Abstract
For half a century, the human brain was believed to contain about 100 billion neurons and one trillion glial cells, with a glia:neuron ratio of 10:1. A new counting method, the isotropic fractionator, has challenged the notion that glia outnumber neurons and revived a question that was widely thought to have been resolved. The recently validated isotropic fractionator demonstrates a glia:neuron ratio of less than 1:1 and a total number of less than 100 billion glial cells in the human brain. A survey of original evidence shows that histological data always supported a 1:1 ratio of glia to neurons in the entire human brain, and a range of 40–130 billion glial cells. We review how the claim of one trillion glial cells originated, was perpetuated, and eventually refuted. We compile how numbers of neurons and glial cells in the adult human brain were reported and we examine the reasons for an erroneous consensus about the relative abundance of glial cells in human brains that persisted for half a century. Our review includes a brief history of cell counting in human brains, types of counting methods that were and are employed, ranges of previous estimates, and the current status of knowledge about the number of cells. We also discuss implications and consequences of the new insights into true numbers of glial cells in the human brain, and the promise and potential impact of the newly validated isotropic fractionator for reliable quantification of glia and neurons in neurological and psychiatric diseases.
INDEXING TERMS: Glia number, Neuron number, Glia-neuron ratio, Cell counts, Human brain, Quantification, History
INTRODUCTION
“More attention must … be paid to quantitative studies of neuroglia and nerve cells, as opinions are often conflicting and frequently based on faulty technique.”
Paul Glees, mentor of celebrities Paul Wall and Oliver Sacks (Wall, 2001) in his foreword to “Neuroglia”, page ix (Glees, 1955)
Quantification of cells and their ratios in the nervous system is considered an important approach to understand the cellular composition, development, and evolution of the brain, neurological and psychiatric diseases, and aging (Coggeshall and Lekan, 1996; Morrison and Hof, 1997; Azevedo et al., 2009; Hilgetag and Barbas, 2009; Lent et al., 2012; Yuhas and Jabr, 2012; Herculano-Houzel, 2009, 2014; Geuna and Herrera-Rincon, 2015). Quantification adds an essential, new dimension to the topic of investigation, as famously expressed by Lord Kelvin (Thomson, 1889; von Bartheld and Wouters, 2015). Recent studies have shown that the cellular composition of the human brain is very different than was believed and taught for almost half a century (Azevedo et al., 2009; Hilgetag and Barbas, 2009; Lent et al., 2012; Yuhas and Jabr, 2012; Herculano-Houzel, 2009, 2014). A major motivation for our work is to provide a comprehensive analysis of the events and circumstances that delayed recognition of the true cellular composition of the human brain.
We envision that our review will be utilized in multiple ways. Foremost, our review examines from a historical perspective the efforts that have been made to estimate and report cell numbers and ratios in the human brain. As such, it reviews the origin, perpetuation, and recent refutation of the claim of one trillion glial cells, compares different counting methods, and emphasizes the importance of proper citation of relevant previous work. We attempt to provide a comprehensive account of previous studies that quantified cells in the human brain, to serve as a useful reference for current and future investigations.
Cell counting in the human brain has had a complex history. Cells in the brain can be quantified and reported in three different ways: Total neuron numbers; total glia numbers; and the ratio of glia to neurons (“GNR”), which refers not only to astrocytes but to all glial cells (astrocytes, oligodendrocytes and microglia) in the tissue. Historically, these three ways of numerical accounting have followed surprisingly distinct trajectories that seemed to co-exist, on superficial inspection, in agreement. Although they are linked in a simple mathematical formula (G/N = GNR, where G is the number of glia, N is the number of neurons, and GNR is the ratio of G/N for any given structure), this relationship was neglected on multiple occasions.
Brain cell counting can be roughly divided into three historical phases. In the first phase, data were collected only for parts of the human brain, in particular the cerebral cortex. Some investigators admitted uncertainty about absolute numbers for the whole brain, while others calculated or postulated GNRs for the whole brain (Hyden, 1960; Kuffler and Nicholls, 1976; Kandel and Schwartz, 1981). This phase lasted until about the 1970s. A second phase witnessed the first publications of serious estimates of total numbers, for both glial cells (40–130 billion: compiled by Blinkov and Glezer, 1968, and Haug, 1986) and neurons (70–85 billion: compiled by Haug, 1986, also reviewed in Williams and Herrup, 1988). Even though these cell density-based estimates supported a total GNR of about 1:1, this was either not recognized or not effectively communicated, thus allowing statements of a 10:1 or 50:1 GNR in major textbooks and reviews to remain essentially unchallenged from the 1960s until 2009 (Phase 2, Kandel et al., 1991, 2000; Nicholls et al., 1992; Bear et al., 2001, 2007). In this phase, most textbooks reiterated the view of a 10:1 abundance of glia, while neglecting the few, but existing published primary data that conflicted with this notion. The 10:1 GNR had – prematurely and mistakenly – attained the status of “common knowledge.” The third and most recent phase began with the study by Azevedo et al. (2009) that revealed the discrepancy with “textbook knowledge” and essentially confirmed the numbers published by Blinkov and Glezer (1968) and Haug (1986).
There was a disconnect between published reports on actual counts of cells in the human brain, and how such numbers were reported in review articles and text books. Inconsistencies in reports of neuron content in the human brain were first documented for psychology textbooks and reviews in the 1980s (Soper and Rosenthal, 1988). We here provide a similar analysis for neuroscience reviews and textbooks, but we compile, besides neuron counts, also reports about glia counts and the GNR, and add trends and insights from a more longitudinal, long-term perspective over several decades.
We also review the different types of counting methods that have been developed and have been employed for estimating cell numbers in human brains. Numerical ranges based on these different methods will be discussed, as well as the advantages and limitations of each of these methods.
With the benefit of hindsight, we examine the origin of the claim of a 10:1 or 50:1 glia-neuron ratio (GNR), with a corresponding total number of between 1 and 5 trillion glial cells in the human brain. We also examine reasons for the longevity (more than half a century) of the notion of one trillion or more glial cells in human brains. Surprisingly, the main reason for the origin and persistence of the notion of one trillion glial cells was not the technical disadvantage of the histological (and other) counting methods for global cell counts in heterogeneous tissue, but rather the failure to notice that published numbers for all three components: neuron counts, glia counts and the assumed GNR of 10:1 contradicted each other, and therefore one or more components had to be false. Major textbooks consistently presented the notion as a well-established fact, thereby allowing circumvention of the normal mechanism of peer validation of new claims. Additional sections give examples of the impact of cell counting and discuss the potential role of the new counting method, the IF, on obtaining and verifying glial and neuronal cell numbers and their ratios in human diseases. For reasons of space, we restrict our review primarily to the literature on cell counts in adult human brains.
OVERVIEW OF CELL COUNTING METHODS
It is useful to briefly review the three types of counting methods that have been employed to quantify cells in the human brain. The unit that is being counted is the cell body with its nucleus, the building blocks of the brain. For the purpose of this review, we do not take into account that neurons have different sizes, shapes, or their varying dendritic or axonal morphology, or that they belong to different cell types. To determine numbers of glial cells, most studies have similarly combined astrocytes, oligodendrocytes and microglia. Thus, the GNR reflects the ratio of numbers of all glial cells to all neurons in a structure, regardless of their sizes. The three different principal approaches to estimate the number of cells in the brain are: (1) Either model-based or design-based counting of stained cells, nuclei or nucleoli or their fragments in histological sections; (2) DNA extraction and measurement of total DNA content to calculate cell numbers; and (3) “direct enumeration” of cells in homogenized brain tissue by counting cell nuclei in suspension (a rudimentary precursor of the isotropic fractionator), and the isotropic fractionator itself.
Histology/stereology
This is the most often used approach, and it has been detailed in numerous reports (Abercrombie, 1946; Ebbesson and Tang, 1965; Cragg, 1967; Blinkov and Glezer, 1968; Konigsmark, 1970; Haug et al., 1984; Haug, 1987; Howard and Reed, 1998; Schmitz and Hof, 2005; Lyck et al., 2009). Tissues are fixed, usually in a formaldehyde-based fixative, embedded in a suitable medium, sectioned into thin slices, stained with a dye, and cells or subcellular particles are counted under the microscope (Fig. 1). There are two major types of the histology approach: model-based and design based. The traditional model-based approach (profile counting) relies on analysis of thin sections (of 5–15 microns thickness), spaced 10 or 20 sections apart. Subcellular particles (usually nuclei or nucleoli) are counted in those thin sections, then one extrapolates for the sections in between the ones used for counting, and applies correction factors to account for the fact that larger particles appear in multiple sections (Abercrombie, 1946; Ebbesson and Tang, 1965; Blinkov and Glezer, 1968; Konigsmark, 1970; Clarke and Oppenheim, 1995). This requires knowledge or assumptions about the size and shape of particles. The design-based approach (stereology) uses thicker sections of 20–100 microns, takes random samples within these sections so that the samples are representative of the particle density, and applies the random sampling scheme to the entire reference space (Haug et al., 1984; Haug, 1987; Gundersen et al., 1988; Williams and Rakic, 1988; Howard and Reed, 1998; Schmitz and Hof, 2005). Such a method is unbiased in theory, although bias can arise due to tissue deformation and loss of particles during tissue processing and other errors (von Bartheld, 1999, 2002; Guillery, 2002). For this reason, investigators have recommended calibration of both methods against the ultimate standard, i.e. 3-dimensional serial section reconstructions of an entire region or a sample thereof (Coggeshall et al., 1990; Hatton and von Bartheld, 1999; von Bartheld, 2001; von Bartheld, 2002; Williams et al., 2003; Kaplan et al., 2010).
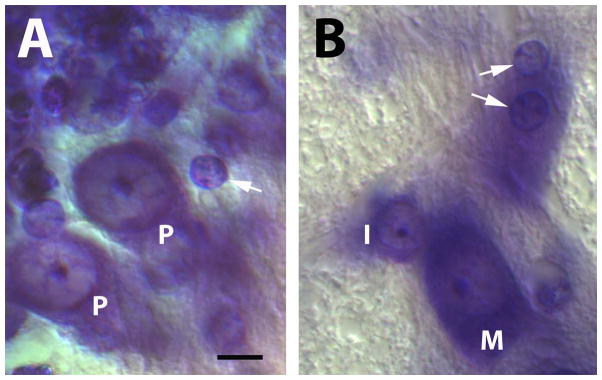
Fig. 1.
A–B Photomicrographs of Nissl-stained neurons and glial cells. A. Purkinje cells (P) and granule cells (arrow) in the cerebellum of an adult mouse brain. B. Motoneuron (M), interneuron (I) and glial cells (arrows) in the trochlear nucleus of an adult mouse brain. Note that the neuronal granule cell (arrow) in panel A is virtually indistinguishable in size and shape from glial cells (arrows) in panel B. Thionin stains of 40 μm paraffin sections. Digital images obtained on a Nikon Eclipse E600 microscope, with no digital adjustments or manipulations of the images. Scale bar = 10 μm. Histological sections kindly provided by Dr. Larisa M. Wiggins.
Major challenges of the histological approach are to make sure that samples are truly representative of the reference volume, to prevent double counting of particles that appear in multiple sections, to account for differential shrinkage that changes with age and tissue composition (white matter vs. grey matter), to distinguish correctly between neurons and glia (Fig. 1) (discussed in more detail below), to identify the true borders and dimensions of the reference volume, and to measure the true height of tissue sections (von Bartheld, 2001, 2002; Guillery, 2002; Schmitz and Hof, 2005). The importance of counting absolute numbers of cells rather than cell densities was underscored by the finding that tissues shrink differentially with age (Haug et al., 1984). Neglect of the fact of differential shrinkage of brain tissue with age led to the false belief that neuron number declines steadily and significantly in human brains during normal aging (Brody, 1955; Haug et al., 1984; Morrison and Hof, 1997; Peters et al., 1998; Mouton, 2002; Peters, 2002). It is important to assess absolute numbers of cells as opposed to cell densities within unclear reference volumes – densities can be misleading when such volumes change due to confounding variables – and can give rise to the so-called “reference trap” (Haug et al., 1984; West, 1993a; Mayhew and Gundersen, 1996; Mouton, 2002). The histology/stereology approach is considered a valuable method for analysis of well-defined regions with precise borders, but has limitations when large tissues with heterogeneous composition or components and/or fuzzy borders need quantification (Peters et al., 1998; Benes and Lange, 2001; Lent et al., 2012; Herculano-Houzel et al., 2015).
DNA extraction
An alternative approach to histology is to extract and measure DNA content and to calculate cell numbers based on knowledge of DNA content per cell nucleus (Heller and Elliott, 1954; Hess, 1961; Zamenhof et al., 1964; Margolis, 1969; Bass et al., 1971; Hess and Thalheimer, 1971; Dobbing and Sands, 1973; Zamenhof, 1976; Mares et al., 1985; Jacobson, 1991; Yuhas and Jabr, 2012). However, this technique also has its drawbacks: complete recovery of DNA is required; there can be contamination with other nucleic acids; not all cells are euploid, and only total cell number, but not cell type is revealed.
DNA extraction has been used mostly in the 1950s through the 1970s, primarily to determine changes or trends, by applying the known amount of DNA per cell nucleus in a given species and to make relative comparisons rather than to obtain absolute numbers (Robins et al., 1956; Hess, 1961; Zamenhof et al., 1964; Margolis, 1969; Hess and Thalheimer, 1971; Dobbing and Sands, 1973; Zamenhof, 1976; Jacobson, 1991). Some of these studies compared DNA content in primate cortex with glial and neuronal densities as obtained by histological techniques (Brizzee et al., 1964; Cragg, 1967; Bass et al., 1971; Ling and Leblond, 1973; Leuba and Garey, 1989), but these comparisons were done in animal models, and not in the human brain. While theoretically an elegant solution (Jacobson, 1991), the DNA approach has been criticized for a number of reasons, as recently compiled (Bahney and von Bartheld, 2014): (1) many initial reports relied on DNA-P measurement, but P may not necessarily be derived exclusively from DNA (Drasher, 1953); (2) it requires complete DNA extraction and recovery; (3) there are concerns that the large and more fragile neuronal nuclei may be preferentially destroyed during the isolation procedures (Nurnberger and Gordon, 1957); (4) mitochondria also contain a small amount of DNA (Nass and Nass, 1963); (5) DNA extraction is problematic when lipids and lipoproteins are abundant in the tissue of interest, as is the case in white matter (Zamenhof et al., 1964; Penn and Suwalski, 1969; Saldanha et al., 1984); (6) aldehyde fixation causes DNA denaturation (Srinivasan et al., 2002) and possibly irreversible crosslinking of peptides to DNA, thereby decreasing the yield of DNA that can be measured by spectrophotometry (Savioz et al., 1997); (7) euploidy in brain cells is assumed, yet as many as 20% of adult human neurons are hyperploid (Mosch et al., 2007).
Despite these caveats, some of the reports based on the DNA method were used to support notions about human cell numbers or GNRs (Nurnberger and Gordon, 1957, Discussion pages 129–138; Hess, 1961; Hess and Thalheimer, 1971; Yuhas and Jabr, 2012), and therefore contributed to the development of an apparent consensus about the GNR.
Homogenization and counting cells in suspension (“brain soup”) – also called “direct enumeration” and more recently “isotropic fractionator”
This approach was originally designed in the 1950s (Nurnberger and Gordon, 1957; Nurnberger, 1958). Dissected chilled tissue was weighed, homogenized, diluted in a known volume of medium, stained with methylene blue, mixed, and aliquots of the diluted medium were counted on a hemocytometer. The original paper suggested that neuronal nuclei could be distinguished from vascular and glial cell nuclei on the basis of centrally located single nucleoli as opposed to multiple eccentric nucleoli, and differences in intensity of staining (Nurnberger and Gordon, 1957). However, these and subsequent investigators (Brizzee et al., 1964) also stated that nuclei of small neurons (such as cerebellar granule cells) were misidentified as glial cells (page 112), so that the neuron counts may be too low, in particular for the cerebellum. The original version of the “direct enumeration” method suffered from several shortcomings, primarily rapid degradation of unfixed cells and lack of distinction between cell types, and therefore it was rarely applied. Comparisons with histological cell counts on rat, monkey and human brains revealed discrepancies, and it remained unclear how to resolve them (Nurnberger and Gordon, 1957; Brizzee et al., 1964).
Subsequent modifications introduced a formalin fixation step for the dissected tissue, used disintegration in water, ultrasonication, followed by dilution, resuspension and staining with thionine (Zamenhof, 1976; Zamenhof and Klimuszko, 1977). These modifications allowed to easily recognize larger cerebellar neurons, but the distinction between granule cells and glial cells remained problematic. Comparison with histological counts suggested that numbers obtained with the “direct enumeration” method were too low, by at least one third (Clarke and Oppenheim, 1995), possibly due to rupturing of cells during the mechanical disintegration and sonication steps.
Without knowledge of Zamenhof’s attempts to improve Nurnberger’s method, significant further modifications of this method were introduced in 2005, and the greatly improved method was called the “isotropic fractionator” (Herculano-Houzel and Lent, 2005; Zorzetto, 2012) (Fig. 2). The new modifications included fixation of animal brains by perfusion with buffered 4% paraformaldehyde of tissues or immersion fixation of human brains, followed by perfusion through the carotid arteries within 24 hours post mortem (Azevedo et al., 2009; Andrade-Moraes et al., 2013), detergent-assisted mechanical dissociation, centrifugation to collect nuclei in the pellet, visualization of nuclei with a fluorescent nuclear stain (4′,6-Diamidino-2-Phenylindole, Dihydrochloride, DAPI), and distinction between neuronal and non-neuronal cell nuclei by use of a neuron-specific antibody, anti-NeuN (Herculano-Houzel and Lent, 2005). This solved some of the major limitations of previous versions of this approach. Furthermore, the method has the advantages of being easy, fast, and accurate, generating estimates of numbers of cells that are independent of tissue volume or cell density, and overcoming problems of heterogeneity of tissues. However, there are also limitations of the IF: the use of antibodies against nuclear proteins (to distinguish neurons from non-neuronal cells) does not identify cell types among the non-neuronal cells, NeuN antigens are not expressed by a small number of neuronal populations (Mullen et al., 1992), and only regions and volumes of tissues that can be dissected macroscopically can be analyzed (Lent et al., 2012). Automated versions of the IF have been reported, both for the homogenization procedure (Azevedo et al., 2013) and for the counting procedure, using flow cytometry (Collins et al., 2010; Young et al., 2012; Herculano-Houzel et al., 2015). Long-standing concerns about loss of nuclei when using a biochemical homogenization approach (Brizzee et al., 1964; Hadjiolov et al., 1965; Lovtrup-Rein and McEwen, 1966; Cragg, 1967; Kato and Kurokawa, 1967; Clarke and Oppenheim, 1995; Yuhas and Jabr, 2012; Carlo and Stevens, 2013; Verkhratsky and Butt, 2013; Charvet et al., 2015) have recently been addressed and dispelled in two studies that directly compared the IF, in side-by-side experiments, with results obtained by stereology (Bahney and von Bartheld, 2014; Miller et al., 2014). These studies, as well as others (Brautigam et al., 2012; Andrade-Moraes et al., 2013; Walloe et al., 2014) indicate equivalency between the IF and stereology (Herculano-Houzel et al., 2015).
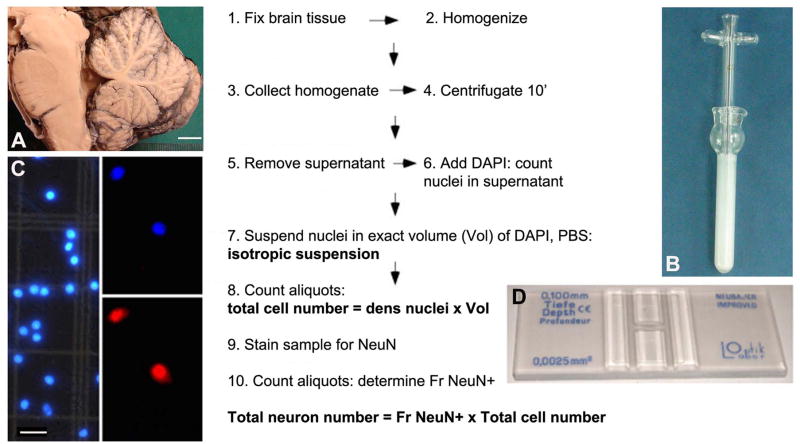
Fig. 2.
A–D Flow chart of the isotropic fractionator (IF) cell counting method. The major steps of the procedure are illustrated. A. Example of fixed brain tissue. Scale bar = 1 cm. B. Tenbroek glass homogenizer. C. Appearance of DAPI-stained nuclei (left) and two nuclei double-labeled with DAPI (upper panel) and NeuN (lower panel). Scale bar = 20 μm. D. Neubauer counting chamber. DAPI, 4,6-diamidino-2-phenylindole; Fr, fraction; NeuN+, neuronal nuclear antigen positive; PBS, phosphate-buffered saline; Vol, volume; Modified from Herculano-Houzel and Lent (2005), Bahney and von Bartheld (2014), and Herculano-Houzel et al. (2015).
HISTORY OF CELL COUNTS AND ESTIMATES OF CELL NUMBERS
There has been considerable interest in quantitative aspects of the human brain for nearly 150 years. Despite the technical limitations of early microscopes’ optical resolution and the need to develop, refine and optimize fixation and staining methods (Mühlmann, 1936; Glees, 1955; Blinkov and Glezer, 1968; Brodal, 1969; Iniguez et al., 1985; Glees, 1988; Gittins and Harrison, 2004a), plausible numbers of cells were estimated in the 1900s for animal brains and for major parts of the human brain, in particular the cerebral cortex. Overall, and considering that results were obtained by different investigators using different methods, most of the data are relatively consistent. For example, the majority of studies estimated total neuron numbers for the entire human cerebral cortex at 10–20 billion (Table 1).
TABLE 1.
Estimates of numbers of neurons (N), non-neurons (nN) and glia (G) in human cerebral cortex (in billion) – Cortex comprises only grey matter, but does not include white matter (WM), unless specifically indicated [ ].
| Author | Year | N One side | N Total | nN Total | G Total |
|---|---|---|---|---|---|
| Meynert | 1868/1872 | 0.612 | 1.224 | ||
| Donaldson | 1895 | 1.200 | |||
| Thompson | 1899 | 9.282 | |||
| Berger | 1921 | 5.512 | |||
| von Economo & Koskinas | 1925 | 14 | |||
| von Economo | 1926 | 13.653 | |||
| Agduhr | 1941 | 5.0 | |||
| Shariff | 1953 | 6.9 | |||
| Sholl | 1956 | 5.000 | 10.000 | ||
| Haug & Rebhan | 1956 | 16.5 | |||
| Haug | 1959 | 8.200 | 16.400 | ||
| Pakkenberg | 1966 | 2.6 | |||
| Gallatz et al. | 1982 | 10.030 | |||
| Haug | 1985 | 13.8 ± 2.4 | |||
| Haug | 1987 | 10–19 | |||
| Pakkenberg et al. | 1989 | ~20 | |||
| Braendgaard et al. | 1990 | 13.7 | [27.4] | ||
| Pakkenberg | 1992 | 25.1 | |||
| Jensen & Pakkenberg | 1993 | 23.2 | |||
| Pakkenberg | 1993 | 22.1 | |||
| Regeur et al. | 1994 | 18.1 | |||
| Pakkenberg & Gundersen | 1997 | 19.3–22.8 [range: 14.7 – 32.0] | |||
| Gredal et al. | 2000 | 22.3 | |||
| Pakkenberg et al. | 2003 | 19.3–22.8 | 39 | ||
| Pelvig et al. | 2003 | 21.2 | 29.1 | ||
| Koch | 2004 | 20 | |||
| Pedersen et al. | 2005 | 18.8 | |||
| Pelvig et al. | 2008 | 21.4 – 26.3 | 27.9 – 38.9 | ||
| Azevedo et al. | 2009 | 6.18 | 12.36 | ||
| Azevedo et al. | 2009 | [16.34] | [60.84] | ||
| Lyck et al. | 2009 | [15–19.7] | [35.4–40.6] | [18.5–20.3] | |
| Karlsen & Pakkenberg | 2011 | 17.9 | 18.2 | ||
| Andrade-Moraes et al. | 2013 | [12.7] | [54.9] | ||
[ ] includes white matter (WM)
Since the cerebral cortex comprises by volume about 80–85% of the adult human brain (Stephan et al., 1981; Rilling and Insel, 1999), quantitative data for the cortex was often equated with or taken to be equivalent to the whole brain. This turned out to be a consequential over-simplification, because the contribution of the cerebellum (which contains about 80% of all neurons in the human brain; Azevedo et al. (2009)) was neglected, and this helped to support the myth of one trillion glial cells in human brains, as discussed in more detail later in this review. The following sections examine the history of numerical reports for the three major components of the human brain – cerebral cortex (80–85% of total brain volume or 1,200 g), the cerebellum (10% of volume or 150 g), the remaining components, the brainstem, diencephalon and striatum, sometimes called “the rest of brain” or “remaining regions” (2–8% of volume or 75–110 g; Blinkov and Glezer, 1968; Azevedo et al. (2009); Andrade-Moraes et al., 2013), and the entire human brain.
