Abstract
Exposures to seizure-inducing chemical threat agents represent a major public health concern. Of particular need is improved treatments to terminate convulsions and to prevent the long-term neurological sequelae in survivors. We are studying the organophosphorus (OP) cholinesterase inhibitor diisopropylfluorophosphate (DFP) and the GABA receptor inhibitor tetramethylenedisulfotetramine (TETS), which arguably encompass the mechanistic spectrum of seizure-inducing chemical threats, with the goal of identifying therapeutic approaches with broad-spectrum efficacy. Research efforts have focused on developing translational models and translational diagnostic approaches including (1) in vivo models of DFP- and TETS-induced seizures for studying neuropathological mechanisms and identifying treatment approaches; (2) in vivo imaging modalities for noninvasive longitudinal monitoring of neurological damage and response to therapeutic candidates; and (3) higher-throughput in vitro platforms for rapid screening of compounds to identify potential antiseizure and neuroprotective agents, as well as mechanistically relevant novel drug targets. This review summarizes our progress towards realizing these goals and discusses best practices and mechanistic insights derived from our modeling efforts.
Keywords: diisopropylfluorophosphate, organophosphate, seizures, status epilepticus, tetramethylenedisulfotetramine
Introduction
A collaborative effort was begun at the University of California (UC), Davis in 2012 to improve the treatment of chemical-induced seizures under the auspices of the CounterACT program of the National Institutes of Health (NIH). The long-term objective of the UC Davis CounterACT Center of Excellence is to identify improved medical countermeasures for terminating seizures and mitigating persistent neurological sequelae triggered by acute intoxication with chemical threat agents. Preclinical models are critical tools for identifying potential therapeutic agents. Therefore, the initial goal of the center was to develop preclinical models to study the acute and persistent effects of the organophosphorus (OP) cholinesterase inhibitor diisopropylfluorophosphate (DFP) and the GABAA receptor (GABAAR) inhibitor tetramethylenedisulfotetramine (TETS). OP neurotoxins are among the most potent of the chemical warfare agents and have been used by terrorists against civilian populations.1 DFP, while less potent than the nerve agents in the Chemical Weapons Convention toxic chemicals list, has nearly identical effects and is widely used as a nerve agent surrogate for academic research.1 TETS is a highly toxic convulsant poison, which, on the basis of dosage (mg/kg), is as potent as or more potent than OP nerve agents. Although its production has been banned worldwide since 1991, clinical reports of TETS intoxication continue to appear regularly in Chinese-language academic journals.2 There has been a large number of mass poisonings in China and elsewhere, and it is likely that tens of thousands of people have been poisoned with TETS, many deliberately.3,4 The ease and low cost of synthesizing TETS from readily available materials heightens concerns about its possible use as a threat agent. DFP and TETS act on different molecular targets in the brain to produce dramatically different seizures: OPs inhibit cholinesterases to initiate continuous limbic status epilepticus,5 whereas TETS inhibits GABAAR to trigger clusters of clonic seizures and, at higher exposure levels, tonic–clonic seizures.6–8 Survivors of acute OP or TETS intoxication present with a variety of neurological dysfunctions ranging from mild to severe decline in memory, affective disorders, and chronic epilepsy.1,4,5,9
Our rationale for studying mechanistically diverse seizure-inducing chemical threat agents is to provide a platform for the identification of broad-spectrum antiseizure agents used singly or in combination to treat victims of exposures before the offending toxin is definitively identified. Specifically, we are investigating whether there are shared mechanisms of seizure-induced neuropathology triggered by diverse classes of seizurogenic chemicals that present an opportunity for developing broad-spectrum treatments to prevent the persistent neurological effects of chemically induced seizures. Additionally, comparisons of toxic profiles and responses to pharmacological probes across these models are likely to provide insights into convergent and divergent therapeutic targets by which these seizure-inducing agents cause progressive neurological damage. Here, we describe our efforts to develop preclinical models of acute OP and TETS intoxication that replicate clinical observations of chemical-induced status epilepticus. Models that have a relatively high survival rate will enable identification of therapeutic windows for effective pharmacological intervention following acute intoxication and testing of novel neuroprotective strategies. In addition, we discuss the development of in vitro platforms for more rapid screening of compounds, singly and in combination, for anticonvulsant and neuroprotective potential, as well as identification of novel mechanistically relevant drug targets.
In vivo models of acute TETS intoxication
We have developed several rodent models of TETS exposure to allow treatment agents to be tested at various times after TETS poisoning and to provide an opportunity to study the short- and long-term consequences of TETS-induced seizures.8,10,11 Our initial studies with NIH Swiss albino mice and Sprague Dawley albino rats revealed that TETS evokes seizures in both species. However, because the seizures are usually rapidly lethal, they do not provide an adequate model of persistent seizure activity (status epilepticus) as observed in humans.8,10,11 TETS is thought to cause seizures via noncompetitive antagonism of GABAAR.12 As is the case with other GABAAR antagonists, such as picrotoxin or pentylenetetrazol, a single intraperitoneal (IP) injection of TETS in rodents induces a characteristic sequence of seizure behaviors beginning with immobility, followed by myoclonic twitches, clonic seizures, and tonic–clonic seizures. The latter are characterized by wild running, loss of righting reflex, and forelimb tonic extension with hind limb tonic contraction and/or extension that in some cases may be followed by clonic movements of all limbs. The severity, incidence and time to onset of seizure behavior depend on the dose of TETS administered. At low doses (0.1 mg/kg, IP), only the initial signs are observed, with most animals exhibiting immobility and/or myoclonic body twitches, although some also exhibit clonic seizures. Higher doses of TETS (0.15 mg/kg, IP) produce clonic seizures that in most cases progress to tonic–clonic seizures that are nearly always associated with death within 1 h after TETS exposure. TETS-intoxicated animals that fail to exhibit tonic–clonic seizures survive indefinitely without apparent long-term impairment.13 At the lower IP doses, the onset of clonic seizures is delayed (mean time to onset is 54 min), whereas with higher doses, the onset to clonic seizures is rapid (~2 min). Oral administration of TETS, which better mimics the most common route of human exposure,4,6 produces a similar sequence of seizure signs, but higher doses are required.8 A similar pattern of seizures occurs in adult male C57BL/6 mice, although this mouse strain seems to be less sensitive to TETS than the NIH Swiss mouse.14,15
