currents (mIPSCs) obtained from dentate gyrus granule cells in hippocampal slices from 4- to8-week-old Wistar rats after 1 h of lithium–pilocarpine CSE and compared them to slices obtained from sham control rats.27 Miniature IPSCs recorded from granule cells in slices prepared 1 h into SE showed a decrease peak amplitude to 61.8 ± 11.9% of controls (–31.5 ± 6.1 pA for SE versus –51.0 ± 17.0 pA for controls; P < -.001) (Fig. 1A) and an increase of decay time to 127.9 ± 27.6% of controls (7.75 ± 1.67 ms for SE versus 6.06 ± 1.17 ms for controls; P < 0.001). These changes suggest a reduction of the postsynaptic response to a quantum of GABA released from a single vesicle. Possible explanations include GABAA receptor internalization, changes in receptor kinetics, and alteration of GABA release/uptake during SE. Exposing hippocampal slices to micromolar GABA resulted in a rapid reduction of mIPSCs, suggesting that the changes observed in SE may be triggered by the massive GABA release during seizures. Mathematical modeling of GABAA synapses using mean-variance fluctuation analysis and seven-state GABAA receptor models suggested that SE reduced the number of postsynaptic GABAA receptors per granule cell soma synapse by 47%, from 38 ± 15 (control) to 20 ±6 (SE) receptors per synapse (P < 0.001). This may underestimate the acute changes, since slices collected from animals in SE were examined after 1–2 seizure-free hours in vitro.
Figure 1.
Trafficking of GABAA receptors, loss of inhibition, and a mechanism for pharmacoresistance in status epilepticus. (A) In hippocampal slices prepared from rats in lithium–pilocarpine–induced status epilepticus (SE) for 1 h, γ-aminobutyric acid (GABA)A miniature inhibitory postsynaptic currents (IPSCs) recorded from granule cells display reduced amplitude that can be attributed primarily to a loss of synaptic receptors (reduced number of GABAA receptors from 38 ± 15 to 20 ± 6 per granule cell synapse). (B) Tonic currents generated by extrasynaptic GABAA receptors are increased in slices from rats in SE, reflecting (at least in part) increased extracellular GABA concentration during SE. (C) Subcellular distribution of β2–3 subunits of GABAA receptors after SE. In control granule cells (left) the β2–3 subunits of GABAA receptors (red) localize to the vicinity of the presynaptic marker synaptophysin (green), whereas after 1 h of SE induced by lithium and pilocarpine (right), many have moved to the cell interior. (D) The graph shows an increase in β2–3 subunit internalization following SE in the hilus and in the dendate gyrus granule cell layer. (E) Model of our hypothesis of GABAA receptor trafficking during the transition of single seizures to status epilepticus. After repeated seizures, the synaptic membrane of GABAA receptors forms clathrin-coated pits, which internalize as clathrin-coated vesicles, inactivating the receptors because they are no longer within reach of the neurotransmitter. These vesicles develop into endosomes, which can deliver the receptors to lysosomes, where they are destroyed, or to the Golgi apparatus, from where they are recycled to the membrane.
Immunocytochemistry was performed in rats perfused after 60 min of seizures induced by lithium–pilocarpine or intrahippocampal injection of neurokinin B. Sections through the hippocampus were double-labeled with antibodies for the β2/β3 subunits (which are the most abundant subunits of those receptors) and for the presynaptic marker synaptophysin, and viewed by confocal microscopy. These studies indicate that the decrease in number of synaptic receptors observed physiologically reflects, at least in part, receptor internalization (Fig. 1C). They show colocalization of the β2/β3 subunits with the presynaptic marker synaptophysin on the surface of soma and proximal dendrites of dentate granule cells and CA3a pyramids in controls, with internalization of those subunits in SE: in the lithium–pilocarpine model at 60 min, 12 ± 17% of β2/β3 subunits are internalized in control CA3 compared to 54 ± 15% in slices from rats in SE (P < 0.001). Numbers in CA1 were similar. We also found that the γ2 subunits are internalized during SE: because of the high cell packing density and relatively low γ2 subunit concentration on their soma, those measurements were difficult in granule cells, but the proportion of internalized endosome-like structures with γ2-like immunoreactivity in the soma of basket cells at the edge of the granule cell layer increased from 19 ± 4% to 86 ± 23% after 1 h of lithium–pilocarpine SE.27,28
Unlike mIPSCs, tonic currents (Fig. 1B) increased in amplitude to a mean of –130.0 (± 73.6) pA in SE versus –44.8(± 19.2) pA in controls (P < 0.05; GABA uptake blocked). Tonic currents in dentate gyrus granule cells are thought to be mediated by extrasynaptic receptors containing δ subunits, which are known to display low levels of desensitization and internalization. The persistence of tonic currents during SE might suggest the use of drugs with a strong affinity for extrasynaptic receptors, such as neurosteroids (which prefer δ-containing receptors) or THIP (which prefers α4-δ–containing receptors).
