Abstract
Aim
To evaluate the relationship between periodontal diseases and subclinical atherosclerosis in a younger and lean South Asian population.
Methods
We conducted a cross-sectional study in 917 subjects (mean age 46 years and mean body mass index 21.1 kg/m2) from the Health Effects of Arsenic Longitudinal Study in Bangladesh. Multivariate linear regression models were used to assess the associations between multiple clinical measures of periodontal diseases and carotid intima-media thickness (IMT).
Results
Mean attachment loss (AL) and percentage of sites with AL ≥ 4 mm (% AL ≥ 4) were associated with increased IMT. The IMT was 20.0-μm (95% CI: 2.2, 37.8) and 26.5-μm (95% CI: 8.9, 44.1) higher in those in the top quartile of mean AL (> 3.72 mm) and % AL ≥ 4 (> 58.4%), respectively, compared to those in the bottom quartile. In a subset of 366 subjects, mean AL was positively associated with plasma levels of matrix metalloproteinase-9 (P < 0.05) and soluble intercellular adhesion molecule-1 (P < 0.01).
Conclusions
Attachment loss was associated with subclinical atherosclerosis in this young and lean Bangladeshi population. Future prospective studies are needed to confirm this association.
Keywords: atherosclerosis, Bangladesh, cardiovascular disease, carotid intima-media thickness, periodontal diseases
Introduction
Accumulated epidemiologic evidence suggests a positive association between periodontal diseases and cardiovascular disease (CVD) (Lockhart et al., 2012). The effect of periodontal diseases has been extended to subclinical measures of CVD, particularly the intima-media thickness (IMT) of the carotid artery. Carotid IMT is a surrogate marker for subclinical atherosclerosis and predicts CVD risk (de Groot et al., 2004, Stein et al., 2008). Although a number of studies have reported an association between periodontal diseases and increased IMT levels or odds of having carotid atherosclerosis (defined as carotid IMT ≥1 mm) as summarized in a recent systemic review and meta-analysis (Orlandi et al., 2014), most of the previous studies were conducted among older adults, with limited evidence in younger populations, populations with low body mass index (BMI, < 23 kg/m2) (WHO, 2004), or in South Asians. Whether the association between periodontal diseases and atherosclerosis can be generalizable to these populations remains controversial.
The prior literature presents several methodological challenges that have not been adequately addressed. Firstly, definitions of periodontal diseases were based on one or a few clinical periodontal measures. As specific periodontal measures may represent current or cumulative inflammatory burden and convey differential risk for atherosclerosis (Demmer et al., 2008, Beck and Offenbacher, 2002), multiple measures from full mouth exams should be considered. Secondly, few studies have explored whether the association between periodontal diseases and atherosclerosis differs by the status of smoking, an established risk factor for both periodontal diseases and CVD. In addition, systemic inflammation has been raised as one of the mechanisms connecting periodontal diseases and atherosclerosis. However, only a limited number of inflammatory markers have been investigated (Paraskevas et al., 2008, Loos, 2005, Nakajima et al., 2010).
We aimed to evaluate the association between various clinical measures of periodontal diseases and carotid IMT in a rural Bangladeshi population. In a subset, we also assessed whether the strongest periodontal predictor of carotid IMT was associated with plasma levels of a panel of inflammatory markers.
Methods
Study population
The parent study, the Health Effects of Arsenic Longitudinal Study (HEALS), is an ongoing prospective cohort study designed to investigate the health effects of arsenic exposure from drinking water in Araihazar, Bangladesh. Since it is a population-based study, the HEALS is also appropriate for assessing other CVD risk factors in South Asians. Study details have been presented previously (Ahsan et al., 2006). Briefly, between October 2000 and May 2002, we recruited 11 746 participants (original cohort) from a well-defined 25-km2 geographical area, who met the following eligibility criteria: married (to reduce loss to follow-up); aged 18–75 years; user of a tube well as a primary water supply and living in the study area for at least five years before recruitment. During 2006–2008, the cohort was expanded to include an additional 8287 participants (expansion cohort) following the same methods. The overall participation rate was 97%.
The cohort has been followed up with in-person home visits at 2-year intervals. Informed consent was obtained from the study participants and study procedures were approved by the Ethical Committee of the Bangladesh Medical Research Council and the Institutional Review Boards of Columbia University and the University of Chicago.
Between April 2010 and January 2012, we randomly selected 800 participants from the 11 224 original cohort members as well as 700 participants from the 5136 participants over 30 years of age in the expansion cohort, as part of a previous study on urinary arsenic and carotid IMT (Chen et al., 2013). In total, IMT was measured for 1206 individuals, and 294 participants did not complete IMT measurements due to deaths, move, serious illness, or time constraints (McClintock et al., 2014). A comprehensive oral examination was performed on the same day between April 2010 and September 2011 for the first 951 of the 1206 participants, consisting of 585 from the original cohort and 366 from the expansion cohort. Comparison of demographics and lifestyle factors between the total participants with IMT measurements and the 951 participants included in this study did not show significant difference (data not shown).
Questionnaire data
Demographic and lifestyle characteristics were collected at baseline using a standardized questionnaire. Physicians measured height, weight and blood pressure with standard protocols and equipments (Chen et al., 2007a, Pierce et al., 2010). Diabetes status was defined by self-report of physician-diagnosed diabetes which was validated by comparison with results from glycosylated haemoglobin and glucosuria tests. Questions on tobacco smoking included cigarettes and bidis (filterless, locally-produced cigarettes) smoked alone or together, past and current use, number of cigarettes or bidis smoked per day, and years of tobacco smoking. Smoking intensity in past and current smokers was calculated as pack-years (Wu et al., 2013). Information on betel quid chewing included past and current use, the number of times per day betel was used and years of betel use. We also estimated the intensity of betel quid chewing (quid-years) as the product of times used per day and years of use (Wu et al., 2015, McClintock et al., 2014). All covariate data were derived from the time of IMT measurement, except sex and educational attainment (years) which were ascertained at baseline only since they do not change over time.
Measurement of carotid IMT
Detailed methodology for IMT measurements have been described previously (Chen et al., 2013). Briefly, one designated physician who had extensive training in carotid sonography performed all measurements using a SonoSite MicroMaxx ultrasound machine (SonoSite, Inc., Bothell, Washington) equipped with an L38e/10-5-MHz transducer according to a specific research protocol developed and implemented in the Oral Infections and Vascular Disease Epidemiology Study (INVEST) (Desvarieux et al., 2005). IMT was examined in the near and far walls of the common carotid artery (CCA), bifurcation and internal carotid artery (ICA) of both sides of the neck. Overall IMT was calculated as a mean of the maximum measurements of the 12 carotid sites in mm, which was found to be more strongly associated with coronary atherosclerosis than IMT from individual segments (Crouse et al., 2002).