In NIH Swiss mice, the CD97 and LD97 (convulsive dose and lethal dose in 97% of animals) for TETS-induced tonic–clonic seizures and mortality is 0.2 mg/kg, as calculated using the method of Litchfield and Wilcoxon.16 At this dose, the time to seizure onset ranges from 7 to 20 min, providing a sufficient time window to evaluate the efficacy of “rescue treatment paradigms” that would be administered shortly after exposure, for example, to soldiers and first responders. Pretreatment with diazepam before administration of TETS (0.2 mg/kg, IP) effectively prevents tonic seizures and mortality during the first hour postexposure.17 In animals administered a lower dose of TETS (0.15 mg/kg, IP) to delay the time to onset of tonic–clonic seizures, treatment with diazepam immediately following the second clonic seizure (approximately 20 min after TETS exposure) effectively stopped subsequent electrographic seizures for at least 1 h posttreatment.11 However, the high dose of diazepam (5 mg/kg, IP) required to terminate seizures caused motor impairment and hypotension while not preventing TETS-induced neuroinflammation in the brain.11 Subsequent studies revealed that co-administration of lower doses of diazepam and the neurosteroid allopregnanolone (0.03–0.1 mg/kg, IP) either 10 min before TETS or immediately following the second clonic seizure increased survival from 10% to 90% with no effects on motor function or blood pressure.10 Interestingly, this drug combination also significantly reduced TETS-induced microglial activation.10
As noted, systemic administration of TETS at doses sufficient to cause clonic seizure activity is almost invariably followed by tonic seizures and death. To obtain a more clinically relevant model that mimics the persistent seizure activity (status epilepticus) reported in humans poisoned with TETS, we adapted a previously described approach for obtaining status epilepticus in mice exposed to the GABAAR antagonist pentylenetetrazol, which also ordinarily causes lethality.18 Drugs, such as phenytoin, that block voltage-gated sodium channels are known to prevent the tonic hind limb extension phase of tonic–clonic seizures evoked by electrical stimulation in the maximal electroshock test or by GABAAR antagonists like pentylenetetrazol.19,20 However, such agents are not highly effective in protecting against clonic seizures. Therefore, pretreatment with a sodium channel–blocking antiseizure agent can prolong survival by protecting against the tonic seizures that are associated with lethality in mice without inhibiting ongoing seizure activity. Parenteral phenytoin has a delayed onset of action, exhibiting its maximum effect 2 h after intraperitoneal injection.20 This is inconvenient, since pretreatment with phenytoin would require dosing hours before TETS injection. Therefore, in our adaptation of this status epilepticus model we used riluzole (2-amino-6-(trifluoromethoxy)benzothiazole) as the pretreatment agent, which we found to be protective against tonic seizures in the maximal electroshock test within 10 min of parenteral administration.21 Riluzole is well known to inhibit neuronal voltage-gated sodium channels in a use-dependent manner.22 More recently, it has been found to activate apamin-sensitive small-conductance Ca2+-activated KCa2 (SK) channels,23,24 which are widely expressed in the nervous system and are responsible for the medium after hyperpolarization (AHP) that regulates tonic, burst, and rhythmic neuronal firing. We have found that administration of riluzole (10 mg/kg) 10 min before a lethal dose of TETS (0.2 mg/kg) protects NIH Swiss mice from tonic extension and reproducibly results in continuous seizure activity, characterized by a progression of behavioral seizure signs and EEG seizure discharges (Fig. 1). In this model, abnormal EEG activity begins approximately 3 min after TETS administration. During the initial 20 min following TETS exposure, animals exhibit myoclonic twitches associated with isolated spikes, sharp waves, and spike and slow-wave complexes or clusters that are followed by isolated clonic seizures and, finally, tonic–clonic seizures. Subsequently, merging seizures and continuous ictal discharges are observed for nearly 1 h. The EEG then typically transitions into spike and slow-wave complexes and clusters. In this model, 70–80% of TETS-intoxicated animals survive 1 h after receiving TETS; however, administration of antiseizure treatments within 1 h of TETS exposure allows the animals to survive for 24 h or longer.
Figure 1.
Behavioral seizure events and EEG signatures at various stages of TETS-induced status epilepticus. An NIH Swiss mouse was treated with riluzole followed 10 min later by a lethal dose of TETS. The top trace in each pair is the EEG signal from the right frontal area and the bottom trace is the EMG signal recorded from the neck. The pair of traces at the bottom indicate the overall time course of the recording. Samples of activity at the points indicated by the bracketed letters are shown at the top. The regions designated by bars are shown on an expanded time scale in the middle pair of traces.
In vivo model of acute DFP intoxication
Both OP nerve agents and OP pesticides cause acute toxicity by inhibiting acetylcholinesterase, the enzyme that hydrolyzes the neurotransmitter acetylcholine in the central and peripheral nervous systems. The consequent accumulation of acetylcholine at muscarinic and nicotinic receptors results in an acute cholinergic syndrome characterized by autonomic dysfunction, involuntary movements, muscle fasciculations, respiratory distress, and seizures, including status epilepticus.25–27 OP toxicity is not limited to these acute responses, and persistent debilitating neurological effects have been reported in individuals who survive the acute cholinergic crisis.9,28,29 Consistent with these clinical and epidemiological observations, experimental animal models of acute OP nerve agent intoxication demonstrate brain injury consequent to early convulsive seizures.30–34 The nerve agent simulant DFP has been used to model seizures and convulsions with subsequent behavioral deficits in rodents.35–39 DFP rapidly inhibits AChE, produces seizures and status epilepticus as determined by electroencephalography,36,37 and causes a high rate of mortality if animals are not treated aggressively to eliminate peripheral symptoms of cholinergic toxicity.37 In animals pretreated with atropine, acute DFP intoxication has been shown to cause delayed apoptotic cell death in the CNS 24 and 48 h after DFP exposure, as detected using terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL).37,40 However, the spatiotemporal pattern of cell injury and the cell types injured following acute DFP intoxication remain poorly understood.
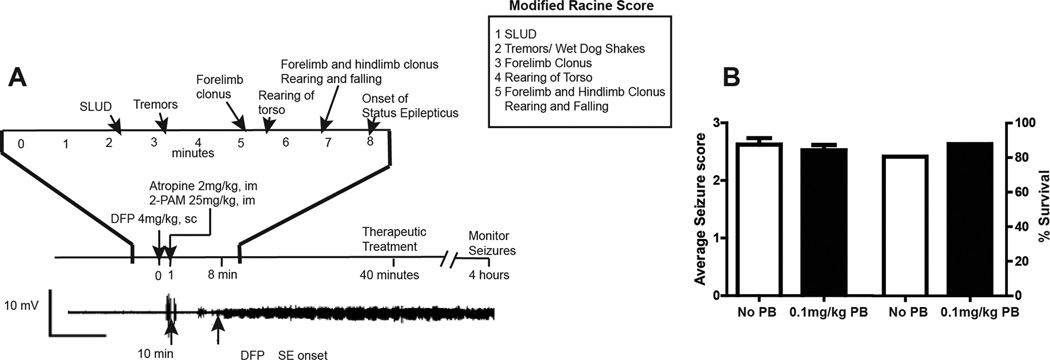
We have compared two previously described exposure paradigms for inducing seizures with DFP in the adult male Sprague Dawley rat: (1) pyridostigmine bromide (0.1 mg/kg, intramuscular (IM)) 30 min before DFP, atropine methyl nitrate (20 mg/kg, IM) 10 min before DFP, and DFP (9 mg/kg, IP);39,41 and (2) DFP (4 mg/kg, subcutaneous (SC)), atropine sulfate (2 mg/kg, IP) and 2-PAM (25 mg/kg, IM) 1 min after DFP exposure.36,42 While we observed persistent seizure activity (status epilepticus) using either exposure paradigm, in our experience, the second model yields more reliable and consistent results in terms of seizure incidence and survival, and it has the added benefit of not requiring pretreatments for the animals to survive. Thus, we have observed that a single subcutaneous injection of DFP at 4 mg/kg rapidly induces persistent seizures and SE in more than 90% of exposed adult male Sprague Dawley rats (Fig. 2). There is good correlation between seizure behaviors, which are scored using a modified Racine scale (Fig. 2), and EEG activity. Postexposure treatment with atropine sulfate and 2-PAM is required for DFP-exposed animals to survive: absent these posttreatments, survival is less than 10%, whereas with post-treatment survival is greater than 75%. We determined that pretreatment with pyridostigmine bromide did not significantly decrease seizure severity or enhance 24-h survival in this model (Fig. 2). Long-term survival is significantly enhanced if animals receive 10% dextrose in saline (10 mL, SC) within 4–6 h after DFP exposure to replace fluids lost as a result of the parasympathomimetic effects associated with acute DFP intoxication. Additionally, to ensure survival beyond 24 h, animals require soft food (DietGel Recovery, Clear H20, Portland, ME) and moistened chow as well as assistance with locating both food and water during the first 3 days following DFP exposure. Using these husbandry practices, more than 90% of DFP animals that survive the first 4 h postexposure will survive indefinitely.
Figure 2.
