Abstract
The new civil wars and waves of terrorism are causing crucial social changes, with consequences in all fields, including health care. In particular, skin injuries are evolving as an epidemic issue. From a physiological standpoint, although wound repair takes place more rapidly in the skin than in other tissues, it is still a complex organ to reconstruct. Genetic and clinical variables, such as diabetes, smoking, and inflammatory/immunological pathologies, are also important risk factors limiting the regenerative potential of many therapeutic applications. Therefore, optimization of current clinical strategies is critical. Here we summarize the current state of the field by focusing on stem cell therapy applications in wound healing, with an emphasis on current clinical approaches being developed at Sapienza University. These involve protocols for the ex vivo expansion of adipose tissue–derived mesenchymal stem cells by means of a patented GMP-compliant platelet lysate, Mesengen™. A combination of multiple strategies, including genetic modifications of stem cells, biomimetic scaffolds, or novel vehicles such as nanoparticles, are also discussed as future approaches.
Keywords: wound healing, skin, mesenchymal stem cells, platelet lysate
Introduction
Managing scarring in chronic wounds represents one of the most relevant clinical burdens in the United States and in Europe,1 particularly when they occur as a consequence of exposure to chemicals used in a terrorist attack. These can cause severe physical damage, predominantly resulting in insults to the skin. Cutaneous injuries can also combine with mechanical trauma, resulting in exacerbated pathologies. Victims with preexisting diseases are of particular concern, as treatment and resolution of injury frequently requires long-term care. Healing is usually compromised in these individuals owing to the presence of diabetes, metabolic syndrome, chronic renal failure, and aging,2 since the ability to rapidly re-epithelialize and re-vascularize injured tissue is impaired. Both clinical and genetic features of individual patients must be considered in wound healing, as well as variation in medical responses based on the type of chemical weapon employed and the nature and extent of the injured area. In fact, large wounds, under either adverse local or systemic conditions, respond poorly to treatments, and they can frequently reopen. A number of strategies have been developed recently to treat dermal wounds resulting from chemical exposures. One of the most efficient methods to lower bacterial load and reduce the incidence of sepsis is debridement of the wound.3 Cleansing agents and topical antibiotics are also useful to decrease microbial growth and to reduce invasive infection.4 Additionally, treatment of the wound with autologous leukocytes seeded into a proangiogenic matrix and enriched with a platelet concentrate preparation, has been reported to induce the release of growth factors, cytokines, and chemokines, thus increasing the in situ recruitment of endothelial precursor cells and promoting the resolution of microbial infections.5 Despite these improvements, treatment of dermal wounds has not always yielded positive outcomes. Major drawbacks include the fact that the skin is highly complex and thus difficult to reconstruct after injury. In fact, the physiological re-epithelialization phase is a multistep process involving several cell types and molecular mechanisms, and the presence of a favorable environment for bacterial colonization is mostly undesirable.6,7 As a consequence, most current treatments have been only palliative, mainly aiming to accelerate the healing time and to limit additional clinical complications due to adventitious bacterial infection. Therefore, alternative strategies are required in order to balance treatment of patients, economic costs, and safety of civilians.