Cerebral cortex
We first review the published estimates for neuronal numbers, then the GNR, and finally glial numbers. Unless indicated otherwise, “cortex” refers to the grey matter only, and excludes underlying white matter tracts.
Numbers of neurons
Several investigators have estimated numbers of neurons in the human cerebral cortex, mostly based on histological methods, as compiled in Table 1. The prevailing approach was to measure cell densities in histological sections, and to cope with the challenge of differential tissue shrinkage (Nurnberger and Gordon, 1957; Crabb, 1967; Blinkov and Glezer, 1968). There has been some confusion whether “cerebral cortex” means only the grey matter, or also includes the underlying white matter. Indeed, the large majority of studies excluded white matter. The number of neurons in white matter is relatively small – estimated to be 250–1,000 per mm3 (Garcia-Marin et al., 2010) which is less than 1% of the number of glial cells, with 20,000–200,000 glial cells per mm3 white matter, see below: “The number of glial cells.” Therefore, inclusion of white matter does not make a significant difference for neuron numbers, although it does make a difference for total cell numbers discussed later. Blinkov and Glezer (1968) and Haug (1986) reviewed the early history of counting neurons and reporting of numerical estimates in human cerebral cortex, but to our knowledge there have been no comprehensive reviews of this topic published since that time.
As can be seen in Table 1, the estimates ranged from 1.2–32 billion neurons for the entire cortex (right and left hemispheres combined), with a majority of studies reporting between 10 and 20 billion neurons. It should be noted that some investigators (e.g., Meynert, 1868/1872; Shariff, 1953) were ambiguous in whether their estimates were applicable to one or both hemispheres, as mentioned for the Meynert study by von Economo (1926). This type of confusion explains why Blinkov and Glezer (1968) listed Shariff’s numbers incorrectly for only one hemisphere, while Haug (1986) correctly listed those numbers for total cortex. There has been similar confusion whether numerical reports apply to one or both sides in the 1990s (e.g., Mufson and Benzing, 1994; Regeur et al., 1994b; Peters et al., 1998).
Table 1 shows that von Economo’s studies (von Economo and Koskinas, 1925; von Economo, 1926) were the first to correctly estimate the total number of neurons at about 14 billion. Ironically, their numbers became highly controversial and prompted a harsh rebuttal by Agduhr (1941). Ultimately, this was one of several controversies where von Economo and Koskinas were vindicated in history (Triarhou, 2005, 2006).
Table 1 also shows three apparent outliers on the low end by Meynert (the very first report in 1868/1872), Donaldson (1895), and H. Pakkenberg (1966), with estimates between 1.2 and 2.6 billion neurons. On the high end, the group of B. Pakkenberg reported 20–32 billion neurons (Pakkenberg et al., 1989; Braendgaard et al., 1990; Pakkenberg, 1992, 1993; Pakkenberg and Gundersen, 1997; Pelvig et al., 2003, 2008). This range appears too high, based on the previous histological studies and also the results from the isotropic fractionator (IF) (Azevedo et al. (2009); Andrade-Moraes et al., 2013). There are additional examples where numbers reported by the group of B. Pakkenberg, one of the pioneers of stereological counting methods, had to be revised; this is not surprising, given the large biological variability among human brains and the difficulties of working with human tissues. Another potential source of error pertains to sampling issues such as the controversial notion that counting only 100–200 neurons is sufficient (Gundersen, 1986; Andersen et al., 1992; Coggeshall and Lekan, 1996), while more recent work employing computer simulations indicates that considerably more neurons should be counted (Schmitz and Hof, 2000; Geuna and Herrera-Rincon, 2015). Examples of discrepancies of results include lack of cortical neuron loss in Alzheimer’s disease (Regeur et al., 1994a; Mufson and Benzing, 1994; Peters et al., 1998; Andrade-Moraes et al., 2013), numbers of neurons in the cerebellum – apparently over-estimated by about 50% (Andersen et al., 1992; see below), and the initial underestimation of the number of neurons in the dorsomedial thalamic nucleus (1.8–7.29 x106 neurons, see “Brainstem, Diencephalon and Striatum,” below).
Nevertheless, it is remarkable that the large majority of the histology-derived estimates converge at 10–20 billion neurons, which is furthermore supported by estimates obtained by the IF (Azevedo et al. (2009); Andrade-Moraes et al., 2013). Several studies have documented the surprisingly wide range of neurons in human cerebral cortex between individuals (biological variance, Haug, 1986, 1987; Terry et al., 1987; West, 1993a). There appears to be a normal biological variation in the number of neocortical neurons by a factor of more than 2; this represents a variance of more than eight times the variance of human body height (Haug, 1987; Pakkenberg and Gundersen, 1997). The notion that large numbers of neurons (30–50%) are lost during decades of normal human aging (“Neuronal Fall-Out”, Brody, 1955; Hanley, 1974; Devaney and Johnson, 1980; Curcio et al., 1982) has been refuted, primarily through Haug’s pioneering work and others’ (Haug et al., 1984; Haug, 1987; Terry et al., 1987; West, 1993b; Anderton, 1997, see also EXAMPLES SHOWING IMPACT OF CELL COUNTING). Actual losses appear to be of a much lesser scale and region-specific (Curcio et al., 1982; West, 1993b; Peters et al., 1998). It still is controversial whether women have a smaller number of neurons than men and whether neocortex loses a small amount of neurons (less than 10% over 80 years, Haug, 1987; Pakkenberg and Gundersen, 1997). Given the large biological variation (over 100%) vs. the small effect size (West, 1993a), an apparent decrease of less than 10% may be due, at least in part, to secular (generational) changes in body height, brain size and neuron number (Haug, 1984; Haug, 1987; Pakkenberg, 1989), and furthermore may be functionally insignificant (Peters et al., 1998). Indeed, recent work indicates that very old women have no reduction in cortical neuron numbers (Fabricius et al., 2013; Walloe et al., 2014). Overall, excluding the extreme outliers, the numbers compiled in Table 1 provide a plausible range of estimates for neuronal numbers in cerebral cortex.
The GNR
The GNR in the human cerebral cortex (grey matter, unless indicated otherwise) was first established in the 1930s (Mühlmann, 1936; Arutyunova, 1938). Mühlmann measured densities of glia and neurons in Giemsa-stained samples from the frontal lobe, and he estimated the GNR to be ~ 1.5 in the adult human cortex (Mühlmann, 1936; Arutyunova, 1938). The GNR of 1.5 in the grey matter of adult human cortex was confirmed by numerous subsequent investigations as listed in Table 2. Considering well-established neuronal numbers of 10–20 billion in the human cerebral cortex, this would place the number of glial cells in the human cortex at about 15–30 billion. The median of this range is close to the average of 17.4–19.4 billion non-neuronal cells in the human cortical grey matter estimated with the isotropic fractionator (IF, Azevedo et al. (2009); Andrade-Moraes et al., 2013). The number of non-neuronal cells provides a maximum estimate for the number of glial cells, since non-neuronal cells comprise both glial cells and endothelial cells. Endothelial cells in the human forebrain and other CNS parts are estimated to make up about 30% of the non-neuronal cells (equivalent to a ratio of ~2:1 glia:endothelial cells, Nurnberger, 1958; Blinkov and Glezer, 1968; Brasileiro-Filho et al., 1989; Bjugn and Gundersen, 1993; García-Amado and Prensa, 2012), leaving 70% glial cells, and reducing the non-neuronal to neuron ratio (nNNR) from 1.48 to a true GNR of 1.04 in Azevedo et al. (2009) and from 1.64 to 1.15 in Andrade-Moraes et al. (2013) (Table 2). It should be noted that endothelial cells in white matter appear to comprise a somewhat lower percentage (10–20% of non-neuronal cells, Bahney and von Bartheld, 2014) than they do in cerebral cortex grey matter and other parts of the CNS (about 30%, see below). The only two discrepancies to the findings of a ~1.5 GNR in human cerebral cortex (Table 2, with none of these specifying the extent of white matter inclusion) seem to be a 10:1 statement by Hyden and Pigon (1960) and an anecdotal suggestion of a 5:1 ratio made by J. Olszewski as cited in Heller and Elliott (1954), yet Olszewski published just three years later a 1.78:1 GNR for human cerebral cortex grey matter (Hawkins and Olszewski, 1957 – see Table 2). Hyden and Pigon’s claim of a 10:1 ratio in human cortex (unclear whether this referred to grey matter only) was not backed by any data of their own or other’s original data. In fact, the discrepancy between Hyden’s 10:1 ratio and those of other investigators was already noted by Glees (1988).
TABLE 2.
Reports of glia-neuron ratios (GNRs) and non-neuron-neuron ratios (nNNRs) in human cerebral cortex, grey matter (GM) only, unless indicated.
| GNR | nNNR | Comments | Author | Year |
|---|---|---|---|---|
| _ | ||||
| ~2 | adult: 1.04 – 2.3, newborn: 0.14 – 0.2 | Mühlmann | 1936 | |
| 1.2 – 2.1 | Adult Superior Frontal Gyrus, all layers | Arutyunova | 1938 | |
| 1.24 – 1.98 | Human Cortex | Friede | 1953 | |
| 1.24 – 1.98 | Human Cortex, layers II – VI | Friede | 1954 | |
| 1.78 | Human cortex, layers II – VI | Hawkins & Olszewski | 1957 | |
| 2.9 – 3.5 | 4.4–5.2 | Striate cortex, GM+WM | Nurnberger & Gordon | 1957 |
| 0.74 – 6.6 | tabulated by Blinkov and Glezer, 1968 (p. 416) | Schlote | 1959 | |
| 10 ** | Hyden & Pigon | 1960 | ||
| 2 | “Human Cortex” | Cragg | 1968 | |
| 2.3 | Frontal cortex | Hess & Thalheimer | 1971 | |
| 0.49 – 0.57 | Frontal/parietal cortex (control) | Diamond et al. | 1985 | |
| 0.86 – 1.09 | Frontal/parietal cortex (Albert Einstein) | Diamond et al. | 1985 | |
| 1 – 1.5 | Visual cortex | Leuba & Garey | 1989 | |
| 1.56 – 2.02 | Males and Females, 18–98 years old | Pakkenberg et al. | 2003 | |
| 1.37 | Neocortex without archicortex, 60–98 years old | Pelvig et al. | 2003 | |
| 1.65 | Frontal cortex, layers II/III | Sherwood et al. | 2006 | |
| 1.32 – 1.49 | Females – Males, 18–93 years old | Pelvig et al. | 2008 | |
| 1.48 – 1.05* | 3.72 | in GM only | Azevedo et al. | 2009 |
| 2.48 | in GM+WM | Azevedo et al. | 2009 | |
| 1.64–1.15* | 4.31 | in GM only | Andrade-Moraes et al. | 2013 |
| 3.01 | in GM+WM | Andrade-Moraes et al. | 2013 | |
| 1.2–3.6 | for GM, not including WM | Ribeiro et al. | 2013 |
GM, grey matter; GNR, glia-neuron ratio; nN, non-neuronal cells; N, neurons; nNNr, non-neuronal-neuron ratio; WM, white matter.
Based on a 2:1 ratio of glia to endothelial cells (References: 27–30%: Nurnberger, 1958; Blinkov and Glezer, 1968; Brasileiro-Filho et al., 1989; Lyck et al., 2009; García-Amado and Prensa, 2012).
No primary data or reference provided
Taken together, we conclude that based on all available primary data, the GNR of human (and other primate’s) grey matter of prefrontal cerebral cortex is about 1.5 (Sherwood et al., 2006; Hilgetag and Barbas, 2009; Ribeiro et al., 2013), and varies locally in the grey matter between 1.2 in occipital and 3.6 in frontal areas of the human cortical grey matter (Ribeiro et al., 2013). When white matter is included along with grey matter, then the GNR in cerebral cortex increases from 1–2 to about 3–4 (Table 2). The average GNR of 1–2 for grey matter cerebral cortex has been known since 1936 and has to our knowledge never been seriously disputed (Table 2).
The number of glial cells
Glial cell densities of 200,000 per 1 mm3 in white matter and about 100,000 per 1 mm3 in grey matter were reported for adult human cortex (Blinkov and Ivanitskii, 1965), while Schlote (1959) counted 40,000–90,000 per 1 mm3, Hess (1961) counted 108,000 in white matter, and Blinkov and Glezer (1968) list 48,000 cells (glia and neurons) per 1 mm3, which is close to Haug’s (1987) report of about 20,000–25,000 glial cells per 1 mm3, assuming a GNR of between 1 and 2. Applying a total volume of about 250 cm3 per cortical hemisphere grey matter (Blinkov and Glezer, 1968), the number of glial cells in human cerebral cortex (500 cm3) amounts to 10 billion (Haug, 1987), 20–45 billion (Schlote, 1959) or 50–100 billion (Blinkov and Ivanitskii, 1965). The number of glial cells in the grey matter of the human cerebral cortex was more recently reported by using stereological methods (Pakkenberg et al., 2003; Pelvig et al., 2003, 2008; Karlsen and Pakkenberg, 2011); these studies estimated between 18.2 and 38.9 billion glial cells (Table 2), while studies using the IF determined the number of non-neuronal (NN) cells at an average 17.4–19.1 billion in the grey matter of the cerebral cortex (Azevedo et al. (2009); Andrade-Moraes et al., 2013) (Table 2).
One of the major – if not most serious – problems in the histology-based counting methods is the technical difficulty of recognizing glia and distinguishing them from small neurons (Fig. 1). This problem has a long history (Mühlmann, 1936; Kryspin-Exner, 1943; Glees, 1955; Nurnberger and Gordon, 1957; Braitenberg and Atwood, 1958; Palay, 1958; Schlote, 1959; Iniguez et al., 1985; Andersen et al., 1992; Gittins and Harrison, 2004a), and still awaits resolution, since immunostaining with the NeuN antibody in tissue sections appears to be incomplete and variable (Lyck et al., 2009). The difficulty of distinguishing small neurons from glia may explain some of the conflicting results that have been obtained in human neuropathology (see: ROLE OF THE ISOTROPIC FRACTIONATOR IN FUTURE RESEARCH). Therefore, the design of methods that can accurately determine neuronal and glial cell numbers is important.
Among the glia, numerous investigators have determined the relative contributions of astrocytes, oligodendrocytes and microglia, mostly in cerebral cortex, as compiled in Table 3. Not surprisingly, oligodendrocytes are more frequent than other glial cell types in white matter (Table 3). There is also considerable, but not unanimous agreement across primary sources that in different brain regions, including neocortical grey matter, oligodendrocytes are the most frequent at 45–75% of glial cells, followed by astrocytes (19–40%), while microglia contribute 10% or less, although some textbooks and reviews have reported differently, unfortunately without references (Verkhratsky and Butt, 2007; Pastor and Sola, 2008; Bayraktar et al., 2015). Statements that microglia alone are about as numerous as neurons (Streit, 1999; Fields, 2009) are incorrect, because they were based on the mistaken belief of a 10:1 GNR. In conclusion, all three methods: histology, DNA extraction, and the IF method support numbers of about 10–20 billion neurons and at most a 2-fold larger number of glial cells (20–40 billion) in the human cerebral cortical grey matter, thus supporting an average GNR of approximately 1.5. Inclusion of the white matter (that underlies the grey matter of cerebral cortex) increases the GNR to about 3.0.
TABLE 3.
Types of Glial Cells contributing to the Total Number of Glia in the Human Brain.
| Oligodendrocytes | Astrocytes | Microglia | Comments | Authors | Year |
|---|---|---|---|---|---|
| 29% | 61.5% | 9.5% | Visual Cortex | Kryspin-Exner | 1952 |
| 40% | 54% | Caudatum | Kryspin-Exner | 1952 | |
| 57% | Pallidum | Kryspin-Exner | 1952 | ||
| 52–74% | 30–40% | 6–8% | Thalamus | Kryspin-Exner | 1952 |
| 77.5% | Nucleus ruber | Kryspin-Exner | 1952 | ||
| 62% | Substantia nigra, pc | Kryspin-Exner | 1952 | ||
| 29–77.5% | 30–61.5% | 6–9.5% | various regions | Glees | 1955 |
| Review of Kryspin-Exner’s work | |||||
| 51% | 40% | 9% | Motor Cortex, layer V | Brownson | 1956 |
| 45% | 45% | 10% | GM | Pope | 1958 |
| <67% | >23% | 10% | WM | Pope | 1958 |
| 52% | 39% | 9% | Motor Cortex | Windle (Brownson) | 1958 |
| 45% | 45% | 10% | GM | Windle (Pope) | 1958 |
| 67% | WM | Windle (Pope) | 1958 | ||
| 36.6% | 46.5% | 16.8% | Frontal Cortex GM | Pope | 1959 |
| 69% | 24% | 6.9% | Frontal Cortex WM | Pope | 1959 |
| 50.9% * | 40.8% * | 16.7% ** | Frontal Cortex | Schlote | 1959 |
| 45% | 45% | 10% | Cortex | Blinkov & Glezer | 1968 |
| 24.5–69.2% * | 25.6–63.2% * | 9–28.1% ** | Data: Schlote, 1959 | Hess & Thalheimer | 1971 |
| 75% | 19% | 6% | Neocortex GM | Pelvig et al. | 2003 |
| 5% | 80% | 10–15% | CNS | Verkhratsky & Butt | 2007 |
| 74.6–75.6% | 17.3–20.2% | 5.2–6.5% | Males, females Neocortex (GM) Pelvig et al. | 2008 | |
| 15–18% | Males, Neocortex | Lyck et al. | 2009 | ||
| 75% | 20% | 5% | Neocortex (GM) | Verkhratsky & Butt | 2013 |
GM, grey matter; WM, white matter; studies reporting primary data are shaded in
 .
.
Note: These numbers from Schlote’s 1959 data are compiled according to Hess and Thalheimer (1971), and adjusted for the percentages among glial cells (microglia and endothelial cells are assumed at a 1:1 ratio). As pointed out by Hess and Thalheimer (1971), the figure legends in Schlote (1959) erroneously switched the symbols for astroglia and oligodendroglia. This may explain some text books reporting of an abundance of astroglia vs. oligodendrocytes (e.g., Verkhratsky and Butt, 2007).
Note: This percentage includes microglia plus endothelial cells.
Cerebellum
The cerebellum is another part of the human brain in which cell numbers were estimated throughout the last century. Initially, only numbers for the large cerebellar neuronal types were reported – in particular the easily recognized Purkinje cells with most estimates (8/13) between 14–26 x 106 (Fig. 1; Table 4). Braitenberg and Atwood (1958) were the first to also report the number of granule cells (small cerebellar neurons) which alone were estimated to be “of the order of 10–100 billion.” In 1975, Lange reported the density of neurons in the human cerebellum as 1,610 cells/0.001 mm3 in the granular layer, with an average 720.8 neurons/0.001 mm3 in cerebellar cortex (Lange, 1975). Applying the reference volume from other studies, Lange’s neuronal densities in the cerebellum translate to a total of 65–70 billion neurons in the human cerebellar cortex (Williams and Herrup, 1988). In contrast, Haug estimated about 50 billion neurons in cerebellar cortex (Haug, 1986), based on Lange’s work and his own counts. A very large number of neurons in the cerebellum had been suspected by earlier investigators (Elliott in: Nurnberger and Gordon, 1957; Kuffler and Nicholls, 1976), as well as a very low number of non-neuronal (glial) cells (Elliott in: Nurnberger and Gordon, 1957), but the study of Andersen et al. (1992) provided for the first time direct evidence for a very low number of glial cells in the human cerebellum. Andersen et al. estimated that among a total of 105 billion cells in the human cerebellum, there were 101 billion granule cells, with most of the remainder, about 3 billion, being glial cells (see their Figure 8, Andersen et al., 1992). This implied that the GNR of the human cerebellum had to be extremely low, about 0.03. However, Andersen et al. (1992) did not comment on how the cellular composition of the cerebellum (GNR of less than 0.1) compared with other brain structures such as the cerebral cortex (GNR of ~ 2–3, when white and grey matter are combined). Accordingly, the implications for total neuron and glia numbers in human brains and the differences between GNRs in the cerebellum and the cerebral cortex remained hidden. In the meantime, the group of B. Pakkenberg revised their stereological estimates of the human cerebellum from 101 billion granule cells (Andersen et al., 1992) to about 70 billion granule cells (Andersen et al., 2012), a number that is much closer to the numbers obtained by using the IF methodology as well as Lange’s and Haug’s estimates, implying that 50–70 billion is a most plausible range (Table 4).
TABLE 4.
Estimates of cell numbers in the human cerebellum (both sides together).
| Number | Method | Author and Year |
|---|---|---|
| Purkinje cells | ||
| 14 × 106 | Histology | Kreuzfuchs, 1902 |
| 25–26 × 106 | Histology | Lojda, 1955 |
| 15 × 106 | Histology | Braitenberg & Atwood, 1958 |
| 15.4 × 106 | Stereology | Nairn et al., 1989 |
| 0.88 × 106 | Histology | Riedel et al., 1989 |
| 15.6 × 106 | Stereology | Mayhew et al., 1990 |
| 30.5 × 106 | Stereology | Andersen et al., 1992 |
| 30.5 × 106 | Stereology | Korbo and Andersen, 1995 |
| 28.5 × 106 | Stereology | Andersen & Pakkenberg, 2003 |
| 28 × 106 | Stereology | Andersen et al., 2003 |
| 22.3 × 106 | Stereology | Agashiwala et al., 2008 |
| 26 × 106 | Stereology | Andersen et al., 2012 |
| 26 × 106 * | Stereology | Kiessling et al., 2014 |
| Granule cells (granule neurons) | ||
| 10–100 × 109 | Histology | Braitenberg & Atwood, 1958 |
| 19.8 ×109 | Histology | Riedel et al., 1989 |
| 101 × 109 | Stereology | Andersen et al., 1992 |
| 112.3 × 109 | Stereology | Andersen & Pakkenberg, 2003 |
| 109 × 109 | Stereology | Andersen et al., 2003 |
| 70 × 109 | Stereology | Andersen et al., 2012 |
| 75.2 × 109 | Stereology | Kiessling et al., 2014 |
| Total neurons | ||
| 65–70 × 109 | Histology | Lange, 1975; Williams & Herrup, 1988 |
| 50 × 109 | Stereology | Haug, 1986 |
| 105 × 109 | Stereology | Andersen et al., 1992 |
| 69 × 109 | IF | Azevedo et al., 2009 |
| 54 × 109 | IF | Andrade-Moraes et al., 2013 |
| Glial cells | ||
| 3 × 109 | Stereology | Andersen et al., 1992 |
| Non-neuronal cells | ||
| 16 × 109 | IF | Azevedo et al., 2009 |
| 15.4 × 109 | IF | Andrade-Moraes et al., 2013 |
Abbreviations: IF, isotropic fractionator
Data from 10–11 month old infants
Based on the study of Andersen et al. (1992), and also taking into account the numbers of glial cells in the white matter of the cerebellum (Bahney and von Bartheld, 2014), Andersen and colleagues’ counts of 30,000–40,000 glial cells per mm3 appear plausible, resulting in a total of about 3 billion glial cells in the cerebellum. Compared with the number of neurons (about 65 billion), the GNR for the entire human cerebellum appears to be about 0.05. Studies using the IF have estimated the average number of cells in the cerebellum to be between 55 and 70 billion (Azevedo et al. (2009); Andrade-Moraes et al., 2013), with granule cells (granule neurons) constituting the overwhelming majority (Azevedo et al. (2009)). The same method yields an upper estimate of around 16 billion glial cells; this counts all non-neuronal cells which comprise the combined total of glial and endothelial cells in the cerebellum, and therefore amounts to a maximal GNR of 0.23 (Azevedo et al. (2009); Andrade-Moraes et al., 2013).