Of the 951 participants, 14 participants were excluded from the present analysis due to missing IMT at all 4 sites of the CCA and bifurcation, as IMT data were largely complete at these two segments. An additional 20 subjects were excluded because of unsuccessful IMT measurements at all sites of the ICA. The final study population included 917 participants with all measurements of the CCA and bifurcation and at least one measurement of the ICA.
Oral examination
Participants underwent a comprehensive dental examination by two trained and calibrated examiners also following the INVEST protocol (Desvarieux et al., 2005). The number of missing teeth was recorded. The periodontal examination was performed at six sites (mesiobuccal, midbuccal, distobuccal, mesiolingual, midlingual, and distolingual) per tooth of all retained teeth excluding the third molars, using a UNC-15 manual probe (Hu-Friedy, Chicago, IL) (Loe, 1967). Several clinical measures of periodontal diseases were recorded in millimeters for each of the six sites, including probing depth (PD), defined as the distance from the gingival margin to the base of the gingival sulcus, and clinical attachment loss (AL), defined as the distance from the cementoenamel junction to the base of the sulcus. Bleeding on probing (BOP) was recorded dichotomously for each tooth and deemed positive if it occurred within 15 s of probing. The percentage of sites with PD ≥ 4 mm (% PD ≥ 4), AL ≥ 4 mm (% AL ≥ 4), and BOP (% BOP) was calculated by dividing the number of sites with PD ≥ 4 mm, AL ≥ 4 mm, and BOP by the total number of sites measured.
Prevalence of periodontitis was also reported according to the CDC-AAP case definitions (Page and Eke, 2007). Severe periodontitis was defined as the presence of 2 or more interproximal sites with ≥ 6 mm AL (not on the same tooth) and 1 or more interproximal site(s) with ≥ 5 mm PD. Moderate periodontitis was defined as 2 or more interproximal sites with ≥ 4 mm AL (not on the same tooth) or 2 or more interproximal sites with PD ≥ 5 mm, also not on the same tooth. No or mild periodontitis was applied if neither moderate nor severe periodontitis was diagnosed. Based on these definitions, only 7 subjects were defined as having no or mild periodontitis. We therefore did not investigate the difference in IMT in the multivariate model by case status of periodontitis.
Measurements of plasma levels of CVD markers
Five inflammatory markers, including matrix metalloproteinase-9 (MMP-9), myeloperoxidase (MPO), soluble E-selectin (sE-selectin), soluble intercellular adhesion molecule-1 (sICAM-1), and soluble vascular adhesion molecule-1 (sVCAM-1), were measured in baseline blood samples for 500 subjects from the expansion cohort for a prior investigation of arsenic exposure and CVD markers (Wu et al., 2012). The data were available for all the 366 (40%) expansion cohort participants who also had data on IMT and periodontal measures. sICAM-1 and sVCAM-1 are highly reproducible (Wu et al., 2012, Pai et al., 2002, Chen et al., 2007b) and one-time measurement can represent long-term level to be associated with periodontal diseases. In brief, plasma levels of MMP-9, MPO, sE-selectin, sICAM-1, and sVCAM-1 were analyzed by multiplex assays using MILLIPLEX MAP Human CVD Panel 1 kits (Millipore, Billerica, MA). The range of the inter-plate, inter-individual, and intra-individual coefficient of variation for the five markers were 0-2.1%, 3.5-11%, and 1.9-5.6%, respectively, and the range of intraclass correlation was 0.75-0.90.
Statistical analyses
We excluded participants with missing data on the periodontal measures in their respective analyses (n = 2 for % BOP and n = 3 for mean PD, mean AL, % PD ≥ 4, and % AL ≥ 4). We first conducted descriptive analyses to assess the associations of demographic, lifestyle, and periodontal measures with carotid IMT levels using Chi-square tests and analysis of variance (ANOVA). Multiple linear regression models using IMT as the response variable were used to estimate the difference in IMT comparing each of the higher three quartiles of the periodontal measures with the bottom quartile. We also considered periodontal measures as continuous variables and examined the difference in IMT in association with a difference of 1-mm in mean PD and AL, 1 missing tooth in number of missing teeth, as well as 10% in % PD ≥ 4, % AL ≥ 4, and % BOP.
We first adjusted for sex and age (model 1); we then adjusted for BMI, educational attainment (years), smoking status (never and ever), and betel quid chewing (model 2). Sensitivity analyses were conducted to include additional adjustment for well water arsenic, smoking intensity (pack-years), intensity of betel quid chewing (quid-years), systolic blood pressure, and diabetes at baseline. Sensitivity analyses were also conducted using the mean of the maximum measurements of the four CCA sites as the response variable. We selected the periodontal measure that demonstrated the strongest association with IMT in the aforementioned regression analyses and conducted stratified analyses to examine whether the association differed by sex, age, BMI, or smoking status (never versus ever). Age and BMI were dichotomized by the median value in the overall population. The P value for the cross-product term between a potential effect modifier and the periodontal measure as a continuous variable was used to judge the statistical significance of the multiplicative interaction. Lastly, in a subset, we examined the associations between the periodontal measures and plasma levels of the CVD markers using multiple linear regression models with each periodontal measure as the independent variable and each marker as the dependent variable. The associations between plasma levels of each marker and carotid IMT were also explored. All analyses were completed using SAS (version 9.3; SAS Institute Inc, Cary, NC).
Results
Characteristics of the study participants are shown in Table 1. Overall, 41.1% were male with a mean age of 46.4 years. The participants were lean with an average BMI of 21.1 and had an average of 3 years of formal education. The proportion of ever smokers was much higher in men (76.9%) than in women (10.4%). The prevalence of betel quid chewing was high (55.8%) while diabetes was not common (1.9%). The prevalence of moderate and severe periodontitis was 60.1% and 39.1%, respectively, and only less than 1% of the participants had healthy periodontal condition or mild disease status.
Table 1.