Adipose tissue–derived mesenchymal stem cells
The potential use of different types of stem cells for regenerative applications to repair skin injuries has recently received considerable attention.8 Several protocols have been established aimed at ensuring the resolution of wounds by targeting different phases of the healing process, namely the control of inflammation in a suitable microenvironment, the enhancement of stem cell engraftment after implantation, an efficient and terminal transdifferentiation of progenitors towards the dermal lineages, and the reconstruction of the vasculature system around the wound.9,10 Mesenchymal stem cells (MSCs) have recently been proposed as a promising solution to enhance the re-epithelialization phase.11 Studies using mouse models have shown that intradermal injection of human MSCs or adipose tissue derived stromal cells (ASCs) accelerates skin wound healing in nude mice.1 Similarly, results from clinical trials have demonstrated the benefits derived by the employment of both autologous or heterologous MSCs, especially in chronic wounds.12–15 Defined as adult multipotent cells, MSCs can be easily obtained from multiple sources, including adipose tissue depots, localized in different body compartments during major and/or aesthetic surgical procedures.16,17 Multiple mechanisms underlying the potential of both populations to positively influence wound repair have been proposed; these include modulation of inflammatory states, stimulation of angiogenesis, cell proliferation and fibroblast activity, activation and enhanced migration of keratinocytes to sites of injury in a paracrine fashion, the possible direct transdifferentiation of MSCs towards the dermal lineage (including fibroblasts and keratinocytes), and, finally, the recruitment of host cells.12,18–19 After in vivo administration, the immunotolerance generated by ASCs, defined as the ability to modulate the immunosurveillance system in the recipient, has been largely reported as the main biological property, thus highlighting a major advantage of their use.20,21 Moreover, cross talk between ASCs and inflammatory cells at the site of injury is a major contributory factor. Soluble factors released by MSCs and ASCs, such as vascular endothelial growth factor, interleukin-6, or transforming growth factors, are known to regulate local cellular responses during cutaneous injury.11 Of note, MSCs may also exert antibacterial effects at the wound site by both directly secreting IL-37, an antimicrobial protein, and positively influencing phagocytosis by the immune system.11,22 The proliferative and transdifferentiative potential of MSCs has been also highlighted in tissue engineering–based applications, specifically with regard to skin graft reconstruction, where MSCs are employed either alone, as a feeder layer for keratinocytes, or seeded in combination with gelatin-, collagen/chitosan–, or fibrin polymer–based scaffolds.23–25 Of note, among suitable substrates, synthetic polymers have exhibited a strong ability to absorb and transport fluids and protect from bacterial exposure.26 Other methods used to deliver MSCs to the wound site have included injection and topical or systemic administration, by employing a range of vehicles such as scaffolds, matrix, and human amniotic membrane grafts.27–30
Novel strategies developed at Sapienza University