Rat model of acute DFP Intoxication. (A) Adult male Sprague Dawley rats administered DFP at 4 mg/kg SC rapidly progress to seizures with status epilepticus beginning within 10 min after DFP exposure. Seizures were scored using a modified Racine score every 5 min during the first 2 h post-DFP and then every 20 min from 2 to 4 h post-DFP. Seizure severity scores were calculated as the time-weighted average of the modified Racine score over the 4-h period. To increase survival, atropine sulfate and 2-PAM are administered within 1 min after DFP injection to block peripheral cholinergic symptoms and regenerate functional acetylcholinesterase, respectively. (B) Pretreatment with pyridostigmine bromide (0.1 mg/kg, IM) 30 min before DFP injection had no effect on either the average time-weighted seizure score or the percent of DFP-intoxicated animals that survived to 24 h. Data presented as the mean ± S.E.M. (n = 10 animals per group).
Consistent with previous studies,39,41,43 neuropathological analyses of DFP rats demonstrate >50% increase in the number of FluoroJade-stained neurons in multiple brain regions relative to vehicle controls as early as 12 h postexposure. Our data suggests that this neurodegeneration persists up to 14 days postexposure but returns to control levels by 21 days postexposure.44 Also consistent with previous studies,39,45,46 we observed a robust neuroinflammatory response, as measured by GFAP immunoreactivity, a biomarker of reactive astrocytes, and the number of cells immunopositive for both Iba-1 and CD68, a biomarker of activated microglia. Our preliminary data suggests that this neuroinflammatory response persists up to 60 days after DFP exposure in some brain regions.44 This neuropathology coincides with significant deficits in the contextual fear conditioning task to assess learning and memory that persist for up to 2 months postexposure, as well as EEG evidence of spontaneous recurrent seizures in approximately 25–30% of DFP-exposed animals.44 Collectively, these findings suggest that this rat model of DFP intoxication recapitulates key features of acute OP intoxication observed in humans, including status epilepticus, spontaneous recurrent seizures, and behavioral deficits,9,25–29 suggesting that it will be a rigorous platform for identifying mechanisms by which OP-induced status epilepticus causes persistent neurological effects and for testing candidate therapeutics for neuroprotection.
A major challenge in preclinical studies of neuroprotection is longitudinal monitoring of the progressive damage caused by acute intoxication and the efficacy of candidate therapeutic agents in altering the course of this damage. Current approaches largely rely on histopathologic assessments and behavioral readouts. The former permits very detailed structural and histochemical analyses of the brain; however, only a single snapshot in time can be obtained from any given animal. Tracking damage or therapeutic rescue over time would, therefore, require a significant number of animals. Conversely, behavioral readouts allow multiple measures in the same animal over time but do not provide structural or biochemical information about the brain. Thus, we are actively exploring in vivo imaging as a tool for overcoming these challenges. Specifically, we are evaluating positron emission tomography (PET) imaging of the 18-kDa translocator protein TSPO (formerly known as the peripheral benzodiazepine receptor or PBR) as an in vivo biomarker of neuroinflammation47–49 and magnetic resonance imaging (MRI) to assess neuroanatomical changes in the intact brain. An additional advantage of these noninvasive, non-destructive techniques is that they are already being used in humans. Thus, results obtained in animal models can be readily translated to the clinic, enabling clinicians to better identify individuals at risk for delayed TETS or OP neurotoxicity and to assess therapeutic efficacy. Correlational analysis of histopathology with PET and MRI data from the same animals support the feasibility of using these in vivo modalities for longitudinal monitoring of seizure-induced neurological damage. Specifically, there is remarkable spatiotemporal registry between the imaging data and the histopathology.44 Brain regions with neuropathology (neuronal necrosis as determined by H&E staining or NeuN immunohistochemistry or neuroinflammation evidenced as increased Iba-1 and GFAP immunohistochemistry) exhibited damage as determined by MRI and by PET, and the spatial extent and timing of the damage is comparable between the in vivo imaging and histopathology.44 Brain regions that did not exhibit neuropathology as determined using histopathology (e.g., the cerebellum) also did not exhibit damage as assessed by in vivo imaging. Analyses of PET TSPO imaging relative to histopathology from the same animal identified the global average of standardized [18F]PBR111 PET scores as a quantitative imaging variable with a moderate to high level of correlation with a biologically relevant histopathological variable, while similar analyses of MRI data identified the variance of the apparent diffusion coefficient (ADC) as a quantitative imaging variable with a high level of correlation with a biologically relevant histopathological variable.44
Rapid-throughput in vitro models of neural network hyperexcitation by TETS and DFP
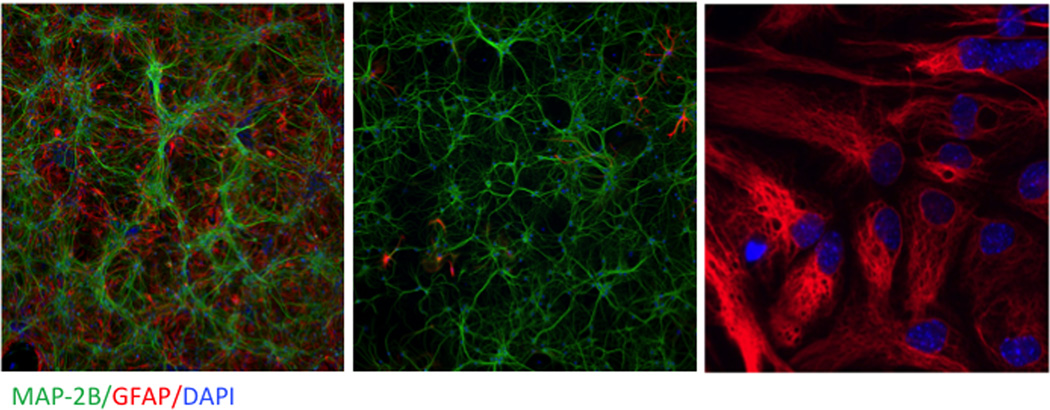
One goal of the center is to establish imaging and electrophysiological techniques for acquiring real-time rapid-throughput information about cellular physiology and pathophysiology before, during, and subsequent to exposure to chemical threat agents. Two experimental models for predicting neuronal network excitability in response to challenges with the seizure-inducing agents TETS and DFP are currently being developed and tested for face validity to the seizure activity and persistent neuroinflammation triggered by acute intoxication with these chemicals in vivo and, more importantly, for identifying promising new chemical entities that may serve to prevent seizures and/or mitigate neuropathological consequences. Both models rely on primary neuronal cell cultures isolated from newborn mice or rats. For consistency with in vivo models being developed by the UC Davis CounterACT Center, mouse hippocampal cultures serve to test the acute and chronic excitotoxicity of TETS, whereas rat cortical cultures are the focus of parallel studies with DFP. Primary cultures offer significant advantages and flexibility for not only defining temporal patterns of network excitation and neuropathology but also enabling systematic analysis of the inherent physiological responses of neurons and astrocytes in isolation or in co-culture that protect against or promote toxicity of threat agents (Fig. 3). Such cultures develop elaborate neuronal networks with functional synapses whose activity can be quantitatively monitored in real time using three complementary approaches: two that use fluorescent indicators to monitor intracellular Ca2+ or membrane potential using the 96-well FLIPR® Tetra imager and a third that measures electrical spike activity in cultures plated on multiwell microelectrode arrays (MEAs).
Figure 3.
Representative photomicrographs of cultures used for in vitro studies. Enriched neurons (left panel), enriched astrocytes (middle panel), and neuron–astrocyte co-cultures (right panel) immunostained for the dendritic biomarker MAP-2b (green), the astrocyte biomarker GFAP (right), and the nuclear stain DAPI (blue). Magnification differs between images.