Distribution of population characteristics by quintiles of carotid IMT*
| Overall (n = 917) | Quintiles of carotid IMT† |
p ‡ | |||||
|---|---|---|---|---|---|---|---|
| 1 (n = 182) | 2 (n = 185) | 3 (n = 184) | 4 (n = 183) | 5 (n = 183) | |||
| IMT, μm | 807.2 ± 96.6 | 694.9 ± 26.6 | 749.8 ± 12.2 | 789.9 ± 12.3 | 845.3 ± 18.0 | 956.3 ± 74.4 | – |
| Male, % | 41.1 | 21.4 | 27.0 | 40.2 | 50.8 | 66.1 | < 0.01 |
| Age, years | 46.4 ± 8.7 | 39.8 ± 6.6 | 43.6 ± 7.3 | 46.1 ± 7.2 | 49.0 ± 7.3 | 53.5 ± 8.6 | < 0.01 |
| Body mass index, kg/m2 | 21.1 ± 4.4 | 21.1 ± 4.5 | 20.9 ± 4.3 | 20.9 ± 4.0 | 21.4 ± 5.5 | 21.0 ± 3.8 | 0.75 |
| Ever smoking, % | |||||||
| Men | 76.9 | 74.4 | 58.0 | 77.0 | 78.5 | 84.2 | < 0.01 |
| Women | 10.4 | 4.2 | 7.4 | 12.8 | 13.3 | 23.0 | < 0.01 |
| Education, years§ | 3.0 ± 3.6 | 2.8 ± 3.4 | 3.2 ± 3.6 | 2.7 ± 3.3 | 3.0 ± 3.6 | 3.5 ± 4.2 | 0.24 |
| Betel quid chewing, % | 55.8 | 43.6 | 50.5 | 54.6 | 63.9 | 66.3 | < 0.01 |
| Systolic blood pressure, mmHg | 122.3 ± 15.8 | 117.7 ± 13.3 | 118.2 ± 13.3 | 121.2 ± 15.6 | 125.3 ± 17.1 | 129.0 ± 16.8 | < 0.01 |
| Diastolic blood pressure, mmHg | 76.6 ± 12.3 | 73.4 ± 11.6 | 75.1 ± 10.5 | 76.1 ± 11.8 | 78.1 ± 13.3 | 80.2 ± 13.0 | < 0.01 |
| Diabetes, %§ | 1.9 | 1.6 | 0.0 | 0.0 | 2.7 | 4.9 | < 0.01 |
| Well water arsenic, μg/L§ | 80.0 ± 102.0 | 82.5 ± 106.0 | 85.1 ± 95.6 | 70.9 ± 95.0 | 72.7 ± 85.4 | 88.6 ± 123.2 | 0.38 |
| Mean probing depth, mm | 2.5 ± 0.4 | 2.4 ± 0.3 | 2.4 ± 0.3 | 2.5 ± 0.3 | 2.5 ± 0.4 | 2.6 ± 0.4 | < 0.01 |
| Mean attachment loss, mm | 3.1 ± 1.2 | 2.5 ± 0.9 | 2.8 ± 0.9 | 3.0 ± 1.0 | 3.5 ± 1.3 | 3.7 ± 1.3 | < 0.01 |
| Percentage of sites with bleeding on probing | 18.0 ± 12.9 | 23.0 ± 14.1 | 19.8 ± 12.3 | 18.6 ± 12.9 | 15.0 ± 11.3 | 13.7 ± 11.4 | < 0.01 |
| Percentage of sites with ≥ 4 mm probing depth | 8.5 ± 10.4 | 6.7 ± 8.5 | 6.6 ± 7.3 | 8.8 ± 10.0 | 10.0 ± 12.5 | 10.5 ± 12.3 | < 0.01 |
| Percentage of sites with ≥ 4 mm attachment loss | 38.3 ± 28.2 | 23.9 ± 22.2 | 31.3 ± 24.5 | 36.7 ± 27.0 | 47.5 ± 29.3 | 51.9 ± 28.1 | < 0.01 |
| Number of missing teeth | 3.3 ± 5.0 | 1.9 ± 2.8 | 2.5 ± 3.1 | 2.7 ± 4.0 | 4.4 ± 5.7 | 5.0 ± 7.2 | < 0.01 |
| CDC-AAP periodontitis∥ | |||||||
| No/mild | 0.8 | 1.6 | 0.0 | 0.5 | 0.6 | 1.1 | < 0.01 |
| Moderate | 60.1 | 80.8 | 69.2 | 57.6 | 48.6 | 44.2 | |
| Severe | 39.1 | 17.6 | 30.8 | 41.9 | 50.8 | 54.7 | |
Data were missing on body mass index, ever smoking, betel quid chewing, systolic blood pressure, and diastolic blood pressure at the time of IMT measurement for 10, 4, 5, 4, and 4 subjects, respectively; on diabetes and water arsenic for 3 and 13 subjects; on mean probing depth, mean attachment loss, percentage of sites with ≥ 4 mm probing depth, and percentage of sites with ≥ 4 mm attachment loss on 3 subjects; and on percentage of sites that bled on probing for 2 subjects.
Quintile 1: 606.4-729.2 μm; Quintile 2: 729.3-771.7 μm; Quintile 3: 771.8-814.6 μm; Quintile 4: 814.7-876.7 μm; Quintile 5: 876.8-1423.3 μm.
P values were computed with the chi-square test or analysis of variance.
Assessed at baseline.
Periodontitis according to the Centers for Disease Control and Prevention (CDC) and the American Academy of Periodontology (AAP) case definitions.
Male sex, increasing age, ever smoking among both men and women, betel quid use, and elevated blood pressure were associated with higher levels of carotid IMT (Table 1). Prevalence of diabetes at baseline was significantly higher in the higher two quintiles of IMT. There was no association of BMI, educational attainment, and well water arsenic with IMT. Mean PD, mean AL, % PD ≥ 4, % AL ≥ 4, and number of missing teeth were increasing whereas % BOP was decreasing with increasing quintiles of IMT. The prevalence of severe periodontitis based on the CDC-AAP definition was increasing with increasing levels of IMT.
IMT in participants with > 3.72-mm mean AL was 20.0-μm [95% confidence interval (CI): 2.2, 37.8] higher than that in participants with ≤ 2.30-mm mean AL (Table 2). There was a significant dose-response relationship between mean AL and IMT (model 2). Every 1-mm difference in mean AL was associated with a 6.4-μm (95% CI: 0.9, 11.9) difference in IMT. The association remained significant with additional adjustment for smoking intensity [6.9-μm (95% CI: 1.1, 12.7)] and did not materially change with additional adjustment for other variables (data not shown). For instance, every 1-mm difference in mean AL was associated with a difference of 6.2-μm (95% CI: 0.8, 11.5) IMT with the inclusion of systolic blood pressure in the model. We observed a similar pattern of associations between % AL ≥ 4 and IMT. There was an inverse association between % BOP and IMT (model 1), which was attenuated in the full model. On the other hand, mean PD, % PD ≥ 4, and number of missing teeth were not associated with IMT. In sensitivity analyses using the average IMT of four sites of the CCA, we observed a similar association; IMT was 18.8-μm (95% CI: 1.0, 36.5) higher among participants in the highest quartile of % AL ≥ 4, compared with those in the lowest quartile.
Table 2.