In conclusion, a decrease in synaptic GABAA currents and an increase in extrasynaptic tonic currents are observed with SE. Internalization of postsynaptic GABAA receptors can explain the decreased amplitude of synaptic mIPSCs. These changes at GABAergic synapses may represent important events in the transition from single seizures to self-sustaining SE (Fig. 1E). Since internalized receptors are not available to function at synapses, this internalization may reduce the response of inhibitory synapses to additional seizures and may in part explain the failure of inhibitory GABAergic mechanisms that characterizes the initiation phase of SSSE. Internalized receptors may be recycled to the synaptic membrane through the Golgi apparatus or may be destroyed in lysosomes (Fig. 1E). The reduced number of synaptic receptors may explain the diminished effect of benzodiazepines and other GABAergic drugs as SE proceeds.17,22 Preventing or reversing internalization of GABAA receptors might be attempted with osmotic agents such as mannitol29 or by targeting the intracellular signal pathways for GABAA receptor trafficking, but our limited attempts in that direction have had no success so far.
NMDA receptor trafficking, synaptic potentiation, and the maintenance phase of SE
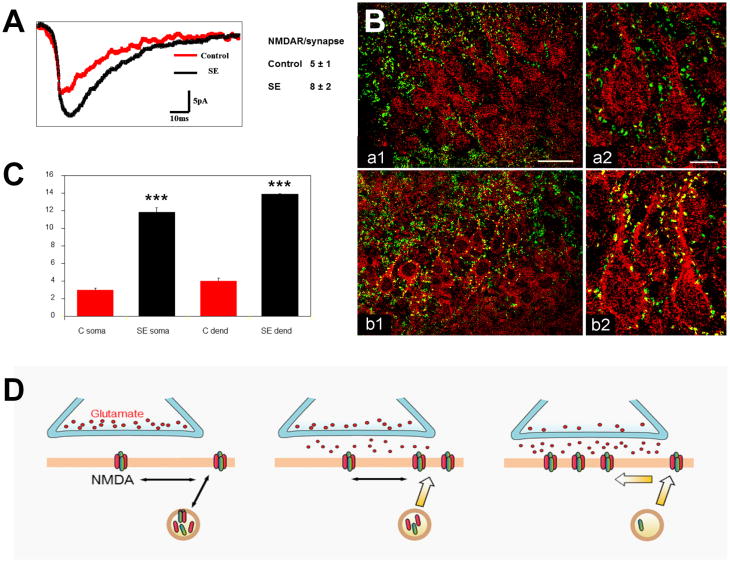
The self-perpetuating nature of SE suggests that synaptic potentiation (e.g., a form of long-term potentiation (LTP) and/or posttetanic potentiation) may account for some of the maintenance mechanisms of SE. Indeed, SE induced by perforant path stimulation is accompanied by increased LTP in the perforant path–dentate gyrus pathway.30 Several mechanisms may underlie facilitation of LTP during SSSE. The first is impaired GABAergic inhibition, as discussed above. Lack of GABA inhibition facilitates LTP. Thus, SE-induced loss of GABA inhibition, which occurs at a very early stage of stimulation, may contribute to facilitation of LTP. However, direct changes affecting excitatory NMDAR also seem to be involved.20,31 We compared hippocampal slices from 4- to 8-week-old rats in lithium–pilocarpine SE for 1 h to controls.32 Physiological measurements included NMDA miniature excitatory postsynaptic currents (mEPSCs) recorded from granule cells in the hippocampal slice with visualized whole-cell patch-clamp. The mEPSCs showed an increased peak amplitude (Fig. 2A) from –16.2 ± 0.4 pA for controls to –19.5 ± 2.4 for SE (P < 0.001). Mean-variance analysis of the mEPSCs showed an increase from 5.2 ± 1.2 NMDARs per synapse in controls to 7.8 ± 1.2 receptors during SE (50% increase; P < 0.001). Immunocytochemical analysis with antibodies to the NR1 subunit of NMDARs showed a movement of NR1 subunits from cytoplasmic sites to the neuronal surface and an increase in colocalization with the presynaptic marker synaptophysin, suggesting a mobilization of “spare” subunits to the synapse (Fig. 2B & 2C).