When the cerebellum and the cerebral cortex are considered together, the GNR for these two major parts of the brain amounts to a value of 0.8–0.9, much less than the GNR of the cerebral cortex alone, without the cerebellum. This difference is so substantial, because the cerebellum has not only a very large number of neurons, but also a number of glial cells that is extremely low in comparison. However, the human cerebellum is not an outlier in its GNR or glial cell composition; if the GNR appears abnormally low, it is because of the very large density of neurons in this structure (Herculano-Houzel, 2014). The large number of cerebellar neurons was recognized by early investigators (e.g., Elliott in: Nurnberger and Gordon, 1957; Kuffler and Nicholls, 1976); Kuffler and Nicholls remarked on the “staggering numbers of neurons” in the human cerebellum, but the relatively low number of cerebellar glial cells remained obscure and largely unrecognized even after the report by Andersen et al. (1992). Without the numbers in the cerebellum, the human brain would have a GNR of at most 4 (using values from Azevedo et al. (2009)). The unusual cellular composition of the cerebellum was a key factor in failed attempts to calculate the true GNR for the total human brain, and a major reason for the persistence of the notion of one trillion glial cells.
Brainstem, Diencephalon and Striatum
These parts of the brain, primarily the brainstem, have been measured to comprise between 2 and 8% of the volume of the entire brain, but accommodate less than 1% of its neurons (Azevedo et al. (2009)). The brainstem contains a variety of neuronal nuclei and fiber tracts. Until 2009 (Azevedo et al. (2009)), there had been no attempts made to estimate the total number of neurons or glial cells in this part of the brain, although Blinkov (1963) reported on the glia index for several structures in the human brainstem. A select number of nuclei or regions was investigated with histological techniques for neuron numbers, including the reticular formation (5.2 x 106 neurons, Blinkov and Glezer, 1968), corpora quadrigemina (inferior colliculi: 1.2 x 106 neurons Blinkov and Glezer, 1968), and lateral geniculate nucleus (on one side: 570,000 neurons, Balado and Franke, 1934; 1.2 x106 neurons, Chacko, 1948; 3.5 x 106, Selemon and Begovic, 2007; 2.0 x 106 neurons, Dorph-Petersen et al., 2009). The reason for the discrepancies for the lateral geniculate nucleus is unknown, but both the 2007 and the 2009 studies employed the same stereological method. The supraoptic nucleus contains about 75,000 neurons and the paraventricular nuclei 85,000 neurons (various sources, reviewed in Blinkov and Glezer, 1968). The mammillary bodies (medial nuclei) contain about 800,000 neurons, and there are about 1.3 x106 neurons in the anteroventral and medial nuclei of the thalamus (Powell et al., 1957). The basal ganglia have been reported to contain 816 x 106 neurons (Karlsen and Pakkenberg, 2011), with about 100 x 106 small neurons and 570,000–670,000 large neurons in the striatum (Schröder et al., 1975), 7.8 x 106 neurons in the anterior striatum (Weise et al., 2015), about 700,000 neurons in the globus pallidus (Thörner et al., 1975), and 300,000 in the subthalamic nucleus (Lange et al., 1975). The number of glial cells was estimated at 400 x 106 in the striatum (Schröder et al., 1975) and at 63–82 x 106 in the globus pallidus (Thörner et al., 1975). The number of neurons in the substantia nigra was reported to be about 450,000 pigmented neurons (McGeer et al., 1977), 500,000–600,000 neurons (Mann, 1986) and 550,000 pigmented and 260,000 non-pigmented neurons (Pakkenberg et al., 1991; Stark and Pakkenberg, 2004), while the subthalamic nucleus has 286,000–306,000 neurons (Lange et al., 1975), and the locus coeruleus contains 32,000–38,000 pigmented neurons (Mouton et al., 1994; Ohm et al., 1997).
It is in the brainstem and diencephalon where some large GNR values are indeed found. The superior colliculus has a GNR of about 10 (Blinkov and Glezer, 1968), and the lateral vestibular nucleus a GNR of about 30–50 (Blinkov, 1963; Ponomarev, 1966; Blinkov and Glezer, 1968). The GNR was reported near 160 for the globus pallidus (89–114 x106 glial cells; 688,000–711,000 neurons, Thörner et al., 1975), but is only 3.7 in the striatum (380–408 x 106 glial cells; 100.7–105.6 x 106 neurons, Schröder et al., 1975). Pakkenberg and Gundersen (1988) reported neuron and glia numbers for the ventral pallidum (3.97 x 106 neurons; GNR = 12.2) and the dorsomedial thalamic nucleus (1.8 x 106 neurons; GNR = 17). However, the initially reported number of neurons in the dorsomedial thalamic nucleus turned out to be an underestimate: subsequent studies, also using stereology, reported ~ 3.5 x 106 (Popken et al., 2000), 7.29 x 106 (Dorph-Petersen, 2004), and more recently ~ 6.43 x 106 (Abitz et al., 2007) and ~ 6.4 x 106 (Nielsen et al., 2008). The discrepancies between studies – even when using the same stereological counting method – illustrate the difficulty encountered by efforts to determine the true number of neurons in just one small nucleus in the brainstem.
Regardless of the precise numbers, it is obvious that the total number of neuronal and glial cells in the brainstem, diencephalon and striatum does not add up to numbers that are even close to those in cerebral cortex or cerebellum. Since the volume is small and the total number of cells is relatively low, this part of the brain contains only about 700 million neurons and about 6.6–7.7 billion non-neuronal cells, with a GNR of maximal 10:1, as determined by the isotropic fractionator (Azevedo et al. (2009); Andrade-Moraes et al., 2013). Therefore, the fluctuations in GNR between specific nuclei or tracts in the brainstem and diencephalon add little to the overall GNR when compared with the numbers provided by the cerebral cortex and cerebellum. When the number of neurons in these two structures together was determined to be between about 80–100 billion, it should have become apparent that a 10:1 GNR, with the implied 1 trillion or more glial cells, was impossible. There are not nearly enough glial cells in either the cerebral cortex or in the cerebellum to arrive at such a number (Azevedo et al. (2009)).
Discrepancies of estimates
Attempts to pinpoint the cause(s) of discrepancies between studies have proven difficult, not only because most investigators do not provide sufficiently detailed information (Schmitz and Hof, 2005), but also because a multitude of potential factors can generate biases. This was shown by studies designed to quantify biases, by comparison with the gold standard, serial section reconstruction, by changing distinct variables, and by ultrastructural verification of particle identity (Coggeshall et al., 1990; Hatton and von Bartheld, 1999; Baryshnikova et al., 2006; Ward et al., 2008; Lyck et al., 2009; Kaplan et al., 2010). Sources of bias may be in opposite directions, may even cancel each other, or may skew estimates in the same direction, and then be additive. Without full access to primary data, to all aspects of tissue processing, and an independent re-examination of counting, it is impossible to identify sources of bias with any certainty. For these reasons, it has been recommended, as a practical approach, to calibrate counting methods against a small sample of serial section reconstructions, still considered the ultimate standard (Coggeshall et al., 1990; von Bartheld, 2002; Kaplan et al., 2010).
Entire human brain
Based on actual counts of neuronal densities using histological methods, the number of neurons in the entire human brain was estimated by experts in quantitative neuroscience at 30 billion (Szentagothai, 1983), 70–80 billion (Haug, 1986), and 85 billion (Williams and Herrup, 1988). Investigators using the isotropic fractionator confirmed these latter neuron numbers at 67–86 billion neurons (Azevedo et al. (2009); Andrade-Moraes et al., 2013). Based on glial cell densities, Blinkov and Glezer (1968) estimated the number of glial cells in the entire human brain to be 100–130 billion, while Haug, using his own densities and volume measurements, estimated 40–50 billion glial cells for the entire human brain (Haug, 1986). The current estimates of numbers of non-neuronal cells in the entire human brain, as revealed by the IF, place the total glial numbers well below 85 billion (since these 85 billion include approximately 20–25 billion endothelial cells), and therefore are closer to the estimates of Haug (40–50 billion glial cells) rather than those of Blinkov and Glezer (100–130 billion glial cells) (Fig. 3).
Fig. 3.
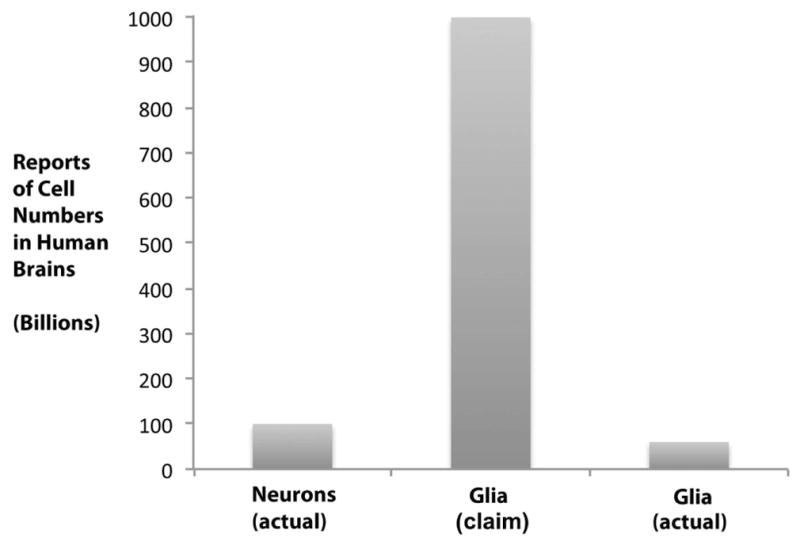
This graph summarizes the essence of Table 6. From the 1960s until 2009, the number of glial cells in human brains was reported to be about one trillion, 10 times more than neurons (100 billion), as detailed in Table 6. The number of glia, based on published data, is in fact lower than the number of neurons, resulting in a glia-neuron ratio of less than 1 rather than 10:1.
HISTORY OF THE GLIA-NEURON RATIO (OR “GLIA INDEX”)
The GNR or “glia index” is defined as the ratio between the number of glial cells and the number of neurons in the same volume of brain substance. The GNR and its implications have attracted interest among investigators for more than a century. The notion of the GNR was conceived by Nissl (Nissl, 1898), but first applied and studied in a systematic way in the 1930s. While some scientists question the utility of the GNR – or of any cell quantification (Yuhas and Jabr, 2012), many current investigators conclude that the GNR informs about brain development, physiology, diseases, aging, and brain evolution (Sherwood et al., 2006; Hilgetag and Barbas, 2009; Herculano-Houzel, 2014), as detailed below in EXAMPLES SHOWING THE IMPACT OF CELL COUNTING. The GNR is most useful in a comparative context and when applied to comparable brain regions. Technically, the GNR is easier to establish than total absolute numbers, especially for distinct brain parts, because no absolute values are required. Rather, for any given volume, the number of glia and neurons can be estimated and compared with some certainty in relationship to each other. Thus, the GNR can be calculated as the ratio between the density of glia and the density of neurons in any structure or volume, without ever estimating absolute numbers of cells (e.g., Friede, 1954; Hawkins and Olszewski, 1957; Haug 1987; Stolzenburg et al. 1989). Persistent problems were how to define precise borders between grey and white matter, to clearly distinguish small neurons from glial cells, and to extrapolate to the whole brain from the data obtained in spatially restricted samples. Since the GNR was recently discussed in the context of glial cells and phylogeny (showing a remarkable and evolutionarily conserved scaling of GNRs with neuronal density between structures and species, Herculano-Houzel, 2014), we focus here on a brief history of the GNR as it relates to human brains and the claims of glial cell numbers.
Recent work (Fields, 2009; Verkhratsky and Butt, 2013) stated that Fridtjof Nansen was the first to associate an increasing GNR with increasing intelligence. Unfortunately, this statement is based on a mis-quotation. Nansen (1886) attributed such increasing mental abilities to increasing amounts of what he called “dotted substance” which is essentially neuropil made up of neuronal and glial processes (Table 5). Fields (2009) and Verkhratsky and Butt (2013) recently adopted Galambos’ (1961) misquote, implying that Nansen was not referring to the “dotted substance,” but rather to glia exclusively (Table 5; Nansen, 1886, page 171). The dotted substance was later termed “neuropil” by von Apathy (1897), as reviewed in detail by Florey (1985).
TABLE 5.
Mis-quotations of Nansen’s original opinion about neuropil [“Leydig’s dotted substance]” being the seat of intelligence to claims of glia or neuroglia being the seat of intelligence and increasing during evolution in size or number.
| Nansen, 1886: “… the more complicated the structure of dotted substance [neuropil consisting of neuronal and glial processes*] is – the more highly is the animal mentally developed; in other words, we may conclude that the more the inteligence of an animal is developed – the more intricate becomes the web of plaiting of nerve-tubes and fibrillae in its dotted substance … and this web is probably the principal seat of inteligence.” (page 171, Nansen, 1886, his italics). |
| Glees, 1955: “It is worth mentioning Nansen’s opinion … that this substance [Leydig’s dotted substance = ‘plaiting of nerve-tubes and fibrillae’] was the seat of intelligence as it increases in size from the lower to the higher forms of animal.” (cites Nansen, 1886) |
| Galambos, 1961: “Nansen … said neuroglia was ‘the seat of intelligence, as it increases in size from the lower to the higher forms of animal.’ “ (cites Glees’ 1955 footnote) |
| Fields, 2009: “Nansen … observed in 1886 that glia might be ‘the seat of intelligence, as [their number] increase in size from the lower to the higher forms of animal.’ ” (cites Galambos, 1961) |
| Verkhratsky and Butt, 2013: “Nansen … postulated that neuroglia was ‘the seat of intelligence, as it increases in size from the lower to the higher forms of animal’ “ (cites Galambos, 1961). |
“Nerve-tubes are …present in great plenty in the dotted substance” (Nansen, 1886, page 124)
Accordingly, Franz Nissl was the first to note the prevalence of glial cells in mammalian cortices (Nissl, 1898; also reviewed in Herculano-Houzel, 2014), while the GNR was first calculated and reported for a major part of the human brain by Mühlmann (1936). Mühlmann established that the approximate GNR (“Prozentgehalt der Nerven und der Gliazellen”) of the grey matter of the human cerebral cortex is about 1.5, a value that since has been widely confirmed (Table 2). He also conducted a detailed developmental study that revealed how the GNR in cortex changes from the newborn (GNR = 0.3:1) to the aged adult (GNR = 2:1). This showed that the GNR is age-specific and that glia-neuron relations change as the brain matures. From the 1950s until the 1980s, the GNR was called “glia index” (Friede, 1953, 1954), glia/neuron index (Brizzee and Jacobs, 1959), or glia/nerve cell index (Hawkins and Olszewski, 1957). Altman (1967) was the first to use interchangeably the terms glia index and glia-neuron ratio (GNR), while Bass et al. (1971) and some subsequent investigators advocated the use of the reciprocal of the GNR: the “neuron/glia ratio” (Thörner et al., 1975; Diamond et al., 1985; Terry et al., 1987; Leuba and Garey, 1989), the rationale being that the neuronal density varies much more than the glial cell density (Bass et al., 1971; Reichenbach, 1989). Bass et al. (1971) – incorrectly as it turned out – assumed that the number of endothelial cells in brains was negligible: “since the vascular cell fraction is relatively small, the neuron/non-neuron ratio(n) essentially equals the neuron/glia ratio.” Other’s work showed that as much as one third of non-neuronal cells were endothelial cells in mammalian, including human, CNS (Blinkov and Glezer, 1968; Brasileiro-Filho et al., 1989; Bjugn and Gundersen, 1993; Davanlou and Smith, 2004; Lyck et al., 2009; García-Amado and Prensa, 2012).
Work by Friede and others in the 1950s rapidly confirmed Nissl’s suspicion and revealed that the GNR differs between species in what appeared to be a “phylogenetic” trend. This prompted Friede to propose that the GNR serves as an indicator of the “developmental advancement” of a species – culminating in humans (Friede, 1954; Pfrieger and Barres, 1995; Araque et al., 2001). Brizzee and Jacobs (1959) concluded that brain weight as well as brain complexity contributed to the GNR. When investigators examined brains larger than those of humans, they found even larger GNRs (Hawkins and Olszewski, 1957; Tower and Young, 1973; Haug, 1987; Eriksen and Pakkenberg, 2007). They concluded that the GNR was associated with brain size rather than with “developmental advancement” or cognitive abilities. However, the hypothesis originally formulated by Nissl and Friede of glia as being correlated with increasing intelligence persisted in the literature due to the intuitively appealing idea that a relatively large GNR in human cerebral cortex compared with other animals might be related to this species’ cognitive abilities (Jerison, 1973; Diamond et al., 1985; Witelson et al., 1995; Araque et al., 2001; Fields, 2009; Koob, 2009; Verkhratsky and Butt, 2013).
It was recognized in the 1960s that differences in GNRs are largely determined by changes in neuronal densities rather than changes in glial cell densities – glial cell densities remain remarkably constant between species and even brain structures, at 50,000–130,000 cells per mm3, while neuronal densities in different parts of the human brain vary between 0 and over 400,000 per mm3 (Blinkov and Glezer, 1968; Bass et al., 1971; Tower and Young, 1973; Haug, 1987; Herculano-Houzel, 2014). Accordingly, the GNR largely reflects differences in neuronal density, but not, or only to a very minor extent, differences in glial density (Blinkov and Glezer, 1968). The GNR was shown not to increase universally with brain mass or cortical mass, but rather with decreasing neuronal density, which may or may not coincide with increasing brain mass (Herculano-Houzel, 2014). However, it is still not resolved how much increasing axon length, dendritic arbor size, and somatic size contribute to increasing neuronal cell size and thus decreasing neuron density (Friede and van Houten, 1962; Jehee and Murre, 2008; Herculano-Houzel, 2014). These are crucial questions from an engineering perspective: how to optimize information processing within finite spaces. While the GNR is easier to determine from a sampling standpoint than absolute numbers of glia or neurons, investigators have to deal with one major technical issue: how to distinguish glia from small neurons.
How to best distinguish glia from small neurons
Small neurons are difficult to distinguish from glial cells (Fig. 1). Virtually all investigators using histology encountered and recognized this as a major problem, especially in the granular layer of the cerebellum (Kryspin-Exner, 1943; Glees, 1955; Nurnberger and Gordon, 1957; Braitenberg and Atwood, 1958; Andersen et al., 1992; Lyck et al., 2009). Mühlmann tested several different stains and recommended the Giemsa stain as the best way to distinguish glia and neurons (in paraffin sections, Mühlmann, 1936). Kryspin-Exner (1943) and Schlote (1959) preferred to study glia in Nissl-stained material. Glees (1955) routinely used silver impregnation and Nissl stain in adjacent sections to confirm cell types. Braitenberg and Atwood (1958) were “not fully satisfied with any of the methods available” and acknowledge the “serious difficulty presented by the small size of the granular cells.” Even at the ultrastructural level, glial cells can be difficult to identify and classify (Palay, 1958).
The Giemsa stain is a mixture of dyes (methylene blue and eosin yellow) with the capacity to stain not only ribonucleic acid in the cytoplasm (neurons), but also nuclear chromatin (glia), in a temperature- and pH-dependent manner (Iniguez et al., 1985). The utility of the Giemsa stain and long tradition in distinguishing neurons and glia is often overlooked (Mufson and Benzing, 1994), and it has been stated that the Giemsa stain was introduced in neurohistology only in the 1970s (e.g., Scheff and Baldwin, 1996), even though Mühlmann described in the 1930s in considerable detail the use of the Giemsa stain to distinguish glia and neurons (Mühlmann, 1936). A method paper devoted to the Giemsa stain in brain sections further confirmed that this stain is well suited to visualize both neurons and glia (Iniguez et al., 1985). Thus, utilization of the Giemsa stain predates the adoption of this stain by Gundersen, West and Pakkenberg for their resin sections in the 1980s and 1990s (e.g., Gundersen et al., 1988; West and Gundersen, 1990; Regeur et al., 1994a). Mufson and Benzig (1994) discuss in their commentary the importance of distinguishing neurons and glia, and types of stains that have been used to reach this goal.
A breakthrough seemed to have been achieved by utilizing an antibody against a neuron-specific nuclear antigen (NeuN; Mullen et al., 1992). This was first applied in histology to distinguish small neurons from non-neuronal cells in tissue sections (Gittins and Harrison, 2004a). A side-by-side analysis of NeuN and Nissl stains in the cerebral cortex showed that cell counts using Nissl stain underestimated numbers of neurons, apparently because small interneurons can be confused with glial cells (Gittins and Harrison, 2004a), while another study found that only a fraction (18–57%) of neurons were identified as NeuN-positive in histological sections from human cortex, and a panel of neuron-specific antibodies was recommended for future work (Lyck et al., 2009). On the other hand, the NeuN antibody was proven a highly efficient tool to separate neuronal from non-neuronal cell nuclei in the isotropic fractionator method (Herculano-Houzel and Lent, 2005). Additional suitable antibodies are now becoming available that can be used to further classify neurons into subtypes, and to separate the non-neuronal cells unambiguously into glial cell types and endothelium. Currently, however, the IF does not distinguish glia from endothelial cells, but rather pools both types together as non-neuronal cells. The ratio obtained with the IF is therefore not a GNR, but a “nN-NR” ratio (non-neuronal cells/neurons ratio) – which, however, serves as a useful upper limit to the GNR. Given that the vasculature represents a constant (and small, 1–5%) fraction of brain tissue (and cerebral cortex in particular; Buchweitz and Weiss, 1986; Lawers et al., 2008; Tsai et al., 2009; Karbowski, 2011), values of nN-NR likely translate into GNR by the same proportion across species.
Reports of the GNR and estimates of absolute numbers
In the context of the notion of one trillion glial cells, Table 6 compiles reports of the GNR as well as estimates of absolute cell numbers in the entire human brain from 1895 until 2015 (see also Fig. 3). We attempted to include all major reviews and textbooks. It is interesting that in the late 1950s through the 1970s, qualifiers such as “perhaps” and “about” were often associated with the numbers given, but in the 1980s and beyond, such caution was largely replaced by an assertiveness that seemed to convey knowledge and evidence rather than a “best guess” or possible range. Several scientists reported wide ranges in the 1970s, e.g., Hubel (1979) and Nauta and Feirtag (1979). “The number of nerve cells, or neurons, that make up man’s three pounds or so of brain is on the order of 1011 (a hundred billion) give or take a factor of 10” (Hubel, 1979); and Nauta and Feirtag (1979) wrote: “… there are classes of neurons so small and densely crowded that it is difficult to judge their number … There are so many granule cells … that the estimate of 1010 neurons in the entire central nervous system becomes suspect. The total could easily be an order of magnitude, perhaps two orders of magnitude, higher.”