Associations [β* (95% CI)] between periodontal indices and carotid IMT (μm)
| n | Model 1† | Model 2‡ | |
|---|---|---|---|
| Mean probing depth, mm | |||
| ≤ 2.25 | 226 | Reference | Reference |
| 2.26–2.46 | 226 | −6.2 (−20.5, 8.0) | −3.4 (−17.5, 10.8) |
| 2.47–2.69 | 226 | −1.2 (−15.4, 13.0) | 3.2 (−11.1, 17.4) |
| > 2.69 | 225 | −0.4 (−14.9, 14.2) | 4.8 (−9.8, 19.4) |
| P for trend§ | 0.87 | 0.37 | |
| Per 1-mm increase | 2.3 (−12.1, 16.8) | 7.7 (−6.8, 22.3) | |
| Mean attachment loss, mm | |||
| ≤ 2.30 | 224 | Reference | Reference |
| 2.31–2.85 | 228 | 2.1 (−12.3, 16.5) | 6.4 (−8.0, 20.8) |
| 2.86–3.72 | 226 | 8.5 (−7.1, 24.1) | 12.0 (−3.9, 27.9) |
| > 3.72 | 225 | 12.9 (−4.3, 30.0) | 20.0 (2.2, 37.8)∥ |
| P for trend§ | 0.12 | 0.03 | |
| Per 1-mm increase | 3.7 (−1.6, 9.1) | 6.4 (0.9, 11.9)∥ | |
| Percentage of sites with bleeding on probing | |||
| ≤ 7.8 | 225 | Reference | Reference |
| 7.9–15.6 | 223 | −11.5 (−25.8, 2.8) | −9.8 (−24.1, 4.5) |
| 15.7–25.6 | 227 | −5.7 (−20.3, 8.8) | −3.1 (−17.7, 11.5) |
| > 25.6 | 229 | −18.1 (−33.2, −3.1)∥ | −11.4 (−26.8, 3.9) |
| P for trend§ | 0.05 | 0.27 | |
| Per 10% increase | −4.6 (−8.8, −0.4)‡ | −2.6 (−6.9, 1.7) | |
| Percentage of sites with ≥ 4 mm probing depth | |||
| ≤ 2.0 | 227 | Reference | Reference |
| 2.1–4.8 | 226 | −6.9 (−21.1, 7.2) | −6.2 (−20.2, 7.8) |
| 4.9–11.1 | 224 | −7.2 (−21.5, 7.0) | −5.9 (−20.1, 8.2) |
| > 11.1 | 226 | 5.8 (−8.6, 20.3) | 8.6 (−5.9, 23.0) |
| P for trend§ | 0.49 | 0.29 | |
| Per 10% increase | 2.3 (−2.7, 7.3) | 3.4 (−1.6, 8.3) | |
| Percentage of sites with ≥ 4 mm attachment loss | |||
| ≤ 14.3 | 225 | Reference | Reference |
| 14.4–29.4 | 227 | 4.7 (−9.7, 19.1) | 9.6 (−4.7, 24.0) |
| 29.5–58.4 | 226 | 6.0 (−9.5, 21.5) | 9.5 (−6.1, 25.1) |
| > 58.4 | 225 | 18.4 (1.3, 35.5)∥ | 26.5 (8.9, 44.1)# |
| P for trend§ | 0.05 | < 0.01 | |
| Per 10% increase | 1.4 (−0.8, 3.7) | 2.4 (0.1, 4.7)∥ | |
| Number of missing teeth | |||
| 0 | 265 | Reference | Reference |
| 1–2 | 268 | 2.3 (−10.9, 15.5) | 1.1 (−12.0, 14.2) |
| 3–4 | 177 | −4.0 (−18.9, 10.9) | −1.4 (−16.1, 13.3) |
| > 4 | 196 | 2.0 (−13.6, 17.6) | 5.4 (−10.2, 21.0) |
| P for trend§ | 0.96 | 0.63 | |
| Per 1-tooth increase | 0.1 (−1.0, 1.3) | 0.4 (−0.7, 1.5) |
For categorical variables, β represents the adjusted difference in average carotid IMT values between the comparison and reference groups. For continuous variables, β represents the change in IMT for every 1-unit increase in the exposure variable.
Adjusted for sex and age at the time of IMT measurement.
Adjusted for sex, age, BMI, smoking status (never and ever), and betel quid chewing at the time of IMT measurement, as well as educational attainment at baseline.
Based on ordered variables for quartile categories of periodontal indices.
P < 0.05.
P < 0.01.
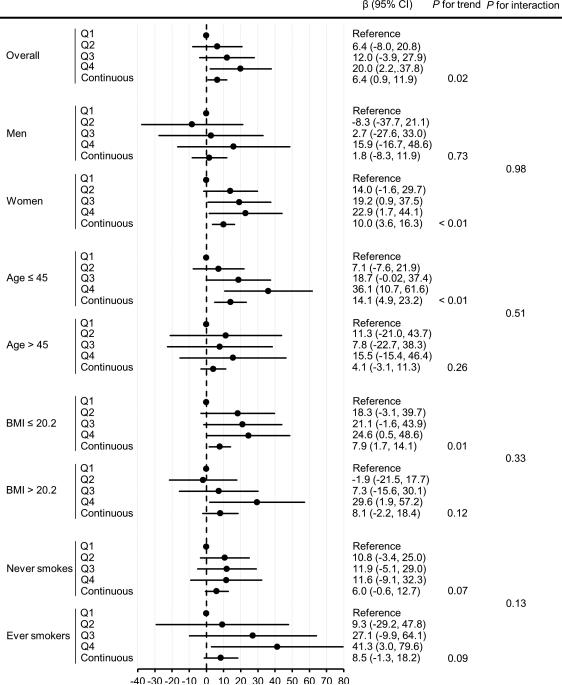
In stratified analyses (Figure 1), although there was no statistically significant interaction between sex and mean AL or between age and mean AL in IMT (all P's for interaction > 0.05), the positive association between mean AL and IMT was stronger in women and in younger individuals aged ≤ 45 years. The positive association between mean AL and IMT remained similar in never smokers and did not differ substantially by smoking status (P for interaction = 0.13). For instance, among never smokers, every 1-mm difference in mean AL was associated with a 6.0-μm (95% CI: −0.6, 12.7) difference in IMT; among ever smokers, those with the highest quartile of mean AL had a higher level of IMT by 41.3-μm (95% CI: 3.0, 79.6).
Figure 1.
Carotid intima-media thickness (μm) in relation to mean attachment loss (mm) overall and stratified. Cut-off points for mean attachment loss were determined by quartiles (Q1: ≤ 2.30; Q2: 2.31−2.85; Q3: 2.86−3.72; Q4: > 3.72) in the overall population. β represents the adjusted difference in average carotid IMT values between the comparison and reference groups, adjusting for sex, age, BMI, smoking status (never and ever), and betel quid chewing at the time of IMT measurement, as well as educational attainment at baseline (except for the variable stratified by in the stratified analyses). P for trend was based on continuous mean attachment loss. P for interaction was based on the cross-product term between each effect modifier and continuous mean attachment loss.