Maturing neurons display synchronous Ca2+ oscillations (SCOs) that are easily detected by FLIPR and whose amplitude and frequency reflect long-range network activity.50,51 SCO patterns influence growth, complexity, and plasticity over the entire developmental time frame of the cultures (~ 3 weeks). Since astrocytes only display spontaneous asynchronous Ca2+ oscillations,52 which are not detected by the FLIPR imager, the FLIPR method provides a convenient readout of neuronal network activity, even with neuron/astrocyte co-cultures. Microscopic imaging techniques are used to test how threat agents and interventions directly influence asynchronous Ca2+ events from pure astrocytic cultures.52 We discovered that acute challenge of mouse hippocampal cultures enriched in neurons with TETS, a GABAA receptor blocker; kainate, an AMPA/kainate receptor agonist; 4-aminopyridine (4-AP), a K+ channel blocker; or pilocarpine, a muscarinic acetylcholine receptor agonist, caused distinct changes in SCO dynamics.51,53 Importantly, TETS-triggered changes in SCO patterns have provided a basis for screening compounds, including benzodiazepines and neurosteroids, as novel therapeutic interventions that may be more efficacious than the current standard of care for TETS intoxication.10,22,54
We have also integrated recent advances in multiwell multielectrode array (MEA) technology to measure the frequency, amplitude, bursting, and synchronicity of spontaneous electrical spike activity from the cultures described above.54 It is clear that TETS51 and some organophosphates potently alter spike parameters in very different ways. Thus, measures of SCO and electrical spike activity represent two highly complementary approaches for screening chemicals for antiseizure and neuroprotective efficacy to be prioritized for testing in the center’s in vivo models, which has already proved successful in predicting therapeutic benefit from the combination diazepam plus allopregnanolone compared to either alone.10,51 Recently, polytherapy with diazepam and the N-methyl-d-aspartate (NMDA) blocker MK-801 was shown to confer synergistic protection against TETS-induced tonic–clonic seizures and lethality at 24 h,14,15 supporting the concept that coordinated therapeutic interventions that allosterically amplify GABAA receptor activity and normalize Ca2+ dynamics may be more effective than either strategy alone to treat TETS intoxication.
Challenges associated with model development
A major challenge when working with both DFP and TETS includes compound availability and purity. TETS production and use is banned worldwide. The UC Davis CounterACT Center therefore synthesizes its own TETS, confirms chemical identity, determines purity as described,8 and handles it as a controlled, highly toxic substance according to the center’s standard operating procedures. The organophosphate DFP is currently only available from a limited number of commercial suppliers, including Sigma-Aldrich, which obtains DFP from an undisclosed third-party manufacturer, most likely in India. Both our center and the NIH Anticonvulsant Screening Program have observed that different batches of DFP, which is only available in 1-g quantities, can vary significantly in their potency for seizure induction. Our center therefore now uses a combination of 1H-, 13C-, 19F- and 31P-NMR to detect impurities in commercially available DFP. DFP batches in which additional signals are detected in the 31P-NMR are rejected. Since there was no reliable literature data on the stability of DFP in DMSO or in aqueous buffers used for tissue culture or imaging experiments, we also investigated the stability of DFP in solution and the rate at which it hydrolyses into diisopropyl phosphoric acid (DIPA) through nucleophilic substitution of the F. This hydrolysis can be easily monitored by the diminishing octet at 4.77 ppm and the appearance of a shifted octet at 4.51 ppm in the 800 Hz 1H-NMR spectrum or the change of the 31P signal from a doublet induced by the coupling to the F in DFP to a singlet in the hydrolysis product that no longer contains F. While DFP was highly stable in DMSO and showed no signs of hydrolysis even after 19 days, it hydrolyzed quickly in unbuffered deionized water, which is typically acidic. DFP was reasonably stable in phosphate buffered solutions, where it showed no signs of hydrolysis over 20 h, suggesting that it would be sufficiently stable in tissue culture experiments or imaging experiments that last less than 24 h.55 Therefore, we believe that DFP is stable in the Ca2+-imaging experiments described above.
Center investigations incorporate study design, statistical analysis, and reporting guidelines to promote the reproducibility of preclinical research.56–58 Targeted statistical support and training is provided through the center’s own statistics and data management research support core, which is staffed by faculty with substantial experience in statistical design and analysis of preclinical and clinical research. An international group of researchers recently identified important elements for reporting and, by implication, for the design and statistical analysis of preclinical research.57 Of particular concern to us in our animal model development work is the use of appropriate statistical analysis techniques for clustered data that arise from repeated measures from the same animal across time (i.e., longitudinal data) and/or space (i.e., brain regions). Appropriate techniques include mixed-effects regression models,59 methods for clustered survey data,60 and generalized estimating equations.61 These methods avoid the pitfall of pseudoreplication while also permitting precise and robust estimation of treatment effects by permitting the pooling of correlated data to be analyzed in a single model.62,63 To maximize predictive value, we use parsimony-favoring model goodness-of-fit criteria such as the Akaike information criterion for model selection (i.e., selecting interaction terms to include as fixed effects in the mixed-effects analysis of a multifactorial experiment).64 We prefer reporting effect-size estimates with 95% confidence intervals (CI) as a complement or replacement for P-values, noting the irreproducibility and misinterpretation of P-values and the usefulness of confidence intervals for decision makers in conveying which hypothetical effect sizes are plausible given the sample data.65,66 To help ensure that estimated standard errors are robust against mistaken regression modeling assumptions, we prefer robust sandwich estimators, such as those available in the mixed-effects modeling procedures in Stata67 and SAS.68
Our preferred modeling strategy is illustrated in a recent publication where we compared histological measures of neuroinflammation in TETS-intoxicated mice treated with allopregnanolone versus vehicle.10 To estimate the effect of treatment, we applied mixed-effects models to natural logarithm–transformed immunoreactivity luminescence area measures from 4–7 samples of cortical and hippocampal brain tissue per animal with five animals from each of these two treatment groups for each of two time points post-TETS. In addition, a third treatment group of TETS-intoxicated mice treated only with vehicle were included in the analysis. Pairwise contrasts in mean log-transformed measures and 95% CI were back-transformed by applying the inverse natural logarithm transformation y=ex, resulting in geometric mean rations and 95% CI reported in the original scale of measurement. The contrasts depicted in the original paper are summarized in Table 1.
Table 1.
Geometric mean ratios and 95% CI from previous study and sample-size inflation factors to use in planning future studies
| Immunostain | Brain region | Days postexposure |
Effect of allopregnanolone geometric mean ratio (95% CI) |
Sample-size inflation factor for future studies to achieve 80% power to detect given true value of GMR |
|
|---|---|---|---|---|---|
| GMR = 1.5 | GMR = 2 | ||||
|
GFAP (for reactive astrogliosis) |
Cortex | 2 | 2.67 (1.19–6.02) | 8.26 | 2.82 |
| Cortex | 3 | 5.39 (3.10–9.37) | 3.83 | 1.31 | |
| Hippocampus | 2 | 1.28 (0.94–1.75) | 1.19 | 0.41 | |
| Hippocampus | 3 | 1.33 (1.05–1.69) | 0.69 | 0.23 | |
|
Iba-1 (for microglial activation) |
Pooled | Pooled | 0.61 (0.40–0.94) | 2.30 | 0.79 |
Note: Geometric mean ratios and 95% CI were estimated using mixed-effect regression analysis of log-transformed outcomes and reported previously in Figures 6 and 7 of Ref. 9. Sample-size inflation factors describe the factor to apply to the sample size from the published study (five animals per group) to approximate the sample size needed in future studies needed to achieve 80% power (under two-sided testing with type-1 error rate = 5%) for detecting hypothesized true geometric mean ratio.
