Abstract
Threats by fundamentalist leaders to use chemical weapons have resulted in renewed interest in cyanide toxicity. Relevant insights may be gained from studies on cyanide mass intoxication in populations relying on cyanogenic cassava as the main source of food. In these populations, sublethal concentrations (up to 80 µmol/L) of cyanide in the blood are commonplace and lead to signs of acute toxicity. Long-term toxicity signs include a distinct and irreversible spastic paralysis, known as konzo, and cognition deficits, mainly in sequential processing (visual–spatial analysis) domains. Toxic culprits include cyanide (mitochondrial toxicant), thiocyanate (AMPA-receptor chaotropic cyanide metabolite), cyanate (protein-carbamoylating cyanide metabolite), and 2-iminothiazolidine-4-carboxylic acid (seizure inducer). Factors of susceptibility include younger age, female gender, protein-deficient diet, and, possibly, the gut functional metagenome. The existence of uniquely exposed and neurologically affected populations offers invaluable research opportunities to develop a comprehensive understanding of cyanide toxicity and test or validate point-of-care diagnostic tools and treatment options to be included in preparedness kits in response to cyanide-related threats.
Keywords: cassava, cyanide, paralysis, neurocognition, warfare
Introduction
A substantial body of literature suggests that cyanide or its precursors could be used as chemical asphyxiants in mass killings during military operations or terrorist attacks.1 The unpredictable nature of such events imposes a sustained attention on the research community, as type, source, and timing of exposure may significantly vary, and preparedness kits must be developed accordingly. Exposure scenarios that naturally or accidently occur may serve as conceptual frameworks to develop research lines relevant to the field. Exposure to cyanide or its precursors may occur via inhalation of smoke, notably during the combustion of fabrics containing nylon, silk, or wool, and plastics, such as melamine, polyurethane, and polyacrylonitrile; or hydrogen cyanide during murder attempts.1–4 Other sources of exposure include drinking water contaminated with cyanurated industrial wastes or spills, and ingestion of cyanogenic food, such as cassava.5,6 Additional lessons can also be learned from studies of individual cases of cyanide poisoning, which may occur in patients on laetrile or sodium nitroprusside.4
The toxicity of cyanide occurs as a result of its chemical binding to cytochrome c oxidase, blocking the mitochondrial electron transport chain with subsequent inhibition of tissue aerobic respiration.4,7 Acute toxicity may lead to depression of the central nervous system and death. Survival with or without sequelae remains possible, and individual susceptibility factors have yet to be uncovered.4 While animal studies and clinical experience have led to a robust understanding of the metabolism of cyanide, finding effective therapies to deploy in the context of mass intoxication of a terror type has remained a daunting task.1,8,9 Studies of the efficacy of antidotes or treatment options are hampered by ethics that prohibit randomized trials in those needing medical attention for acute exposure or less common scenarios of mass exposures. Also, the source and type of exposure (e.g., hydrogen cyanide alone or in combination with chemical stabilizing agents), as well as the timing of the intervention, are among other factors that determine success in treatment protocols.1 These limitations underscore the importance of toxicity models that can allow testing and/or validation of point-of-care diagnostic tools, testing of treatment options, and understanding of both acute and long-term impacts of cyanide on human health and functioning.
This article reviews the impact of cyanide on the human brain, as well as the susceptibility factors and the long-term neurocognitive impact, from a model of cyanide poisoning in populations that almost exclusively rely on cyanogenic cassava as a staple food. Current understanding of the neurotoxic effects associated with cassava remains limited, as most of the features of toxicity in humans have not been fully reproduced in experimental models.10–13
Epidemiology of food (cassava) cyanide poisoning and related neurological disease
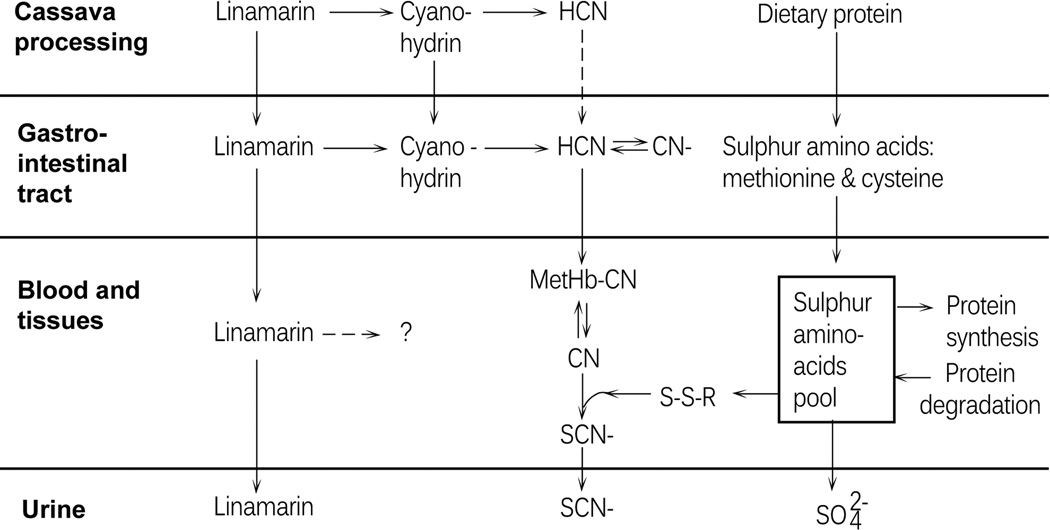
Food (cassava) cyanide poisoning has often resulted in death or survival with permanent neurological deficits, such as the paralytic disease known as konzo, in thousands of people in sub-Saharan African countries, notably in Angola, Cameroon, the Central African Republic, the Democratic Republic Of Congo (DRC), Tanzania, Uganda, and Mozambique. In these countries, populations heavily rely on cyanogenic cassava as the main source of food.14–21 Cassava (Manihot esculenta Crantz) is a drought-tolerant tropical shrub that is cultivated for its starchy storage roots and leafy vegetation and is believed to be a staple for more than 600 million people in the tropics, half of whom live in Africa.22–24 Cassava cultivars are either “bitter” or “sweet.” The former generally contain higher amounts of cyanogenic glycosides, mainly linamarin and structurally similar lotaustralin (in a ~93:7 concentration ratio).23 Traditional processing methods are used to remove cyanogenic glycosides