Figure 2.
Rapid surface accumulation of NMDA receptors increases glutamatergic excitation during SE. (A) NMDA-mEPSC mean traces from a typical granule cell from a control (red) and a SE animal (black), demonstrating larger amplitude and area under the curve (AUC) in the latter. (B) Subcellular distribution of NMDA NR1 subunit–LI during SE. Hippocampal sections through CA3 of control (a1) and SE (b1) brains stained with antibodies against the NR1 subunit–LI (red) and against the presynaptic marker synaptophysin–LI (green), with overlaps appearing yellow. Hippocampal sections of CA3 at higher magnification are shown in a2 and b2. Note increased NR1 subunit–LI colocalization with synaptophysin–LI in pyramidal cells for SE rat (bar: 40 μm left panel; 10 μm right panel). (C) The number of colocalizations increases with NKB SE at both the soma and proximal dendrites of CA3 pyramidal cells (error bars show ± SEM). (D) Model of our hypothesis of NMDAR trafficking during the transition of single seizures to SE. After repeated seizures, in NMDA synapses, subunits are mobilized to the synaptic membrane and assemble into additional receptors. They may move initially to the perisynaptic area, then laterally to the synaptic area. As a result of this trafficking, the number of functional NMDARs per synapse increases.
In conclusion, during SE, endocytosis/internalization of GABAA postsynaptic receptors is accompanied by an increase in number of excitatory NMDARs per somatic synapse on dentate granule cells (Fig. 2D). Under physiological conditions, glutamatergic excitation sufficient to remove the magnesium block of the NMDAR channel is known to increase trafficking of AMPA and NMDA receptors to synapses (Fig. 2D, middle). Seizures, with their massive and repetitive glutamatergic stimulation, seem to have a similar but much larger effect on NMDARs (Fig. 2D, right) (and on AMPA receptors,33 but in our hands the latter effect is small). Receptor trafficking may regulate the balance between excitatory and inhibitory postsynaptic receptor numbers and may be an important element in the transition to and maintenance of SE and CSE.
Other changes associated with initiation and maintenance of SE and CSE
Many other biochemical and physiological changes occur that are beyond the scope of this review and may play important roles in the transition from single seizures to SE and in the maintenance of SE: extracellular ion concentrations, including K+ and Ca2+; changes in gap junctions between glial cells and between interneurons; changes in inhibitory and excitatory peptides; and changes in protein kinases including PKC and calmodulin kinase II. For example, during SE, increased intraneuronal Ca2+ causes autophosphorylation of calmodulin kinase II (CaMKII), greatly increasing its Ca-independent kinase activity.34 This continues to phosphorylate proteins even when the cell is not firing and intracellular Ca is not elevated.35 This increases the rate of phosphorylation of synapsin I, resulting in separation of phosphosynapsin I from the vesicle wall and increasing the likelihood of presynaptic transmitter release.
Therapeutic implications of seizure-induced receptor trafficking
Polytherapy versus monotherapy
Standard treatment (benzodiazepine monotherapy) allosterically stimulates the remaining synaptic GABAAR.5,36 This can restore inhibition as long as a sufficient number of receptors remain on the postsynaptic membrane. However, even if GABAergic inhibition is successfully restored, this only addresses half the problem. The increase in functional NMDARs and the resulting runaway excitation and potential excitotoxicity remain untreated. Treating both changes induced by seizure-induced receptor trafficking would require using at least two drugs from the outset. This may be why, in some models of SE, NMDA antagonists have been reported to remain effective late in the course of SE:20 they correct maladaptive changes that are usually untreated. Optimal treatment to reverse the results of seizure-induced receptor trafficking would include at least two drugs: a GABAAR agonist (e.g., a benzodiazepine) and an NMDAR antagonist. More generally, since at least two receptor types undergo maladaptive changes in the brain, we should use drugs which target at least two receptor types in order to correct those maladaptive changes.