A further challenge confronting researchers is determining appropriate sample sizes to yield adequate power to detect meaningful effects. For complex data collection and analysis strategies where previous studies are available, a practical approach is to use standard error estimates from analyses of those studies as a basis for informing the design of the future study. To illustrate such an approach, we have included sample size inflation factors in Table 1 that could be applied to the original sample size (five animals per contrasted group per time point) to approximate a sample size that would provide 80% power to detect true geometric mean ratios (GMR) of 1.5 and 2.0. These were computed under the standard assumption that the logarithm of the geometric mean ratio has an approximately normal sampling distribution with a standard deviation equal to the estimated standard error (SE(previous)) from the previous study whose effective sample size was n.59,69 The value of this standard error can be recovered by dividing the difference between the upper and lower limits of the 95% CI for log(GMR) by 2 × z0.975 = 2 × 1.96, where zp is the 100p–th percentile of the standard normal distribution: SE(previous) = (log(UCL of GMR) – log(LCL of GMR)) / 2 × 1.96. For a future study with sample size m, we assume that the standard error for this estimated contrast would be SE(future) = SE(previous) / √(m/n), where the ratio (m/n) is defined as the inflation factor. Hence, the inflation factor necessary to achieve a given power 1–β to detect a specified effect size D = log(GMR) under two-sided testing with type-1 error α for the contrast in mean log-transformed outcomes is approximated by solving the following inequality:
From Table 1, we can make many inferences and decisions that would be difficult or impossible to make had only P-values been reported. For example, from the given data, it is plausible that the geometric mean reactive astrogliosis in the treated group is as low as 1.19 to as high as 6.02 on day 2. Hypothesizing that a GMR of 2 in reactive astrogliosis would be minimally clinically significant, a future study should prescribe approximately 2.8 times as many animals per group (e.g., 2.8 × 5 = 14 animals per group per day of sacrifice) in order to have 80% power to detect such a difference, assuming similar methods would otherwise be followed. The increases in reactive astrogliosis in the hippocampus at day 3 are statistically significant, with plausible values for the GMR ranging from 1.05 to 1.69. Because this confidence interval excludes 2, we can conclude with 95% confidence that the true GMR is not as high as 2.0. If we consider a true GMR of 1.5 to be clinically significant, then we can detect that with at least 80% power in a future study of similar or even slightly lower sample size per group. For microglial activation, the effects of treatment are similar (on a multiplicative scale) in the cortex and hippocampus on both postexposure times. Plausible true reductions in geometric mean microglial activation range from 6% to 60%. To detect a 50% reduction in a future study, which corresponds in magnitude to log(GMR) = −log(1.5), the sample size would need to be increased 2.3 fold (i.e., 2.3 × 5 = 11.5, rounded up to 12 animals per group) to ensure 80% power.
Mechanistic insights derived from model development efforts
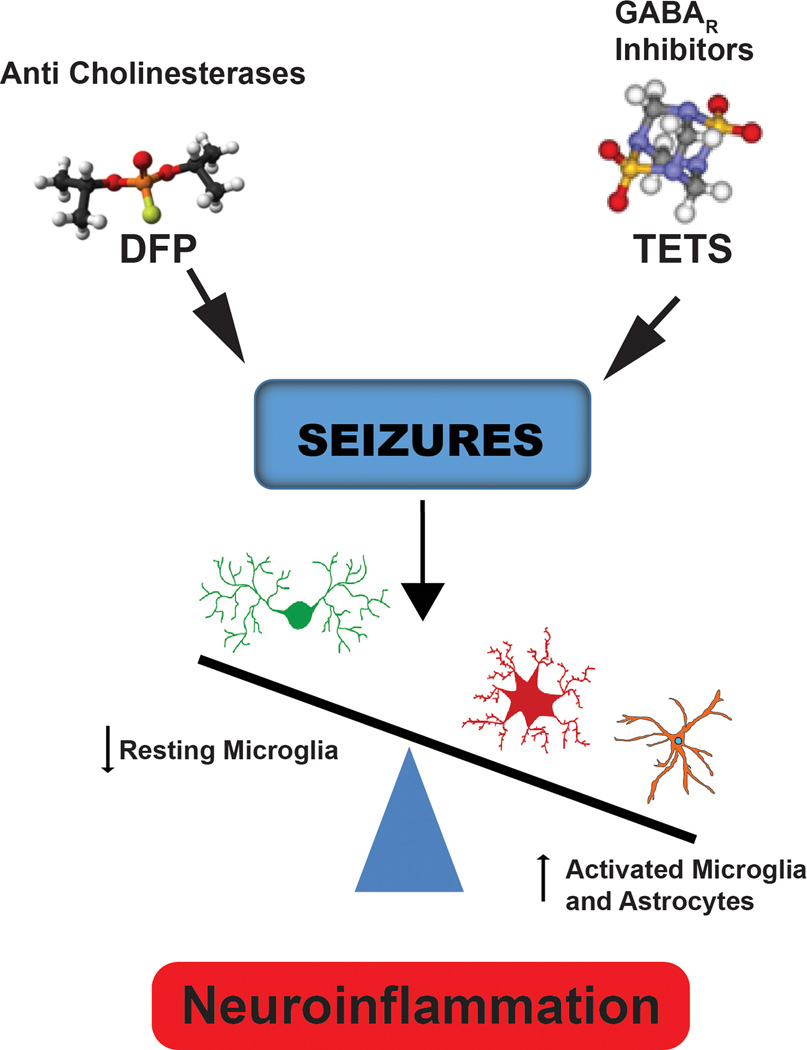
A major goal of the center is to identify convergent mechanisms of seizure-induced neuropathology. Our results thus far suggest that neuroinflammation is a common neuropathological mechanism associated with seizures triggered by OPs and GABAAR antagonists. We have observed delayed and persistent reactive astrogliosis and microglial cell activation in the hippocampus and cortex of mice following TETS-induced seizures.8,10,11 In addition, we have demonstrated that acute OP intoxication triggers massive activation of microglia and astrocytes coincident with increased brain levels of proinflammatory mediators, including arachidonic acid metabolites (Fig. 4). Whether neuroinflammation contributes to or mitigates neuropathology triggered by DFP or TETS remains controversial. However, a variety of lines of evidence raise the possibility that the neuroinflammatory response can have adverse consequences. Thus, it has been found that proinflammatory mediators significantly impair spatial memory,70 experimental induction of inflammation significantly downregulates cortical and hippocampal expression of neurotrophins that are critically important in synaptic plasticity,71 and glial-derived IL-1β contributes to the etiopathogenesis of seizures and the establishment of chronic epileptic foci.72,73 These observations raise the possibility that targeting neuroinflammation could be a viable therapeutic strategy for mitigating the long-term neurological sequela of acute intoxication with seizure-inducing chemical threat agents.
Figure 4.
Convergent mechanism of seizure-induced neuropathology. Data collected during the development of a rat model of acute DFP intoxication and a mouse model of acute TETS intoxication have identified persistent microglial activation and reactive astrogliosis as common neurologic sequelae in both models.