Despite great improvements in the use of ASCs and MSCs for skin-regenerative applications, current use is limited by the presence of fetal bovine serum (FBS) in the cultures during their ex vivo expansion. According to the European Good Manufacturing Practice (GMP) guidelines, the employment of FBS is discouraged, as it is a potential source of zoonoses.16,31–32 In light of this, platelet lysate (PL), a hemoderivate enriched with soluble mitogenic factors,16,31,33 represents a superior alternative to FBS. Reported to enhance the biological stem cell properties of ASCs, such as proliferation, clonogenic capacity, and migration,15,33,34 PL has been also recently been demonstrated to promote ASCs pluripotency and commitment towards specific phenotypes.33–36 Interestingly, PL, manufactured in an injectable form or gel,5,6 embedded in scaffolds3 or incorporated into nanoparticles,4 also represents a widely investigated clinical strategy to accelerate wound healing in chronic ocular and diabetic dermal ulcers. Because of the large amounts of cytokines and growth factors contained in PL, it has multiple and significant advantages if locally applied to skin wounds, such as enhancement of angiogenesis and fibroblast migration, restoration of collagen synthesis, and reduction of oxidative stess.33 In addition, PL has been demonstrated to efficiently reestablish skin integrity.37
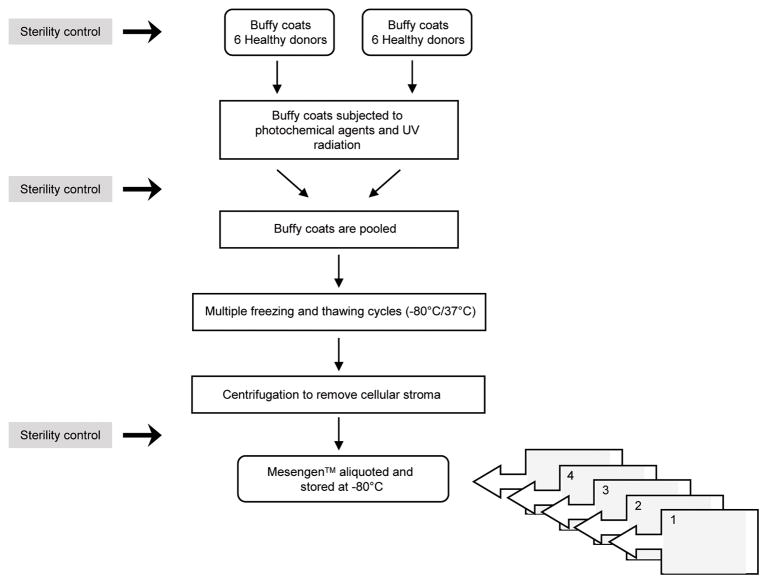
Recently, a GMP-compliant PL (Mesengen™, Pub. No. WO/2013/042095) has been developed as an adjuvant for culturing human ASCs, endothelial progenitor cells, and fibroblasts.16,33,38,39 The method to generate Mesengen has been standardized and optimized, including determining the amounts of cytokines and growth factors in the preparation. Importantly, potential fungi, viruses, and bacteria known to contaminate human emoderivates are avoided by rapidly inactivating the Mesengen™ through a combination of a photochemical agent and UV radiation. A summary of the basic steps in the preparation of PL is summarized in Figure 1. Of note, researchers at Sapienza have exploited the biological and molecular properties of Mesengen by concurrently establishing a standardized protocol (Fig. 2) to isolate and expand ex vivo ASCs from alternative fat depots, such as the mediastinum (Fig. 3).16,34 Recent studies on Mesengen by our team have also elucidated its ability to influence the commitment of ASCs by inducing epigenetic modifications,34 as well as to positively alter the in vitro microenvironment by decreasing oxidative stress.33 These studies highlight the ability of PL to boost the biological and functional properties of mesenchymal-like cell populations. Therefore, it is plausible that the combination of Mesengen and ASCs or other progenitor cell populations could be successfully employed to target wound repair and regeneration. Moreover, PL has been reported to maintain its properties as either a liquid formulation or frozen, highlighting an important clinical advantage. In the future, this approach could be considered complementary to routine strategies developed at Sapienza University, where a Center of Excellence for the in vitro culturing of skin substitutes is already available, including the treatment of a wide range of dermal disorders, such as burns, chronic ulcers, giant congenital melanocytic nevi, and even the reconstruction of epithelial mucosa.40–45 Specifically, the epithelial “organoid” developed by our research group is based on a combination of transplanted autologous cells seeded on biomimetic scaffolds. This methodology has been successfully established and clinically available at several hospitals collaborating with Sapienza, and it has already shown to significantly reduce the hospitalization time and costs.
Figure 1.
Overview of the major steps in the manufacturing of platelet lysate (Mesengen™).
Figure 2.
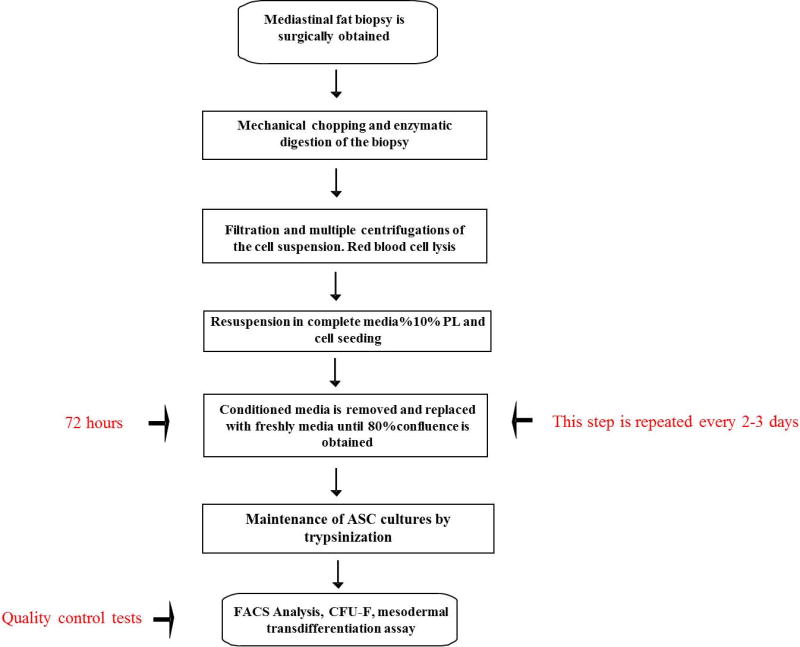
Flow diagram showing the optimization and standardization phases to isolate and expand in vitro ASCs derived from the mediastinal fat depots.
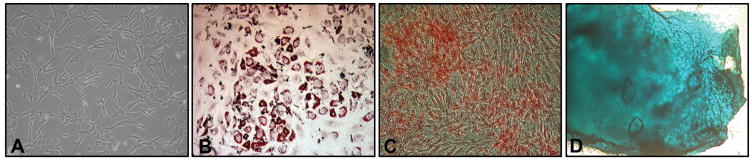
Figure 3.
Optical image of ASCs at passage 3 cultured in PL and displaying the typically spindle-shaped morphology (A). Note that platelet lysate is able to preserve the mesodermal transdifferentiation of ASCs towards the adipogenic (B), osteogenic (C), and chondrogenic (D) lineages. Magnification 5×.