and their degradation products from cassava before human consumption. Methods to remove cyanogens include soaking raw cassava in water for up to 4 days or grating the tuber followed by sun drying or heating.16,25,20 Once the physical integrity of the cassava tissue has been disrupted, linamarin is brought in contact with the β-glycosidase linamarase, which is located in the plant cell walls, and hydrolyzed to glucose and cyanohydrins. At pH > 5, the cyanohydrins spontaneously break down into ketones, and hydrogen cyanide (HCN) gas escapes. Processed cassava roots are then used to produce flour and other types of food items for household consumption. However, in times of famine that may be caused by flood, drought, pestilence, or war, poor populations are forced to reduce food-processing times. Under these conditions, higher residual amounts of cyanogenic compounds in poorly processed foodstuffs are ingested and metabolized to cyanide, a highly toxic compound, which will be converted into the less toxic thiocyanate (Fig. 1).14,26–28
Figure 1.
Metabolic fate of linamarin and cyanide during cassava processing and after ingestion of poorly processed cassava foodstuffs. Once the physical integrity of the cassava tissue is disrupted, linamarin is hydrolyzed to glucose and cyanohydrins. At pH > 5, the cyanohydrins spontaneously break down into ketones, and hydrogen cyanide (HCN) gas escapes. Lower pH leads to persistence of cyanohydrins in the finished food product, with the result that cyanide may be released by bacterial enzymatic cleavage in the gastrointestinal tract and enter the bloodstream. Once in the bloodstream, cyanide is either trapped by methemoglobin (MetHB–CN) or converted into thiocyanate (SCN). The human body may then excrete either intact linamarin or reportedly less toxic SCN in urine.
Patterns of low-level chronic cyanogenic exposure punctuated by rises and peaks in exposure are common in those populations relying on cyanogenic cassava as the main source of food. For example, peaks in exposure are seen during the dry season, due to shortcuts in cassava-processing methods because of limited availability of water and pressing need for cassava flour. Under these circumstances, rise in cyanide exposure has often been followed by outbreaks of konzo (vide infra).15,27,29 A number of epidemiological studies have shown that the disease mainly affects children and women of childbearing age––mostly those with poor dietary intake of animal protein, a major source of sulfur amino acids needed to provide sulfur for the detoxification of cyanide. Whether the greater susceptibility of children or women is explained by factors other than poor nutrition is still unknown. 30
Konzo was named after a local designation in kiyaka, a local language spoken in the DRC, and means “tied legs,” in reference to the scissoring spastic gait of affected subjects. The disease was first documented in 1938 in the southwestern region of Zaire, the former "Belgian Congo," presently known as the DRC. The literature indicates that konzo was already known to the local population of the Bandundu province in the DRC in the late 1800s.31,32 Since the beginning of the 20th century, outbreaks have occurred in several other countries.14,15,18,20,26–28,33,34 While isolated cases of the cyanide-related spastic paralysis may be seen, the disease often occurs in an outbreak manner, with a point prevalence of up to 10% in certain areas. The total number of cases has been underestimated owing to the lack of reliable demographic censuses and surveillance systems. A single major outbreak in the Kahemba district of the DRC accounted for nearly 2000 children affected by the disease during the dry season that spanned June–September 2009.21 The exposure patterns were similar to those reported in outbreaks that previously occurred in all of the above-mentioned countries.21,35
Cassava cyanide–associated neurological deficits and phenotypes
There are numerous reports of acute toxicity and death following consumption of toxic cassava. Acute signs of toxicity include headache, dizziness, lethargy, and sometimes seizing events. Gastrointestinal (GI) symptoms are also common and may include nausea, vomiting, and abdominal pain.36–39 These symptoms are commonplace in konzo areas, as cyanogenic cassava is the main source of food. Whether they reflect a direct toxicity of cyanide to the GI tract smooth muscle or the autonomic nervous plexi is unknown. Subacute exposure to cassava cyanogens, often punctuated by a rise in exposure when shortcuts are undertaken during the processing of cassava, is followed by a sudden onset of konzo. Subjects with konzo present with signs of dysfunction in the motor system, with a clear and distinct type of spastic paraparesis (leg paralysis), or tetraparesis (both legs and arms are paralyzed) in those severely affected by the disease (Fig. 2).19,40 Symptoms at onset often occur after a long walk and include sudden trembling in the legs, sometimes in association with paresthesia; sensations of electrical discharges in the spine and legs; and loss of visual acuity. There are no signs of peripheral nerve involvement. The mechanisms underlying the spasticity observed immediately at the onset of the disease, as well as those preferentially targeting the upper motor system, have yet to be uncovered.19,41–43
Figure 2.