Mean AL was positively associated with both MMP-9 and sICAM-1 (Table 3). The highest tertile of mean AL was associated with a difference of 28.5-ng/mL (95% CI: 6.9, 50.1) MMP-9 and 55.5-ng/mL (95% CI: 14.6, 96.4) sICAM-1, respectively, compared to the bottom tertile. The associations between other periodontal measures and plasma levels of the CVD markers were shown in Supplemental Table 1. In line with mean AL, % AL ≥ 4 was dose-dependently related to plasma levels of MMP-9 and sICAM-1. Mean PD and % PD ≥ 4 were also significantly related to sICAM-1.
Table 3.
Associations [β* (95% CI)] between mean attachment loss and plasma levels of MMP-9, MPO, sE-selectin, sICAM-1, and sVCAM-1
| Mean attachment loss, mm | n | MMP-9 (ng/ml) | MPO (ng/ml) | sE-selectin (ng/ml) | sICAM-1 (ng/ml) | sVCAM-1 (ng/ml) |
|---|---|---|---|---|---|---|
| ≤ 2.68 | 115 | Reference | Reference | Reference | Reference | Reference |
| 2.69–3.53 | 116 | 7.6 (−11.1, 26.4) | 0.7 (−3.1, 4.6) | −3.6 (−8.3, 1.2) | 22.9 (−12.5, 58.4) | −46.3 (−139.8, 47.2) |
| > 3.53 | 118 | 28.5 (6.9, 50.1)† | 0.9 (−3.6, 5.4) | −3.0 (−8.5, 2.5) | 55.5 (14.6, 96.4)† | 30.7 (−77.2, 138.5) |
| P for trend§ | < 0.01 | 0.70 | 0.29 | < 0.01 | 0.55 | |
| Per 1-mm increase | 11.0 (2.5, 19.5)‡ | 0.3 (−1.4, 2.1) | 0.8 (−1.3, 3.0) | 27.0 (11.0, 42.9)† | 22.2 (−20.1, 64.6) |
For categorical mean attachment loss, β represents the adjusted difference in average plasma levels of the markers between the comparison and reference groups. For continuous mean attachment loss, β represents the change in plasma levels of the markers for every 1-unit increase in mean attachment loss. β was adjusted for sex, age, BMI, smoking status (never and ever), and betel quid chewing at the time of IMT measurement, as well as educational attainment at baseline.
P < 0.01.
P < 0.05.
Based on ordered variables for tertile categories of mean attachment loss.
Discussion
In the present study, we found a dose-response relationship of mean AL and % AL ≥ 4 with carotid IMT. This association was stronger in women and younger individuals (≤ 45 years). Furthermore, AL was related to plasma levels of MMP-9 and sICAM-1 in a dose-response manner. To the best of our knowledge, this is the first epidemiologic study to report the association between periodontal disease and carotid IMT in a South Asian population.
Several previous cross-sectional studies have assessed the association between AL and continuous IMT measurement (Hayashida et al., 2013, Jung et al., 2014, Yu et al., 2014). To date, all these studies have been conducted in middle-aged to elderly population (mean age > 60 years) with a higher BMI (mean 23-29 kg/m2). The current study adds to the weight of the evidence linking AL to subclinical atherosclerosis in a younger (mean age 46 years) and largely lean (mean BMI 21.1) population. In our study, the magnitude of IMT difference in association with mean AL persisted in those with a BMI ≤ 20.2 kg/m2, suggesting that the effect of periodontal diseases on subclinical atherosclerosis is independent of obesity. We also observed that the positive association between mean AL and IMT was stronger in women and in younger individuals. The contribution of periodontal diseases might have been masked by other risk factors among men (such as smoking) and older individuals (such as aging). AL, which represents an individual's cumulative history of periodontal diseases, may be the most appropriate measure of long-term periodontal diseases for atherosclerosis, which also develops over a long period of time as a result of lifelong exposure to cardiovascular risk factors (Demmer et al., 2008, Beck and Offenbacher, 2002). In our study, severe AL (the highest quartile of mean AL and % AL ≥ 4) was associated with a difference of 20-30-μm carotid IMT. Prior literature has shown a 100-μm difference in IMT being associated with a 50% increased risk of coronary heart disease (Chambless et al., 1997). Therefore, the IMT difference associated with severe AL in this Bangladeshi population could translate into a substantial proportion of CVD risk, given the high prevalence of moderate and severe periodontitis (Corbet et al., 2002, Petersen and Ogawa, 2012, van Palenstein Helderman et al., 1996, WHO, 2013) and the existence of a broad range of social and behavioral risk factors for periodontitis (Kalam, 1996), in particular cigarette smoking (76.9% in men) and betel quid chewing (55.8%), lack of oral health care, as well as an average of low education attainment (mean 3 years).
Previous studies have documented an association between periodontal diseases and higher circulating levels of C-reactive protein and interleukin-6—general markers for inflammation (Loos, 2005). Our finding on MMP-9 and sICAM-1 is in concert with the concept that cumulative history of periodontal diseases measured as higher levels of AL is related to systemic inflammation pertaining to atherosclerosis. MMP-9 is expressed in vulnerable atherosclerotic plaques (Galis et al., 1994) while sICAM-1 mediates the attachment of circulating leukocytes to activated endothelium early in atherosclerotic plaque formation (Springer, 1994). Both markers are positively associated with CVD risk (Ridker et al., 1998, Hansson et al., 2011). The association between periodontal diseases and MMP-9 has previously been observed in a small European study (80 periodontitis cases and 31 healthy controls) (Soder et al., 2009). In a large US study (n = 5410), several clinical periodontal measures including AL were associated with serum levels of sICAM-1 (Beck and Offenbacher, 2002). However, these studies are limited in that they reported univariate association and that they assessed one or two inflammatory markers. The present study, although modest in sample size, investigated a panel of five inflammatory markers for CVD and depicted a dose-dependent relationship between mean AL and plasma levels of MMP-9 and sICAM-1. Interestingly, sICAM-1 was also positively associated with IMT and every 1-SD difference in sICAM-1 was associated with a difference of 6.5-μm (95% CI: -1.4, 14.4) IMT in the present study (P = 0.11; Supplemental Table 2). However, our sample size is limited to conduct mediation analyses. Future larger studies are warranted to explore the biological importance of these markers as mechanistic mediators of the association between periodontal diseases and subclinical atherosclerosis.
We did not find an association of PD with carotid IMT, consistent with a recent study in an Australian population with a median age of 39 years, and studies in China and Korea in older adults, all of which failed to show an association between mean PD or % PD ≥ 4 and carotid IMT (Jung et al., 2014, Kapellas et al., 2014, Yu et al., 2014). The Japanese study was the one single study that reported a dose-response relationship between mean PD and carotid IMT (Hayashida et al., 2013). PD represents current periodontal inflammatory activity and is modifiable. As expected, compared with AL, mean PD demonstrated a stronger association with plasma levels of sICAM-1 (Supplemental Table 2), which is a relatively short-lived marker of vascular stress (Beck and Offenbacher, 2002). However, the lack of a significant association between PD and IMT highlights that this periodontal measure may not be informative for outcomes with long induction periods like atherosclerosis (Beck and Offenbacher, 2002, Demmer et al., 2008).