The approaches to finding more potent and effective medical countermeasures for acute intoxication with TETS (as well as chemically related cage convulsants) or organophosphates (with DFP serving as a chemical surrogate for an OP nerve agent) have traditionally been based on a paradigm of “primary toxicological target.” In this respect, DFP and TETS are believed to cause seizures primarily, if not exclusively, by blocking brain AChE and central GABAAR, respectively.1,12,74 A significant challenge to these current mechanistic dogmas is the quantitative disconnect between the relative in vitro potencies of TETS and DFP at their intended primary targets and their acute potencies demonstrated in the relevant in vivo models. In this regard, both TETS (IC50 10 µM) and DFP (IC50 2 µM) are relatively weak direct blockers of GABAAR Cl− current and AChE, respectively. Although the in vitro potencies of TETS and DFP are consistent across multiple endpoints (SCO, MEA, and whole-cell current), they seem to grossly underestimate in vivo potency. Pharmacokinetic factors could contribute to the discrepancy between in vitro IC50 and in vivo LD50. Our initial investigations with DFP, paraoxon, and chlorpyrifos oxon have revealed similar large discrepancies between the potency for AChE inhibition in vitro and in vivo acute toxicity. Clearly, other primary toxicological targets need to be identified, as we have begun to explore for other classes of insecticides and threat agents.36,50,75
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Jett DA, Yeung DT. The CounterACT Research Network: basic mechanisms and practical applications. Proc Am Thorac Soc. 2010;7:254–256. doi: 10.1513/pats.201001-003SM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li Y, Gao Y, Yu X, Peng J, Ma F, Nelson L. Tetramine poisoning in China: changes over a decade viewed through the media's eye. BMC Public Health. 2014;14:842. doi: 10.1186/1471-2458-14-842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu YQ, Sun CY. Poison control services in China. Toxicology. 2004;198:279–284. doi: 10.1016/j.tox.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 4.Li JM, Gan J, Zeng TF, Sander JW, Zhou D. Tetramethylenedisulfotetramine intoxication presenting with de novo Status Epilepticus: a case series. Neurotoxicology. 2012;33:207–211. doi: 10.1016/j.neuro.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 5.Chen Y, Garcia GE, Huang W, Constantini S. The involvement of secondary neuronal damage in the development of neuropsychiatric disorders following brain insults. Front Neurol. 2014;5:22. doi: 10.3389/fneur.2014.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Whitlow KS, Belson M, Barrueto F, Nelson L, Henderson AK. Tetramethylenedisulfotetramine: old agent and new terror. Ann Emerg Med. 2005;45:609–613. doi: 10.1016/j.annemergmed.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 7.Zhao C, Hwang SH, Buchholz BA, Carpenter TS, Lightstone F, Yang J, Hammock BD, Casida JE. GABAA receptor target of tetramethylenedisulfotetramine. Proc Natl Acad Sci U S A. 2014;111:8607–8612. doi: 10.1073/pnas.1407379111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zolkowska D, Banks CN, Dhir A, Inceoglu B, Sanborn JR, McCoy MR, Bruun DA, Hammock BD, Lein PJ, Rogawski MA. Characterization of seizures induced by acute and repeated exposure to tetramethylenedisulfotetramine. J Pharmacol Exp Ther. 2012;341:435–446. doi: 10.1124/jpet.111.190579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Y. Organophosphate-induced brain damage: mechanisms, neuropsychiatric and neurological consequences, and potential therapeutic strategies. Neurotoxicology. 2012;33:391–400. doi: 10.1016/j.neuro.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 10.Bruun DA, Cao Z, Inceoglu B, Vito ST, Austin AT, Hulsizer S, Hammock BD, Tancredi DJ, Rogawski MA, Pessah IN, Lein PJ. Combined treatment with diazepam and allopregnanolone reverses tetramethylenedisulfotetramine (TETS)-induced calcium dysregulation in cultured neurons and protects TETS-intoxicated mice against lethal seizures. Neuropharmacology. 2015;95:332–342. doi: 10.1016/j.neuropharm.2015.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vito ST, Austin AT, Banks CN, Inceoglu B, Bruun DA, Zolkowska D, Tancredi DJ, Rogawski MA, Hammock BD, Lein PJ. Post-exposure administration of diazepam combined with soluble epoxide hydrolase inhibition stops seizures and modulates neuroinflammation in a murine model of acute TETS intoxication. Toxicol Appl Pharmacol. 2014;281:185–194. doi: 10.1016/j.taap.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bowery NG, Brown DA, Collins JF. Tetramethylenedisulphotetramine: an inhibitor of gamma-aminobutyric acid induced depolarization of the isolated superior cervical ganglion of the rat. Br J Pharmacol. 1975;53:422–424. doi: 10.1111/j.1476-5381.1975.tb07379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flannery BM, Silverman JL, Bruun DA, Puhger KR, McCoy MR, Hammock BD, Crawley JN, Lein PJ. Behavioral assessment of NIH Swiss mice acutely intoxicated with tetramethylenedisulfotetramine. Neurotoxicol Teratol. 2015;47:36–45. doi: 10.1016/j.ntt.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shakarjian MP, Ali MS, Veliskova J, Stanton PK, Heck DE, Velisek L. Combined diazepam and MK-801 therapy provides synergistic protection from tetramethylenedisulfotetramine-induced tonic-clonic seizures and lethality in mice. Neurotoxicology. 2015;48:100–108. doi: 10.1016/j.neuro.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shakarjian MP, Veliskova J, Stanton PK, Velisek L. Differential antagonism of tetramethylenedisulfotetramine-induced seizures by agents acting at NMDA and GABA(A) receptors. Toxicol Appl Pharmacol. 2012;265:113–121. doi: 10.1016/j.taap.2012.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Litchfield JT, Jr, Wilcoxon F. A simplified method of evaluating dose-effect experiments. J Pharmacol Exp Ther. 1949;96:99–113. [PubMed] [Google Scholar]
- 17.Zolkowska DD, A. Banks C, Inceoglu B, Sanborn JR, McCoy MR, Bruun D, Hammock B, Lein PJ, Rogawski MA. Perampanel, a potent AMPA receptor antagonist, protects against tetramethylenedisulfotetramine-induced seizures. Epilepsy Curr. 2012;12:304. [Google Scholar]
- 18.Raines A, Henderson TR, Swinyard EA, Dretchen KL. Comparison of midazolam and diazepam by the intramuscular route for the control of seizures in a mouse model of status epilepticus. Epilepsia. 1990;31:313–317. doi: 10.1111/j.1528-1157.1990.tb05381.x. [DOI] [PubMed] [Google Scholar]
- 19.Loscher W, Fassbender CP, Nolting B. The role of technical, biological and pharmacological factors in the laboratory evaluation of anticonvulsant drugs. II. Maximal electroshock seizure models. Epilepsy Res. 1991;8:79–94. doi: 10.1016/0920-1211(91)90075-q. [DOI] [PubMed] [Google Scholar]
- 20.Loscher W, Honack D, Fassbender CP, Nolting B. The role of technical, biological and pharmacological factors in the laboratory evaluation of anticonvulsant drugs. III. Pentylenetetrazole seizure models. Epilepsy Res. 1991;8:171–189. doi: 10.1016/0920-1211(91)90062-k. [DOI] [PubMed] [Google Scholar]