Spastic stance in a child severely affected by the cassava-associated spastic paraparesis known as konzo (child left) and a woman with a moderate form of the disease (woman with walking stick).
The World Health Organization (WHO) has adopted the following definition criteria for the disease konzo: (1) a visible symmetric spastic abnormality of gait while walking or running; (2) a history of onset of less than 1 week followed by a nonprogressive course in a formerly healthy person; and (3) bilaterally exaggerated knee or ankle jerks without signs of disease of the spine. Once the subject is capable of standing and/or walking, the disease severity is graded as follows: mild konzo (able to walk with no support), moderate konzo (needs support to walk), and severe konzo (unable to walk).44 Severely affected subjects may present with speech and swallowing difficulties. Motor symptoms are permanent and irreversible. However, somatosensory and visual symptoms tend to regress over the course of the disease, and genitourinary functions remain normal. Deficits in fine motor control, in association with exaggerated deep tendon reflexes or ankle clonus, are commonly found in the general population of konzo-affected areas, suggesting the existence of subclinical and preclinical konzo.19,43,45,46
Clinical electrophysiological studies indicate slowing of nerve conduction in the motor, somatosensory, and visual pathways, as well as a general slowing of activities in the electroencephalogram (Table 1).42,47–49 A recent study using the Kaufman Assessment Battery for Children, 2nd edition (KABC-II) for cognition and the Bruininks/Oseretsky Test, 2nd Edition (BOT-2) measure for motor proficiency revealed that cassava cyanide poisoning is associated with cognitive deficits and severe impairments of fine motor control and coordination (Table 2).35 Experimental studies suggest that neurocognitive deficits may be caused by cassava cyanogens and/or their metabolites, including cyanate. Whether the motor and cognitive deficits observed in children from konzo areas share the same pathogenic mechanisms has yet to be determined.35,50–52
Table 1.
Clinical electrophysiology of the cassava cyanide–associated spastic paraparesis konzo
| Explorations | Abnormalities |
|---|---|
| Motor evoked potentials (MEPs) | Frequent inability to elicit MEP.a When present, central motor conduction time is often increased.b |
| Peripheral nerve conduction studies | Normal motor and sensory nerve conduction. Increased amplitude of F-waves. |
| Somatosensory evoked potentials (SEPs) | Cortical responses following tibial stimulation frequently absent. If present, the latency is prolonged. Median SEP often normal. |
| Visual evoked potentials (VEPs) | Frequent delay and decreased amplitude of P100. |
| Electroencephalography (EEG) | Frequent generalized slowing of background activity and nonspecific paroxysmal activities. |
Consistent with reduction of the upper motor neuron pool.
Consistent with loss of pyramidal conductivity from spinal tract (axonal) damage.
Table 2.
Performance scores of konzo and non-konzo children at neuropsychological testing.