We observed a significant inverse association between % BOP and IMT in the model adjusting for sex and age, but not in the fully adjusted model (Table 2). This can be explained by confounding due to other factors such as cigarette smoking, which has a strong, chronic effect on vasoconstriction of the gingival vasculature that suppresses the normal gingival inflammatory response and bleeding on probing (Dietrich et al., 2004, Amarasena et al., 2003, Bergstrom and Bostrom, 2001). In the full model (model 2 in Table 2), despite that the point estimates were negative, there was not much an association between % BOP and IMT, since there wasn't a consistent trend in the estimates and the confidence intervals were wide.
No significant relationship between number of missing teeth and carotid IMT was found in this study. An earlier study of 1710 subjects aged 45-75 years in Germany demonstrated a significantly increased IMT among edentulous men relative to those with 0 to 8 missing teeth (Desvarieux et al., 2004). A Korean study also documented a dose-dependent effect of number of missing teeth on CCA IMT, which was robust in never smokers (Jung et al., 2014). As our participants were relatively young, their median number of missing teeth was 2 teeth, much lower than the number reported in the Korea (median 7 teeth) or Germany (mean 13 teeth) study. Our cohort may have a narrow range of missing teeth to detect a positive effect.
Strengths of our study include a large population from South Asia that has received little epidemiologic attention, the use of standardized IMT protocols, and the extensive data on periodontal measures and potential confounders. Our study is primarily limited by its cross-sectional design, which prevents us from establishing a temporal relationship between periodontal diseases and subclinical atherosclerosis. However, the possibility of reverse causation was largely reduced as all the participants were free of clinical CVD conditions and unaware of their periodontal conditions. In fact, the oral examination was the first periodontal exam for most of the participants. Our study extended the generalizability of the association between AL and IMT observed in studies largely consisting of older subjects to a younger (mean age 46 years) and mostly lean (mean BMI 21.1) South Asian population residing in rural Bangladesh. Measurements of the inflammatory markers and the clinical periodontal examination were performed at different time points, hence we could not evaluate the impact of periodontal diseases on the markers at the same time of periodontal exam or after periodontal exam. However, given the short time gap (mean 4 years) between baseline blood collection (2006–08) and periodontal exam (2011), we do not believe our findings would have been significantly biased for markers that have good long-term reproducibility, such as sICAM-1 and sVCAM-1. Nevertheless, future studies are needed to confirm a cause-effect relationship between periodontal diseases and systemic inflammation and to evaluate other markers. We did not include lipid profiles in the multivariate model as the data were not collected. However, lipids may serve as pathological mediators underpinning the association between periodontal diseases and atherosclerosis (Schenkein and Loos, 2013, Iacopino and Cutler, 2000), which should not have been controlled in the analyses. Nevertheless, we acknowledge that it could have been helpful to have such data to assess the extent that the effect of periodontal diseases on IMT can be accounted for by the effect of periodontal diseases on lipid profiles.
In conclusion, we found that AL was dose-dependently related to carotid IMT. Our findings add to the growing body of epidemiologic evidence that periodontal diseases may be an important and independent risk factor for atherosclerosis.
Supplementary Material
Clinical Relevance.
Scientific rationale for study: A number of studies have reported that periodontal diseases are associated with carotid intima-media thickness (IMT), a surrogate marker for subclinical atherosclerosis. However, most of these studies were conducted among older adults, with limited evidence in younger populations, populations with low body mass index (< 23 kg/m2), or in South Asians. Further, previous studies often did not conduct stratified analyses by smoking, and few studies have investigated systemic inflammatory markers as potential underpinnings linking periodontal diseases and subclinical atherosclerosis.
Principal findings: Attachment loss was positively associated with IMT in this young and lean Bangladesh population and among never smokers. Attachment loss was also positively associated with plasma levels of matrix metalloproteinase-9 and soluble intercellular adhesion molecule-1.
Practical implications: Periodontal diseases may be an important and independent risk factor for atherosclerosis.
Acknowledgments
Funding: This study was supported by grants from the National Institutes of Health (R01 ES017541, P42 ES010349, and P30 ES000260).
Footnotes
Conflict of Interest: The authors declare that there are no conflicts of interest in this study.
Disclosures
None
References
- Ahsan H, Chen Y, Parvez F, Argos M, Hussain AI, Momotaj H, Levy D, van Geen A, Howe G, Graziano J. Health Effects of Arsenic Longitudinal Study (HEALS): description of a multidisciplinary epidemiologic investigation. J Expo Sci Environ Epidemiol. 2006;16:191–205. doi: 10.1038/sj.jea.7500449. doi:10.1038/sj.jea.7500449. [DOI] [PubMed] [Google Scholar]
- Amarasena N, Ekanayaka AN, Herath L, Miyazaki H. Association between smoking, betel chewing and gingival bleeding in rural Sri Lanka. J Clin Periodontol. 2003;30:403–408. doi: 10.1034/j.1600-051x.2003.20010.x. [DOI] [PubMed] [Google Scholar]
- Beck JD, Offenbacher S. Relationships among clinical measures of periodontal disease and their associations with systemic markers. Ann Periodontol. 2002;7:79–89. doi: 10.1902/annals.2002.7.1.79. doi:10.1902/annals.2002.7.1.79. [DOI] [PubMed] [Google Scholar]
- Bergstrom J, Bostrom L. Tobacco smoking and periodontal hemorrhagic responsiveness. J Clin Periodontol. 2001;28:680–685. doi: 10.1034/j.1600-051x.2001.028007680.x. [DOI] [PubMed] [Google Scholar]