If treatment is delayed, triple therapy may be needed
Internalization of GABAAR increases with time (or more likely with seizure burden, which during SE increases with time), so that, if treatment is delayed, a high percentage of synaptic GABAARs may be internalized into endosomes and inactivated. As a result, even maximal stimulation with benzodiazepines may not be able to fully restore GABAergic inhibition. In addition to midazolam and ketamine, a third drug (e.g., an antiseizure drug) is then needed to enhance inhibition at a non-benzodiazepine site. Since nerve agent–induced SE in terrorist situations is likely to encounter significant treatment delays, triple therapy should be routinely considered in that situation. The choice of the best drug that works synergistically with midazolam and ketamine is critical and is the focus of our current research.
Sequential versus simultaneous polytherapy
Standard treatment of SE and CSE uses sequential polytherapy, since each drug that fails to stop seizures is rapidly followed by another drug or treatment. Typically, a benzodiazepine (midazolam, lorazepam, or diazepam) is followed by another antiseizure drug (e.g., fosphenytoin), then by a newer antiseizure drug (e.g., valproate, levetiracetam, or lacosamide), then by general anesthesia, and, after several anesthetics fail, by ketamine or other less commonly used drugs. However, sequential polytherapy takes time, since one has to wait for a drug to fail before starting the next treatment. During that time, receptor changes that are not treated by the initial drug (e.g., NMDAR changes if the first drug is a benzodiazepine) are likely to get worse and may be intractable by the time a drug that targets them (e.g., ketamine) is used many hours or even days later. We should consider simultaneous polytherapy in order to reverse the effects of receptor trafficking early, before they become irreversible.
Early treatment is essential
The progressive nature of receptor changes and the indirect evidence that they probably occur quite early27,28 suggest that time is of the essence. One should treat as early in the course of SE as possible. Indeed, the success of prehospital treatment,25,37 and the benefit of small gains in early delivery of drugs intramuscularly25 support the applicability of that principle to clinical SE.
Conclusions
In summary, recent progress in our understanding of the pathophysiology of SE and CSE require a drastic reevaluation of the way we treat those syndromes. The unquestionable benefits of monotherapy for chronic epilepsy may not apply to SE/CSE, an acute, life- and brain-threatening condition. Polytherapy with drug cocktails (a benzodiazepine combined with a NMDA antagonist and an appropriate antiseizure drug) addressing the seizure-induced maladaptive changes that occur needs to be evaluated and may provide at least a partial solution to the problem of overcoming pharmacoresistance during SE.
Acknowledgments
This work was supported by Merit Review Award #I01 BX000273-07 from the United States Department of Veterans Affairs, by NINDS (NIH Counteract Program; Grant UO1 NS074926; CW), and by the James and Debbie Cho Foundation.
Footnotes
Conflicts of interest: The authors declare no conflicts of interest.
References
- 1.DeLorenzo RJ, Hauser WA, Towne AR, et al. A prospective, population-based epidemiologic study of status epilepticus in Richmond, Virginia. Neurology. 1996;46:1029–1035. doi: 10.1212/wnl.46.4.1029. [DOI] [PubMed] [Google Scholar]
- 2.Lowenstein DH, Alldredge BK. Status epilepticus. N Engl J Med. 1998;338:970–976. doi: 10.1056/NEJM199804023381407. [DOI] [PubMed] [Google Scholar]
- 3.Fountain NB. Status epilepticus: risk factors and complications. Epilepsia. 2000;41 Suppl 2:S23–30. doi: 10.1111/j.1528-1157.2000.tb01521.x. [DOI] [PubMed] [Google Scholar]