| Kaufman Assessment Battery (2nd ed.) (KABC-II) standardized global scales and their subtests and Bruininks- Oseretsky Test (2nd ed.) (BOT- 2) standardized global scales |
Konzo groups median (interquartile range) |
Kruskal- Wallis ANOVA K score |
Kruskal-Wallis P values for between-group konzo/non- konzo comparisons for all and by gender |
|||
|---|---|---|---|---|---|---|
| Nonkonzo n = 87 |
Konzo n = 123 |
K All DF = 1 |
P All n = 210 |
P Female n = 93 |
P Male n = 117 |
|
| KABC-II sequential processing | 74 (22) | 71 (17) | 2.73 | 0.098 | 0.193 | 0.430 |
| Number recall | 7 (5) | 6 (5) | 1.05 | 0.306 | 0.441 | 0.439 |
| Word order | 4 (4) | 3 (3) | 4.40 | 0.036 | 0.063 | 0.017 |
| Hand movements | 4 (4) | 6 (4) | 3.14 | 0.077 | 0.236 | 0.035 |
| KABC-II simultaneous processing | 64 (16) | 58 (16) | 8.78 | 0.003 | 0.501 | 0.003 |
| Face recognitiona | 8 (6) | 5 (6) | 15.90 | < 0.001 | 0.362 | < 0.001 |
| Rover | 7 (6) | 5 (6) | 8.31 | 0.004 | 0.478 | 0.589 |
| Triangles | 1 (2) | 1 (1) | 3.34 | 0.068 | 0.540 | 0.543 |
| KABC-II learning | 70 (14) | 70 (11) | 0.30 | 0.582 | 0.596 | 0.875 |
| Atlantis | 5 (3) | 5 (3) | 0.11 | 0.738 | 0.974 | 0.923 |
| Rebus | 4 (3) | 4 (4) | 1.02 | 0.312 | 0.231 | 0.112 |
| KABC-II delayed learning | 72 (14) | 74 (14) | 0.19 | 0.666 | 0.646 | 0.257 |
| Atlantis delayed | 5 (3) | 5 (3) | 2.09 | 0.149 | 0.800 | 0.854 |
| Rebus delayed | 4 (3) | 4 (4) | 1.19 | 0.275 | 0.317 | 0.464 |
| KABC-II planning | 57 (11) | 51 (16) | 3.71 | 0.054 | 0.742 | 0.037 |
| Conceptual thinkinga | 8 (7) | 6 (8) | 6.18 | 0.013 | 0.836 | 0.002 |
| Pattern recognition | 2 (2) | 1 (2) | 2.24 | 0.135 | 0.899 | 0.975 |
| KABC-II mental processing index (MPI) | 60 (14) | 58 (13) | 4.56 | 0.033 | 0.420 | 0.087 |
| BOT-2 fine motor control | 32 (13) | 29 (12) | 8.44 | 0.004 | 0.146 | 0.032 |
| BOT-2 manual coordination | 36 (10) | 26 (10) | 58.30 | < 0.001 | < 0.001 | < 0.001 |
| BOT-2 body coordination | 40 (15) | 25 (10) | 111.43 | < 0.001 | < 0.001 | < 0.001 |
| BOT-2 strength & agility | 38 (9) | 24 (11) | 104.13 | < 0.001 | < 0.001 | < 0.001 |
| BOT-2 motor proficiency total | 34 (10) | 22 (9) | 83.26 | < 0.001 | < 0.001 | < 0.001 |
NOTE: Group medians and interquartile ranges (IQR) are presented for the Kaufman Assessment Battery for Children (2nd ed) (KABC-II) standardized (age-adjusted) global scales and their corresponding subtests, as well as for the Bruininks-Oseretsky Test of Motor Proficiency (2nd ed) (BOT-2) standardized (adjusted for age and gender) global scores. The Kruskal-Wallis test P values for the non-konzo/konzo between-group comparisons are stratified by gender (far right columns). Statistically significant P values are in bold. DF, degrees of freedom.
KABC-II subtest raw score used because scaled scores only available for children under 7 years.
On the biomarkers and mechanisms of cassava cyanide–associated neurological disease
Biomarkers of exposure
In most studies, the exposure to cyanogenic compounds is ascertained by measuring the concentrations of thiocyanate (SCN), the main cyanide metabolite in urine (U-SCN) or plasma (P-SCN).30 Levels of U-SCN or P-SCN may be as high as 1720 and 426 µmol/L, respectively, in konzo-affected populations. A recent study found a concentration of 520.4 ± 355.7 (mean ± SD) µmol/l of U-SCN in children with konzo, which was significantly higher than 382.5± 226.3 µmol/L, in those with no konzo (P < 0.05).35 Samples of cassava flour from 18 consenting households were collected and found to have cyanide concentrations from 30–200 ppm with a mean of 92.2 (± 56.2) ppm, well above the 10-ppm safe limit proposed by the WHO (Joint Food and Agriculture Organization/World Health Organization report on food contaminants, Rotterdam, 2009).35 The introduction of a new food-processing method has helped reduce the cyanogenic content of cassava and, hence, the levels of exposure to its cyanogenic compounds, and a subsequent decrease in the number of incident cases of konzo was noted in select areas of the DRC and Tanzania.16,20,53,54 The only study that measured the concentration of cyanide in the blood of konzo subjects found concentrations that reached ~ 20 times the "accumulation level" of 4 µmol/L in three subjects within the first week of konzo onset.14 In general, a fatal level is considered to exceed 100–115 µmol/L.