TABLE 6.
Reports of glia-neuron ratios (GNRs), numbers of total cells, glia, and neurons in human brain
| GNR | Total cell number | Glia # | Neuron # | Method | Reference |
|---|---|---|---|---|---|
| 3 bn | Donaldson, 1895 | ||||
| 10:1 (“perhaps”) | Glees, 1958 | ||||
| 10:1 (“perhaps”) | Pope, 1958 | ||||
| 10:1 (“around”) | Hyden, 1960 | ||||
| 10:1 (“perhaps”) | Galambos, 1961 | ||||
| 10:1 (“about”) | Hyden, 1961 | ||||
| 10:1 | 110 bn | 100 bn | 10 bn | Asimov, 1963 | |
| 10:1 | Maron, 1963 | ||||
| (glia “more abundant” than neurons) | Kuffler & Nicholls, 1966 | ||||
| 100–130 bn | Histology | Blinkov & Glezer, 1968 | |||
| “glia …outnumber neurones by several fold” | Dobbing & Sands, 1970 | ||||
| 5:1 – 10:1 | Noback & Demarest, 1975 | ||||
| 10:1 (“at least”) >10 bn | Kuffler & Nicholls, 1976 | ||||
| ~10:1 | Ganong, 1977 | ||||
| “Glia … far outnumber(s) neurons” | 20–200 bn | Wittrock, 1977 | |||
| 50 bn | Edelman & Mountcastle 1978 | ||||
| ~10:1 | Ganong, 1979 | ||||
| 10bn – 1 trn | Hubel, 1979 | ||||
| 10 bn – 100 bn or 1 trn | Nauta & Feirtag, 1979 | ||||
| 100 bn | Stevens, 1979 | ||||
| 5:1 | Jensen, 1980 | ||||
| 5:1 – 10:1 | Snell, 1980 | ||||
| 9:1 | [10 trn] | [~9 trn] | ~1 trn | Kandel & Schwartz, 1981 | |
| 10:1 (“perhaps”) | [~1 trn] | 100 bn | Nolte, 1981 | ||
| 30 bn (“roughly”) | Szentagothai, 1983 | ||||
| 10:1 | Damask & Swenberg, 1984 | ||||
| 10:1 – 50:1 | [11–51 trn] | [10–50 trn] | 1 trn (“best estimate”) | Kandel & Schwartz, 1985 | |
| 10:1 | Nicholls et al., 1985 2nd ed | ||||
| 0.7:1 | 40–50 bn | 70–80 bn | Histology | Haug, 1986 | |
| 85 bn | Williams & Herrup, 1988 | ||||
| 10:1 | Steward, 1989 | ||||
| 10:1 | Bignami, 1991 | ||||
| 1:1 or 10:1 | Jacobson, 1991 | ||||
| 10:1 – 50:1 | 1.1–5.1 trn | [1–5 trn] | 100 bn (“best estimate”) | Kandel et al., | |
| 1991 | |||||
| 5:1 – 10:1 (“depending on region”) | Noback et al., 1991 | ||||
| G≫N (glia # “much higher” than neuron #) | Brodal, 1992 | ||||
| 10:1 (“at least”) 10 bn – 1 trn | Nicholls et al., 1992 | ||||
| (“several times that many glial cells”) | 100 bn | Nolte, 1993 | |||
| 10:1 (glial cells … outnumber neurons 10 to 1”) | Black and Ransom, 1999 | ||||
| 10:1 – 50:1 | [1.1–5.1 trn] | [1–5 trn] | 100 bn (“on the order of …”) | Kandel et al., 2000 | |
| 10:1 | Steward, 2000 | ||||
| 10:1 | [~1 trn] | ~100 bn | Bear et al., 2001, 2nd ed | ||
| (“glial cells … vastly outnumber neurons”) | Lemke, 2001 | ||||
| 10:1 (“thought to be at least ten glia per neuron”) | Haydon, 2001 | ||||
| 10:1 (“or more”) | Levitan & Kaczmarek, 2002 | ||||
| 100 bn | Haines, 2002 | ||||
| (“glia are the most numerous cells in the brain”) | Doetsch, 2003 | ||||
| 10:1 – 50:1 | [1.1 – 5.1 trn] | [1–5 trn] | 100 bn | Hatton & Parpura, 2004 | |
| 10:1 | Bear et al., 2007, 3rd ed | ||||
| 10:1 | > “several trillions” (probably) | Verkhratsky & Butt, 2007 | |||
| 1:1 | 170 bn | <85 bn | 86 bn | IF | Azevedo et al., 2009 |
| 6:1 | [767 bn] | [667 bn] (85%) | 100 bn (15%) | Fields, 2009 | |
| G≫N (glia # “much higher” than neuron #) | Brodal, 2010 | ||||
| 3:1 or 4:1 | 100 bn | Purves, 2010 | |||
| ~1:1 “human brain contains roughly equal numbers of glia and neurons” | Smith, 2010 | ||||
| 10:1 (“at least”) | Nicholls et al., 2012, 5th ed | ||||
| 2:1 – 10:1 | [200 bn −1 trn] | 100 bn | Kandel et al., 2013, 5th ed | ||
| 1:1 | Verkhratsky & Butt, 2013 | ||||
| ~1:1 | <78.6 bn | 67.3 bn | IF | Andrade-Moraes et al., 2013 | |
| ~1:1 “glial cells are as abundant as neurons” | Kettenmann and Verkhratsky, 2013 | ||||
| ~1:1 “roughly … equal numbers of neurons and glia” | Streit, 2013, p. 86 | ||||
| 4:1 “glia … constitute … the majority of cells …, 80% in the human brain” | Barres et al., 2015 | ||||
| ~1:1 “neuroglial cells … are about as numerous as neurons in the brain as a whole” | Gundersen et al., 2015 | ||||
[ ], implied numbers - not stated explicitly; bn, billion; ed, edition; GNR, glia-neuron ratio; IF, isotropic fractionator; trn, trillion;

As can be seen in Table 6, nearly all authors surveyed endorse a 5:1 – 50:1 abundance of glia over neurons, with very few exceptions. The exceptions are authors who actually did the counting (shaded in grey in Table 6: Blinkov and Glezer, 1968; Szentagothai, 1983; Haug, 1986; Azevedo et al. (2009); Andrade-Moraes et al., 2013) or authors who were intimately familiar with the relevant primary literature (e.g., Jacobson, 1991). Only five publications report a much lower GNR of 0.7:1 – 1:1 for the whole brain (Haug, 1986; Azevedo et al. (2009); Andrade-Moraes et al., 2013; Streit, 2013; Verkhratsky and Butt, 2013). Table 6 shows that the range of neuronal numbers in the human brain is by and large within one order of magnitude, with 20/23 authors giving numbers or a median between 10 and 100 billion. Two texts say one trillion (Kandel and Schwartz, 1981, 1985), and the authors did not correct this mistake for neuron numbers until subsequent editions of their textbook (Kandel et al., 1991, 2000, 2013). Remarkably, such errors, in neuron number, glia number and GNR, were contained in the most prestigious textbook of its generation (Darlington, 2009). For example, the 2000 edition of Kandel et al was praised: “The bible of neuroscience and the singular source for all things brain. It is 1500 pages of facts, information, data, theory, and on a level of scholarship unparalleled. Ever since its first edition came out in the early 1980s, this book has set the standard for erudition in the sciences and is probably on the bookshelf of almost every neuroscientist in the world …” (Lambert and Kinsley, 2004).
Several authors implicitly postulate a number of 1–50 trillion glial cells in human brains, because they provide the GNR as well as estimates of the total number of neurons (Kandel and Schwarz, 1981, 1985; Kandel et al., 1991, 2000; Bear et al., 2001; Hatton and Parpura, 2004). We calculated those implicit numbers and indicated them in brackets in Table 6 (“[…]”). Accordingly, two editions of the Principles of Neural Science (Kandel and Schwarz, 1981 and 1985) suggested that human brains contain as many as 50 trillion glial cells, despite the fact that the largest number ever suggested in the primary literature was 130 billion (Blinkov and Glezer, 1968). The overwhelming number of claims of a 10:1 or higher GNR (the origin of which will be examined next) outweighed the few original reports showing a 1:1 GNR (only three publications prior to 2009, Table 6).
None of the textbooks or reviews listed in Table 6 provides a primary reference – or any valid reference – for the claim of a 10:1 GNR. The lack of citations for the notion of a 10:1 GNR over a 50 year period is an example of a major failure in the scientific process that is supposed to self-correct invalid claims or reports (Committee on the Conduct of Science, 1989; Neville, 2007; Firestein, 2012; Ioannidis, 2012), as explained in more detail below. Not surprisingly, the first response of many brain scientists to the “maverick” report by Azevedo et al. (2009) was disbelief (see below and Yuhas and Jabr, 2012), and it has taken several years for the new evidence to become accepted (Table 6). The refutation of the notion of one trillion glial cells is also an example where a new (or substantially improved) technique, the IF, initiated a paradigm shift, but subsequent scrutiny showed – surprisingly – that the new paradigm had been supported all along, for decades, by traditional (histological) techniques. The problem appears to have been disregard of conflicting primary data and a failure to recognize the lack of supporting data for the prevailing consensus. The false belief was enabled and facilitated by presenting the 10:1 GNR as a “fact” and as “common knowledge” not requiring citations (Committee on the Conduct of Science, 1989; Neville, 2007).
EXAMPLES SHOWING THE IMPACT OF CELL COUNTING
There are numerous examples of how cell counting has informed and impacted progress in the field, with classical studies documenting the loss of neurons in degenerative diseases, for example correlating the extent of neuron loss with disease severity (Damier et al., 1999; Stark and Pakkenberg, 2004; Kordower et al., 2013). However, cell quantification is fundamentally important not only in pathology and in the clinical area. We selected here three examples that illustrate how cell counting has had a significant impact in areas beyond clinical medicine. The first example is from the aging human brain, the second is from the evolution of the brain, and the last is from developmental neuroscience.
Is there a significant loss of neurons in the normal aging brain?
Based on studies in the 1950s to 1980s, it was reported and generally believed that the normal aging brain loses large numbers of neurons each day after 30 years of age (Brody, 1955; Devaney and Johnson, 1980), so that “a 60-year span of adulthood would mean loss of half the cerebral neurons” (Hanley, 1974; see also Curcio et al., 1982; Kausler et al., 2007). Reports of this “neuronal fall-out” with normal aging provided a depressing outlook for octogenarians: loss of neurons was thought to be the cause of senile dementia, and senile dementia was thought to be an inevitable part of growing old. Thus, the above-mentioned cell counting studies may have contributed to the fear of dementia among the elderly (“greatest cause of distress,” Jorm, 1987; Pitt, 1998), a segment of the population with high rates of suicides (Meehan et al., 1991; McKeown et al., 2006; Schmutte et al., 2009). In this context, the innovative and diligent quantitative work of Haug and colleagues (1984) demonstrated that the studies indicating a constant and significant loss of neurons in the normal aging brain were flawed. The shrinkage of brains after fixation depends on the person’s age, and accordingly the reference volumes of brains from older people differ from those of younger brains. When this was taken into account, there was very little if any normal loss of neurons in most parts of the brain (Haug et al., 1984; West, 1993b; Morrison and Hof, 1997; Stark and Pakkenberg, 2004; Fabricius et al., 2013). The new view, that mental decline is not an imminent or inevitable fate, changed the elderly’s outlook on their remaining life span rather dramatically, even though the old dogma of continuous age-dependent neuronal death can still be found in recent literature (Rodriguez-Arellano et al., 2015; see also Verkhratsky et al., 2004; Kausler et al., 2007). The misconception of the extent of neuron loss in normal aging brains had profound implications beyond the quality of life for octogenarians: it complicated and delayed research into the causes of the real problem: the pathological loss of neurons in Alzheimer’s and related dementias. It took major efforts to correct this view (Morrison and Hof, 1997; Hof and Mobbs, 2009). As revealed in our review, once a myth has found its way into textbooks, curricula and common knowledge, it becomes difficult to rectify.
Evolution of the human brain – insights from the GNR
Throughout much of the 20th century the notion prevailed that the cellular composition of the human brain was exceptional among species and likely responsible for the superior cognitive abilities of humans (Gazzaniga, 2008). Previous work had suggested that the human brain and in particular the human neocortex showed an abnormally high GNR when compared with other mammals with lesser cognitive abilities (Friede, 1954; Jerison, 1973; Araque et al., 2001; Fields, 2009; Koob, 2009; Verkhratsky and Butt, 2013). Examination of Albert Einstein’s post-mortem brain, showing an increased GNR in some regions of his cortex, appeared to support this idea (Diamond et al., 1985; Witelson et al., 1995; Fields, 2009; Koob, 2009). The development of a more efficient cell counting method, the IF, made it possible to re-examine GNRs and to survey a much larger number of species, and in more detail (Azevedo et al. (2009); Herculano-Houzel, 2009; Herculano-Houzel, 2011; Herculano-Houzel, 2012; Herculano-Houzel, 2014). The results of these studies, comparing cell numbers and GNRs among a wide range of species, has shown that brain size does not scale universally with neuron number, that different mammalian species such as primates and rodents scale differently, that cell numbers in cerebral cortex and cerebellum evolve in a coordinated fashion, and that glia density and sizes vary much less than neuronal density and sizes. The GNR is highly conserved between structures and species, pointing to an important and close regulation of glia numbers (scaling) in response to, or regulated by, neuron density and neuron sizes (Herculano-Houzel, 2012; Herculano-Houzel, 2014; Mota and Herculano-Houzel, 2014). Most importantly, a GNR of 10 would indeed have made the human brain extraordinary – but that is not the case: The human ratio of non-neuronal to neuronal cells of 1 is similar to that of other primates, firmly establishing humans as non-outliers (Herculano-Houzel, 2012). Thus, the new studies comparing GNRs of different primate brains have shown that the human brain and its neocortex have “hardware” and cellular contents that are expected for its body size and are not extraordinary in their cellular composition. Accordingly, efforts to explain underlying mechanisms of humans’ cognitive abilities must look elsewhere (Dicke and Roth, 2016). Such new insights and new directions depended upon the development and implementation of accurate and efficient counting methods.
How are glia and neuron numbers controlled during development?
Neuron and glia numbers and their ratios fluctuate within relatively narrow ranges even in different species and different adult brain structures, emphasizing the importance of optimal quantitative relations between cell types. How these ratios are accomplished during development has been unclear, although it has been shown that the GNR increases markedly during early postnatal development (Mühlmann, 1936; Brizee et al., 1964; Bandeira et al., 2009). Using a combination of the DNA extraction and stereological axon counting methods, Martin Raff’s group counted retinal ganglion cell axons and quantified glial cells in the optic nerve and tract; they made significant advances by showing that mice with genetically increased numbers of retinal ganglion cells and axons caused corresponding glial cells to increase their numbers proportionally (Burne et al., 1996). These results implied that the neurons (retinal ganglion cells) communicated signals either to glial cell precursors to proliferate or to existing glia to allow more of the already produced glial cells to survive, so that a constant (presumably optimal) ratio between neurons or axons and supporting glial cells was maintained in the mice with increased neuron numbers (Burne et al., 1996). Thus, cell counting studies helped to advance a new field of study: neuron-glia interactions and signaling between these two types of cells in the brain, leading to a better understanding of how neurons and glia interact, communicate, and depend on each other during normal development of brains, as well as during abnormal development and disorders of the brain (Araque et al., 2001; Kettenmann and Ransom, 2013).
ORIGIN OF THE CLAIM OF ONE TRILLION GLIAL CELLS
The notion of a 10:1 GNR dates back to the 1950s, as can be seen in Table 6. We found that the earliest published accounts by brain and glia scientists – Glees (1958), Pope (1958) and Galambos (1961) included qualifiers (such as “perhaps”) in their estimates of a 10-fold abundance of glia over neurons, or they used vague terms such as “glia cells … in higher animals are extremely numerous” (Bullock, 1967). On the other hand, Hyden, a glia researcher (Hyden, 1960, 1961, 1967a) was more assertive and proclaimed: “The glial cells outnumber the nerve cells by a factor of around 10,” and this was stated in the context of “the central nervous tissue,” quoted from the chapter “The Neuron” in the influential series “The Cell” (Hyden, 1960), among other texts (e.g., “the glia are by far the most numerous cells”, Hyden, 1967b). This makes it sound as if a 10:1 GNR was a known fact. How does a new finding become a “scientific fact”? This process has been described as follows: “At each stage, researchers submit their work to be examined by others with the hope that it will be accepted. This process of public, systematic skepticism is critical in science.” … “Bypassing the standard routes of validation can short-circuit the self-correcting mechanisms of science.” (Committee on the Conduct of Science, 1989). In the case of the GNR, the normal scientific process of peer review and gradual validation was essentially “short-circuited.” One researcher or a small group of researchers convinced their contemporaries and their successors by making a claim (that should have been worded as a testable hypothesis) sound as if it was common knowledge and therefore did not need a primary reference or other citations. Neuroscientists then, with very few exceptions, copied it from review to review and from textbook to textbook for over half a century (Table 6), before it was exposed as one of the most persistent scientific myths of recent history (Firestein, 2012).
What made Hyden so convinced about a global 10:1 GNR? The key to understanding this conviction may lie in the context of Hyden’s own research area, which were the large Deiters neurons in the lateral vestibular nucleus (Hyden and Pigon, 1960). These brainstem nuclei indeed have a very large GNR – later determined and verified to be about 30–50 (Blinkov, 1963; Ponomarev, 1966; Blinkov and Glezer, 1968). Accordingly, Hyden was used to seeing neurons surrounded by a large number of glial cells, and it is likely that this contributed to his and others’ belief that such an arrangement was representative for the entire mammalian and human brain (Nicholls, 1991). As revealed by the work of Blinkov and Glezer (1968) as well as Thörner and colleagues (1975), the GNR can vary substantially among different brainstem nuclei – thus, the assumption that the distribution in one small nucleus of the brain was representative for the whole brain likely contributed to the widely overstated GNR in reviews and textbooks (Table 6). Unfortunately, we cannot ask Hyden what made him believe in the 10fold GNR – he died in 2000 (Hertz et al., 2001; Delgado and Estanol, 2013). Although Hyden appears to have been the driving force behind the initial formulation of the myth, he was not the only one who propagated the abundance of glial cells. In the late 1950s and early 1960s, it was widely believed that glial cells, and in particular oligodendrocytes, were the most numerous among the cell types in the human brain (Pope, 1958), although Schlote (1959) found fewer oligodendrocytes than neurons in most layers of human cortex. It is obvious that there seemed to be a general consensus in the late 1950s and early 1960s that glia far outnumbered neurons, as also stated in a memorandum of the RAND corporation (Maron, 1963), as well as in the popular book first published in 1963 by Isaac Asimov, a science fiction writer and professor at Columbia University (Asimov, 1963) (Table 6). The notion appears to have originated as an inadvertent mistake, with no evidence of deliberate manipulation, as in other instances of mis-information in science (Proctor and Schiebinger, 2008).
PERPETUATION OF THE CLAIM
In the previous section, we examined how the claim originated. Here we examine the question “how did that first, wrong number become so widespread?” (Firestein, 2012). Is it true, as Firestein surmises, that “textbook writers … just picked it up from one another and kept passing it around?” Once the notion of an overabundance of glia relative to neurons had formed and had been incorporated into early influential textbooks (Hyden, 1960; Kuffler and Nicholls, 1976; Kandel and Schwartz, 1981), the notion was treated as a fact, and the abundance itself was rarely questioned; rather, it became largely reduced to the question of by exactly how much glia outnumbered neurons, whether it was 5:1, 10:1, or 50:1.
There is a certain irony in that the perpetuation of the claim was to a large part due to errors in Kandel’s textbook editions (Table 6), which helped glia biologists to advance their arguments of glial neglect (e.g., Fields, 2009; Koob, 2009), yet at the same time, Kandel was criticized for promoting the “neural dogma” and ignoring the importance of glial cells (Merrill, 2009).
Our review of textbooks and other published reports on the GNR and neuron and glia numbers shows that reports can be divided into three types. (1) A few authors remained cautious and stated a wide range, used non-specific terms (“large number”) or said that numbers or ratios were unknown (Hubel, 1979; Nauta and Feirtag, 1979; Jacobson, 1991). (2) Other authors reported numbers based on particular studies and data sets (their own or others) and properly cited the original reference(s) – this was also relatively rare (Blinkov and Glezer, 1968; Szentagothai, 1983; Haug, 1986; Williams and Herrup, 1988; Azevedo et al. (2009); Andrade-Moraes et al., 2013; Verkhratsky and Butt, 2013, Table 6). (3) A large majority of reports cited a specific number or small range, making it sound as if the exact number or ratio was known, but did not provide any reference (Table 6).
The claim of an overabundance of glial cells spread beyond quantitative brain science and reached diverse areas of society: the policies of federal funding agencies that decide on brain research funding, the National Institute of Neurological Disorders & Stroke (NINDS); public educational databases (BrainFacts.org) established by major neuroscience societies and foundations (Society for Neuroscience, The Kavli Foundation, GATSBY); the curricula of medical, graduate and undergraduate students, and the media such as National Public Radio (NPR).
For example, the director of NINDS stated during an NPR interview that was nationally broadcast in the USA in 2013 that the human brain contained “trillions” of nerve cells. NINDS publishes an annual narrative for justification of neuroscience research funding to the legislature. These narratives mirror the misleading statements about glia-neuron ratios in the textbooks, and are factually wrong, but reflect the “textbook knowledge” of their times: “glial cells far outnumber nerve cells in the brain” (NINDS, 2001); “non-neuronal cells … far outnumber nerve cells in the brain” (NINDS, 2011); “non-nerve cells, called glial cells, outnumber nerve cells in the brain” (NINDS, 2015) (years indicate the fiscal year of the narratives). Several BrainFacts articles, some as recent as 2012 (http://www.brainfacts.org/Brain-Basics/Neuroanatomy/Articles/2012/The-Neuron) and 2013 (http://www.brainfacts.org/Brain-Basics/Cell-Communication/Articles/2013/Neuroglia-and-the-Brain) repeat the old, incorrect information as “brain facts.”
Missed opportunities to refute the notion
Blinkov and Glezer (1968) compiled a wealth of data, but failed to realize that the GNR, the neuron numbers, and their own glial cell numbers for the whole brain “did not add up.” Maximally 130 billion glia with a 10:1 GNR could not be true, because that would cap the total number of neurons at 13 billion, but there were 10–16 billion neurons in cerebral cortex alone, plus at least 10 billion neurons in cerebellar cortex (granule neurons alone), and possibly up to a total of 100 billion neurons in cerebellar cortex. This should have been a warning signal – that the GNR could not exceed 6.5:1 (130:20 = 6.5), and probably was much lower, possibly as low as a GNR of 100:116 which equals 0.86:1. Haug (1986) had calculated the numbers of glia and neurons to be equally low, with a low GNR – with a remarkable accuracy, as revealed in hindsight. He was a founding member of the International Society of Stereology and a prolific worker with more than 160 publications (Kühnel, 2003), yet he does not appear to have made any efforts to refute the prevailing numbers. Most influential for the propagation of those numbers appear to have been the textbooks of prominent neuroscientists such as Kuffler and Nicholls and the textbook by Nobel laureate Kandel and his colleagues. While at Harvard, Kuffler was a mentor not only to Kandel, but also to Hubel and Wiesel (both of them also Nobel laureates). Kuffler, the founder of the department of neurobiology at Harvard, is admired as the “father of modern neuroscience” and the “most dominant figure in experimental neuroscience in the 1960s and ‘70s” (McMahan, 1990), and Kandel’s textbook editions have been praised as the “bible of neuroscience” (Lambert and Kinsley, 2004; Darlington, 2009). Endorsement of the prevailing numbers by the most accomplished neuroscientists thus was a formidable influence. We conclude that there is not one single predominant reason, but a combination of factors that contributed to the notion of one trillion glia and its perpetuation: These factors include failure to realize that the numbers did not add up; focus on parts of the human brain that were not representative; neglecting the role of the cerebellum; missing primary literature; copying information from previous reviews without scrutiny; inaccurate quoting of others’ work, and reluctance to challenge the prevailing dogma (Ioannidis, 2012; Nuzzo, 2015).