- Chambless LE, Heiss G, Folsom AR, Rosamond W, Szklo M, Sharrett AR, Clegg LX. Association of coronary heart disease incidence with carotid arterial wall thickness and major risk factors: the Atherosclerosis Risk in Communities (ARIC) Study, 1987-1993. Am J Epidemiol. 1997;146:483–494. doi: 10.1093/oxfordjournals.aje.a009302. [DOI] [PubMed] [Google Scholar]
- Chen Y, Factor-Litvak P, Howe GR, Graziano JH, Brandt-Rauf P, Parvez F, van Geen A, Ahsan H. Arsenic exposure from drinking water, dietary intakes of B vitamins and folate, and risk of high blood pressure in Bangladesh: a population-based, cross-sectional study. Am J Epidemiol. 2007a;165:541–552. doi: 10.1093/aje/kwk037. doi:10.1093/aje/kwk037. [DOI] [PubMed] [Google Scholar]
- Chen Y, Santella RM, Kibriya MG, Wang Q, Kappil M, Verret WJ, Graziano JH, Ahsan H. Association between arsenic exposure from drinking water and plasma levels of soluble cell adhesion molecules. Environ Health Perspect. 2007b;115:1415–1420. doi: 10.1289/ehp.10277. doi:10.1289/ehp.10277 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Wu F, Graziano JH, Parvez F, Liu M, Paul RR, Shaheen I, Sarwar G, Ahmed A, Islam T, Slavkovich V, Rundek T, Demmer RT, Desvarieux M, Ahsan H. Arsenic exposure from drinking water, arsenic methylation capacity, and carotid intima-media thickness in Bangladesh. Am J Epidemiol. 2013;178:372–381. doi: 10.1093/aje/kwt001. doi:10.1093/aje/kwt001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbet EF, Zee KY, Lo EC. Periodontal diseases in Asia and Oceania. Periodontol 2000. 2002;29:122–152. doi: 10.1034/j.1600-0757.2002.290107.x. [DOI] [PubMed] [Google Scholar]
- Crouse JR, 3rd, Tang R, Espeland MA, Terry JG, Morgan T, Mercuri M. Associations of extracranial carotid atherosclerosis progression with coronary status and risk factors in patients with and without coronary artery disease. Circulation. 2002;106:2061–2066. doi: 10.1161/01.cir.0000033833.54884.34. [DOI] [PubMed] [Google Scholar]
- de Groot E, Hovingh GK, Wiegman A, Duriez P, Smit AJ, Fruchart JC, Kastelein JJ. Measurement of arterial wall thickness as a surrogate marker for atherosclerosis. Circulation 109, III33-38. 2004 doi: 10.1161/01.CIR.0000131516.65699.ba. doi:10.1161/01.CIR.0000131516.65699.ba. [DOI] [PubMed] [Google Scholar]
- Demmer RT, Kocher T, Schwahn C, Volzke H, Jacobs DR, Jr., Desvarieux M. Refining exposure definitions for studies of periodontal disease and systemic disease associations. Community Dent Oral Epidemiol. 2008;36:493–502. doi: 10.1111/j.1600-0528.2008.00435.x. doi:10.1111/j.1600-0528.2008.00435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desvarieux M, Demmer RT, Rundek T, Boden-Albala B, Jacobs DR, Jr., Sacco RL, Papapanou PN. Periodontal microbiota and carotid intima-media thickness: the Oral Infections and Vascular Disease Epidemiology Study (INVEST). Circulation. 2005;111:576–582. doi: 10.1161/01.CIR.0000154582.37101.15. doi:10.1161/01.CIR.0000154582.37101.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desvarieux M, Schwahn C, Volzke H, Demmer RT, Ludemann J, Kessler C, Jacobs DR, Jr., John U, Kocher T. Gender differences in the relationship between periodontal disease, tooth loss, and atherosclerosis. Stroke. 2004;35:2029–2035. doi: 10.1161/01.STR.0000136767.71518.36. doi:10.1161/01.STR.0000136767.71518.36. [DOI] [PubMed] [Google Scholar]
- Dietrich T, Bernimoulin JP, Glynn RJ. The effect of cigarette smoking on gingival bleeding. J Periodontol. 2004;75:16–22. doi: 10.1902/jop.2004.75.1.16. doi:10.1902/jop.2004.75.1.16. [DOI] [PubMed] [Google Scholar]
- Galis ZS, Sukhova GK, Lark MW, Libby P. Increased expression of matrix metalloproteinases and matrix degrading activity in vulnerable regions of human atherosclerotic plaques. J Clin Invest. 1994;94:2493–2503. doi: 10.1172/JCI117619. doi:10.1172/JCI117619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson J, Vasan RS, Arnlov J, Ingelsson E, Lind L, Larsson A, Michaelsson K, Sundstrom J. Biomarkers of extracellular matrix metabolism (MMP-9 and TIMP-1) and risk of stroke, myocardial infarction, and cause-specific mortality: cohort study. PLoS One. 2011;6:e16185. doi: 10.1371/journal.pone.0016185. doi:10.1371/journal.pone.0016185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashida H, Saito T, Kawasaki K, Kitamura M, Furugen R, Iwasaki T, Hayashida Y, Nakazato M, Sekita T, Takamura N, Maeda T. Association of periodontitis with carotid artery intima-media thickness and arterial stiffness in community-dwelling people in Japan: the Nagasaki Islands study. Atherosclerosis. 2013;229:186–191. doi: 10.1016/j.atherosclerosis.2013.04.002. doi:10.1016/j.atherosclerosis.2013.04.002. [DOI] [PubMed] [Google Scholar]
- Iacopino AM, Cutler CW. Pathophysiological relationships between periodontitis and systemic disease: recent concepts involving serum lipids. J Periodontol. 2000;71:1375–1384. doi: 10.1902/jop.2000.71.8.1375. doi:10.1902/jop.2000.71.8.1375. [DOI] [PubMed] [Google Scholar]
- Jung YS, Shin MH, Kim IS, Kweon SS, Lee YH, Kim OJ, Kim YJ, Chung HJ, Kim OS. Relationship between periodontal disease and subclinical atherosclerosis: the Dong-gu study. J Clin Periodontol. 2014;41:262–268. doi: 10.1111/jcpe.12204. doi:10.1111/jcpe.12204. [DOI] [PubMed] [Google Scholar]
- Kalam MA. Periodontal disease: Its risk factors among Bangladeshi populations. In: Bartold PM, editor. Risk factors in Asian Pacific populations. Asian Pacific Society of Periodontology; Brisbane: 1996. pp. 16–21. [Google Scholar]
- Kapellas K, Jamieson LM, Do LG, Bartold PM, Wang H, Maple-Brown LJ, Sullivan D, O'Dea K, Brown A, Celermajer DS, Slade GD, Skilton MR. Associations between periodontal disease and cardiovascular surrogate measures among Indigenous Australians. Int J Cardiol. 2014;173:190–196. doi: 10.1016/j.ijcard.2014.02.015. doi:10.1016/j.ijcard.2014.02.015. [DOI] [PubMed] [Google Scholar]
- Lockhart PB, Bolger AF, Papapanou PN, Osinbowale O, Trevisan M, Levison ME, Taubert KA, Newburger JW, Gornik HL, Gewitz MH, Wilson WR, Smith SC, Jr., Baddour LM. Periodontal disease and atherosclerotic vascular disease: does the evidence support an independent association?: a scientific statement from the American Heart Association. Circulation. 2012;125:2520–2544. doi: 10.1161/CIR.0b013e31825719f3. doi:10.1161/CIR.0b013e31825719f3. [DOI] [PubMed] [Google Scholar]