Biomarkers of susceptibility
Current knowledge of cassava toxicity clearly indicates that konzo is associated with chronic reliance on improperly processed cyanogenic (bitter) cassava as the main source of food. It is possible that outbreaks of the disease and/or its sudden onset are triggered by a peak in the exposure to the cassava cyanogens and, hence, to their toxic metabolites (vide infra).17,26,27,34 While most subjects from konzo-affected areas rely on cyanogenic cassava as staple food, only a fraction (i.e., up to 10%) suffers from overt gait abnormalities, suggesting that there may be individual factors that dictate susceptibility to the disease. The latter include malnutrition and younger age or female gender, for reasons that are not clearly understood, as well as poor cyanide detoxification capability and, possibly, genetics. Impaired cyanide detoxification may be seen as a result of poor nutrition (insufficient protein intake and/or availability of sulfur donors) and/or possible genetic polymorphisms.30
Putative biomarkers of neuropathology
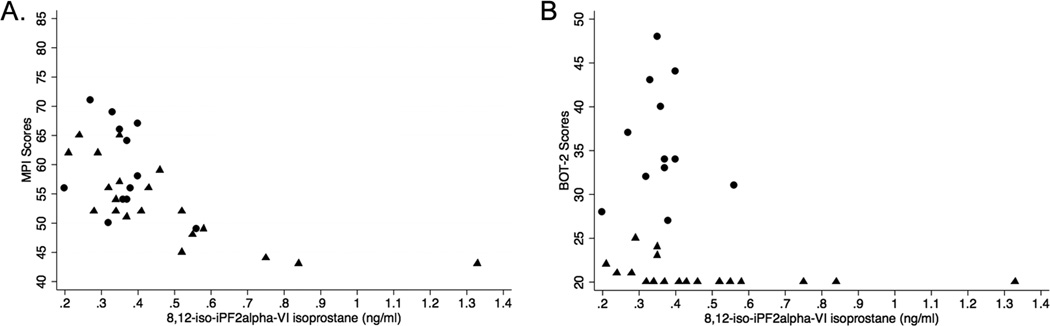
To date, the only observation performed on an autopsied brain, in the 1930s, was not conclusive.31 Most insight on the site of the lesion in konzo has been provided by electrophysiological studies, which suggest that both motor and somatosensory pathways, as well as visual pathways are affected (Table 1).42,48,49 Additional insights have been gained from experimental studies, which suggest that the neuropathology of konzo may be mediated through mechanisms of oxidative damage or protein carbamoylation induced by cyanide (pro-oxidant) or cyanate (cyanide metabolite, motor system toxicant, and protein-carbamoylating agent).11,55–57 We recently confirmed the association between the serum levels of 8,12-iso-iPF2α-VI F2-isoprostane isomer, a marker of lipid peroxidation and thus oxidative damage, and the extent of neurocognitive deficits found in children with konzo (Fig. 3).51 However, these peripheral markers of oxidative stress may not reflect the exact neuropathogenic mechanisms taking place in the central nervous system in response to the toxicity of cyanogenic cassava.
Figure 3.
Low motor or cognition performance scores significantly correlate with high serum concentrations of 8,12-iso-iPF2α-VI isoprostane. Neuropsychological assessments were done using the Kaufman Assessment Battery for Children, 2nd edition (KABC-II) for cognition and the Bruininks/Oseretsky Test, 2nd Edition (BOT-2) measure for motor proficiency. (A) MPI (KABC-II) scores relative to serum concentration of 8,12-iso-iPF2α-VI isoprostane (triangles = konzo children, r = −0.78, P = 0.00; circles = non-konzo children, r = −0.24, P = 0.47). (B) BOT-2 scores relative to serum level of 8,12-iso-iPF2α-VI isoprostane (triangles = konzo children, r = −0.63, P < 0.01; circles = non-konzo children, r = −0.06, P = 0.86).51
Future perspectives
The human model of food (cassava) cyanide poisoning offers invaluable opportunities to explore treatment options (e.g., antidotes for cyanide poisoning) and elucidate the neuropathogenic mechanisms underlying the long-term impact of cyanide exposure on the brain. Other opportunities to be exploited include testing and validation of point-of-care diagnostic tools to measure and monitor levels of cyanide exposure and metabolites in relation to risks for neurological disease. Further studies should explore the differential roles of cyanide (mitochondrial toxicant), thiocyanate (AMPA-receptor chaotropic metabolite), cyanate (protein-carbamoylating metabolite), and 2-iminothiazolidine-4-carboxylic acid (seizure inducer) in the pathogenesis of cassava-associated neurological damage.57 Whole-genome or exome sequencing, metagenomics, and epigenetics may unveil other factors of individual susceptibility to cyanide-related neurological disease.
There is no effective treatment for the motor sequelae associated with the neurotoxicity of cassava cyanogens. Once the neurodamaging process has stabilized, the disability (konzo) remains unchanged. Centrally acting spasmolytics, dorsal rhizotomy, or intramuscular injection of botulinum toxin, used with success to reduce adductor spasticity in patients with cerebral palsy, could be tested to alleviate symptoms. Prospects on cognitive rehabilitation have yet to be defined and tested.