RELUCTANT ACCEPTANCE OF THE REFUTATION OF THE CLAIM
The first challenge to the statements of one trillion cells in the brain and a GNR of 10:1 came with the estimation of a total number of no more than 130 billion glial cells (Blinkov and Glezer, 1968). However, the significance was not realized. The second challenge came with Haug’s estimate of less than 50 billion glial cells and a GNR of less than 1 (Haug, 1986). Again, these estimates were not placed in context, numbers were not compared with those taught in textbooks, and discrepancies therefore remained hidden. The third challenge was based on data obtained by the isotropic fractionator which showed that the cellular composition of the human brain comprised an average of 86 billion neurons, 85 billion non-neuronal cells, and thus rendered a GNR of less than 1:1 (Azevedo et al. (2009)). This time, the significance of the findings was realized, and the authors drew attention to the discrepancies and made considerable efforts to locate the source of the prevailing erroneous estimates (Herculano-Houzel, 2009; Hilgetag and Barbas, 2009; Firestein, 2012; Yuhas and Jabr, 2012) (Fig. 3).
Some gliabiologists and neuroscientists, however, disagreed: the IF had not yet been validated against the current standard in the field, stereology (Yuhas and Jabr, 2012; Carlo and Stevens, 2013; Verkhratsky and Butt, 2013; Charvet et al., 2015), although the estimates obtained with the IF for the human brain were very close to those in the literature, where they existed (Azevedo et al. (2009)). Indeed, the possibility that a significant fraction of cell nuclei were not recovered or were damaged in the isotropic fractionator method was not addressed in the initial publication, despite concerns that the dissociation, isolation and purification methods might damage glial or neuronal cell nuclei preferentially. In fact, notions have been controversial whether destruction of nuclei may affect primarily the larger, neuronal nuclei (Lovtrup-Rein and McEwen, 1966; Clarke and Oppenheim, 1995) or the smaller, glial cell nuclei (Hadjiolov et al., 1965; Kato and Kurokawa, 1967). Initial concerns were for unfixed tissues and nuclei (Hadjiolov et al., 1965; Lovtrup-Rein and McEwen, 1966; Kato and Kurokawa, 1967), but also for fixed tissues (Clarke and Oppenheim, 1995). For example, Hadjiolov et al. (1965) wrote that “According to an analysis of the nuclear size distribution, a considerable loss of smaller nuclei (10 to 20μ2), mainly from glial cells, occurs during the purification procedure” (Hadjiolov et al., 1965), and that “the purification procedure results in a considerable loss of smaller nuclei (10 to 20/~2) which most probably originate from oligodendroglial and microglial cells“ (Hadjiolov et al., 1965). “The greater number of smaller nuclei were lost during the ordinary isolation procedure” (Kato and Kurokawa, 1967). Other researchers, however, were concerned that the larger, neuronal nuclei were more fragile: “Because of their extreme fragility, … brain nuclei, mainly those from neurons and astrocytes, are easily disrupted during the homogenization procedure“ (Lovtrup-Rein and McEwen, 1966). And: “large numbers of cells might be ruptured by the dissociation procedure … this concern is supported by the fact that Zamenhof’s total large cells … was 1.75 x 105 on average, whereas counts in histological sections of only the Purkinje cells … came to 2.62 x 105 ” (Clarke and Oppenheim, 1995). More recent concerns stated: “This ‘isotropic fractionation’ technique can not be considered flawless, of course. We do not know how many nuclei are lost in the process …” (Verkhratsky and Butt, 2013, p. 95; see also: Yuhas and Jabr, 2012).
Recent calibration studies have dispelled these concerns and validated the IF against other counting methods, including stereology (Bahney and von Bartheld, 2014; Miller et al., 2014). Approaches used were to examine adjacent samples of white matter to directly compare the methods of IF, histology/stereology, and DNA retrieval. In addition, the two cerebral hemispheres of the same non-human primate were examined with IF and stereology, again showing equivalency between methods (Miller et al., 2014). Furthermore, original data based on histological sections are consistent with a 1:1 ratio.
Still, some researchers remain unconvinced. Barres (cited in Yuhas and Jabr, 2012) maintains that glia make up at least 80 percent of cells in the human brain, because growing numbers of glia in the forebrain explain the increase in total forebrain DNA, based on a report of Dobbing and Sands (1973). Yet these DNA data are entirely consistent with the finding that the human forebrain (cerebral cortex, including white matter) has a ratio of about 4:1 between non-neuronal:neuronal cells (3.72:1, Azevedo et al. (2009)). The problem in the Barres argument is that the data are from the forebrain (containing only 19% of the brain’s neurons), but he makes conclusions about the entire brain. The newest (5th) edition of Kandel’s textbook has revised the chapter on the cellular composition of the brain, which is now co-authored by Barres, from the original “10–50 more glia” statement to “2 to 10 times more glia than neurons” (Kandel et al., 2013). This is an improvement, but still incorrect, as is the claim of an abundance of glia over neurons in the human brain (4:1 according to Barres et al., 2015). Nevertheless, as shown in Table 6, there now is gradual acceptance of the IF and its conclusions by many neuroscientists and also glia biologists (Brautigam et al., 2012; Devinsky et al., 2013; Streit, 2013; Verkhratsky and Butt, 2013).
We wish to emphasize that just because glia are less numerous in the brain than neurons, and far less numerous than previously thought, this does not mean in any way that glia are less important. On the contrary, glial cells perform a long list of essential functions (Baumann and Pham-Dinh, 2001; Haydon, 2001; Ullian et al., 2001; Doetsch, 2003; Nedergaard et al., 2003; Allen and Barres, 2009; Fields, 2010; Han et al., 2013; Kettenmann and Ransom, 2013; Verkhratsky and Butt, 2013). The extraordinarily conserved numerical relationship between glia and neurons over at least 90 million years of evolution alone indicates that glia cells and their relation with neurons and brain function must be extremely important (Herculano-Houzel, 2014). A precise balance of glia to neurons in human brain regions seems essential for normal function and this balance is disturbed in disease and trauma (see below). The isotropic fractionator may prove to be a reliable and efficient tool to not only provide insights into brain evolution (Herculano-Houzel, 2009), but also to probe suspected changes in glia and neuron numbers within dissectable regions of the human brain of patients with neurological and psychiatric diseases (Andrade-Moraes et al., 2013; Herculano-Houzel et al., 2015).
ROLE OF THE ISOTROPIC FRACTIONATOR IN FUTURE RESEARCH
A large number of neurological and psychiatric diseases have been implicated with abnormal glia numbers or GNRs. The earliest such reports originated in the 19th century (Hammarberg, 1895; Ferrero, 1947; Friede, 1953; Hempel and Treff, 1959; Schlote, 1959). While the degree and localization of abnormalities differed considerably between studies (Ferrero, 1947; Rowland and Mettler, 1949; Hempel and Treff, 1959; Benes, 1993; Ongür et al., 1998; Harrison, 1999; Vawter et al., 2000; Todtenkopf et al., 2005; Bernstein et al., 2015; Elsayed and Magistretti, 2015), changes in glial cell number, densities or GNRs in discrete brain regions have been confirmed in more recent studies employing stereological or IF methods for diseases including autism spectrum disorders, mood disorder, depression, schizophrenia, and Alzheimer’s disease (Rajkowska, 2000; Cotter et al., 2001; Hof et al., 2003; Vostrikov et al., 2007; Morgan et al., 2010; Karlsen and Pakkenberg, 2011; Andrade-Moraes et al., 2013; Verkhratsky et al., 2014).
Early research into glia abnormalities (as described above) was much forgotten – so much so, that the significance of glial changes in psychiatric diseases had to be re-discovered in 2000 (Coyle and Schwarcz, 2000). “… for too long, glial cells have been grossly neglected when thinking about the neurobiological features of psychiatric disorders”; “… in the next century glia will no longer remain the silent majority of brain cells but will assume a major focus of interest in the study of the causes and treatment of neuropsychiatric disorders.”
However, the lack of reliability, validity and therefore trust in quantitative data has been a major impediment to progress in defining the potential roles of numerical glia abnormalities in neurological and psychiatric diseases. As already mentioned in previous sections of this review, there have been multiple examples where initial reports of numbers or ratios of glial cells and neurons could not be replicated or had to be substantially revised, even within the same group of investigators or when using the same type of counting technique (Pakkenberg and Gundersen, 1988; Guillery and Herrup, 1997; Schmitz et al., 2001; von Bartheld, 2001; Dorph-Petersen, 2004; Abitz et al., 2007; Nielsen et al., 2008; Dorph-Petersen et al., 2009; Herculano-Houzel et al., 2015). Therefore, meta-analyses have become common to explore the status and validity of previously published quantitative studies (Harrison, 1999; Rajkowska, 2000, 2002; Hof et al., 2003; Lyness et al., 2003; Palmen et al., 2004; Todtenkopf et al., 2005; Courchesne et al., 2007; Amaral et al., 2008). Unfortunately, meta-analyses have not been able to resolve all controversies about glia numbers and ratios in human neurological and psychiatric diseases, especially when the primary data was based on densities or ratios, rather than absolute numbers, and using profile counting or even stereology. Use of design-based stereology does not, unfortunately, guarantee unbiased results – there can be significant numerical differences between studies, indicating that these techniques are not infallible (Herculano-Houzel et al., 2015).
For this reason, there is hope that the recently developed alternative to histological counting methods, the isotropic fractionator, may emerge as a more robust option to obtain and validate quantitative data about glia and neuron numbers and their ratios in deceased patient’s brains. Isotropic fractionator technology is a relatively fast and simple procedure, and compatible with a large range of fixatives, which makes this approach more versatile than histological approaches (Bahney and von Bartheld, 2014). It is yet too early to tell, but this alternative counting technique may provide a much-needed verification and validation of previously reported numerical abnormalities in glia and neurons in various neurological and psychiatric diseases.
It is important that quantitative studies of glia and neuron composition refer to the whole body of published information, take into account all relevant studies, and compare new data with previously published work. Too often in the history of cell quantification have discrepancies between investigators, studies, and techniques remained hidden. We hope that our review will help to facilitate comparison with previous work. More careful scrutiny of relevant studies, including primary sources, would increase transparency to better compare studies, data, and techniques, and would contribute to resolve conflicting opinions and uncover faulty techniques, as Paul Glees forewarned more than 60 years ago (Glees, 1955).
Acknowledgments
GRANT SUPPORT: NIH grants NS079884 (CSvB, JB), GM104944 and GM103554 (CSvB); CNPq, Faperj, and the James S. McDonnell Foundation (SHH).
We thank Rob Williams and Glenn Rosen for sharing unpublished data. Our work was supported by NIH grants NS079884, GM104944 and GM103554 (Center of Biomedical Research Excellence, funded by the National Institute for General Medical Science, CSvB). One of the authors (CSvB) acknowledges collaborations on quantitation of cells with Stefano Geuna, Suleyman Kaplan, Jon Kaas, Ron Oppenheim, Glenn Rosen, Oliver von Bohlen und Halbach, and Rob Williams. SHH thanks Roberto Lent for collaboration in developing the IF and in early work on the human brain. Her work was supported by CNPq, Faperj, and the James S. McDonnell Foundation.
Abbreviations
- DAPI
4′,6-Diamidino-2-Phenylindole, Dihydrochloride
- DNA-P
desoxyribonucleic acid-phosphorus
- GNR
glia-neuron ratio
- IF
isotropic fractionator
Footnotes
CONFLICT OF INTEREST
There are no conflicts of interest.
AUTHOR CONTRIBUTIONS
All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: CSvB and SHH. Acquisition of data: CSvB and JB. Analysis and interpretation of data: CSvB and SHH. Drafting of the manuscript: CSvB. Critical revision of the manuscript for important intellectual content: CSvB and SHH. Obtained funding: CSvB, JB and SHH. Administrative, technical, and material support: JB. Study supervision: CSvB.
LITERATURE CITED
- Abercrombie M. Estimation of nuclear populations from microtome sections. Anat Rec. 1946;94:239–247. doi: 10.1002/ar.1090940210. [DOI] [PubMed] [Google Scholar]
- Abitz M, Nielsen RD, Jones EG, Laursen H, Graem N, Pakkenberg B. Excess of neurons in the human newborn mediodorsal thalamus compared with that of the adult. Cereb Cortex. 2007;17:2573–2578. doi: 10.1093/cercor/bhl163. [DOI] [PubMed] [Google Scholar]
- Agashiwala RM, Louis ED, Hof PR, Perl DP. A novel approach to non-biased systematic random sampling: a stereologic estimate of Purkinje cells in the human cerebellum. Brain Res. 2008;1236:73–78. doi: 10.1016/j.brainres.2008.07.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agduhr E. A contribution to the technique of determining the number of nerve cells per volume unit of the tissue. Anat Rec. 1941;80:191–202. [Google Scholar]
- Allen NJ, Barres BA. Neuroscience: Glia - more than just brain glue. Nature. 2009;457:675–677. doi: 10.1038/457675a. [DOI] [PubMed] [Google Scholar]
- Altman J. Postnatal growth and differentiation of the mammalian brain, with implication for a morphological theory of memory. In: Qarton GQ, Melnechuk T, Schmitt FO, editors. The Neurosciences: A Study Program. New York: The Rockefeller Univ Press; 1967. pp. 721–743. [Google Scholar]
- Amaral DG, Schumann CM, Nordahl CW. Neuroanatomy of autism. Trends Neurosci. 2008;31:137–145. doi: 10.1016/j.tins.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Andersen BB, Korbo L, Pakkenberg B. A quantitative study of the human cerebellum with unbiased stereological techniques. J Comp Neurol. 1992;326:549–560. doi: 10.1002/cne.903260405. [DOI] [PubMed] [Google Scholar]
- Andersen BB, Gundersen HJ, Pakkenberg B. Aging of the human cerebellum: a stereological study. J Comp Neurol. 2003;466:356–365. doi: 10.1002/cne.10884. [DOI] [PubMed] [Google Scholar]
- Andersen BB, Pakkenberg B. Stereological quantitation in cerebella from people with schizophrenia. Br J Psychiatry. 2003;182:354–361. doi: 10.1192/bjp.182.4.354. [DOI] [PubMed] [Google Scholar]
- Andersen K, Andersen BB, Pakkenberg B. Stereological quantification of the cerebellum in patients with Alzheimer’s disease. Neurobiol Aging. 2012;33:197, e11–20. doi: 10.1016/j.neurobiolaging.2010.06.013. [DOI] [PubMed] [Google Scholar]
- Anderton BH. Changes in the ageing brain in health and disease. Philos Trans R Soc Lond B Biol Sci. 1997;352:1781–1792. doi: 10.1098/rstb.1997.0162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade-Moraes CH, Oliveira-Pinto AV, Castro-Fonseca E, da Silva CG, Guimarães DM, Szczupak D, Parente-Bruno DR, Carvalho LR, Polichiso L, Gomes BV, Oliveira LM, Rodriguez RD, Leite RE, Ferretti-Rebustini RE, Jacob-Filho W, Pasqualucci CA, Grinberg LT, Lent R. Cell number changes in Alzheimer’s disease relate to dementia, not to plaques and tangles. Brain. 2013;136:3738–3752. doi: 10.1093/brain/awt273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araque A, Carmignoto G, Haydon PG. Dynamic signaling between astrocytes and neurons. Annu Rev Physiol. 2001;63:795–813. doi: 10.1146/annurev.physiol.63.1.795. [DOI] [PubMed] [Google Scholar]
- Armstrong RC, Clarke PG. Neuronal death and the development of the pontine nuclei and inferior olive in the chick. Neuroscience. 1979;4:1635–1647. doi: 10.1016/0306-4522(79)90025-3. [DOI] [PubMed] [Google Scholar]
- Arutyunova AY. Age changes in the number of nerve and neuroglial cells in man. Azerb Med Zh. 1938;1:150. [Google Scholar]
- Asimov I. The Human Brain: Its Capacities and Functions. The University of Michigan; Houghton Mifflin: 1963. p. 357. [Google Scholar]
- Azevedo FAC, Carvalho LRB, Grinberg LT, Farfel JM, Ferretti REL, Leite REP, Jacob Filho W, Lent R, Herculano-Houzel S. Equal numbers of neuronal and nonneuronal cells make the human brain an isometrically scaled-up primate brain. J Comp Neurol. 2009;513:532–541. doi: 10.1002/cne.21974. [DOI] [PubMed] [Google Scholar]
- Azevedo FAC, Andrade-Moraes CH, Curado MR, Oliveira-Pinto AV, Guimarães DM, Szczupak D, Gomes BV, Alho AT, Polichiso L, Tampellini E, Lima L, de Lima DO, da Silva HA, Lent R. Automatic isotropic fractionation for large-scale quantitative cell analysis of nervous tissue. J Neurosci Methods. 2013;212:72–78. doi: 10.1016/j.jneumeth.2012.09.015. [DOI] [PubMed] [Google Scholar]
- Bahney J, von Bartheld CS. Validation of the isotropic fractionator: Comparison with unbiased stereology and DNA extraction for quantification of glial cells. J Neurosci Methods. 2014;222:165–174. doi: 10.1016/j.jneumeth.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balado M, Franke E. Das Corpus Geniculatum Externum. In: Foerster O, Rudin E, Spatz H, editors. Monographien aus dem Gesamtgebiete der Neurologie und Psychiatrie. Berlin: Julius Springer; 1937. pp. 1–118. [Google Scholar]
- Bandeira FC, Lent R, Herculano-Houzel S. Changing numbers of neuronal and non-neuronal cells underlie postnatal brain growth in the rat. Proc Natl Acad Sci USA. 2009;106:14108–14113. doi: 10.1073/pnas.0804650106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barres BA, Freeman MR, Stevens B. Cold Spring Harbor Perspectives in Biology. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 2015. Glia. [Google Scholar]
- Baryshnikova LM, von Bohlen und Halbach O, Kaplan S, von Bartheld CS. Two distinct events, section compression and loss of particles (“lost caps”), contribute to z-axis distortion and bias in optical disector counting. Microsc Res Tech. 2006;69:738–756. doi: 10.1002/jemt.20345. [DOI] [PubMed] [Google Scholar]
- Bass NH, Hess HH, Pope A, Thalheimer C. Quantitative cytoarchitectonic distribution of neurons, glia, and DNA in rat cerebral cortex. J Comp Neurol. 1971;143:481–490. doi: 10.1002/cne.901430405. [DOI] [PubMed] [Google Scholar]
- Baumann N, Pham-Dinh D. Biology of oligodendrocyte and myelin in the mammalian central nervous system. Physiol Rev. 2001;81:871–927. doi: 10.1152/physrev.2001.81.2.871. [DOI] [PubMed] [Google Scholar]
- Bayraktar OE, Fuentealba LC, Alvarez-Buylla A, Rowitch DH. Astrocyte development and heterogeneity. In: Barres BA, Freeman MR, Stevens B, editors. Glia. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 2015. pp. 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bear MF, Connors BW, Paradiso MA. Exploring the Brain. 2. Philadelphia: Lippincott Williams & Wilkins; 2001. Neuroscience. [Google Scholar]
- Bear MF, Connors BW, Paradiso MA. Exploring the Brain. 3. Philadelphia: Lippincott Williams & Wilkins; 2007. Neuroscience. [Google Scholar]
- Benes FM. Neurobiological investigations in cingulate cortex of schizophrenic brain. Schizophr Bull. 1993;19:537–549. doi: 10.1093/schbul/19.3.537. [DOI] [PubMed] [Google Scholar]
- Benes FM, Lange N. Two-dimensional versus three-dimensional cell counting: a practical perspective. Trends Neurosci. 2001;24:11–17. doi: 10.1016/s0166-2236(00)01660-x. [DOI] [PubMed] [Google Scholar]
- Berger H. Untersuchungen uber den Zellgehalt der menschlichen Grosshirnrinde. Z Ges Neurol Psychiat. 1921;69:45–69. [Google Scholar]
- Bernstein HG, Steiner J, Guest PC, Dobrowolny H, Bogerts B. Glial cells as key players in schizophrenia pathology: recent insights and concepts of therapy. Schizophr Res. 2015;161:4–18. doi: 10.1016/j.schres.2014.03.035. [DOI] [PubMed] [Google Scholar]
- Bignami A. Glial cells in the central nervous system. Discussions Neurosci. 1991;8:7–45. [Google Scholar]
- Bjugn R, Gundersen HJ. Estimate of the total number of neurons and glial and endothelial cells in the rat spinal cord by means of the optical disector. J Comp Neurol. 1993;328:406–414. doi: 10.1002/cne.903280307. [DOI] [PubMed] [Google Scholar]
- Black JA, Ransom BR. Glial cells: Morphology, development and function in the CNS. In: Adelman G, Smith BH, editors. Encyclopedia of Neuroscience. 2. Vol. 1. Elsevier; Amsterdam: 1999. pp. 805–809. [Google Scholar]
- Blinkov SM. The glial index and distribution density of glial cells in the human brain stem. Arkh Anat Gistol Embriol. 1963;45:42–47. [PubMed] [Google Scholar]
- Blinkov SM, Glezer II. A Quantitative Handbook. New York: Plenum Press; 1968. The Human Brain in Figures and Tables; p. 482. [Google Scholar]
- Blinkov SM, Ivanitski GR. On the number of glial cells in the human brain. (Calculations on the computer) Biofizika. 1965;10:817–825. [PubMed] [Google Scholar]
- Braendgaard H, Evans SM, Howard CV, Gundersen HJ. The total number of neurons in the human neocortex unbiasedly estimated using optical disectors. J Microsc. 1990;157:285–304. doi: 10.1111/j.1365-2818.1990.tb02967.x. [DOI] [PubMed] [Google Scholar]
- BrainFacts. A Primer on the brain and nervous system. A companion to BrainFacts.org. The Kavli Foundation, GATSBY, and the Society for Neuroscience. http://www.brainfacts.org/~/media/Brainfacts/Article%20Multimedia/About%20Neuroscience/Brain%20Facts%20book.ashx.