- 21.Coleman N, Nguyen HM, Cao Z, Brown BM, Jenkins DP, Zolkowska D, Chen YJ, Tanaka BS, Goldin AL, Rogawski MA, Pessah IN, Wulff H. The riluzole derivative 2-amino-6-trifluoromethylthio-benzothiazole (SKA-19), a mixed KCa2 activator and NaV blocker, is a potent novel anticonvulsant. Neurotherapeutics. 2015;12:234–249. doi: 10.1007/s13311-014-0305-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coleman N, Nguyen HM, Cao Z, Brown BM, Jenkins DP, Zolkowska D, Chen YJ, Tanaka BS, Goldin AL, Rogawski MA, Pessah IN, Wulff H. The Riluzole Derivative 2-Amino-6-trifluoromethylthio-benzothiazole (SKA-19), a Mixed K2 Activator and Na Blocker, is a Potent Novel Anticonvulsant. Neurotherapeutics. 2015;12:234–249. doi: 10.1007/s13311-014-0305-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grunnet M, Jespersen T, Angelo K, Frokjaer-Jensen C, Klaerke DA, Olesen SP, Jensen BS. Pharmacological modulation of SK3 channels. Neuropharmacology. 2001;40:879–887. doi: 10.1016/s0028-3908(01)00028-4. [DOI] [PubMed] [Google Scholar]
- 24.Sankaranarayanan A, Raman G, Busch C, Schultz T, Zimin PI, Hoyer J, Kohler R, Wulff H. Naphtho[1,2-d]thiazol-2-ylamine (SKA-31), a new activator of KCa2 and KCa3.1 potassium channels, potentiates the endothelium-derived hyperpolarizing factor response and lowers blood pressure. Mol Pharmacol. 2009;75:281–295. doi: 10.1124/mol.108.051425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kwong TC. Organophosphate pesticides: biochemistry and clinical toxicology. Ther Drug Monit. 2002;24:144–149. doi: 10.1097/00007691-200202000-00022. [DOI] [PubMed] [Google Scholar]
- 26.Leikin JB, Thomas RG, Walter FG, Klein R, Meislin HW. A review of nerve agent exposure for the critical care physician. Crit Care Med. 2002;30:2346–2354. doi: 10.1097/00003246-200210000-00026. [DOI] [PubMed] [Google Scholar]
- 27.McDonough JH, Jr, Shih TM. Neuropharmacological mechanisms of nerve agent-induced seizure and neuropathology. Neurosci Biobehav Rev. 1997;21:559–579. doi: 10.1016/s0149-7634(96)00050-4. [DOI] [PubMed] [Google Scholar]
- 28.Miyaki K, Nishiwaki Y, Maekawa K, Ogawa Y, Asukai N, Yoshimura K, Etoh N, Matsumoto Y, Kikuchi Y, Kumagai N, Omae K. Effects of sarin on the nervous system of subway workers seven years after the Tokyo subway sarin attack. J Occup Health. 2005;47:299–304. doi: 10.1539/joh.47.299. [DOI] [PubMed] [Google Scholar]
- 29.Nishiwaki Y, Maekawa K, Ogawa Y, Asukai N, Minami M, Omae K Sarin Health Effects Study G. Effects of sarin on the nervous system in rescue team staff members and police officers 3 years after the Tokyo subway sarin attack. Environ Health Perspect. 2001;109:1169–1173. doi: 10.1289/ehp.011091169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McDonough JH, Jr, Dochterman LW, Smith CD, Shih TM. Protection against nerve agent-induced neuropathology, but not cardiac pathology, is associated with the anticonvulsant action of drug treatment. Neurotoxicology. 1995;16:123–132. [PubMed] [Google Scholar]
- 31.McLeod CG., Jr Pathology of nerve agents: perspectives on medical management. Fundam Appl Toxicol. 1985;5:S10–S16. doi: 10.1016/0272-0590(85)90110-1. [DOI] [PubMed] [Google Scholar]
- 32.Shih TM, Duniho SM, McDonough JH. Control of nerve agent-induced seizures is critical for neuroprotection and survival. Toxicol Appl Pharmacol. 2003;188:69–80. doi: 10.1016/s0041-008x(03)00019-x. [DOI] [PubMed] [Google Scholar]
- 33.Lemercier G, Carpentier P, Sentenac-Roumanou H, Morelis P. Histological and histochemical changes in the central nervous system of the rat poisoned by an irreversible anticholinesterase organophosphorus compound. Acta Neuropathol. 1983;61:123–129. doi: 10.1007/BF00697391. [DOI] [PubMed] [Google Scholar]
- 34.de Araujo Furtado M, Rossetti F, Chanda S, Yourick D. Exposure to nerve agents: from status epilepticus to neuroinflammation, brain damage, neurogenesis and epilepsy. Neurotoxicology. 2012;33:1476–1490. doi: 10.1016/j.neuro.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 35.Auta J, Costa E, Davis J, Guidotti A. Imidazenil: a potent and safe protective agent against diisopropyl fluorophosphate toxicity. Neuropharmacology. 2004;46:397–403. doi: 10.1016/j.neuropharm.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 36.Deshpande LS, Carter DS, Blair RE, DeLorenzo RJ. Development of a prolonged calcium plateau in hippocampal neurons in rats surviving status epilepticus induced by the organophosphate diisopropylfluorophosphate. Toxicol Sci. 2010;116:623–631. doi: 10.1093/toxsci/kfq157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim YB, Hur GH, Shin S. Organophosphate-induced brain injuries: delayed apoptosis mediated by nitric oxide. Environ Toxicol Pharmacol. 1999;7:147–152. doi: 10.1016/s1382-6689(99)00006-x. [DOI] [PubMed] [Google Scholar]
- 38.Wright LK, Liu J, Nallapaneni A, Pope CN. Behavioral sequelae following acute diisopropylfluorophosphate intoxication in rats: comparative effects of atropine and cannabinomimetics. Neurotoxicol Teratol. 2010;32:329–335. doi: 10.1016/j.ntt.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rojas A, Ganesh T, Lelutiu N, Gueorguieva P, Dingledine R. Inhibition of the prostaglandin EP2 receptor is neuroprotective and accelerates functional recovery in a rat model of organophosphorus induced status epilepticus. Neuropharmacology. 2015;93:15–27. doi: 10.1016/j.neuropharm.2015.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kadriu B, Guidotti A, Costa E, Auta J. Imidazenil, a non-sedating anticonvulsant benzodiazepine, is more potent than diazepam in protecting against DFP-induced seizures and neuronal damage. Toxicology. 2009;256:164–174. doi: 10.1016/j.tox.2008.11.021. [DOI] [PubMed] [Google Scholar]
- 41.Li Y, Lein PJ, Liu C, Bruun DA, Tewolde T, Ford G, Ford BD. Spatiotemporal pattern of neuronal injury induced by DFP in rats: a model for delayed neuronal cell death following acute OP intoxication. Toxicol Appl Pharmacol. 2011;253:261–269. doi: 10.1016/j.taap.2011.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reddy DS, Kuruba R. Experimental models of status epilepticus and neuronal injury for evaluation of therapeutic interventions. Int J Mol Sci. 2013;14:18284–18318. doi: 10.3390/ijms140918284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li Y, Lein PJ, Liu C, Bruun DA, Giulivi C, Ford GD, Tewolde T, Ross-Inta C, Ford BD. Neuregulin-1 is neuroprotective in a rat model of organophosphate-induced delayed neuronal injury. Toxicol Appl Pharmacol. 2012;262:194–204. doi: 10.1016/j.taap.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hobson B, Rowland D, Dhakal K, Bruun D, Tancredi D, Cherry S, Garbow J, Lein P. Quantitative magnetic resonance imaging (MRI) of brain lesions predicts cognitive impairment following acute organophosphate intoxication in rats. Abstract no. 2409. The Toxicologist: Supplement to Toxicological Sciences. 2016;150 [Google Scholar]
- 45.Li Y, Lein PJ, Ford GD, Liu C, Stovall KC, White TE, Bruun DA, Tewolde T, Gates AS, Distel TJ, Surles-Zeigler MC, Ford BD. Neuregulin-1 inhibits neuroinflammatory responses in a rat model of organophosphate-nerve agent-induced delayed neuronal injury. J Neuroinflammation. 2015;12:64. doi: 10.1186/s12974-015-0283-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu C, Li Y, Lein PJ, Ford BD. Spatiotemporal patterns of GFAP upregulation in rat brain following acute intoxication with diisopropylfluorophosphate (DFP) Curr Neurobiol. 2012;3:90–97. [PMC free article] [PubMed] [Google Scholar]