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Rodgers GC, Jr, Condurache CT. Antidotes and treatments for chemical warfare/terrorism agents: an evidence-based review. Clinical pharmacology and therapeutics. 2010;88:318–327. doi: 10.1038/clpt.2010.152. [DOI] [PubMed] [Google Scholar]
- 2.Alarie Y. Toxicity of fire smoke. Critical reviews in toxicology. 2002;32:259–289. doi: 10.1080/20024091064246. [DOI] [PubMed] [Google Scholar]
- 3.Riordan M, Rylance G, Berry K. Poisoning in children 5: rare and dangerous poisons. Arch Dis Child. 2002;87:407–410. doi: 10.1136/adc.87.5.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Geller RJ, Barthold C, Saiers JA, et al. Pediatric cyanide poisoning: causes, manifestations, management, and unmet needs. Pediatrics. 2006;118:2146–2158. doi: 10.1542/peds.2006-1251. [DOI] [PubMed] [Google Scholar]
- 5.Luque-Almagro VM, Moreno-Vivian C, Roldan MD. Biodegradation of cyanide wastes from mining and jewellery industries. Current opinion in biotechnology. 2015;38:9–13. doi: 10.1016/j.copbio.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 6.Zagury GJ, Oudjehani K, Deschenes L. Characterization and availability of cyanide in solid mine tailings from gold extraction plants. The Science of the total environment. 2004;320:211–224. doi: 10.1016/j.scitotenv.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 7.Isom GE, Burrows GE, Way JL. Effect of oxygen on the antagonism of cyanide intoxication--cytochrome oxidase, in vivo. Toxicology and applied pharmacology. 1982;65:250–256. doi: 10.1016/0041-008x(82)90007-2. [DOI] [PubMed] [Google Scholar]
- 8.Wilson J. Cyanide in human disease: a review of clinical and laboratory evidence. Fundam Appl Toxicol. 1983;3:397–399. doi: 10.1016/s0272-0590(83)80011-6. [DOI] [PubMed] [Google Scholar]
- 9.Wrobel M, Jurkowska H, Sliwa L, et al. Sulfurtransferases and cyanide detoxification in mouse liver, kidney, and brain. Toxicol Mech Methods. 2004;14:331–337. doi: 10.1080/15376520490434683. [DOI] [PubMed] [Google Scholar]
- 10.Rivadeneyra-Dominguez E, Rodriguez-Landa JF. Motor impairments induced by microinjection of linamarin in the dorsal hippocampus of Wistar rats. Neurologia. 2014 doi: 10.1016/j.nrl.2014.10.018. [DOI] [PubMed] [Google Scholar]
- 11.Kassa RM, Kasensa NL, Monterroso VH, et al. On the biomarkers and mechanisms of konzo, a distinct upper motor neuron disease associated with food (cassava) cyanogenic exposure. Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association. 2011;49:571–578. doi: 10.1016/j.fct.2010.05.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mathangi DC, Mohan V, Namasivayam A. Effect of Cassava on motor co-ordination and neurotransmitter level in the albino rat. Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association. 1999;37:57–60. doi: 10.1016/s0278-6915(98)00099-4. [DOI] [PubMed] [Google Scholar]
- 13.Mathangi DC, Namasivayam A. Effect of cassava consumption on open-field behavior and brain neurotransmitters in albino rats. Physiology & behavior. 2000;70:89–93. doi: 10.1016/s0031-9384(00)00252-3. [DOI] [PubMed] [Google Scholar]
- 14.Tylleskar T, Banea M, Bikangi N, et al. Cassava cyanogens and konzo, an upper motoneuron disease found in Africa. Lancet. 1992;339:208–211. doi: 10.1016/0140-6736(92)90006-o. [DOI] [PubMed] [Google Scholar]
- 15.Tylleskar T, Legue FD, Peterson S, et al. Konzo in the Central African Republic. Neurology. 1994;44:959–961. doi: 10.1212/wnl.44.5.959. [DOI] [PubMed] [Google Scholar]
- 16.Banea JP, Bradbury JH, Mandombi C, et al. Effectiveness of wetting method for control of konzo and reduction of cyanide poisoning by removal of cyanogens from cassava flour. Food Nutr Bull. 2014;35:28–32. doi: 10.1177/156482651403500104. [DOI] [PubMed] [Google Scholar]
- 17.Cliff J, Muquingue H, Nhassico D, et al. Konzo and continuing cyanide intoxication from cassava in Mozambique. Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association. 2011;49:631–635. doi: 10.1016/j.fct.2010.06.056. [DOI] [PubMed] [Google Scholar]
- 18.Ciglenecki I, Eyema R, Kabanda C, et al. Konzo outbreak among refugees from Central African Republic in Eastern region, Cameroon. Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association. 2011;49:579–582. doi: 10.1016/j.fct.2010.05.081. [DOI] [PubMed] [Google Scholar]
- 19.Howlett WP, Brubaker GR, Mlingi N, et al. Konzo, an epidemic upper motor neuron disease studied in Tanzania. Brain. 1990;113(Pt 1):223–235. doi: 10.1093/brain/113.1.223. [DOI] [PubMed] [Google Scholar]