- Braitenberg V, Atwood RP. Morphological observations on the cerebellar cortex. J Comp Neurol. 1958;109:1–33. doi: 10.1002/cne.901090102. [DOI] [PubMed] [Google Scholar]
- Brasileiro-Filho G, Guimaraes RC, Pittella JE. Quantitation and karyometry of cerebral neuroglia and endothelial cells in liver cirrhosis and in the hepatosplenic schistosomiasis mansoni. Acta Neuropathol. 1989;77:582–590. doi: 10.1007/BF00687885. [DOI] [PubMed] [Google Scholar]
- Brautigam H, Steele JW, Westaway D, Fraser PE, St George-Hyslop PH, Gandy S, Hof PR, Dickstein DL. The isotropic fractionator provides evidence for differential loss of hippocampal neurons in two mouse models of Alzheimer’s disease. Mol Neurodegener. 2012;7:58. doi: 10.1186/1750-1326-7-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brizzee KR, Jacobs LA. The glia/neuron index in the submolecular layers of the motor cortex in the cat. Anat Rec. 1959;134:97–105. doi: 10.1002/ar.1091340110. [DOI] [PubMed] [Google Scholar]
- Brizzee KR, Vogt J, Kharetchko X. Postnatal changes in glia/neuron index with a comparison of methods of cell enumeration in the white rat. Progr Brain Res. 1964;4:136–149. [Google Scholar]
- Brodal A. Neurological Anatomy. 2. New York: Oxford Univ. Press; 1969. [Google Scholar]
- Brodal P. The Central Nervous System. New York: Oxford Univ. Press; 1992. [Google Scholar]
- Brodal P. Structure and Function. New York: Oxford Univ. Press; 2010. The Central Nervous System. [Google Scholar]
- Brody H. Organization of the cerebral cortex. III. A study of aging in the human cerebral cortex. J Comp Neurol. 1955;102:511–556. doi: 10.1002/cne.901020206. [DOI] [PubMed] [Google Scholar]
- Brody H. Structural changes in the ageing nervous system. Interdiscipl Top Gerontol. 1970;7:9–21. [Google Scholar]
- Brownson RH. Perineuronal satellite cells in the motor cortex of aging brains. J Neuropathol Exp Neurol. 1956;15:190–195. doi: 10.1097/00005072-195604000-00004. [DOI] [PubMed] [Google Scholar]
- Buchweitz E, Weiss HR. Alterations in perfused capillary morphometry in awake vs anesthetized brain. Brain Res. 1986;377:105–111. doi: 10.1016/0006-8993(86)91195-9. [DOI] [PubMed] [Google Scholar]
- Bullock TH. Signals and Neuronal Coding. In: Qarton GQ, Melnechuk T, Schmitt FO, editors. The Neurosciences: A Study Program. New York: The Rockefeller Univ Press; 1967. pp. 347–352. [Google Scholar]
- Burne JF, Staple JK, Raff MC. Glial cells are increased proportionally in transgenic optic nerves with increased numbers of axons. J Neurosci. 1996;16:2064–2073. doi: 10.1523/JNEUROSCI.16-06-02064.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlo CN, Stevens CF. Structural uniformity of neocortex, revisited. Proc Natl Acad Sci USA. 2013;110:1488–1493. doi: 10.1073/pnas.1221398110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chacko LW. The laminar pattern of the lateral geniculate body in the primates. J Neurol Neurosurg Psychiat. 1948;11:211–224. doi: 10.1136/jnnp.11.3.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charvet CJ, Cahalane DJ, Finlay BL. Systematic, cross-cortex variation in neuron numbers in rodents and primates. Cereb Cortex. 2015;25:147–160. doi: 10.1093/cercor/bht214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke PG, Oppenheim RW. Neuron death in vertebrate development: in vitro methods. Methods Cell Biol. 1995;46:277–321. [PubMed] [Google Scholar]
- Coggeshall RE, Lekan HA. Methods for determining numbers of cells and synapses: a case for more uniform standards of review. J Comp Neurol. 1996;364:6–15. doi: 10.1002/(SICI)1096-9861(19960101)364:1<6::AID-CNE2>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Coggeshall RE, La Forte R, Klein CM. Calibration of methods for determining numbers of dorsal root ganglion cells. J Neurosci Methods. 1990;35:187–194. doi: 10.1016/0165-0270(90)90123-w. [DOI] [PubMed] [Google Scholar]
- Collins CE, Young NA, Flaherty DH, Airey DC, Kaas JH. A rapid and reliable method of counting neurons and other cells in brain tissue: a comparison of flow cytometry and manual counting methods. Front Neuroanat. 2010;4:5. doi: 10.3389/neuro.05.005.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Committee on the Conduct of Science, National Academy of Sciences of the United States of America. On being a scientist. Proc Natl Acad Sci USA. 1989;86:9053–9074. doi: 10.1073/pnas.86.23.9053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotter DR, Pariante CM, Everall IP. Glial cell abnormalities in major psychiatric disorders: the evidence and implications. Brain Res Bull. 2001;55:585–595. doi: 10.1016/s0361-9230(01)00527-5. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Pierce K, Schumann CM, Redcay E, Buckwalter JA, Kennedy DP, Morgan J. Mapping early brain development in autism. Neuron. 2007;56:399–413. doi: 10.1016/j.neuron.2007.10.016. [DOI] [PubMed] [Google Scholar]
- Coyle JT, Schwarcz R. Mind glue: implications of glial cell biology for psychiatry. Arch Gen Psychiatry. 2000;57:90–93. doi: 10.1001/archpsyc.57.1.90. [DOI] [PubMed] [Google Scholar]
- Cragg BG. The density of synapses and neurones in the motor and visual areas of the cerebral cortex. J Anat. 1967;101:639–654. [PMC free article] [PubMed] [Google Scholar]
- Cragg BG. Gross, microscopical and ultramicroscopical anatomy of the adult nervous system. In: Davison AN, Dobbing J, editors. Applied Neurochemistry. Oxford: Blackwell; 1968. pp. 3–47. [Google Scholar]
- Curcio CA, Buell SJ, Coleman PD. Morphology of the aging central nervous system: Not all downhill. In: Mortimer JA, Pirozzolo FJ, Maletta GJ, editors. The Aging Motor System. Advances in Neurogerontology. Vol. 3. New York: Praeger; 1982. pp. 7–35. [Google Scholar]
- Dahlstrom A, Hamberger A. Holger Hyden 1917–2000. Neurochem Res. 2001;26:335–336. [Google Scholar]
- Damier P, Hirsch EC, Agid Y, Graybiel AM. The substantia nigra of the human brain. II. Patterns of loss of dopamine-containing neurons in Parkinson’s disease. Brain. 1999;122:1437–1448. doi: 10.1093/brain/122.8.1437. [DOI] [PubMed] [Google Scholar]
- Darlington CL. The Female Brain. 2. Boca Raton: CRC Press; 2009. [Google Scholar]
- Davanlou M, Smith DF. Unbiased stereological estimation of different cell types in rat cerebral cortex. Image Anal Stereol. 2004;23:1–11. [Google Scholar]
- Delgado G, Estañol B. Acido, ergo sum: Holger Hydén--the neuroscientist in Cortázar’s Hopscotch. Arq Neuropsiquiatr. 2013;71:408–410. doi: 10.1590/0004-282X20130048. [DOI] [PubMed] [Google Scholar]
- Devaney KO, Johnson HA. Neuron loss in the aging visual cortex of man. J Gerontol. 1980;35:836–841. doi: 10.1093/geronj/35.6.836. [DOI] [PubMed] [Google Scholar]
- Devinsky O, Vezzani A, Najjar S, De Lanerolle NC, Rogawski MA. Glia and epilepsy: excitability and inflammation. Trends Neurosci. 2013;36:174–184. doi: 10.1016/j.tins.2012.11.008. [DOI] [PubMed] [Google Scholar]
- Diamond MC, Scheibel AB, Murphy GM, Jr, Harvey T. On the brain of a scientist: Albert Einstein. Exp Neurol. 1985;88:198–204. doi: 10.1016/0014-4886(85)90123-2. [DOI] [PubMed] [Google Scholar]
- Dicke U, Roth G. Neuronal factors determining high intelligence. Philos Trans R Soc Lond B Biol Sci. 2016;371:20150180. doi: 10.1098/rstb.2015.0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbing J, Sands J. Timing of neuroblast multiplication in developing human brain. Nature. 1970;226:639–640. doi: 10.1038/226639a0. [DOI] [PubMed] [Google Scholar]
- Dobbing J, Sands J. Quantitative growth and development of human brain. Arch Dis Child. 1973;48:757–767. doi: 10.1136/adc.48.10.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch F. The glial identity of neural stem cells. Nat Neurosci. 2003;6:1127–1134. doi: 10.1038/nn1144. [DOI] [PubMed] [Google Scholar]
- Donaldson HH. The Growth of the Brain. Chicago, New York: Scribner; 1895. [Google Scholar]
- Dorph-Petersen KA, Pierri JN, Sun Z, Sampson AR, Lewis DA. Stereological analysis of the mediodorsal thalamic nucleus in schizophrenia: volume, neuron number, and cell types. J Comp Neurol. 2004;472:449–462. doi: 10.1002/cne.20055. [DOI] [PubMed] [Google Scholar]
- Dorph-Petersen KA, Caric D, Saghafi R, Zhang W, Sampson AR, Lewis DA. Volume and neuron number of the lateral geniculate nucleus in schizophrenia and mood disorders. Acta Neuropathol. 2009;117:369–384. doi: 10.1007/s00401-008-0410-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drasher ML. A criticism of the indiscriminate use of the Schmidt-Thannhauser method for the fractionation of nucleic acids in biological material. Science. 1953;118:181–183. doi: 10.1126/science.118.3059.181. [DOI] [PubMed] [Google Scholar]
- Ebbesson SOE, Tang D. A method for estimating the number of cells in histological sections. J Royal Microsc Soc. 1965;84:449–464. doi: 10.1111/j.1365-2818.1965.tb02146.x. [DOI] [PubMed] [Google Scholar]
- Edelman GM, Mountcastle V. Cortical Organization and the Group-Selective Theory of Higher brain Function. Cambridge: MIT Press; 1978. The Mindful Brain. [Google Scholar]
- Elsayed M, Magistretti PJ. A new outlook on mental illnesses: Glial involvement beyond the glue. Front Cell Neurosci. 2015;9:468. doi: 10.3389/fncel.2015.00468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksen N, Pakkenberg B. Total neocortical cell number in the mysticete brain. Anat Rec (Hoboken) 2007;290:83–95. doi: 10.1002/ar.20404. [DOI] [PubMed] [Google Scholar]
- Fabricius K, Pakkenberg H, Pakkenberg B. No changes in neocortical cell volumes or glial cell numbers in chronic alcoholic subjects compared to control subjects. Alcohol Alcohol. 2007;42:400–406. doi: 10.1093/alcalc/agm007. [DOI] [PubMed] [Google Scholar]
- Fabricius K, Jacobsen JS, Pakkenberg B. Effect of age on neocortical brain cells in 90+ year old human females - a cell counting study. Neurobiol Aging. 2013;34:91–99. doi: 10.1016/j.neurobiolaging.2012.06.009. [DOI] [PubMed] [Google Scholar]
- Ferrero C. Le champ frontal granulaire magnocellulaire de l’ecorce cerebrale. Schweiz Arch Neurol Psychiatr. 1947;59:41–70. [PubMed] [Google Scholar]
- Fields RD. The Other Brain. New York: Simon & Schuster; 2009. [Google Scholar]
- Fields RD. Neuroscience. Change in the brain’s white matter. Science. 2010;330:768–769. doi: 10.1126/science.1199139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florey E. The zoological station at Naples and the neuron: personalities and encounters in a unique institution. Biol Bull. 1985;168(suppl):137–152. [Google Scholar]
- Friede RL. Gliaindex und Hirnstoffwechsel. Wien Zeitschr Nervenh. 1953;7:143–152. [PubMed] [Google Scholar]
- Friede R. Der quantitative Anteil der Glia and der Cortexentwicklung. Acta Anat. 1954;20:290–296. [PubMed] [Google Scholar]
- Friede RL, van Houten WH. Neuronal extension and glial supply: functional significance of glia. Proc Natl Acad Sci USA. 1962;48:817–821. doi: 10.1073/pnas.48.5.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friede RL. The relationship of body size, nerve cell size, axon length, and glial density in the cerebellum. Proc Natl Acad Sci USA. 1963;49:187–193. doi: 10.1073/pnas.49.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firestein S. Ignorance: How it drives Science. New York: Oxford Univ Press; 2012. [Google Scholar]
- Galambos R. A glia-neural theory of brain function. Proc Natl Acad Sci USA. 1961;47:129–136. doi: 10.1073/pnas.47.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallatz K, Palkovits M, Szentágothai J. Ten billion neurons in the human cerebral cortex. Folia Morphol (Praha) 1982;30:133–135. [PubMed] [Google Scholar]
- Ganong WF. The Nervous System. Los Altos: Lange medical Publications; 1977. [Google Scholar]
- Ganong WF. The Nervous System. 2. Los Altos: Lange medical Publications; 1979. [Google Scholar]
- García-Amado M, Prensa L. Stereological analysis of neuron, glial and endothelial cell numbers in the human amygdaloid complex. PLoS One. 2012;7:e38692. doi: 10.1371/journal.pone.0038692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Marín V, Blazquez-Llorca L, Rodriguez JR, Gonzalez-Soriano J, DeFelipe J. Differential distribution of neurons in the gyral white matter of the human cerebral cortex. J Comp Neurol. 2010;518:4740–4759. doi: 10.1002/cne.22485. [DOI] [PubMed] [Google Scholar]
- Gazzaniga MS. Human: The Science Behind What Makes Your Brain Unique. New York: Harper Collins; 2008. [Google Scholar]
- Geuna S, Herrera-Rincon C. Update on stereology for light microscopy. Cell Tissue Res. 2015;360:5–12. doi: 10.1007/s00441-015-2143-6. [DOI] [PubMed] [Google Scholar]
- Gittins R, Harrison PJ. Neuronal density, size and shape in the human anterior cingulated cortex: a comparison of Nissl and NeuN staining. Brain Res Bull. 2004a;63:155–160. doi: 10.1016/j.brainresbull.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Gittins R, Harrison PJ. A quantitative morphometric study of the human anterior cingulate cortex. Brain Res. 2004b;1013:212–222. doi: 10.1016/j.brainres.2004.03.064. [DOI] [PubMed] [Google Scholar]
- Glees P. Morphology and Function. Oxford: Blackwell Scientific Publications; 1955. Neuroglia. [Google Scholar]
- Glees P. The biology of the neuroglia: a summary. In: Windle WF, editor. Biology of Neuroglia. Springfield: Charles C Thomas; 1958. pp. 234–242. [Google Scholar]
- Glees P. The Human Brain. New York: Cambridge Univ Press; 1988. [Google Scholar]
- Gredal O, Pakkenberg H, Karlsborg M, Pakkenberg B. Unchanged total number of neurons in motor cortex and neocortex in amyotrophic lateral sclerosis: a stereological study. J Neurosci Methods. 2000;95:171–176. doi: 10.1016/s0165-0270(99)00175-2. [DOI] [PubMed] [Google Scholar]
- Guillery RW, Herrup K. Quantification without pontification: choosing a method for counting objects in sectioned tissues. J Comp Neurol. 1997;386:2–7. doi: 10.1002/(sici)1096-9861(19970915)386:1<2::aid-cne2>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Guillery RW. On counting and counting errors. J Comp Neurol. 2002;447:1–7. doi: 10.1002/cne.10221. [DOI] [PubMed] [Google Scholar]
- Gundersen HJG, Bagger P, Bendtsen TF, Evans SM, Korbo L, Marcussen N, Møller A, Nielsen K, Nyengaard JR, Pakkenberg B, Sørensen FB, Vesterby A, West MJ. The new stereological tools: Disector, fractionator, nucleator and point sampled intercepts and their use in pathological research and diagnosis. Acta Pathol Microbiol Immunol Scand. 1988;96:857–881. doi: 10.1111/j.1699-0463.1988.tb00954.x. [DOI] [PubMed] [Google Scholar]
- Gundersen V, Storm-Mathisen J, Bergersen LH. Neuroglial transmission. Physiol Rev. 2015;95:695–726. doi: 10.1152/physrev.00024.2014. [DOI] [PubMed] [Google Scholar]
- Hadjiolov AA, Tencheva ZS, Bojadjieva-Mikhailova AG. Isolation and some characteristics of cell nuclei from brain cortex of adult cat. J Cell Biol. 1965;26:383–393. doi: 10.1083/jcb.26.2.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haines D. Fundamental Neuroscience. 2. Philadelphia: Churchill Livingstone; 2002. [Google Scholar]
- Hammarberg RA. Studien uber Klinik und Pathologie der Idiotie, nebst Untersuchungen uber die normale Anatomie der Hirnrinde. Upsala: E.D.v. Berling; 1895. [Google Scholar]
- Han X, Chen M, Wang F, Windrem M, Wang S, Shanz S, Xu Q, Oberheim NA, Bekar L, Betstadt S, Silva AJ, Takano T, Goldman SA, Nedergaard M. Forebrain engraftment by human glial progenitor cells enhances synaptic plasticity and learning in adult mice. Cell Stem Cell. 2013;12:342–353. doi: 10.1016/j.stem.2012.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanley T. ‘Neuronal fall-out’ in the ageing brain: a critical review of the quantitative data. Age Ageing. 1974;3:133–151. doi: 10.1093/ageing/3.3.133. [DOI] [PubMed] [Google Scholar]
- Harrison PJ. The neuropathology of schizophrenia. A critical review of the data and their interpretation. Brain. 1999;122:593–624. doi: 10.1093/brain/122.4.593. [DOI] [PubMed] [Google Scholar]
- Hatton GI, Parpura V. Glia-Neuronal Signaling. Boston: Kluwer Acad. Publishers; 2004. [Google Scholar]
- Hatton WJ, von Bartheld CS. Analysis of cell death in the trochlear nucleus of the chick embryo: calibration of the optical disector counting method reveals systematic bias. J Comp Neurol 1999. 1999;409:169–186. [PubMed] [Google Scholar]
- Haug H. Der Einfluss der sakularen Akzeleration auf das Hirngewicht des Menschen und dessen Anderung wahrend der Alterung. Gegenbaurs Morphol Jahrb. 1984;130:481–500. [PubMed] [Google Scholar]
- Haug H. Gibt es Nervenzellverluste wahrend der biologischen Alterung in der menschlichen Hirnrinde? Ein morphometrischer Beitrag zu dieser Frage. Nervenheilkunde. 1985;4:103–109. [Google Scholar]
- Haug H. History of neuromorphometry. J Neurosci Methods. 1986;18:1–17. doi: 10.1016/0165-0270(86)90110-x. [DOI] [PubMed] [Google Scholar]
- Haug H. Brain sizes, surfaces, and neuronal sizes of the cortex cerebri: a stereological investigation of man and his variability and a comparison with some mammals (primates, whales, marsupials, insectivores, and one elephant) Am J Anat. 1987;180:126–142. doi: 10.1002/aja.1001800203. [DOI] [PubMed] [Google Scholar]
- Haug H, Kühl S, Mecke E, Sass NL, Wasner K. The significance of morphometric procedures in the investigation of age changes in cytoarchitectonic structures of human brain. J Hirnforsch. 1984;25:353–374. [PubMed] [Google Scholar]
- Haug H, Rebhan I. Der Grauzellkoeffizient der menschlichen Hirnrinde. Berechnungen nach dem Zahlenmaterial v. Economos. II. Acta Anat. 1956;28:259–287. [PubMed] [Google Scholar]
- Hawkins A, Olszewski J. Glia/nerve cell index for cortex of the whale. Science. 1957;126:76–77. doi: 10.1126/science.126.3263.76. [DOI] [PubMed] [Google Scholar]
- Haydon PG. GLIA: listening and talking to the synapse. Nat Rev Neurosci. 2001;2:185–193. doi: 10.1038/35058528. [DOI] [PubMed] [Google Scholar]
- Heller IH, Elliott KA. Desoxyribonucleic acid content and cell density in brain and human brain tumors. Can J Biochem Physiol. 1954;32:584–592. [PubMed] [Google Scholar]
- Hempel KJ, Treff WM. The glia cell density in clinically normal persons and schizophrenics. A quantitative-morphological study with reference to glia cell-nerve cell coefficients. J Hirnforsch. 1959;4:371–411. [PubMed] [Google Scholar]
- Herculano-Houzel S. The human brain in numbers: a linearly scaled-up primate brain. Front Hum Neurosci. 2009;3:31. doi: 10.3389/neuro.09.031.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herculano-Houzel S. Not all brains are made the same: new views on brain scaling in evolution. Brain Behav Evol. 2011;78:22–36. doi: 10.1159/000327318. [DOI] [PubMed] [Google Scholar]
- Herculano-Houzel S. The remarkable, yet not extraordinary, human brain as a scaled-up primate brain and its associated cost. Proc Natl Acad Sci USA. 2012;109(Suppl 1):10661–10668. doi: 10.1073/pnas.1201895109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herculano-Houzel S. The glia/neuron ratio: How it varies uniformly across brain structures and species and what that means for brain physiology and evolution. Glia. 2014;62:1377–1391. doi: 10.1002/glia.22683. [DOI] [PubMed] [Google Scholar]