Perspectives
Despite advances in treating wound healing, dermal tissue still remains a difficult organ to regenerate. Our future work will likely consist of multistep approaches rather than single repair strategies, which have shown only partial efficacy.46 The strategy will combine stem cell properties, next-generation scaffolds or vehicles (i.e., nanoparticles), and growth factors or supplements, such as PL. In this way, multiple biological and functional properties can be exploited. In addition, ex vivo gene therapy of adult multipotent stem cells may prove useful to target specific signaling pathways or molecular mechanisms underlying chronic wounds, as well as severe skin diseases resistant to routine therapy. Improvements in our understanding of skin biology and physiological process of wound repair will allow us to better elucidate the healing microenvironment. We will also focus on the mechanical and physical trauma caused by burns or wounding, attempting to clarify whether they might be controlled or redirected towards the resolution of the injury. Our final aim will consist of designing more personalized therapy, which takes into account genetic variability, type of wound, and patients’ clinical and metabolic features.
Acknowledgments
This work was supported by Ateneo (Sapienza University of Rome) and Fondazione Roma and the National Institutes of Health–funded CounterACT Program (NIAMS U54 AR055073).
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Rodriguez J, Boucher F, Lequeux C, et al. Intradermal injection of human adipose-derived stem cells accelerates skin wound healing in nude mice. Stem Cell Res Ther. 2015;6:241. doi: 10.1186/s13287-015-0238-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scuderi N, Ceccarelli S, Onesti MG, et al. Human adipose-derived stromal cells for cell-based therapies in the treatment of systemic sclerosis. Cell Transplant. 2013;22:779–795. doi: 10.3727/096368912X639017. [DOI] [PubMed] [Google Scholar]
- 3.Sandri G, Bonferoni MC, Rossi S, et al. Platelet lysate embedded scaffolds for skin regeneration. Expert Opin Drug Deliv. 2015;12:525–545. doi: 10.1517/17425247.2015.961421. [DOI] [PubMed] [Google Scholar]
- 4.Fontana F, Mori M, Riva F, et al. Platelet lysate-modified porous silicon microparticles for enhanced cell proliferation in wound healing applications. ACS Appl Mater Interfaces. 2016;8:988–996. doi: 10.1021/acsami.5b10950. [DOI] [PubMed] [Google Scholar]
- 5.Fabi S, Sundaram H. The potential of topical and injectable growth factors and cytokines for skin rejuvenation. Facial Plast Surg. 2014;30:157–171. doi: 10.1055/s-0034-1372423. [DOI] [PubMed] [Google Scholar]
- 6.Sun BK, Siprashvili Z, Khavari PA. Advances in skin grafting and treatment of cutaneous wounds. Science. 2014;346:941–945. doi: 10.1126/science.1253836. [DOI] [PubMed] [Google Scholar]
- 7.Eming SA, Martin P, Tomic-Canic M. Wound repair and regeneration: mechanisms, signaling, and translation. Sci Transl Med. 2014;6:265sr6. doi: 10.1126/scitranslmed.3009337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ojeh N, Pastar I, Tomic-Canic M, et al. Stem cells in skin regeneration, wound healing, and their clinical applications. Int J Mol Sci. 2015;16:25476–25501. doi: 10.3390/ijms161025476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caramella CM, Sandri G, Rossi S, et al. New therapeutic platforms for the treatment of epithelial and cutaneous lesions. Curr Drug Deliv. 2013;10:18–31. doi: 10.2174/1567201811310010005. [DOI] [PubMed] [Google Scholar]
- 10.Kasuya A, Tokura Y. Attempts to accelerate wound healing. J Dermatol Sci. 2014;76:169–172. doi: 10.1016/j.jdermsci.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 11.Li X, Hamada T, Ohata C. Potential mesenchymal stem cell therapy for skin diseases. Exp Dermatol. 2013;22:515–516. doi: 10.1111/exd.12194. [DOI] [PubMed] [Google Scholar]
- 12.Wu Y, Chen L, Scott PG, et al. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells. 2007;25:2648–2659. doi: 10.1634/stemcells.2007-0226. [DOI] [PubMed] [Google Scholar]