- 47.Boutin H, Chauveau F, Thominiaux C, Gregoire MC, James ML, Trebossen R, Hantraye P, Dolle F, Tavitian B, Kassiou M. 11C-DPA-713: a novel peripheral benzodiazepine receptor PET ligand for in vivo imaging of neuroinflammation. J Nucl Med. 2007;48:573–581. doi: 10.2967/jnumed.106.036764. [DOI] [PubMed] [Google Scholar]
- 48.Chen MK, Guilarte TR. Translocator protein 18 kDa (TSPO): molecular sensor of brain injury and repair. Pharmacol Ther. 2008;118:1–17. doi: 10.1016/j.pharmthera.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Grauer E, Chapman S, Rabinovitz I, Raveh L, Weissman BA, Kadar T, Allon N. Single whole-body exposure to sarin vapor in rats: long-term neuronal and behavioral deficits. Toxicol Appl Pharmacol. 2008;227:265–274. doi: 10.1016/j.taap.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 50.Cao Z, Cui Y, Nguyen HM, Jenkins DP, Wulff H, Pessah IN. Nanomolar bifenthrin alters synchronous Ca2+ oscillations and cortical neuron development independent of sodium channel activity. Mol Pharmacol. 2014;85:630–639. doi: 10.1124/mol.113.090076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cao Z, Hammock BD, McCoy M, Rogawski MA, Lein PJ, Pessah IN. Tetramethylenedisulfotetramine alters Ca(2)(+) dynamics in cultured hippocampal neurons: mitigation by NMDA receptor blockade and GABA(A) receptor-positive modulation. Toxicol Sci. 2012;130:362–372. doi: 10.1093/toxsci/kfs244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cao Z, Hulsizer S, Cui Y, Pretto DL, Kim KH, Hagerman PJ, Tassone F, Pessah IN. Enhanced asynchronous Ca(2+) oscillations associated with impaired glutamate transport in cortical astrocytes expressing Fmr1 gene premutation expansion. J Biol Chem. 2013;288:13831–13841. doi: 10.1074/jbc.M112.441055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cao Z, Zou X, Cui Y, Hulsizer S, Lein PJ, Wulff H, Pessah IN. Rapid throughput analysis demonstrates that chemicals with distinct seizurogenic mechanisms differentially alter Ca2+ dynamics in networks formed by hippocampal neurons in culture. Mol Pharmacol. 2015;87:595–605. doi: 10.1124/mol.114.096701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cao Z, Hulsizer S, Tassone F, Tang HT, Hagerman RJ, Rogawski MA, Hagerman PJ, Pessah IN. Clustered burst firing in FMR1 premutation hippocampal neurons: amelioration with allopregnanolone. Hum Mol Genet. 2012;21:2923–2935. doi: 10.1093/hmg/dds118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gao J, Naughton SX, Wulff H, Singh V, Beck WD, Magrane J, Thomas B, Kaidery NA, Hernandez CM, Terry AV., Jr Diisopropylfluorophosphate Impairs the Transport of Membrane-Bound Organelles in Rat Cortical Axons. J Pharmacol Exp Ther. 2016;356:645–655. doi: 10.1124/jpet.115.230839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Collins FS, Tabak LA. Policy: NIH plans to enhance reproducibility. Nature. 2014;505:612–613. doi: 10.1038/505612a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Landis SC, Amara SG, Asadullah K, Austin CP, Blumenstein R, Bradley EW, Crystal RG, Darnell RB, Ferrante RJ, Fillit H, Finkelstein R, Fisher M, Gendelman HE, Golub RM, Goudreau JL, Gross RA, Gubitz AK, Hesterlee SE, Howells DW, Huguenard J, Kelner K, Koroshetz W, Krainc D, Lazic SE, Levine MS, Macleod MR, McCall JM, Moxley RT, 3rd, Narasimhan K, Noble LJ, Perrin S, Porter JD, Steward O, Unger E, Utz U, Silberberg SD. A call for transparent reporting to optimize the predictive value of preclinical research. Nature. 2012;490:187–191. doi: 10.1038/nature11556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pusztai L, Hatzis C, Andre F. Reproducibility of research and preclinical validation: problems and solutions. Nat Rev Clin Oncol. 2013;10:720–724. doi: 10.1038/nrclinonc.2013.171. [DOI] [PubMed] [Google Scholar]
- 59.Gelman A, Hill J. Analytical Methods for Social Research. New York: Cambridge University Press; 2007. Data Analysis Using Regression and Multilevel/Hierarchical Models. [Google Scholar]
- 60.LaVange LM, Koch GG, Schwartz TA. Applying sample survey methods to clinical trials data. Statistics in Medicine. 2001;20:2609–2623. doi: 10.1002/sim.732. [DOI] [PubMed] [Google Scholar]
- 61.Diggle PJ, Liang K-Y, Zeger SL. Oxford Statistical Science Series. Oxford: Oxford University Press; 1994. Analysis of Longitudinal Data. [Google Scholar]
- 62.Lazic SE. The problem of pseudoreplication in neuroscientific studies: is it affecting your analysis? BMC Neurosci. 2010;11:5. doi: 10.1186/1471-2202-11-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McCulloch CE, Searle SR, Neuhaus JM. Generalized, Linear, and Mixed Models. Hoboken, NJ: Wiley; 2008. [Google Scholar]
- 64.Burnham KP, Anderson DR. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach. Second. New York: Springer-Verlag; 2002. [Google Scholar]
- 65.Boos DD, Stefanski LA. P-Value Precision and Reproducibility. American Statistician. 2011;65:213–221. doi: 10.1198/tas.2011.10129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.du Prel JB, Hommel G, Rohrig B, Blettner M. Confidence Interval or P-Value? Part 4 of a Series on Evaluation of Scientific Publications. Deutsches Arzteblatt International. 2009;106:335–339. doi: 10.3238/arztebl.2009.0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stata Corp LP. Stata Statistical Software: Release 14. College Station, TX: StataCorp LP; 2015. [Google Scholar]
- 68.SAS Institute Inc. Cary, NC: Copyright © 2002–2012, SAS Institute Inc.; Version 9.4 of the SAS System for Windows. SAS and all other SAS Institute Inc. product or service names are registered trademarks or trademarks of SAS Institute Inc. [Google Scholar]
- 69.Shao J, Chow SC. Reproducibility probability in clinical trials. Stat Med. 2002;21:1727–1742. doi: 10.1002/sim.1177. [DOI] [PubMed] [Google Scholar]
- 70.Wenk GL, McGann K, Hauss-Wegrzyniak B, Rosi S. The toxicity of tumor necrosis factor-alpha upon cholinergic neurons within the nucleus basalis and the role of norepinephrine in the regulation of inflammation: implications for Alzheimer's disease. Neuroscience. 2003;121:719–729. doi: 10.1016/s0306-4522(03)00545-1. [DOI] [PubMed] [Google Scholar]
- 71.Guan Z, Fang J. Peripheral immune activation by lipopolysaccharide decreases neurotrophins in the cortex and hippocampus in rats. Brain Behav Immun. 2006;20:64–71. doi: 10.1016/j.bbi.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 72.Vezzani A, Balosso S, Ravizza T. The role of cytokines in the pathophysiology of epilepsy. Brain Behav Immun. 2008;22:797–803. doi: 10.1016/j.bbi.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 73.Vezzani A, Ravizza T, Balosso S, Aronica E. Glia as a source of cytokines: implications for neuronal excitability and survival. Epilepsia. 2008;49(Suppl 2):24–32. doi: 10.1111/j.1528-1167.2008.01490.x. [DOI] [PubMed] [Google Scholar]
- 74.Prager EM, Aroniadou-Anderjaska V, Almeida-Suhett CP, Figueiredo TH, Apland JP, Braga MF. Acetylcholinesterase inhibition in the basolateral amygdala plays a key role in the induction of status epilepticus after soman exposure. Neurotoxicology. 2013;38:84–90. doi: 10.1016/j.neuro.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 75.Morisseau C, Merzlikin O, Lin A, He G, Feng W, Padilla I, Denison MS, Pessah IN, Hammock BD. Toxicology in the fast lane: application of high-throughput bioassays to detect modulation of key enzymes and receptors. Environ Health Perspect. 2009;117:1867–1872. doi: 10.1289/ehp.0900834. [DOI] [PMC free article] [PubMed] [Google Scholar]