- Herculano-Houzel S, Messeder DJ, Fonseca-Azevedo K, Pantoja NA. When larger brains do not have more neurons: increased numbers of cells are compensated by decreased average cell size across mouse individuals. Front Neuroanat. 2015;9:64. doi: 10.3389/fnana.2015.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herculano-Houzel S, Lent R. Isotropic fractionator: a simple, rapid method for the quantification of total cell and neuron numbers in the brain. J Neurosci. 2005;25:2518–2521. doi: 10.1523/JNEUROSCI.4526-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herculano-Houzel S, von Bartheld CS, Miller DJ, Kaas JH. How to count cells: the advantages and disadvantages of the isotropic fractionator compared with stereology. Cell Tissue Res. 2015;360:29–42. doi: 10.1007/s00441-015-2127-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertz L, Hansson E, Rönnbäck L. Signaling and gene expression in the neuron-glia unit during brain function and dysfunction: Holger Hydén in memoriam. Neurochem Int. 2001;39:227–252. doi: 10.1016/s0197-0186(01)00017-1. [DOI] [PubMed] [Google Scholar]
- Hess HH. The rates of respiration of neurons and neuroglia in human cerebrum. In: Kety SS, Elkes J, editors. Regional Neurochemistry. Oxford: Pergamon Press; 1961. pp. 200–212. [Google Scholar]
- Hess HH, Thalheimer C. DNA and RNA and the cytoarchitecture of human frontal cortex. J Neurochem. 1971;18:1281–1290. doi: 10.1111/j.1471-4159.1971.tb00227.x. [DOI] [PubMed] [Google Scholar]
- Hilgetag CC, Barbas H. Are there ten times more glia than neurons in the brain? Brain Struct Funct. 2009;213:365–366. doi: 10.1007/s00429-009-0202-z. [DOI] [PubMed] [Google Scholar]
- Hof PR, Haroutunian V, Friedrich VL, Jr, Byne W, Buitron C, Perl DP, Davis KL. Loss and altered spatial distribution of oligodendrocytes in the superior frontal gyrus in schizophrenia. Biol Psychiatry. 2003;53:1075–1085. doi: 10.1016/s0006-3223(03)00237-3. [DOI] [PubMed] [Google Scholar]
- Hof PR, Mobbs CV. Preface. London: Elsevier; 2009. Handbook of the Neuroscience of Aging; pp. xvii–xix. [Google Scholar]
- Howard CV, Reed MG. Three–Dimensional Measurement in Microscopy. New York: Springer; 1998. Unbiased Stereology. [Google Scholar]
- Hubel DH. The Brain. A Scientific American Book. Freeman and Co, San Francisco: Freeman and Co; 1979. The brain; pp. 2–12. [Sci. Am. 241 (3)] [Google Scholar]
- Hyden H. The Neuron. In: Brachet J, Mirsky AE, editors. The Cell: Biochemistry, Physiology, Morphology. Vol. 4. New York: Acad Press; 1960. pp. 215–323. [Google Scholar]
- Hyden H. Satellite cells in the nervous system. Sci Am. 1961;205:62–70. [PubMed] [Google Scholar]
- Hyden H. Dynamic aspects of the neuron-glia relationship - a study with microchemical methods. In: Hyden H, editor. The Neuron. Amsterdam: Elsevier; 1967a. pp. 179–217. [Google Scholar]
- Hyden H. RNA in brain cells. The Neurosciences: A Study Program. 1967b:248–266. [Google Scholar]
- Hyden H, Pigon A. A cytophysiological study of the functional relationship between oligodendroglial cells and nerve cells of Deiters’ nucleus. J Neurochem. 1960;6:57–72. doi: 10.1111/j.1471-4159.1960.tb13449.x. [DOI] [PubMed] [Google Scholar]
- Iñiguez C, Gayoso MJ, Carreres J. A versatile and simple method for staining nervous tissue using Giemsa dye. J Neurosci Methods. 1985;13:77–86. doi: 10.1016/0165-0270(85)90045-7. [DOI] [PubMed] [Google Scholar]
- Ioannidis JP. Why science is not necessarily self-correcting. Perspect Psychol Sci. 2012;7:645–654. doi: 10.1177/1745691612464056. [DOI] [PubMed] [Google Scholar]
- Jacobson M. Developmental Neurobiology. 3. New York: Plenum Press; 1991. [Google Scholar]
- Jehee JF, Murre JM. The scalable mammalian brain: emergent distributions of glia and neurons. Biol Cybern. 2008;98:439–445. doi: 10.1007/s00422-008-0228-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen D. The Human Nervous System. New York: Appleton-Century-Crofts; 1980. [Google Scholar]
- Jensen GB, Pakkenberg B. Do alcoholics drink their neurons away? Lancet. 1993;342:1201–1204. doi: 10.1016/0140-6736(93)92185-v. [DOI] [PubMed] [Google Scholar]
- Jerison H. Evolution of the brain and intelligence. New York: Academic Press; 1973. [Google Scholar]
- Jorm AF. Understanding Senile Dementia. Melbourne: Chapman & Hall; 1987. p. 158. [Google Scholar]
- Kandel ER, Schwartz JH. Principles of Neural Science. 1. New York: Elsevier; 1981. [Google Scholar]
- Kandel ER, Schwartz JH. Principles of Neural Science. 2. New York: Elsevier; 1985. [Google Scholar]
- Kandel ER, Schwartz JH, Jessell TM. Principles of Neural Science. 3. Norwalk, Connecticut: Appleton & Lange; 1991. [Google Scholar]
- Kandel ER, Schwartz JH, Jessell TM. Principles of Neural Science. 4. New York: McGrawhill; 2000. [Google Scholar]
- Kandel ER, Schwartz JH, Jessell TM, Siegelbaum SA, Hudspeth AJ. Principles of Neural Science. 5. New York: McGraw-Hill; 2013. [Google Scholar]
- Kaplan S, Geuna S, Ronchi G, Ulkay MB, von Bartheld CS. Calibration of the stereological estimation of the number of myelinated axons in the rat sciatic nerve: a multicenter study. J Neurosci Methods. 2010;187:90–99. doi: 10.1016/j.jneumeth.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karbowski J. Scaling of brain metabolism and blood flow in relation to capillary and neural scaling. PLoS One. 2011;6:e26709. doi: 10.1371/journal.pone.0026709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsen AS, Pakkenberg B. Total numbers of neurons and glial cells in cortex and basal ganglia of aged brains with Down syndrome - a stereological study. Cereb Cortex. 2011;21:2519–2524. doi: 10.1093/cercor/bhr033. [DOI] [PubMed] [Google Scholar]
- Kato T, Kurokawa M. Isolation of cell nuclei from the mammalian cerebral cortex and their assortment on a morphological basis. J Cell Biol. 1967;32:649–662. doi: 10.1083/jcb.32.3.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kausler DH, Kausler BC, Krupsaw JA. The Essential Guide to Aging in the Twenty-First Century: Mind, Body, and Behavior. St Louis: University of Missouri Press; 2007. [Google Scholar]
- Kettenmann H, Ransom BH. Neuroglia. Oxford: Oxford Univ. Press; 1995. [Google Scholar]
- Kettenmann H, Ransom BR. Neuroglia. 3. Oxford: Oxford Univ. Press; 2013. [Google Scholar]
- Kettenmann H, Verkhratsky A. Glial Cells. In: Pfaff DW, editor. Neuroscience in the 21st Century. New York: Springer; 2013. pp. 475–506. [Google Scholar]
- Koch C. A Neurobiological Approach. Englewood: Roberts & Company; 2004. The Quest for Consciousness. [Google Scholar]
- Konigsmark BW. Methods for the counting of neurons. In: Nauta WJH, Ebbesson SOE, editors. Contemporary Research Methods in Neuroscience. New York: Springer; 1970. pp. 315–340. [Google Scholar]
- Konigsmark BW, Murphy EA. Neuronal populations in the human brain. Nature. 1970;228:1335–1336. doi: 10.1038/2281335a0. [DOI] [PubMed] [Google Scholar]
- Koob A. The Root of Thought: Unlocking Glia – the Brain Cell That Will Help Us Sharpen Our Wits, Heal Injury, and Treat Brain Disease. Upper Saddle River: Pearson Education; 2009. [Google Scholar]
- Kordower JH, Olanow CW, Dodiya HB, Chu Y, Beach TG, Adler CH, Halliday GM, Bartus RT. Disease duration and the integrity of the nigrostriatal system in Parkinson’s disease. Brain. 2013;136:2419–2431. doi: 10.1093/brain/awt192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreuzfuchs S. Die Groesse der Oberflaeche des Kleinhirns. Arb Neur Inst Univ Wien. 1902;9:274–278. [Google Scholar]
- Kryspin-Exner W. Beitraege zur Morphologie der Glia im Nissl-Bilde. Z Anat Entw Gesch. 1943;112:389–416. [Google Scholar]
- Kryspin-Exner W. Über die Architektonik der Glia im Zentralnervensystem des Menschen und der Säugetiere. Proc Ist Internat Congr Neuropath, Rome. 1952;3:504–510. [Google Scholar]
- Kühnel W. In memoriam des Anatomen Herbert W. Haug (1920–2002) Ann Anat. 2003;185:293–295. [Google Scholar]
- Kuffler SW, Nicholls JG. The physiology of neuroglial cells. Ergeb Physiol. 1966;57:1–90. [PubMed] [Google Scholar]
- Kuffler SW, Nicholls JG. From Neuron to Brain. Sunderland: Sinauer Associates; 1976. [Google Scholar]
- Kuffler SW, Nicholls JG. From Neuron to Brain. Sunderland: Sinauer Associates; 1985. [Google Scholar]
- Lambert K, Kinsley CH. Clinical Neuroscience. New York: Worth Publishers; 2005. [Google Scholar]
- Landolt R, Hess HH, Thalheimer C. Regional distribution of some chemical structural components of the human nervous system. I. DNA, RNA and ganglioside sialic acid. J Neurochem. 1966;13:1441–1452. doi: 10.1111/j.1471-4159.1966.tb04305.x. [DOI] [PubMed] [Google Scholar]
- Lange W. Cell number and cell density in the cerebellar cortex of man and some other mammals. Cell Tissue Res. 1975;157:115–124. doi: 10.1007/BF00223234. [DOI] [PubMed] [Google Scholar]
- Lange H, Thorner G, Hopf A. Morphometric-statistical structure analysis of human striatum, pallidum and nucleus su-thalamicus. III. Nucleus subthalamicus. J Hirnforsch. 1976;17:31–41. [PubMed] [Google Scholar]
- Lauwers F, Cassot F, Lauwers-Cances V, Puwanarajah P, Duvernoy H. Morphometry of the human cerebral cortex microcirculation: General characteristics and space-related profiles. Neuroimage. 2008;39:936–948. doi: 10.1016/j.neuroimage.2007.09.024. [DOI] [PubMed] [Google Scholar]
- Lee GM, Thornthwaite JT, Rasch EM. Picogram per cell determination of DNA by flow cytofluorometry. Anal Biochem. 1984;137:221–226. doi: 10.1016/0003-2697(84)90374-9. [DOI] [PubMed] [Google Scholar]
- Lemke G. Glial control of neuronal development. Annu Rev Neurosci. 2001;24:87–105. doi: 10.1146/annurev.neuro.24.1.87. [DOI] [PubMed] [Google Scholar]
- Lent R, Azevedo FA, Andrade-Moraes CH, Pinto AV. How many neurons do you have? Some dogmas of quantitative neuroscience under revision. Eur J Neurosci. 2012;35:1–9. doi: 10.1111/j.1460-9568.2011.07923.x. [DOI] [PubMed] [Google Scholar]
- Leuba G, Garey LJ. Evolution of neuronal numerical density in the developing and aging human visual cortex. Hum Neurobiol. 1987;6:11–8. [PubMed] [Google Scholar]
- Leuba G, Garey LJ. Comparison of neuronal and glial numerical density in primary and secondary visual cortex of man. Exp Brain Res. 1989;77:31–38. doi: 10.1007/BF00250564. [DOI] [PubMed] [Google Scholar]
- Ling EA, Leblond CP. Investigation of glial cells in semithin sections. II. Variation with age in the numbers of the various glial cell types in rat cortex and corpus callosum. J Comp Neurol. 1973;149:73–81. doi: 10.1002/cne.901490105. [DOI] [PubMed] [Google Scholar]
- Lojda Zd. Quantitative study of the Purkinje cells in the brain [Czech. Cs Morfol. 1955;3:66–78. [Google Scholar]
- Lovtrup-Rein H, McEwen BS. Isolation and fractionation of rat brain nuclei. J Cell Biol. 1966;30:405–415. doi: 10.1083/jcb.30.2.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyck L, Santamaria ID, Pakkenberg B, Chemnitz J, Schrøder HD, Finsen B, Gundersen HJ. An empirical analysis of the precision of estimating the numbers of neurons and glia in human neocortex using a fractionator-design with sub-sampling. J Neurosci Methods. 2009;182:143–156. doi: 10.1016/j.jneumeth.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Lyness SA, Zarow C, Chui HC. Neuron loss in key cholinergic and aminergic nuclei in Alzheimer disease: a meta-analysis. Neurobiol Aging. 2003;24:1–23. doi: 10.1016/s0197-4580(02)00057-x. [DOI] [PubMed] [Google Scholar]
- Mann DMA. Dopamine neurons of the vertebrate brain: some aspects of anatomy and pathology. In: Winlow W, Markestein R, editors. The Neurobiology of Dopamine Systems. Manchester: Manchester Univ. Press; 1986. pp. 87–103. [Google Scholar]
- Mares V, Crkovská J, Marsak TL, Stípek S. DNA content in nerve-cell nucleus. A biochemical and cytophotometric study of the rat cerebrum. Neuroscience. 1985;16:45–47. doi: 10.1016/0306-4522(85)90045-4. [DOI] [PubMed] [Google Scholar]
- Margolis FL. DNA and DNA-polymerase activity in chicken brain regions during ontogeny. J Neurochem. 1969;16:447–456. doi: 10.1111/j.1471-4159.1969.tb10385.x. [DOI] [PubMed] [Google Scholar]
- Maron ME. The RAND Corporation, Memorandum RM-3522-PR. 1963. Artificial Intelligence and Brain Mechanisms; pp. 1–35. [PubMed] [Google Scholar]
- Mayhew TM, Gundersen HJ. ‘If you assume, you can make an ass out of u and me’: A decade of the disector for stereological counting of particles in 3D space. J Anat. 1996;188:1–15. [PMC free article] [PubMed] [Google Scholar]
- Mayhew TM, MacLaren R, Henery CC. Fractionator studies on Purkinje cells in the human cerebellum: numbers in right and left halves of male and female brains. J Anat. 1990;169:63–70. [PMC free article] [PubMed] [Google Scholar]
- McGeer PL, McGeer EG, Suzuki JS. Aging and extrapyramidal function. Arch Neurol. 1977;34:33–35. doi: 10.1001/archneur.1977.00500130053010. [DOI] [PubMed] [Google Scholar]
- McKeown RE, Cuffe SP, Schulz RM. US suicide rates by age group, 1970–2002: an examination of recent trends. Am J Public Health. 2006;96:1744–1751. doi: 10.2105/AJPH.2005.066951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahan UJ. Steve: Remembrances of Stephen W. Kuffler. Sunderland: Sinauer Associates; 1990. p. 142. [Google Scholar]
- Meehan PJ, Saltzman LE, Sattin RW. Suicides among older United States residents: epidemiologic characteristics and trends. Am J Public Health. 1991;81:1198–1200. doi: 10.2105/ajph.81.9.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merril BC. A Master’s Thesis in Philosophy. University of Nevada; Reno: 2009. The Neural Dogma; p. 124. Thesis. [Google Scholar]
- Meynert T. Der Bau der Grosshirnrinde und seine ortlichen Verschiedenheiten, nebst einem pathologisch-anatomischen Corollarium. Vierteljahresschr Psych. 1868:88–113. [Google Scholar]
- Meynert T. Der Bau der Grosshirnrinde und seine örtlichen Verschiedenheiten. 1872:18. Reprint in Vierteljahrschrift fur Psychiatrie etc. [Google Scholar]
- Miller DJ, Balaram P, Young NA, Kaas JH. Three counting methods agree on cell and neuron numbers in chimpanzee primary visual cortex. Front Neuroanat. 2014;8:36. doi: 10.3389/fnana.2014.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan JT, Chana G, Pardo CA, Achim C, Semendeferi K, Buckwalter J, Courchesne E, Everall IP. Microglial activation and increased microglial density observed in the dorsolateral prefrontal cortex in autism. Biol Psychiatry. 2010;68:368–376. doi: 10.1016/j.biopsych.2010.05.024. [DOI] [PubMed] [Google Scholar]
- Morrison JH, Hof PR. Life and death of neurons in the aging brain. Science. 1997;278:412–419. doi: 10.1126/science.278.5337.412. [DOI] [PubMed] [Google Scholar]
- Mosch B, Morawski M, Mittag A, Lenz D, Tarnok A, Arendt T. Aneuploidy and DNA replication in the normal human brain and Alzheimer’s disease. J Neurosci. 2007;27:6859–6867. doi: 10.1523/JNEUROSCI.0379-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mota B, Herculano-Houzel S. All brains are made of this: a fundamental building block of brain matter with matching neuronal and glial masses. Front Neuroanat. 2014;8:127. doi: 10.3389/fnana.2014.00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountjoy CQ, Roth M, Evans NJ, Evans HM. Cortical neuronal counts in normal elderly controls and demented patients. Neurobiol Aging. 1983;4:1–11. doi: 10.1016/0197-4580(83)90048-9. [DOI] [PubMed] [Google Scholar]
- Mouton PR. An introduction for bioscientists. Baltimore: Johns Hopkins University Press; 2002. Principles and practices of unbiased stereology. [Google Scholar]
- Mouton PR, Pakkenberg B, Gundersen HJ, Price DL. Absolute number and size of pigmented locus coeruleus neurons in young and aged individuals. J Chem Neuroanat. 1994;7:185–190. doi: 10.1016/0891-0618(94)90028-0. [DOI] [PubMed] [Google Scholar]
- Mufson EJ, Benzing WC. Lack of neocortical nerve cell loss in Alzheimer’s disease: reality or methodological artifact. Neurobiol Aging. 1994;15:361–362. doi: 10.1016/0197-4580(94)90034-5. discussion: 379–380. [DOI] [PubMed] [Google Scholar]
- Mühlmann M. About the question of glia formation. Zur Neurogliabildungsfrage Beitr Pathol Anat Allg Pathol. 1936;96:361–374. [Google Scholar]
- Mullen RJ, Buck CR, Smith AM. NeuN, a neuronal specific nuclear protein in vertebrates. Development. 1992;116:201–211. doi: 10.1242/dev.116.1.201. [DOI] [PubMed] [Google Scholar]
- Nairn JG, Bedi KS, Mayhew TM, Campbell LF. On the number of Purkinje cells in the human cerebellum: unbiased estimates obtained by using the “fractionator”. J Comp Neurol. 1989;290:527–532. doi: 10.1002/cne.902900407. [DOI] [PubMed] [Google Scholar]
- Nansen F. The Structure and Combination of the Histological Elements in the Central Nervous System. Bergens Museums Aarsberetning for 1886. 1887:29–195. [Google Scholar]
- Nass S, Nass MM. Intramitochondrial fibers with DNA characteristics. II. Enzymatic and other hydrolytic treatments. J Cell Biol. 1963;19:613–629. doi: 10.1083/jcb.19.3.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute of Neurological Disorders and Stroke. FY2001 NINDS Congressional Justification Narrative. http://www.ninds.nih.gov/news_and_events/congressional_testimony/ninds_cjrev_testimony.htm.
- National Institute of Neurological Disorders and Stroke. FY2011 NINDS Congressional Justification Narrative. http://www.ninds.nih.gov/news_and_events/congressional_testimony/ninds_fy_2011_cj.htm.
- National Institute of Neurological Disorders and Stroke. FY2015 NINDS Congressional Justification Narrative. http://www.ninds.nih.gov/news_and_events/congressional_testimony/ninds_fy_2015_cj.htm.
- Nauta WJH, Feirtag M. The Brain. A Scientific American Book. San Francisco: Freeman and Co; 1979. The organization of the brain; pp. 41–55. [Sci Am 241 (3)] [DOI] [PubMed] [Google Scholar]
- Nedergaard M, Ransom B, Goldman SA. New roles for astrocytes: redefining the functional architecture of the brain. Trends Neurosci. 2003;26:523–530. doi: 10.1016/j.tins.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Neville C. The Complete Guide to Referencing and Avoiding Plagiarism. 2. Maidenhead: Open UP; 2007. [Google Scholar]
- Nicholls JG. Closing Remarks. Ann New York Acad Sci. 1991;633:633–636. [Google Scholar]
- Nicholls JG. Biographical Memoirs. Vol. 74. Natl Acad Press; 1998. Stephen W. Kuffler, August 24, 1913 – October 11, 1980; pp. 193–208. [PubMed] [Google Scholar]
- Nicholls JG, Martin AR, Wallace BG. From Neuron to Brain. 2. Sunderland: Sinauer; 1985. [Google Scholar]
- Nicholls JG, Martin AR, Wallace BG. From Neuron to Brain. 3. Sunderland: Sinauer; 1992. [Google Scholar]
- Nicholls JG, Martin AR, Fuchs PA, Brown DA, Diamond ME, Weisblat DA. From Neuron to Brain. 5. Sunderland: Sinauer; 2012. [Google Scholar]
- Nielsen RD, Abitz M, Pakkenberg B. Neuron and glial cell numbers in the mediodorsal thalamic nucleus in brains of schizophrenic subjects. Image Anal Stereol. 2008;27:133–141. [Google Scholar]
- Nishiyama A, Yang Z, Butt A. What’s in a name? J Anat. 2005;207:687–693. doi: 10.1111/j.1469-7580.2005.00489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissl F. Nervenzellen und graue Substanz. Munch Med Wochenschr. 1898;45:988–992. 1023–1029, 1060–1062. [Google Scholar]
- NPR. New project would map the human brain. 2013 http://www.npr.org/2013/02/18/172336350/new-project-would-map-the-human-brain.
