Abstract
Background
Social anxiety disorder involves fear of social objects or situations. Social referencing may play an important role in the acquisition of this fear and could be a key determinant in future biomarkers and treatment pathways. However, the neural underpinnings mediating such learning in social anxiety are unknown. Using event-related functional magnetic resonance imaging, we examined social reference learning in social anxiety disorder. Specifically, would patients with the disorder show increased amygdala activity during social reference learning, and further, following social reference learning, show particularly increased response to objects associated with other people’s negative reactions?
Method
A total of 32 unmedicated patients with social anxiety disorder and 22 age-, intelligence quotient- and gender-matched healthy individuals responded to objects that had become associated with others’ fearful, angry, happy or neutral reactions.
Results
During the social reference learning phase, a significant group × social context interaction revealed that, relative to the comparison group, the social anxiety group showed a significantly greater response in the amygdala, as well as rostral, dorsomedial and lateral frontal and parietal cortices during the social, relative to non-social, referencing trials. In addition, during the object test phase, relative to the comparison group, the social anxiety group showed increased bilateral amygdala activation to objects associated with others’ fearful reactions, and a trend towards decreased amygdala activation to objects associated with others’ happy and neutral reactions.
Conclusions
These results suggest perturbed observational learning in social anxiety disorder. In addition, they further implicate the amygdala and dorsomedial prefrontal cortex in the disorder, and underscore their importance in future biomarker developments.
Keywords: Amygdala, emotions, imaging, medial prefrontal cortex, social anxiety
Introduction
Emotional expressions allow the rapid transmission of information on the value of objects/actions from one person to the next. This is seen empirically in social referencing paradigms, where the caregiver’s emotional expressions influence the child’s behavior: the child is more likely to approach a novel object to which the caregiver has smiled than one to which the caregiver has shown fear (Klinnert et al. 1986). Comparable behavior is seen in infant monkeys (Mineka & Cook, 1993). Observational learning has been considered a process by which phobias can be acquired and is thought to contribute to the development of anxiety (Mineka & Zinbarg, 2006). Certainly, anxious parents show greater levels of parental anxiety towards novel objects in front of their children (Muris et al. 1996; Fisak & Grills-Taquechel, 2007; Aktar et al. 2013). However, despite the importance of social referencing in models of the development of anxiety, no previous work has examined the neural correlates of social referencing in adults with and without social phobia.
It has been argued that the amygdala is critical for learning the valence of novel objects from the emotional expressions of others (Blair, 2003) and this has been confirmed in animal and human work (Jeon et al. 2010; Meffert et al. 2015). This is interesting given consistent reports of elevated responses to emotional, and in particular negative emotional, expressions in adults with social phobia relative to healthy adults (Stein et al. 2002; Straube et al. 2004, 2005; Phan et al. 2006; Blair et al. 2008b, 2011; Evans et al. 2008). Considered within a social referencing perspective, these data together could be taken to suggest that an elevated amygdale response may lead patients with social phobia, relative to healthy individuals, to more strongly learn valence information from the facial expressions of others.
The current study investigates this issue using a novel social referencing task. The task had two types of phases. During the social reference learning phases, subjects saw animated faces that changed gaze and/or expression as these faces looked either towards objects (social referencing condition), towards empty space (expression only condition), or back towards the research participant (expression face on condition). The subject was asked to respond, via button press, whether these faces were male or female. The faces displayed fear, anger, happiness or neutral emotion. Subsequent to the social reference learning phases, where the face looked towards and learned about the objects, there were object test phases, where the neural response to the previously displayed objects that had become associated with the four different emotions could be examined. We predicted that the patients with social anxiety would show heightened blood oxygen level-dependent (BOLD) responses within the amygdala during social referencing trials particularly when the facial expression changed to fear or anger. We also predicted that they would show heightened BOLD responses within the amygdala to objects associated with fearful and angry expressions. The current study tests these predictions.
Method
Subjects
This study included 32 patients with social anxiety disorder, generalized subtype, and 22 healthy comparison individuals, group-matched on age, gender and intelligence quotient (see Table 1). Subjects were recruited from National Institute of Mental Health Institutional Review Board-approved advertisements.
Table 1.