- 13.Isakson M, de Blacam C, Whelan D, et al. Mesenchymal stem cells and cutaneous wound healing: current evidence and future potential. Stem Cells Int. 2015;2015:831095. doi: 10.1155/2015/831095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kruglikov IL, Scherer PE. Dermal adipocytes: from irrelevance to metabolic targets? Trends Endocrinol Metab. 2016;27:1–10. doi: 10.1016/j.tem.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen D, Hao H, Fu X, Han XW. Insight into reepithelialization: how do mesenchymal stem cells perform? Stem Cells Int. 2016;2016:6120173. doi: 10.1155/2016/6120173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Siciliano C, Ibrahim M, Scafetta G, et al. Optimization of the isolation and expansion method of human mediastinal-adipose tissue derived mesenchymal stem cells with virally inactivated GMP-grade platelet lysate. Cytotechnology. 2015;67:165–174. doi: 10.1007/s10616-013-9667-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bastianelli D, Siciliano C, Puca R, et al. Influence of Egr-1 in cardiac tissue-derived mesenchymal stem cells in response to glucose variations. Biomed Res Int. 2014;2014:254793. doi: 10.1155/2014/254793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sasaki M, Abe R, Fujita Y, et al. Mesenchymal stem cells are recruited into wounded skin and contribute to wound repair by transdifferentiation into multiple skin cell type. J Immunol. 2008;180:2581–2587. doi: 10.4049/jimmunol.180.4.2581. [DOI] [PubMed] [Google Scholar]
- 19.Ozpur MA, Guneren E, Canter HI, et al. Generation of skin tissue using adipose tissue-derived stem cells. Plast Reconstr Surg. 2016;137:134–143. doi: 10.1097/PRS.0000000000001927. [DOI] [PubMed] [Google Scholar]
- 20.Vériter S, Wivine A, Aouassar N, et al. Human adipose-derived mesenchymal stem cells in cell therapy: safety and feasibility in different “hospital exemption” clinical applications. PLoS One. 2015;10:e0139566. doi: 10.1371/journal.pone.0139566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Condé-Green A, Marano AA, Lee ES, et al. Fat grafting and adipose-derived regenerative cells in burn wound healing and scarring: a systematic review of the literature. Plast Reconstr Surg. 2016;137:302–312. doi: 10.1097/PRS.0000000000001918. [DOI] [PubMed] [Google Scholar]
- 22.Krasnodembskaya A, Song Y, Fang X, et al. Antibacterial effect of human mesenchymal stem cells is mediated in part from secretion of the antimicrobial peptide LL-37. Stem Cells. 2010;28:2229–2238. doi: 10.1002/stem.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fierro FA, O’Neal AJ, Beegle JR, et al. Hypoxic pre-conditioning increases the infiltration of endothelial cells into scaffolds for dermal regeneration pre-seeded with mesenchymal stem cells. Front Cell Dev Biol. 2015;3:68. doi: 10.3389/fcell.2015.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wahl EA, Fierro FA, Peavy TR, et al. In vitro evaluation of scaffolds for the delivery of mesenchymal stem cells to wounds. Biomed Res Int. 2015;2015:108571. doi: 10.1155/2015/108571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodrigues C, de Assis AM, Moura DJ, et al. New therapy of skin repair combining adipose-derived mesenchymal stem cells with sodium carboxymethylcellulose scaffold in a pre-clinical rat model. PLoS One. 2014;9:e96241. doi: 10.1371/journal.pone.0096241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gomathysankar S, Halim AS, Yaacob NS. Proliferation of keratinocytes induced by adipose-derived stem cells on a chitosan scaffold and its role in wound healing, a Review. Arch Plast Surg. 2014;41:452–457. doi: 10.5999/aps.2014.41.5.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gould LJ. Topical collagen-based biomaterials for chronic wounds: rationale and clinical application. Adv Wound Care (New Rochelle) 2016;5:19–31. doi: 10.1089/wound.2014.0595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oyarzun-Ampuero F, Vidal A, Concha M, et al. Secretory profiles and wound healing effects of human amniotic fluid-derived mesenchymal stem cells. Stem Cells Dev. 2010;6:887–902. doi: 10.1089/scd.2009.0138. [DOI] [PubMed] [Google Scholar]