- Noback CR, Demarest RJ. Introduction and Review. New York: McGrawHill; 1972. The Human Nervous System. [Google Scholar]
- Noback CR, Demarest RJ. Introduction and Review. 2. New York: McGrawHill; 1975. The Human Nervous System. [Google Scholar]
- Noback CR, Strominger NL, Demarest RJ. Introduction and Review. 4. Philadelphia: LEA & FEBIGER; 1991. The Human Nervous System. [Google Scholar]
- Nolte J. The Human Brain. St. Louis: The C.V. Mosby Company; 1981. [Google Scholar]
- Nolte J. The Human Brain. 3. St. Louis: The C.V. Mosby Company; 1993. [Google Scholar]
- Nurnberger JI, Gordon MW. The cell density of neural tissues: direct counting method and possible applications as a biologic referent. Prog Neurobiol. 1957;2:100–128. [PubMed] [Google Scholar]
- Nurnberger JI. Direct enumeration of cells of the brain. In: Windle WF, editor. Biology of Neuroglia. Springfield: Charles C Thomas; 1958. pp. 193–202. [Google Scholar]
- Nuzzo R. Fooling ourselves. Nature. 2015;526:182–185. doi: 10.1038/526182a. [DOI] [PubMed] [Google Scholar]
- Ohm TG, Busch C, Bohl J. Unbiased estimation of neuronal numbers in the human nucleus coeruleus during aging. Neurobiol Aging. 1997;18:393–399. doi: 10.1016/s0197-4580(97)00034-1. [DOI] [PubMed] [Google Scholar]
- Ongür D, Drevets WC, Price JL. Glial reduction in the subgenual prefrontal cortex in mood disorders. Proc Natl Acad Sci USA. 1998;95:13290–13295. doi: 10.1073/pnas.95.22.13290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmen SJ, van Engeland H, Hof PR, Schmitz C. Neuropathological findings in autism. Brain. 2004;127:2572–2583. doi: 10.1093/brain/awh287. [DOI] [PubMed] [Google Scholar]
- Pakkenberg B. Pronounced reduction of total neuron number in mediodorsal thalamic nucleus and nucleus accumbens in schizophrenics. Arch Gen Psychiatry. 1990;47:1023–1028. doi: 10.1001/archpsyc.1990.01810230039007. [DOI] [PubMed] [Google Scholar]
- Pakkenberg B. Stereological quantitation of human brains from normal and schizophrenic individuals. Acta Neurol Scand Suppl. 1992;137:20–33. doi: 10.1111/j.1600-0404.1992.tb05034.x. [DOI] [PubMed] [Google Scholar]
- Pakkenberg B. Leucotomized schizophrenics lose neurons in the mediodorsal thalamic nucleus. Neuropathol Appl Neurobiol. 1993;19:373–380. doi: 10.1111/j.1365-2990.1993.tb00457.x. [DOI] [PubMed] [Google Scholar]
- Pakkenberg B. Total nerve cell number in neocortex in chronic schizophrenics and controls estimated using optical disectors. Biol Psychiatry. 1993;34:768–772. doi: 10.1016/0006-3223(93)90065-l. [DOI] [PubMed] [Google Scholar]
- Pakkenberg B, Gundersen HJ. Total number of neurons and glial cells in human brain nuclei estimated by the disector and the fractionator. J Microsc. 1988;150:1–20. doi: 10.1111/j.1365-2818.1988.tb04582.x. [DOI] [PubMed] [Google Scholar]
- Pakkenberg B, Gundersen HJ. New stereological method for obtaining unbiased and efficient estimates of total nerve cell number in human brain areas. Exemplified by the mediodorsal thalamic nucleus in schizophrenics. Acta Pathol Microbiol Immunol Scand. 1989;97:677–681. doi: 10.1111/j.1699-0463.1989.tb00462.x. [DOI] [PubMed] [Google Scholar]
- Pakkenberg B, Gundersen HJ. Neocortical neuron number in humans: effect of sex and age. J Comp Neurol. 1997;384:312–320. [PubMed] [Google Scholar]
- Pakkenberg B, Evans SM, Møller A, Brændgaard H, Gundersen HJG. Total number of neurons in human neocortex related to age and sex estimated by way of optical disectors. Acta Stereologica. 1989;8:251–256. [Google Scholar]
- Pakkenberg B, Møller A, Gundersen HJ, Mouritzen Dam A, Pakkenberg H. The absolute number of nerve cells in substantia nigra in normal subjects and in patients with Parkinson’s disease estimated with an unbiased stereological method. J Neurol Neurosurg Psychiatry. 1991;54:30–33. doi: 10.1136/jnnp.54.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakkenberg B, Pelvig D, Marner L, Bundgaard MJ, Gundersen HJ, Nyengaard JR, Regeur L. Aging and the human neocortex. Exp Gerontol. 2003;38:95–99. doi: 10.1016/s0531-5565(02)00151-1. [DOI] [PubMed] [Google Scholar]
- Pakkenberg H. The number of nerve cells in the cerebral cortex of man. J Comp Neurol. 1966;128:17–20. doi: 10.1002/cne.901280103. [DOI] [PubMed] [Google Scholar]
- Palay SL. An electron-microscopical study of neuroglia. In: Windle WF, editor. Biology of Neuroglia. Springfield: Charles C. Thomas; 1958. pp. 24–38. [Google Scholar]
- Pastor J, Sola RG. The role of astrocytes in epileptogenesis. In: Kaneez FS, editor. Underlying Mechanisms of Epilepsy. Rijeka: InTech; 2008. pp. 19–44. [Google Scholar]
- Pedersen KM, Marner L, Pakkenberg H, Pakkenberg B. No global loss of neocortical neurons in Parkinson’s disease: a quantitative stereological study. Mov Disord. 2005;20:164–171. doi: 10.1002/mds.20289. [DOI] [PubMed] [Google Scholar]
- Pelvig DP, Pakkenberg H, Regeur L, Oster S, Pakkenberg B. Neocortical glial cell numbers in Alzheimer’s disease. A stereological study. Dement Geriatr Cogn Disord. 2003;16:212–219. doi: 10.1159/000072805. [DOI] [PubMed] [Google Scholar]
- Pelvig DP, Pakkenberg H, Stark AK, Pakkenberg B. Neocortical glial cell numbers in human brains. Neurobiol Aging. 2008;29:1754–1762. doi: 10.1016/j.neurobiolaging.2007.04.013. [DOI] [PubMed] [Google Scholar]
- Penn NW, Suwalski R. Quantitative determination of deoxyribonucleic acid in rat brain. Biochem J. 1969;115:563–568. doi: 10.1042/bj1150563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A, Morrison JH, Rosene DL, Hyman BT. Feature article: are neurons lost from the primate cerebral cortex during normal aging? Cereb Cortex. 1998;8:295–300. doi: 10.1093/cercor/8.4.295. [DOI] [PubMed] [Google Scholar]
- Peters A. The effects of normal aging on myelin and nerve fibers: a review. J Neurocytol. 2002;31:581–593. doi: 10.1023/a:1025731309829. [DOI] [PubMed] [Google Scholar]
- Pfrieger FW, Barres BA. What the fly’s glia tell the fly’s brain. Cell. 1995;83:671–674. doi: 10.1016/0092-8674(95)90178-7. [DOI] [PubMed] [Google Scholar]
- Pitt B. Loss in late life. BMJ. 1998;316:1452–1454. doi: 10.1136/bmj.316.7142.1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponomarev VS. Glial index in vestibular nuclei of humans, macacos, and dogs. Arkh Anat Gistol Embriol. 1966;51:100–104. [PubMed] [Google Scholar]
- Pope A. Implications of histochemical studies for metabolism of the neuroglia. In: Windle WF, editor. Biology of Neuroglia. Springfield: Charles C Thomas; 1958. pp. 211–233. [Google Scholar]
- Pope A. The intralaminar distribution of dipeptidase activity in human frontal isocortex. J Neurochem. 1959;4:31–41. doi: 10.1111/j.1471-4159.1959.tb13171.x. [DOI] [PubMed] [Google Scholar]
- Popken GJ, Bunney WE, Jr, Potkin SG, Jones EG. Subnucleus-specific loss of neurons in medial thalamus of schizophrenics. Proc Natl Acad Sci USA. 2000;97:9276–9280. doi: 10.1073/pnas.150243397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell TPS, Guillery RW, Cowan WM. A quantitative study of the fornix mamillo-thalamic system. J Anat. 1957;91:419–435. [PMC free article] [PubMed] [Google Scholar]
- Proctor R, Schiebinger LL. Agnotology: The Making and Unmaking of Ignorance. Stanford: Stanford Univ Press; 2008. [Google Scholar]
- Purves D. How they work and what that tells us about who we are. Upper Saddle River: FT Press; 2010. Brains. [Google Scholar]
- Rajkowska G. Postmortem studies in mood disorders indicate altered numbers of neurons and glial cells. Biol Psychiatry. 2000;48:766–777. doi: 10.1016/s0006-3223(00)00950-1. [DOI] [PubMed] [Google Scholar]
- Rajkowska G. Cell pathology in mood disorders. Semin Clin Neuropsychiatry. 2002;7:281–292. doi: 10.1053/scnp.2002.35228. [DOI] [PubMed] [Google Scholar]
- Regeur L, Jensen GB, Pakkenberg H, Evans SM, Pakkenberg B. No global neocortical nerve cell loss in brains from patients with senile dementia of Alzheimer’s type. Neurobiol Aging. 1994a;15:347–352. doi: 10.1016/0197-4580(94)90030-2. [DOI] [PubMed] [Google Scholar]
- Regeur L, Jensen GB, Pakkenberg H, Evans SM, Pakkenberg B. Author’s response to commentaries. Neurobiol Aging. 1994b;15:379–380. doi: 10.1016/0197-4580(94)90030-2. [DOI] [PubMed] [Google Scholar]
- Reichenbach A. Glia:neuron index: review and hypothesis to account for different values in various mammals. Glia. 1989;2:71–77. doi: 10.1002/glia.440020202. [DOI] [PubMed] [Google Scholar]
- Ribeiro PFM, Ventura-Antunes L, Gabi M, Mota B, Grinberg LT, Farfel JM, Ferretti-Rebustini REL, Leite REP, Jacob Filho W, Herculano-Houzel S. The human cerebral cortex is neither one nor many: neuronal distribution reveals two quantitatively different zones in the gray matter, three in the white matter, and explains local variations in cortical folding. Front Neuroanat. 2013;7:28. doi: 10.3389/fnana.2013.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rilling JK, Insel TR. The primate neocortex in comparative perspective using magnetic resonance imaging. J Hum Evol. 1999;37:191–223. doi: 10.1006/jhev.1999.0313. [DOI] [PubMed] [Google Scholar]
- Robins E, Smith DE, Eydt KM. The quantitative histochemistry of cerebral cortex. J Neurochem. 1956;1:54–67. doi: 10.1111/j.1471-4159.1956.tb12054.x. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Arellano JJ, Parpura V, Zorec R, Verkhratsky A. Astrocytes in physiological aging and Alzheimer’s disease. Neuroscience. 2016 doi: 10.1016/j.neuroscience.2015.01.007. in press. [DOI] [PubMed] [Google Scholar]
- Rowland LP, Mettler FA. Cell concentration and laminar thickness in the frontal cortex of psychotic patients; studies on cortes removed at operation. J Comp Neurol. 1949;90:255–280. doi: 10.1002/cne.900900302. [DOI] [PubMed] [Google Scholar]
- Saldanha J, Gannicliffe A, Itzhaki RF. An improved method for preparing DNA from human brain. J Neurosci Methods. 1984;11:275–279. doi: 10.1016/0165-0270(84)90089-x. [DOI] [PubMed] [Google Scholar]
- Savioz A, Blouin JL, Guidi S, Antonarakis SE, Bouras C. A method for the extraction of genomic DNA from human brain tissue fixed and stored in formalin for many years. Acta Neuropathol. 1997;93:408–413. doi: 10.1007/s004010050632. [DOI] [PubMed] [Google Scholar]
- Scheff SW, Baldwin SA. Preparation and staining of tissue for counting neurons. Kopf Carrier. 1996;45:1–4. [Google Scholar]
- Schlote W. Zur Gliaarchitektonik der menschlichen Grosshirnrinde im Niss-Bild. Arch Psychiatr Nervenkr Z Gesamte Neurol Psychiatr. 1959;199:573–595. doi: 10.1007/BF00342861. [DOI] [PubMed] [Google Scholar]
- Schmitz C, Hof PR. Recommendations for straightforward and rigorous methods of counting neurons based on a computer simulation approach. J Chem Neuroanat. 2000;20:93–114. doi: 10.1016/s0891-0618(00)00066-1. [DOI] [PubMed] [Google Scholar]
- Schmitz C, Hof PR. Design-based stereology in neuroscience. Neuroscience. 2005;130:813–831. doi: 10.1016/j.neuroscience.2004.08.050. [DOI] [PubMed] [Google Scholar]
- Schmutte T, O’Connell M, Weiland M, Lawless S, Davidson L. Stemming the tide of suicide in older white men: a call to action. Am J Mens Health. 2009;3:189–200. doi: 10.1177/1557988308316555. [DOI] [PubMed] [Google Scholar]
- Schröder KF, Hopf A, Lange H, Thörner G. Morphometrical-statistical structure analysis of human striatum, pallidum and subthalamic nucleus. J Hirnforsch. 1975;16:333–350. [PubMed] [Google Scholar]
- Selemon LD, Begovic A. Stereologic analysis of the lateral geniculate nucleus of the thalamus in normal and schizophrenic subjects. Psychiatry Res. 2007;151:1–10. doi: 10.1016/j.psychres.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shariff GA. Cell counts in the primate cerebral cortex. J Comp Neurol. 1953;98:381–400. doi: 10.1002/cne.900980302. [DOI] [PubMed] [Google Scholar]
- Sherwood CC, Stimpson CD, Raghanti MA, Wildman DE, Uddin M, Grossman LI, Goodman M, Redmond JC, Bonar CJ, Erwin JM, Hof PR. Evolution of increased glia-neuron ratios in the human frontal cortex. Proc Natl Acad Sci USA. 2006;103:13606–13611. doi: 10.1073/pnas.0605843103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sholl DA. The Organization of the Cerebral Cortex. Methuen, London, New York: Wiley and Sons; 1956. [Google Scholar]
- Sholl DA. The measurable parameters of the cerebral cortex and their significance in its organization. Prog Neurobiol. 1956;(2):324–333. [PubMed] [Google Scholar]
- Sholl DA. A comparative study of the neuronal packing density in the cerebral cortex. J Anat. 1959;93:143–158. [PMC free article] [PubMed] [Google Scholar]
- Snell RS. Clinical Anatomy for Medical Students. Boston: Little, Brown + Co; 1980. [Google Scholar]
- Snell RS. Clinical Anatomy for Medical Students. 6. Philadelphia: Lippincott Williams & Wilkins; 2006. [Google Scholar]
- Smith K. Settling the great glia debate. Nature. 2010;468:160–162. doi: 10.1038/468160a. [DOI] [PubMed] [Google Scholar]
- Soper B, Rosenthal G. The number of neurons in the brain: how we report what we do not know. Tech Psychol. 1988;15:153–156. [Google Scholar]
- Srinivasan M, Sedmak D, Jewell S. Effect of fixatives and tissue processing on the content and integrity of nucleic acids. Am J Pathol. 2002;161:1961–1971. doi: 10.1016/S0002-9440(10)64472-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark AK, Pakkenberg B. Histological changes of the dopaminergic nigrostriatal system in aging. Cell Tissue Res. 2004;318:81–92. doi: 10.1007/s00441-004-0972-9. [DOI] [PubMed] [Google Scholar]
- Stephan H, Frahm H, Baron G. New and revised data on volumes of brain structures in insectivores and primates. Folia Primatol. 1981;35:1–29. doi: 10.1159/000155963. [DOI] [PubMed] [Google Scholar]
- Steward O. Principles of cellular, molecular and developmental neuroscience. New York: Springer; 1989. [Google Scholar]
- Steward O. Functional Neuroscience. New York: Springer; 2000. [Google Scholar]
- Streit WJ. Role of macrophages and microglia in the injured CNS. In: Berry M, Logan A, editors. CNS Injuries: Cellular Responses and Pharmacological Strategies. Boca Raton, FL: CRC Press; 1999. pp. 81–97. [Google Scholar]
- Streit W. Microglial cells. In: Kettenmann H, Ransom BR, editors. Neuroglia. 3. Oxford: Oxford Univ Press; 2013. pp. 86–97. [Google Scholar]
- Stolzenburg JU, Reichenbach A, Neumann M. Size and density of glial and neuronal cells within the cerebral neocortex of various insectivoran species. Glia. 1989;2:78–84. doi: 10.1002/glia.440020203. [DOI] [PubMed] [Google Scholar]
- Szentágothai J. The modular architectonic principle of neural centers. Rev Physiol Biochem Pharmacol. 1983;98:11–61. doi: 10.1007/BFb0033866. [DOI] [PubMed] [Google Scholar]
- Terry RD, DeTeresa R, Hansen LA. Neocortical cell counts in normal human adult aging. Ann Neurol. 1987;21:530–539. doi: 10.1002/ana.410210603. [DOI] [PubMed] [Google Scholar]
- Thörner G, Lange H, Hopf A. Morphometrical-statistical structure analysis of human striatum, pallidus and subthalamic nucleus. II. Globus pallidus. J Hirnforsch. 1975;16:401–413. [PubMed] [Google Scholar]
- Thompson H. The total number of functional cells in the cerebral cortex of man, and the percentage of the total volume of the cortex composed of nerve cell bodies, calculated from Karl Hammarberg’s data; together with a comparison of the number of giant cells with the number of pyramidal fibers. J Comp Neurol. 1899;9:113–140. [Google Scholar]
- Thomson W. Nature Series. Popular Lectures and Addresses. Vol. 1. London: Macmillan and Co; 1889. The practical applications of electricity. Electrical units of measurement; pp. 73–136. (A lecture delivered at the Institution of Civil Engineers, May 3, 1883) [Google Scholar]
- Thörner G, Lange H, Hopf A. Morphometrical-statistical structure analysis of human striatum, pallidus and subthalamic nucleus. II. Globus pallidus. J Hirnforsch. 1975;16:401–413. [PubMed] [Google Scholar]
- Thune JJ, Pakkenberg B. Stereological studies of the schizophrenic brain. Brain Res Rev. 2000;31:200–204. doi: 10.1016/s0165-0173(99)00038-7. [DOI] [PubMed] [Google Scholar]
- Todtenkopf MS, Vincent SL, Benes FM. A cross-study meta-analysis and three-dimensional comparison of cell counting in the anterior cingulate cortex of schizophrenic and bipolar brain. Schizophr Res. 2005;73:79–89. doi: 10.1016/j.schres.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Tower DB. Structural and functional organization of mammalian cerebral cortex; the correlation of neurone density with brain size; cortical neurone density in the fin whale (Balaenoptera physalus L.) with a note on the cortical neurone density in the Indian elephant. J Comp Neurol. 1954;101:19–52. doi: 10.1002/cne.901010103. [DOI] [PubMed] [Google Scholar]
- Tower DB, Elliott KAC. Activity of acetylcholine system in cerebral cortex of various unanesthetized mammals. Amer J Physiol. 1952;168:747–759. doi: 10.1152/ajplegacy.1952.168.3.747. [DOI] [PubMed] [Google Scholar]
- Tower DB, Young OM. The activities of butyrylcholinesterase and carbonic anhydrase, the rate of anaerobic glycolysis, and the question of a constant density of glial cells in cerebral cortices of various mammalian species from mouse to whale. J Neurochem. 1973;20:269–278. doi: 10.1111/j.1471-4159.1973.tb12126.x. [DOI] [PubMed] [Google Scholar]
- Triarhou LC. Georg N. Koskinas (1885–1975) and his scientific contributions to the normal and pathological anatomy of the human brain. Brain Res Bull. 2005;68:121–139. doi: 10.1016/j.brainresbull.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Triarhou LC. The signalling contributions of Constantin von Economo to basic, clinical and evolutionary neuroscience. Brain Res Bull. 2006;69:223–243. doi: 10.1016/j.brainresbull.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Tsai PS, Kaufhold JP, Blinder P, Friedman B, Drew PJ, Karten HJ, Lyden PD, Kleinfeld D. Correlations of neuronal and microvascular densities in murine cortex revealed by direct counting and colocalization of nuclei and vessels. J Neurosci. 2009;29:14553–14570. doi: 10.1523/JNEUROSCI.3287-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullian EM, Sapperstein SK, Christopherson KS, Barres BA. Control of synapse number by glia. Science. 2001;291:657–660. doi: 10.1126/science.291.5504.657. [DOI] [PubMed] [Google Scholar]
- Vawter MP, Freed WJ, Kleinman JE. Neuropathology of bipolar disorder. Biol Psychiatry. 2000;48:486–504. doi: 10.1016/s0006-3223(00)00978-1. [DOI] [PubMed] [Google Scholar]
- Verkhratsky A, Mattson MP, Toescu EC. Aging in the mind. Trends Neurosci. 2004;27:577–578. doi: 10.1016/j.tins.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Verkhratsky A, Butt A. Glial Neurobiology: A Text Book. Chichester, UK: John Wiley & Sons; 2007. [Google Scholar]
- Verkhratsky A, Butt A. Neuroglia: Definition, Classification, Evolution, Numbers, Development. Chapter 3. Oxford, UK: Wiley-Blackwell; 2013. Glial Physiology and Pathophysiology; pp. 73–104. [Google Scholar]
- Verkhratsky A, Rodríguez JJ, Parpura V. Neuroglia in ageing and disease. Cell Tissue Res. 2014;357:493–503. doi: 10.1007/s00441-014-1814-z. [DOI] [PubMed] [Google Scholar]
- von Apathy S. Das leitende Element des Nervensystems und seine topographischen Beziehungen zu den Zellen. Erste Mitth Zool Stat Neapel. 1897;12:495–748. [Google Scholar]
- von Economo C. Ein Koeffizient fur die Organisationshohe der Grosshirnrinde (Zellanzahl derselben und einige andere Cortex-Masse) Klin Wochenschr. 1926;5:593–595. [Google Scholar]
- von Economo C, Koskinas GN. Die Cytoarchitektonik der Grosshirnrinde des erwachsenen Menschen. Wien/Berlin: Springer; 1925. [Google Scholar]
- von Bartheld CS. Systematic bias in an “unbiased” neuronal counting technique. Anat Rec. 1999;257:119–120. doi: 10.1002/(SICI)1097-0185(19990815)257:4<119::AID-AR2>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- von Bartheld CS. Comparison of 2-D and 3-D counting: the need for calibration and common sense. Trends Neurosci. 2001;24:504–506. doi: 10.1016/s0166-2236(00)01960-3. [DOI] [PubMed] [Google Scholar]
- von Bartheld CS. Counting particles in tissue sections: choices of methods and importance of calibration to minimize biases. Histol Histopathol. 2002;17:639–648. doi: 10.14670/HH-17.639. [DOI] [PubMed] [Google Scholar]
- von Bartheld CS, Wouters FS. Quantitative techniques for imaging cells and tissues. Cell Tissue Res. 2015;360:1–4. doi: 10.1007/s00441-015-2149-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Bonin G. The frontal lobes of primates: cytoarchitectural studies. Res Publ Assoc Res Nerv Ment Dis. 1948;27:67–83. [PubMed] [Google Scholar]
- Vostrikov VM, Uranova NA, Orlovskaya DD. Deficit of perineuronal oligodendrocytes in the prefrontal cortex in schizophrenia and mood disorders. Schizophr Res. 2007;94:273–280. doi: 10.1016/j.schres.2007.04.014. [DOI] [PubMed] [Google Scholar]
- Wall PD. In: The History of Neuroscience in Autobiography. Squire LR, editor. San Diego: Academic Press; 2001. pp. 472–500. Patrick D. Wall. [Google Scholar]
- Walloe S, Pakkenberg B, Fabricius K. Stereological estimation of total cell numbers in the human cerebral and cerebellar cortex. Front Hum Neurosci. 2014;8:508. doi: 10.3389/fnhum.2014.00508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward TS, Rosen GD, von Bartheld CS. Optical disector counting in cryosections and vibratome sections underestimates particle numbers: effects of tissue quality. Microsc Res Tech. 2008;71:60–68. doi: 10.1002/jemt.20525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weise CM, Mouton PR, Eschbacher J, Coons SW, Krakoff J. A post-mortem stereological study of striatal cell number in human obesity. Obesity (Silver Spring) 2015;23:100–104. doi: 10.1002/oby.20897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West MJ. New stereological methods for counting neurons. Neurobiol Aging. 1993a;14:275–285. doi: 10.1016/0197-4580(93)90112-o. [DOI] [PubMed] [Google Scholar]
- West MJ. Regionally specific loss of neurons in the aging human hippocampus. Neurobiol Aging. 1993b;14:287–293. doi: 10.1016/0197-4580(93)90113-p. [DOI] [PubMed] [Google Scholar]
- West MJ, Gundersen HJ. Unbiased stereological estimation of the number of neurons in the human hippocampus. J Comp Neurol. 1990;296:1–22. doi: 10.1002/cne.902960102. [DOI] [PubMed] [Google Scholar]
- Williams RW, Herrup K. The control of neuron number. Annu Rev Neurosci. 1988;11:423–453. doi: 10.1146/annurev.ne.11.030188.002231. [DOI] [PubMed] [Google Scholar]
- Williams RW, Rakic P. Three-dimensional counting: accurate and direct method to estimate numbers of cells in sectioned material. J Comp Neurol. 1988;278:344–352. doi: 10.1002/cne.902780305. [DOI] [PubMed] [Google Scholar]
- Williams RW, von Bartheld CS, Rosen GD. Counting cells in sectioned material: a suite of techniques, tools, and tips. Curr Protoc Neurosci. 2003;Chapter 1(Unit 1.11) doi: 10.1002/0471142301.ns0111s24. [DOI] [PubMed] [Google Scholar]
- Windle WF. Biology of Neuroglia. Springfield, Ill: C.C. Thomas; 1958. [Google Scholar]
- Winick M. Changes in nucleic acid and protein content of the human brain during growth. Pediatr Res. 1968;2:352–355. doi: 10.1203/00006450-196809000-00003. [DOI] [PubMed] [Google Scholar]
- Witelson SF, Kigar DL, Harvey T. The exceptional brain of Albert Einstein. Lancet. 1999;35:2149–2153. doi: 10.1016/S0140-6736(98)10327-6. [DOI] [PubMed] [Google Scholar]
- Wittrock MC. The Human Brain. Englewood Cliffs, NJ: Prentice Hall, Inc; 1977. [Google Scholar]
- Young NA, Flaherty DK, Airey DC, Varlan P, Aworunse F, Kaas JH, Collins CE. Use of flow cytometry for high-throughput cell population estimates in fixed brain tissue. Front Neuroanat. 2012;6:27. doi: 10.3389/fnana.2012.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuhas D, Jabr F. [Accessed October 30, 2013];Know your neurons Chapter 4: What is the ratio of glia to neurons in the brain? 2012 http://blogs.scientificamerican.com/brainwaves/2012/06/13/know-your-neurons-what-is-the-ratio-of-glia-to-neurons-in-the-brain/
- Zamenhof S, Bursztyn H, Rich K, Zamenhof PJ. The determination of deoxyribonucleic acid and of cell number in brain. J Neurochem. 1964;11:505–509. doi: 10.1111/j.1471-4159.1964.tb07499.x. [DOI] [PubMed] [Google Scholar]
- Zamenhof S. Final number of Purkinje and other large cells in the chick cerebellum influenced by incubation temperatures during their proliferation. Brain Res. 1976;109:392–394. doi: 10.1016/0006-8993(76)90540-0. [DOI] [PubMed] [Google Scholar]
- Zamenhof S, Klimuszko D. Final number of Purkinje and other large cells in the chick cerebellum increased by oxygen and by glucose. Brain Res. 1977;128:385–388. doi: 10.1016/0006-8993(77)91006-x. [DOI] [PubMed] [Google Scholar]
- Zorzetto R. [Accessed on 4/21/2015];How many neurons do we really have? (And why we may have 10x fewer glia than we previously thought) 2012 http://neuroamer.wordpress.com/2012/05/19/453/