Subject characteristics
| Social anxiety disorder (n = 32) |
Healthy comparison (n = 22) |
p | |
|---|---|---|---|
| Age, years | 30.6 (7.94) | 29.7 (7.94) | n.s. |
| Gender, n | n.s. | ||
| Male | 11 | 13 | |
| Female | 21 | 9 | |
| Race, n | |||
| Caucasian | 21 | 13 | n.s. |
| African-American | 6 | 4 | n.s. |
| Asian | 5 | 2 | <0.001 |
| Unknown | – | 3 | <0.001 |
| IQ | 113.1 (13.14) | 114.3 (13.08) | – |
| LSAS-SR | 67.0 (12.30) | 18.2 (12.30) | |
| IDS-SR | 17.1 (9.04) | 3.9 (4.12) | |
| GAF | 56.7 (15.72) | – |
Data are given as mean (standard deviation) unless otherwise indicated.
n.s., Non-significant; IQ, intelligence quotient; LSAS-SR, Liebowitz Social Anxiety Scale-Self Rated; IDS-SR, Inventory of Depressive Symptomatology-Self Rated; GAF, Global Assessment of Functioning.
Subjects with social anxiety disorder had to meet Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV) criteria for the disorder (1994) based on the Structural Clinical interview for DSM-IV Axis I disorders (First et al. 1997) and a confirmatory clinical interview by a board-certified psychiatrist (D.S.P.). No social anxiety disorder patient had another Axis I diagnosis apart from generalized anxiety disorder (n = 15); all patients were currently medication-free 6+ months. Healthy comparison individuals were excluded if they had a history of any psychiatric illness. All subjects were in good physical health, as confirmed by a complete physical examination, and provided written informed consent.
Further, as part of the assessment, all subjects completed the Liebowitz Social Anxiety Scale – Self Report (LSAS-SR) and the Inventory of Depressive Symptomatology - Self Report (IDS-SR). In addition, for the patients with social anxiety disorder, the level of overall social, occupational and psychological functioning was assessed by the Global Assessment of Functioning. Scores on these measures characterized the social anxiety disorder group as having moderate levels of social anxiety with mild associated impairment in functioning (see Table 1).
Behavioral task
The imaging task involved two phases: a social reference learning phase and an object test phase. These two phases were implemented in four runs, generating four repetitions of the two task phases.
Social reference learning phase
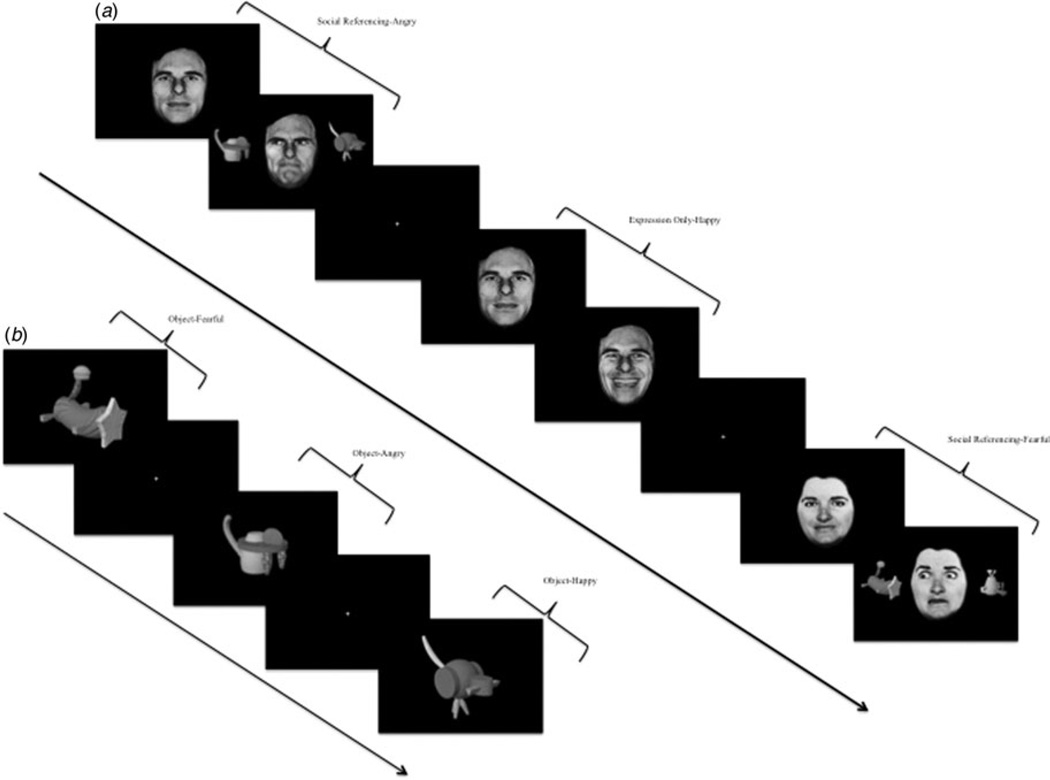
During this phase, subjects viewed stimuli in three different social contexts: social referencing, expression only, and expressions face on. Each trial in all three social contexts began with the presentation of a neutral face looking straight ahead for 800 ms, followed by 1800 ms context-specific presentation (see Fig. 1). The subject’s task was to determine whether the presented face was female or male via button press. The trials across the three phases were created to isolate key experimental variables.