- Loe H. The Gingival Index, the Plaque Index and the Retention Index Systems. J Periodontol. 1967;38(Suppl):610–616. doi: 10.1902/jop.1967.38.6.610. doi:10.1902/jop.1967.38.6.610. [DOI] [PubMed] [Google Scholar]
- Loos BG. Systemic markers of inflammation in periodontitis. J Periodontol. 2005;76:2106–2115. doi: 10.1902/jop.2005.76.11-S.2106. doi:10.1902/jop.2005.76.11-S.2106. [DOI] [PubMed] [Google Scholar]
- McClintock TR, Parvez F, Wu F, Wang W, Islam T, Ahmed A, Shaheen I, Sarwar G, Demmer RT, Desvarieux M, Ahsan H, Chen Y. Association between betel quid chewing and carotid intima-media thickness in rural Bangladesh. Int J Epidemiol. 2014;43:1174–1182. doi: 10.1093/ije/dyu009. doi:10.1093/ije/dyu009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima T, Honda T, Domon H, Okui T, Kajita K, Ito H, Takahashi N, Maekawa T, Tabeta K, Yamazaki K. Periodontitis-associated up-regulation of systemic inflammatory mediator level may increase the risk of coronary heart disease. J Periodontal Res. 2010;45:116–122. doi: 10.1111/j.1600-0765.2009.01209.x. doi:10.1111/j.1600-0765.2009.01209.x. [DOI] [PubMed] [Google Scholar]
- Orlandi M, Suvan J, Petrie A, Donos N, Masi S, Hingorani A, Deanfield J, D'Aiuto F. Association between periodontal disease and its treatment, flow-mediated dilatation and carotid intima-media thickness: a systematic review and meta-analysis. Atherosclerosis. 2014;236:39–46. doi: 10.1016/j.atherosclerosis.2014.06.002. doi:10.1016/j.atherosclerosis.2014.06.002. [DOI] [PubMed] [Google Scholar]
- Page RC, Eke PI. Case definitions for use in population-based surveillance of periodontitis. J Periodontol. 2007;78:1387–1399. doi: 10.1902/jop.2007.060264. doi:10.1902/jop.2007.060264. [DOI] [PubMed] [Google Scholar]
- Pai JK, Curhan GC, Cannuscio CC, Rifai N, Ridker PM, Rimm EB. Stability of novel plasma markers associated with cardiovascular disease: processing within 36 hours of specimen collection. Clin Chem. 2002;48:1781–1784. [PubMed] [Google Scholar]
- Paraskevas S, Huizinga JD, Loos BG. A systematic review and meta-analyses on C-reactive protein in relation to periodontitis. J Clin Periodontol. 2008;35:277–290. doi: 10.1111/j.1600-051X.2007.01173.x. doi:10.1111/j.1600-051X.2007.01173.x. [DOI] [PubMed] [Google Scholar]
- Petersen PE, Ogawa H. The global burden of periodontal disease: towards integration with chronic disease prevention and control. Periodontol 2000. 2012;60:15–39. doi: 10.1111/j.1600-0757.2011.00425.x. doi:10.1111/j.1600-0757.2011.00425.x. [DOI] [PubMed] [Google Scholar]
- Pierce BL, Kalra T, Argos M, Parvez F, Chen Y, Islam T, Ahmed A, Hasan R, Rakibuz-Zaman M, Graziano J, Rathouz PJ, Ahsan H. A prospective study of body mass index and mortality in Bangladesh. Int J Epidemiol. 2010;39:1037–1045. doi: 10.1093/ije/dyp364. doi:10.1093/ije/dyp364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridker PM, Hennekens CH, Roitman-Johnson B, Stampfer MJ, Allen J. Plasma concentration of soluble intercellular adhesion molecule 1 and risks of future myocardial infarction in apparently healthy men. Lancet. 1998;351:88–92. doi: 10.1016/S0140-6736(97)09032-6. doi:10.1016/S0140-6736(97)09032-6. [DOI] [PubMed] [Google Scholar]
- Schenkein HA, Loos BG. Inflammatory mechanisms linking periodontal diseases to cardiovascular diseases. J Clin Periodontol 40 Suppl. 2013;14:S51–69. doi: 10.1111/jcpe.12060. doi:10.1111/jcpe.12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soder PO, Meurman JH, Jogestrand T, Nowak J, Soder B. Matrix metalloproteinase-9 and tissue inhibitor of matrix metalloproteinase-1 in blood as markers for early atherosclerosis in subjects with chronic periodontitis. J Periodontal Res. 2009;44:452–458. doi: 10.1111/j.1600-0765.2008.01145.x. doi:10.1111/j.1600-0765.2008.01145.x. [DOI] [PubMed] [Google Scholar]
- Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- Stein JH, Korcarz CE, Hurst RT, Lonn E, Kendall CB, Mohler ER, Najjar SS, Rembold CM, Post WS. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: a consensus statement from the American Society of Echocardiography Carotid Intima-Media Thickness Task Force. Endorsed by the Society for Vascular Medicine. J Am Soc Echocardiogr. 2008;21:93–111. doi: 10.1016/j.echo.2007.11.011. quiz 189-190. doi:10.1016/j.echo.2007.11.011. [DOI] [PubMed] [Google Scholar]
- van Palenstein Helderman WH, Joarder MA, Begum A. Prevalence and severity of periodontal diseases and dental caries in Bangladesh. Int Dent J. 1996;46:76–81. [PubMed] [Google Scholar]
- WHO Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–163. doi: 10.1016/S0140-6736(03)15268-3. doi:10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- WHO Strategy for oral health in South-East Asia. 2013:2013–2020. [Google Scholar]
- Wu F, Chen Y, Parvez F, Segers S, Argos M, Islam T, Ahmed A, Rakibuz-Zaman M, Hasan R, Sarwar G, Ahsan H. A prospective study of tobacco smoking and mortality in Bangladesh. PLoS One. 2013;8:e58516. doi: 10.1371/journal.pone.0058516. doi:10.1371/journal.pone.0058516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F, Jasmine F, Kibriya MG, Liu M, Wojcik O, Parvez F, Rahaman R, Roy S, Paul-Brutus R, Segers S, Slavkovich V, Islam T, Levy D, Mey JL, van Geen A, Graziano JH, Ahsan H, Chen Y. Association between arsenic exposure from drinking water and plasma levels of cardiovascular markers. Am J Epidemiol. 2012;175:1252–1261. doi: 10.1093/aje/kwr464. doi:10.1093/aje/kwr464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F, Parvez F, Islam T, Ahmed A, Rakibuz-Zaman M, Hasan R, Argos M, Levy D, Sarwar G, Ahsan H, Chen Y. Betel quid use and mortality in Bangladesh: a cohort study. Bull World Health Organ. 2015;93:684–692. doi: 10.2471/BLT.14.149484. doi:10.2471/BLT.14.149484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Qi LT, Liu LS, Wang XY, Zhang Y, Huo Y, Luan QX. Association of Carotid Intima-media Thickness and Atherosclerotic Plaque with Periodontal Status. J Dent Res. 2014;93:744–751. doi: 10.1177/0022034514538973. doi:10.1177/0022034514538973. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.