- 4.DeLorenzo RJ, Pellock JM, Towne AR, et al. Epidemiology of status epilepticus. J Clin Neurophysiol. 1995;12:316–325. [PubMed] [Google Scholar]
- 5.Shih T, McDonough JH, Jr, Koplovitz I. Anticonvulsants for soman-induced seizure activity. J Biomed Sci. 1999;6:86–96. doi: 10.1007/BF02256439. [DOI] [PubMed] [Google Scholar]
- 6.de Araujo Furtado M, Lumley LA, Robison C, et al. Spontaneous recurrent seizures after status epilepticus induced by soman in Sprague-Dawley rats. Epilepsia. 2010;51:1503–1510. doi: 10.1111/j.1528-1167.2009.02478.x. [DOI] [PubMed] [Google Scholar]
- 7.DeGiorgio CM, Tomiyasu U, Gott PS, et al. Hippocampal pyramidal cell loss in human status epilepticus. Epilepsia. 1992;33:23–27. doi: 10.1111/j.1528-1157.1992.tb02278.x. [DOI] [PubMed] [Google Scholar]
- 8.Corsellis JA, Bruton CJ. Neuropathology of status epilepticus in humans. Adv Neurol. 1983;34:129–139. [PubMed] [Google Scholar]
- 9.Trousseau A. Lectures on clinical medicine delivered at the Hotel Dieu, Paris. New Sydenham Society; London: 1868. [Google Scholar]
- 10.Morrisett RA, Jope RS, Snead OC., 3rd Status epilepticus is produced by administration of cholinergic agonists to lithium-treated rats: comparison with kainic acid. Exp Neurol. 1987;98:594–605. doi: 10.1016/0014-4886(87)90268-8. [DOI] [PubMed] [Google Scholar]
- 11.Buterbaugh GG, Michelson HB, Keyser DO. Status epilepticus facilitated by pilocarpine in amygdala-kindled rats. Exp Neurol. 1986;94:91–102. doi: 10.1016/0014-4886(86)90274-8. [DOI] [PubMed] [Google Scholar]
- 12.Suchomelova L, Baldwin RA, Kubova H, et al. Treatment of experimental status epilepticus in immature rats: dissociation between anticonvulsant and antiepileptogenic effects. Pediatr Res. 2006;59:237–243. doi: 10.1203/01.pdr.0000196333.16608.30. [DOI] [PubMed] [Google Scholar]
- 13.Lothman EW, Bertram EH., 3rd Epileptogenic effects of status epilepticus. Epilepsia. 1993;34 Suppl 1:S59–70. doi: 10.1111/j.1528-1157.1993.tb05907.x. [DOI] [PubMed] [Google Scholar]
- 14.Lothman EW, Bertram EH, Bekenstein JW, et al. Self-sustaining limbic status epilepticus induced by ‘continuous’ hippocampal stimulation: electrographic and behavioral characteristics. Epilepsy Res. 1989;3:107–119. doi: 10.1016/0920-1211(89)90038-7. [DOI] [PubMed] [Google Scholar]
- 15.Lothman EW, Bertram EH, Kapur J, et al. Recurrent spontaneous hippocampal seizures in the rat as a chronic sequela to limbic status epilepticus. Epilepsy Res. 1990;6:110–118. doi: 10.1016/0920-1211(90)90085-a. [DOI] [PubMed] [Google Scholar]
- 16.Mazarati A, Liu H, Wasterlain C. Opioid peptide pharmacology and immunocytochemistry in an animal model of self-sustaining status epilepticus. Neuroscience. 1999;89:167–173. doi: 10.1016/s0306-4522(98)00320-0. [DOI] [PubMed] [Google Scholar]
- 17.Mazarati AM, Baldwin RA, Sankar R, et al. Time-dependent decrease in the effectiveness of antiepileptic drugs during the course of self-sustaining status epilepticus. Brain Res. 1998;814:179–185. doi: 10.1016/s0006-8993(98)01080-4. [DOI] [PubMed] [Google Scholar]
- 18.Mazarati AM, Hohmann JG, Bacon A, et al. Modulation of hippocampal excitability and seizures by galanin. J Neurosci. 2000;20:6276–6281. doi: 10.1523/JNEUROSCI.20-16-06276.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mazarati AM, Liu H, Soomets U, et al. Galanin modulation of seizures and seizure modulation of hippocampal galanin in animal models of status epilepticus. J Neurosci. 1998;18:10070–10077. doi: 10.1523/JNEUROSCI.18-23-10070.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mazarati AM, Wasterlain CG. N-methyl-D-asparate receptor antagonists abolish the maintenance phase of self-sustaining status epilepticus in rat. Neurosci Lett. 1999;265:187–190. doi: 10.1016/s0304-3940(99)00238-4. [DOI] [PubMed] [Google Scholar]
- 21.Mazarati AM, Wasterlain CG, Sankar R, et al. Self-sustaining status epilepticus after brief electrical stimulation of the perforant path. Brain Res. 1998;801:251–253. doi: 10.1016/s0006-8993(98)00606-4. [DOI] [PubMed] [Google Scholar]