Fig. 1.
Task illustration. Trials from (a) the social reference learning phase and (b) the object test phase.
The social referencing condition included the main events of interest. There were four types of social referencing trials, each associated with a specific emotion. On each trial, two unrecognizable, neutral objects appeared on either side of the neutral face, and the eye gaze of the face changed direction to ‘look’ at one of these two objects. The face would then ‘react’ to the object by showing a fearful, angry, happy or neutral expression. The objects were paired with each expression with 100% reinforcement; i.e. the happy face would always look towards the same object and always change expression to be happy. Thus, the subject learned to associate four different cued objects with the four different facial expressions (fearful, angry, happy, neutral).
Expression only and expressions face on trials were constructed to isolate the key component of social referencing. Thus, as in the social referencing trials, the eye gaze of faces in the expression only trials also changed direction to look to one side. However, unlike in the social referencing trials, no objects were presented for the face in the expression only trials to ‘look’ at. Thus, the social referencing and expression only trials differed in the presence (social referencing) or absence (expression only) of novel objects to be paired with an expression. Similarly, the expressions face on trials isolated other aspects of social referencing. These trials were identical to the expression only trials except that the eye gaze did not change - the face continued to ‘look’ straight towards the subject. Thus, across the three types of events, the social referencing learning phase consisted of a 3 (social context: social referencing, expression only, expression face on) by 4 (emotion: fearful, angry, happy, neutral) design. Each social reference learning phase lasted 92.8 s and involved two presentations of each of the 12 conditions. In addition, each phase had eight fixation point trials (2900 ms each) to provide a baseline. There were four social reference learning phases in each of the four runs, resulting in a total of 32 presentations per social context condition.
Object test
During this phase, subjects saw the four neutral objects that had previously been paired and become associated with one of the four emotions during the social reference learning phase in the social referencing condition: objects paired with fearful emotion (object-fearful), objects paired with angry emotion (object-angry), objects paired with happy emotion (object-happy), and objects paired with neutral emotion (object-neutral). Subjects indicated by button-press whether they felt like approaching or avoiding the object. Each object test phase lasted 34.8 s and involved two presentations of each of the four associated emotions. In addition, each phase had four fixation point trials (2900 ms each) to provide a baseline. There were four object test phases in each of the four runs, resulting in a total of 32 presentations per paired emotion.
Stimuli
The face stimuli used were selected from the Pictures of Facial Affect (Ekman & Friesen, 1976), and then subjected to digital manipulation to make the faces appear to be looking to either the right- or left-hand side of the screen (Fig. 1). Following Hooker et al. (2006) the objects used throughout the scan for subjects to form associations were from the Michael Tarr library of stimuli, with stimuli images courtesy of Michael J. Tarr, Center for the Neural Basis of Cognition and Department of Psychology, Carnegie Mellon University (http://www.tarrlab.org/).
Functional magnetic resonance imaging (fMRI) parameters
Whole-brain BOLD fMRI data were acquired using a 1.5 Tesla GE MRI scanner. Following sagittal localization, functional T2* weighted images were acquired using an echo-planar single-shot gradient echo pulse sequence [matrix = 64 × 64 mm, repetition time (TR) = 2900 ms, echo time (TE) = 30 ms, field of view (FOV) = 240 mm, 3.75 × 3.75 × 4 mm voxels]. Images were acquired in 46 3 mm axial slices per brain volume. In the same session, a high-resolution T1-weighed anatomical image was acquired to aid with spatial normalization (three-dimensional Spoiled GRASS; TR = 8.1 ms, TE = 3.2 ms, flip angle = 20°; FOV = 240 mm, 124 axial slices, thickness = 1.0 mm, 256 × 256 acquisition matrix).