- 20.Mlingi NL, Nkya S, Tatala SR, et al. Recurrence of konzo in southern Tanzania: rehabilitation and prevention using the wetting method. Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association. 2011;49:673–677. doi: 10.1016/j.fct.2010.09.017. [DOI] [PubMed] [Google Scholar]
- 21.Okitundu Luwa EAD, Bumoko Makila-Mabe G, Ayanne MT, et al. Persistence of konzo epidemics in Kahemba, Democratic Republic of Congo: phenomenological and socio-economic aspects. The Pan African medical journal. 2014;18:213. doi: 10.11604/pamj.2014.18.213.4572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang W, Feng B, Xiao J, et al. Cassava genome from a wild ancestor to cultivated varieties. Nature communications. 2014;5:5110. doi: 10.1038/ncomms6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Siritunga D, Sayre R. Engineering cyanogen synthesis and turnover in cassava (Manihot esculenta) Plant molecular biology. 2004;56:661–669. doi: 10.1007/s11103-004-3415-9. [DOI] [PubMed] [Google Scholar]
- 24.Cock J. Cassava: a basic energy source in the tropics. Science. 1982;218:755–762. doi: 10.1126/science.7134971. [DOI] [PubMed] [Google Scholar]
- 25.Kemdirim OC, Chukwu OA, Achinewhu SC. Effect of traditional processing of cassava on the cyanide content of gari and cassava flour. Plant foods for human nutrition. 1995;48:335–339. doi: 10.1007/BF01088492. [DOI] [PubMed] [Google Scholar]
- 26.Tylleskar T, Banea M, Bikangi N, et al. Epidemiological evidence from Zaire for a dietary etiology of konzo, an upper motor neuron disease. Bull World Health Organ. 1991;69:581–589. [PMC free article] [PubMed] [Google Scholar]
- 27.Tylleskar T, Banea M, Bikangi N, et al. Dietary determinants of a non-progressive spastic paraparesis (Konzo): a case-referent study in a high incidence area of Zaire. Int J Epidemiol. 1995;24:949–956. doi: 10.1093/ije/24.5.949. [DOI] [PubMed] [Google Scholar]
- 28.Cliff J, Nicala D, Saute F, et al. Konzo associated with war in Mozambique. Trop Med Int Health. 1997;2:1068–1074. doi: 10.1046/j.1365-3156.1997.d01-178.x. [DOI] [PubMed] [Google Scholar]
- 29.Banea-Mayambu JP, Tylleskar T, Gitebo N, et al. Geographical and seasonal association between linamarin and cyanide exposure from cassava and the upper motor neurone disease konzo in former Zaire. Trop Med Int Health. 1997;2:1143–1151. doi: 10.1046/j.1365-3156.1997.d01-215.x. [DOI] [PubMed] [Google Scholar]
- 30.Tshala-Katumbay D, Mumba N, Okitundu L, et al. Cassava food toxins, konzo disease, and neurodegeneration in sub-Sahara Africans. Neurology. 2013;80:949–951. doi: 10.1212/WNL.0b013e3182840b81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trolli G. Paraplégie spastique épidémique, "Konzo" des indigènes du Kwango. In: Trolli G, editor. Résumé des observations réunies, au Kwango, au sujet de deux affections d´órigine indéterminée. Brussels: Fonds Reine Elisabeth; 1938. pp. 1–36. [Google Scholar]
- 32.Lucasse C. Le "Kitondji", synonyme de "Konzo", une paralysie spastique. Ann Soc Belg Med Trop. 1952;33:393–401. [PubMed] [Google Scholar]
- 33.Tylleskar T, Banea M, Bottiger B, et al. Konzo, an epidemic spastic paraparesis in Africa, is not associated with antibodies to HTLV-I, HIV, or HIV gag-encoded proteins. J Acquir Immune Defic Syndr Hum Retrovirol. 1996;12:317–318. doi: 10.1097/00042560-199607000-00014. [DOI] [PubMed] [Google Scholar]
- 34.Mlingi N, Kimatta S, Rosling H. Konzo, a paralytic disease observed in southern Tanzania. Tropical doctor. 1991;21:24–25. doi: 10.1177/004947559102100110. [DOI] [PubMed] [Google Scholar]
- 35.Boivin MJ, Okitundu D, Makila-Mabe Bumoko G, et al. Neuropsychological effects of konzo: a neuromotor disease associated with poorly processed cassava. Pediatrics. 2013;131:e1231–e1239. doi: 10.1542/peds.2012-3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cheok SS. Acute cassava poisoning in children in Sarawak. Tropical doctor. 1978;8:99–101. doi: 10.1177/004947557800800303. [DOI] [PubMed] [Google Scholar]
- 37.Akintonwa A, Tunwashe OL. Fatal cyanide poisoning from cassava-based meal. Human & experimental toxicology. 1992;11:47–49. doi: 10.1177/096032719201100107. [DOI] [PubMed] [Google Scholar]
- 38.Ariffin WA, Choo KE, Karnaneedi S. Cassava (ubi kayu) poisoning in children. The Medical journal of Malaysia. 1992;47:231–234. [PubMed] [Google Scholar]