- 22.Kapur J, Macdonald RL. Rapid seizure-induced reduction of benzodiazepine and Zn2+ sensitivity of hippocampal dentate granule cell GABAA receptors. J Neurosci. 1997;17:7532–7540. doi: 10.1523/JNEUROSCI.17-19-07532.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Treiman DM, Meyers PD, Walton NY, et al. A comparison of four treatments for generalized convulsive status epilepticus. Veterans Affairs Status Epilepticus Cooperative Study Group. N Engl J Med. 1998;339:792–798. doi: 10.1056/NEJM199809173391202. [DOI] [PubMed] [Google Scholar]
- 24.Cloyd JC, Lalonde RL, Beniak TE, et al. A single-blind, crossover comparison of the pharmacokinetics and cognitive effects of a new diazepam rectal gel with intravenous diazepam. Epilepsia. 1998;39:520–526. doi: 10.1111/j.1528-1157.1998.tb01415.x. [DOI] [PubMed] [Google Scholar]
- 25.Silbergleit R, Durkalski V, Lowenstein D, et al. Intramuscular versus intravenous therapy for prehospital status epilepticus. N Engl J Med. 2012;366:591–600. doi: 10.1056/NEJMoa1107494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shirasaka Y, Wasterlain CG. Chronic epileptogenicity following focal status epilepticus. Brain Res. 1994;655:33–44. doi: 10.1016/0006-8993(94)91594-6. [DOI] [PubMed] [Google Scholar]
- 27.Naylor DE, Wasterlain CG. GABA synapses and the rapid loss of inhibition to dentate gyrus granule cells after brief perforant-path stimulation. Epilepsia. 2005;46 Suppl 5:142–147. doi: 10.1111/j.1528-1167.2005.01022.x. [DOI] [PubMed] [Google Scholar]
- 28.Naylor DE, Liu H, Wasterlain CG. Trafficking of GABA(A) receptors, loss of inhibition, and a mechanism for pharmacoresistance in status epilepticus. J Neurosci. 2005;25:7724–7733. doi: 10.1523/JNEUROSCI.4944-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heuser JE, Anderson RG. Hypertonic media inhibit receptor-mediated endocytosis by blocking clathrin-coated pit formation. J Cell Biol. 1989;108:389–400. doi: 10.1083/jcb.108.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mazarati AMW, C Loss of hippocampal inhibition and enhanced LTP after self- sustaining status epilepticus. Epilepsia. 1997;38:178. [Google Scholar]
- 31.Bertram EH, Lothman EW. NMDA receptor antagonists and limbic status epilepticus: a comparison with standard anticonvulsants. Epilepsy Res. 1990;5:177–184. doi: 10.1016/0920-1211(90)90036-u. [DOI] [PubMed] [Google Scholar]
- 32.Naylor DE, Liu H, Niquet J, et al. Rapid surface accumulation of NMDA receptors increases glutamatergic excitation during status epilepticus. Neurobiology of disease. 2013;54:225–238. doi: 10.1016/j.nbd.2012.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rajasekaran K, Joshi S, Kozhemyakin M, et al. Receptor trafficking hypothesis revisited: plasticity of AMPA receptors during established status epilepticus. Epilepsia. 2013;54 Suppl 6:14–16. doi: 10.1111/epi.12266. [DOI] [PubMed] [Google Scholar]
- 34.Bronstein JM, Micevych P, Popper P, et al. Long-lasting decreases of type II calmodulin kinase expression in kindled rat brains. Brain Res. 1992;584:257–260. doi: 10.1016/0006-8993(92)90903-m. [DOI] [PubMed] [Google Scholar]
- 35.Wasterlain CG, Bronstein JM, Morin AM, et al. Translocation and autophosphorylation of brain calmodulin kinase II in status epilepticus. Epilepsy Res Suppl. 1992;9:231–238. [PubMed] [Google Scholar]
- 36.Glauser T, Shinnar S, Gloss D, et al. Evidence-Based Guideline: Treatment of Convulsive Status Epilepticus in Children and Adults: Report of the Guideline Committee of the American Epilepsy Society. Epilepsy Curr. 2016;16:48–61. doi: 10.5698/1535-7597-16.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alldredge BK, Venteicher R, Calderwood TS. Stability of diazepam rectal gel in ambulance-like environments. Am J Emerg Med. 2002;20:88–91. doi: 10.1053/ajem.2002.31573. [DOI] [PubMed] [Google Scholar]