Data were analysed within the framework of the general linear model using analysis of functional neuroimages (AFNI) (Cox, 1996). Both individual- and group-level analyses were conducted. The first five volumes in each scan series, collected before equilibrium magnetization was reached, were discarded. Motion correction was performed by registering all volumes in the EPI dataset to a volume collected close to acquisition of the high-resolution anatomical dataset.
The EPI datasets for each subject were spatially smoothed (isotropic 6 mm kernel) to reduce variability among individuals and generate group maps. Next, the time series data were normalized by dividing the signal intensity of a voxel at each time point by the mean signal intensity of that voxel for each run and multiplying the result by 100, producing regression coefficients representing percentage-signal change. Regressors (social referencing-fearful, social referencing-angry, social referencing-happy, social referencing-neutral, expression on-fearful, expression on-angry, expression on-happy, expression on-neutral, expression face on-fearful, expression face on-angry, expression face on-happy, expression face on-neutral, object-fearful, object-angry, object-happy, object-neutral) were created by convolving the train of stimulus events with a γ-variate hemodynamic response function. Linear regression modeling was performed using these regressors plus regressors for a first-order baseline drift function. This produced for each voxel and each regressor, a β coefficient and its associated t statistic.
Voxel-wise group analyses involved transforming single subject β coefficients into the standard coordinate space of Talairach & Tournoux (1988). Subsequently, two analyses of variance (ANOVAs) were performed. The first was a 2 (group: social anxiety disorder, healthy comparison) by 3 (social context: social referencing, expression only, expression face on) by 4 (emotion: fearful, angry, happy, neutral) ANOVA examining the neural responses occurring during the social reference learning phase of the task. The second was a 2 (group: social anxiety disorder, healthy comparison) by 4 (object: object-fearful, object-angry, object-happy, object-neutral) ANOVA examining the neural responses to the objects during the object test phase of the task. These produced statistical maps of the main effects and interactions (p < 0.005). To correct for multiple comparisons for the whole-brain analysis at p < 0.005, we performed a spatial clustering operation using AlphaSim (http://afni.nimh.nih.gov/pub/dist/doc/manual/AlphaSim.pdf) with 1000 Monte Carlo simulations taking into account the entire EPI matrix. This procedure yielded a minimum cluster size with a map-wise false-positive probability of p < 0.05, corrected for multiple comparisons. Precise statistical results, with correction for multiple comparisons, are clearly indicated in Table 2.
Table 2.
Significant areas of activation for the group × social context and group × object interactions†
| Region | Brodmann area | Volume, mm3 | x | y | z | F |
|---|---|---|---|---|---|---|
| Group × social context interaction | ||||||
| Right amygdala* | 173 | 24 | −1 | −18 | 3.50 | |
| Left middle frontal gyrus | 10 | 4520 | −38 | 38 | 13 | 13.28 |
| Right middle frontal gyrus | 6 | 4191 | 34 | 0 | 53 | 12.91 |
| Left rostral medial frontal cortex | 8/9 | 3586 | −9 | 32 | 44 | 11.79 |
| Left caudate | 2432 | −13 | 14 | 0 | 14.94 | |
| Right precuneus | 7 | 1677 | 19 | −68 | 43 | 9.22 |
| Left posterior insula | 13 | 2756 | −35 | −22 | 8 | 12.09 |
| Right superior frontal gyrus | 10 | 3348 | 27 | 51 | 25 | 14.89 |
| Left middle frontal gyrus | 6 | 2525 | −27 | 5 | 47 | 14.39 |
| Left dorsomedial frontal cortex | 6 | 2042 | −1 | 3 | 55 | 11.84 |
| Left precuneus | 19 | 1841 | −33 | −80 | 34 | 12.59 |
| Group × object interaction | ||||||
| Right amygdala* | 21 | 29 | 0 | −15 | 2.71 | |
| Left amygdala* | 62 | −17 | −5 | −12 | 3.08 |
All activations are effects observed in whole-brain analyses significant at p < 0.005 corrected for multiple comparisons (significant at p < 0.05) except
(significant at p < 0.005 unconnected).