- 39.Espinoza OB, Perez M, Ramirez MS. Bitter cassava poisoning in eight children: a case report. Veterinary and human toxicology. 1992;34:65. [PubMed] [Google Scholar]
- 40.Tylleskar T, Howlett WP, Rwiza HT, et al. Konzo: a distinct disease entity with selective upper motor neuron damage. J Neurol Neurosurg Psychiatry. 1993;56:638–643. doi: 10.1136/jnnp.56.6.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mwanza JC, Tshala-Katumbay D, Kayembe DL, et al. Neuro-ophthalmologic findings in konzo, an upper motor neuron disorder in Africa. Eur J Ophthalmol. 2003;13:383–389. doi: 10.1177/112067210301300409. [DOI] [PubMed] [Google Scholar]
- 42.Mwanza JC, Lysebo DE, Kayembe DL, et al. Visual evoked potentials in konzo, a spastic paraparesis of acute onset in Africa. Ophthalmologica. 2003;217:381–386. doi: 10.1159/000073066. [DOI] [PubMed] [Google Scholar]
- 43.Tshala-Katumbay D, Eeg-Olofsson KE, Tylleskar T, et al. Impairments, disabilities and handicap pattern in konzo--a non-progressive spastic para/tetraparesis of acute onset. Disabil Rehabil. 2001;23:731–736. doi: 10.1080/09638280110055075. [DOI] [PubMed] [Google Scholar]
- 44.WHO. Konzo: a distinct type of upper motor neuron disease. Weekly Epidemiological Record. 1996;71:225–232. [Google Scholar]
- 45.Cliff J, Nicala D. Long-term follow-up of konzo patients. Trans R Soc Trop Med Hyg. 1997;91:447–449. doi: 10.1016/s0035-9203(97)90279-0. [DOI] [PubMed] [Google Scholar]
- 46.Cliff J, Nicala D, Saute F, et al. Ankle clonus and thiocyanate, linamarin, and inorganic sulphate excretion in school children in communities with Konzo, Mozambique. J Trop Pediatr. 1999;45:139–142. doi: 10.1093/tropej/45.3.139. [DOI] [PubMed] [Google Scholar]
- 47.Tshala Katumbay D, Lukusa VM, Eeg-Olofsson KE. EEG findings in Konzo: a spastic para/tetraparesis of acute onset. Clin Electroencephalogr. 2000;31:196–200. doi: 10.1177/155005940003100408. [DOI] [PubMed] [Google Scholar]
- 48.Tshala-Katumbay D, Edebol Eeg-Olofsson K, Kazadi-Kayembe T, et al. Abnormalities of somatosensory evoked potentials in konzo--an upper motor neuron disorder. Clin Neurophysiol. 2002;113:10–15. doi: 10.1016/s1388-2457(01)00705-2. [DOI] [PubMed] [Google Scholar]
- 49.Tshala-Katumbay D, Eeg-Olofsson KE, Kazadi-Kayembe T, et al. Analysis of motor pathway involvement in konzo using transcranial electrical and magnetic stimulation. Muscle Nerve. 2002;25:230–235. doi: 10.1002/mus.10029. [DOI] [PubMed] [Google Scholar]
- 50.Kimani S, Sinei K, Bukachi F, et al. Memory deficits associated with sublethal cyanide poisoning relative to cyanate toxicity in rodents. Metabolic brain disease. 2014;29:105–112. doi: 10.1007/s11011-013-9459-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Makila-Mabe BG, Kikandau KJ, Sombo TM, et al. Serum 8,12-iso-iPF2alpha-VI isoprostane marker of oxidative damage and cognition deficits in children with konzo. PloS one. 2014;9:e107191. doi: 10.1371/journal.pone.0107191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bumoko GM, Sombo MT, Okitundu LD, et al. Determinants of cognitive performance in children relying on cyanogenic cassava as staple food. Metabolic brain disease. 2014;29:359–366. doi: 10.1007/s11011-014-9492-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Banea JP, Bradbury JH, Mandombi C, et al. Control of konzo by detoxification of cassava flour in three villages in the Democratic Republic of Congo. Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association. 2013;60:506–513. doi: 10.1016/j.fct.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 54.Banea JP, Nahimana G, Kuwa N, et al. Preventive control of konzo in the Democratic Republic of Congo. Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association. 2012;50:4234–4235. doi: 10.1016/j.fct.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 55.Zottola MA, Beigel K, Soni SD, et al. Disulfides as cyanide antidotes: evidence for a new in vivo oxidative pathway for cyanide detoxification. Chem Res Toxicol. 2009;22:1948–1953. doi: 10.1021/tx900258m. [DOI] [PubMed] [Google Scholar]
- 56.Kimani S, Moterroso V, Lasarev M, et al. Carbamoylation correlates of cyanate neuropathy and cyanide poisoning: relevance to the biomarkers of cassava cyanogenesis and motor system toxicity. Springerplus. 2013;2:647. doi: 10.1186/2193-1801-2-647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Spencer PS. Food toxins, AMPA receptors, and motor neuron diseases. Drug Metab Rev. 1999;31:561–587. doi: 10.1081/dmr-100101936. [DOI] [PubMed] [Google Scholar]