- 29.Sánchez-Sánchez R, Brena-Molina A, Martínez-López V, et al. Generation of two biological wound dressings as a potential delivery system of human adipose-derived mesenchymal stem cells. ASAIO J. 2015;61:718–725. doi: 10.1097/MAT.0000000000000277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chimenti I, Gaetani R, Forte E, et al. Serum and supplement optimization for EU GMP-compliance in cardiospheres cell culture. J Cell Mol Med. 2014;18:624–634. doi: 10.1111/jcmm.12210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Committee for Medicinal Products for Human Use (CHMP) Guideline on the use of bovine serum in the manufacture of human biological medicinal products. 2013 EMA/CHMP/BWP/457920/2012 rev 1. [Google Scholar]
- 32.Carducci A, Scafetta G, Siciliano C, et al. GMP-grade platelet lysate enhances proliferation and migration of tenon fibroblasts. Front Biosci (Elite Ed) 2016;8:84–99. doi: 10.2741/E753. [DOI] [PubMed] [Google Scholar]
- 33.Siciliano C, Chimenti I, Bordin A, et al. The potential of GMP-compliant platelet lysate to induce a permissive state for cardiovascular transdifferentiation in human mediastinal adipose tissue-derived mesenchymal stem cells. Biomed Res Int. 2015;2015:162439. doi: 10.1155/2015/162439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Keating A. Mesenchymal stromal cells. Curr Opin Hematol. 2006;13:419–425. doi: 10.1097/01.moh.0000245697.54887.6f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zuk PA, Zhu M, Mizuno H, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 36.Lima AC, Mano JF, Concheiro A, Alvarez-Lorenzo C. Fast and mild strategy, using superhydrophobic surfaces, to produce collagen/platelet lysate gel beads for skin regeneration. Stem Cell Rev. 2015;11:161–179. doi: 10.1007/s12015-014-9548-6. [DOI] [PubMed] [Google Scholar]
- 37.Tasev D, van Wijhe MH, Weijers EM, et al. Long-term expansion in platelet lysate increases growth of peripheral blood-derived endothelial-colony forming cells and their growth factor-induced sprouting capacity. PLoS One. 2015;10:e0129935. doi: 10.1371/journal.pone.0129935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mirabet V, Solves P, Miñana MD, et al. Human platelet lysate enhances the proliferative activity of cultured human fibroblast-like cells from different tissues. Cell Tissue Bank. 2008;9:1–10. doi: 10.1007/s10561-007-9048-x. [DOI] [PubMed] [Google Scholar]
- 39.Onesti G, Carella S, Ceccarelli S, et al. The Use of Human Adipose-Derived Stem Cells in the Treatment of Physiological and Pathological Vulvar Dystrophies. Stem Cells Int. 2016;2016:2561461. doi: 10.1155/2016/2561461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Benedetti Panici P, Maffucci D, Ceccarelli S, et al. Autologous in vitro cultured vaginal tissue for vaginoplasty in women with Mayer-Rokitansky-Küster-Hauser syndrome: anatomic and functional results. J Minim Invasive Gynecol. 2015;22:205–211. doi: 10.1016/j.jmig.2014.09.012. [DOI] [PubMed] [Google Scholar]
- 41.Nodale C, Vescarelli E, D’Amici S, et al. Characterization of human vaginal mucosa cells for autologous in vitro cultured vaginal tissue transplantation in patients with MRKH syndrome. Biomed Res Int. 2014;2014:201518. doi: 10.1155/2014/201518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nodale C, Ceccarelli S, Giuliano M, et al. Gene expression profile of patients with Mayer-Rokitansky-Küster-Hauser syndrome: new insights into the potential role of developmental pathways. PLoS One. 2014;9:e91010. doi: 10.1371/journal.pone.0091010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fioramonti P, Onesti MG, Marchese C, et al. Autologous cultured melanocytes in vitiligo treatment comparison of two techniques to prepare the recipient site: erbium-doped yttrium aluminum garnet laser versus dermabrasion. Dermatol Surg. 2012;38:809–812. doi: 10.1111/j.1524-4725.2012.02354.x. [DOI] [PubMed] [Google Scholar]
- 44.Mancino R, Aiello F, Ceccarelli S, et al. Autologous conjunctival epithelium transplantation and scleral patch graft for postlensectomy wound leakage in Marfan syndrome. Eur J Ophthalmol. 2012;22:830–833. doi: 10.5301/ejo.5000124. [DOI] [PubMed] [Google Scholar]
- 45.Gurtner GC, Werner S, Barrandon Y, et al. Wound repair and regeneration. Nature. 2008;453:314–321. doi: 10.1038/nature07039. [DOI] [PubMed] [Google Scholar]