After observing hypothesized group differences, post-hoc analyses were performed to facilitate interpretations. For these analyses, average percentage signal change was measured across all voxels within each region of interest generated from the functional mask, and data for main effects and interactions were unpacked and analysed using appropriate follow-up tests, principally one-way repeated-measures ANOVAs, within SPSS (USA).
Results
Behavioral data
Social referencing phase
The 2 (group: social anxiety disorder, healthy comparison) by 3 (social context: social referencing, expression only, expression face on) by 4 (emotion: fearful, angry, happy, neutral) ANOVA performed on gender judgments showed no significant interactions with group (F range = 0.45 to 1.03, n.s.) or main effect of group (F < 1, n.s.). The data from two healthy controls were missing due to technical problems. Overall, the percentage of correct responses was high, with all cells having more than 97% correct responses (see online Supplementary Table S1 for full data).
Object test phase
The second 2 (group: social anxiety disorder, healthy comparison) by 4 (object: object-fearful, object-angry, object-happy, object-neutral) ANOVA performed on wanting to approach v. wanting to avoid judgments also showed no significant interaction with group (F = 1.23, n.s.) or main effect of group (F = 1.25, n.s.). However, it should be noted that results from planned one-way ANOVAs showed a trend towards the social anxiety disorder group being more likely than the healthy comparison group to want to avoid the objects associated with other individuals’ fearful expressions (whereas the social anxiety group wanted to avoid 61.0% of those trials, the healthy comparison group wanted to avoid 51.0%; F = 2.92, p = 0.09).
fMRI data
Social reference learning phase
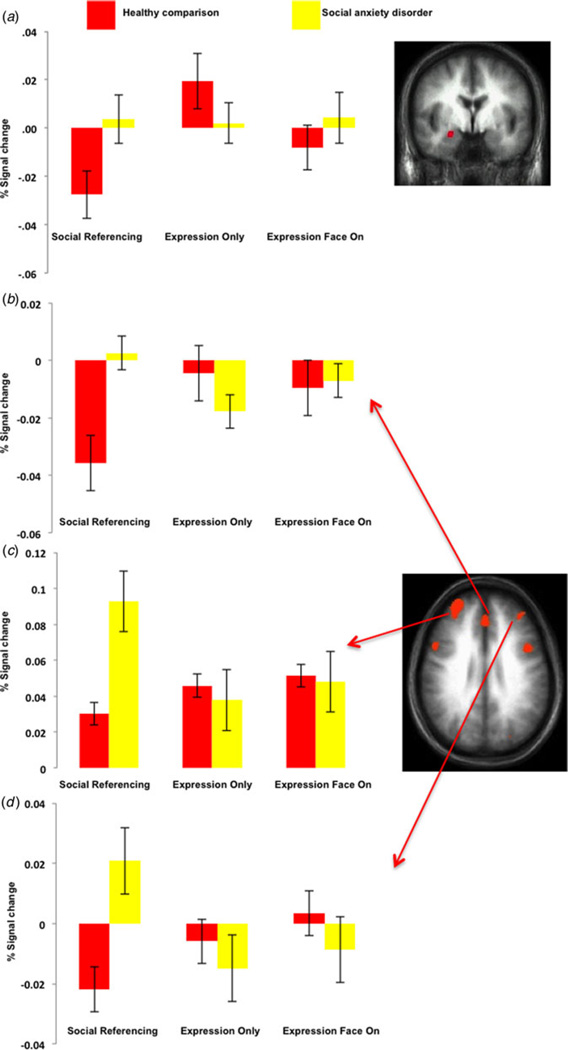
The first 2 (group: social anxiety disorder, healthy comparison) by 3 (social context: social referencing, expression only, expression face on) by 4 (emotion: fearful, angry, happy, neutral) ANOVA revealed significant group × social context interactions not only within the amygdala (Fig. 2) but also the rostral, dorsomedial and lateral frontal and parietal cortices (Table 2). In all regions, the patients with social anxiety disorder showed significantly increased responses to the social referencing trials compared with the healthy comparison individuals (F range = 7.32 to 15.18, p< range 0.001 to 0.01). However, the two groups did not differ significantly in their response within any of the regions to the expression face on trials (F range = 0.028 to 3.81, n.s.), or the expressions only trials (F range = 0.004 to 2.82, n.s.), apart from in the superior frontal cortex where social anxiety disorder < healthy comparison group (F = 14.61, p < 0.001). No regions survived correction for multiple comparisons for the group × social context × emotion or group × emotion interactions.
Fig. 2.
Interactions of group × social context. Blood oxygen level-dependent responses within the (a) right amygdala (24, −1, −18), (b) left medial frontal cortex (−9, 32, 44), (c) right middle frontal gyrus (34, 0, 53) and (d) left middle frontal gyrus (−38, 38, 13) to the social referencing, expression only, and expressions face on trials for the two groups. Values are means, with standard errors represented by vertical bars.
Object test phase
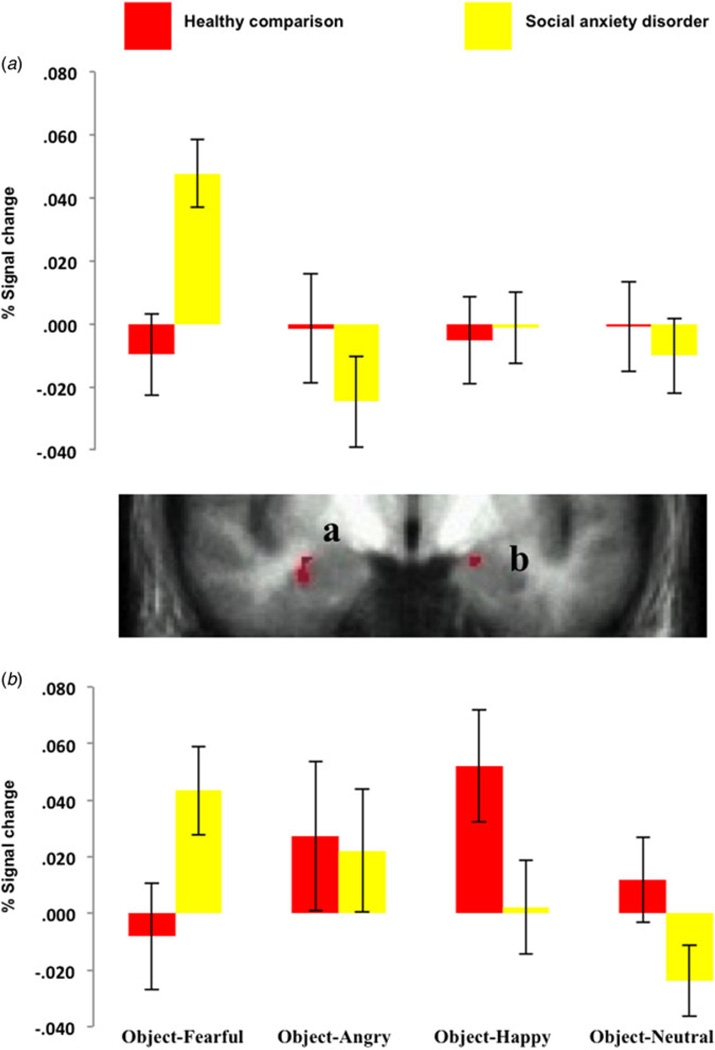
The second 2 (group: social anxiety disorder, healthy comparison) by 4 (object: object-fearful, object-angry, object-happy, object-neutral) ANOVA revealed significant group × object interactions within the bilateral amygdala (corrected for small volume). Within both amygdala, the patients with social anxiety disorder showed significantly increased responses to the stimuli associated with fearful expressions (object-fearful) relative to the healthy comparison group (F = 11.79 and 4.45, respectively, p < 0.001 and 0.05). In addition, they showed a trend towards a significantly decreased response to the stimuli associated with happy (object-happy) and neutral (object-neutral) expressions relative to the healthy comparison individuals in the right amygdala (F = 3.77 and 3.30, respectively, p = 0.058 and 0.075) (see Fig. 3).
Fig. 3.
Interactions of group × object during the object test phase. Blood oxygen level-dependent responses within (a) the right amygdala (29, 0, −15) and (b) left amygdala (−17, −5, −12) to objects associated with fearful, angry, happy and neutral expressions for the two groups. Values are means, with standard errors represented by vertical bars.
Relationship with symptom severity
We examined the relationship between severity of LSAS symptoms in the participants with social anxiety disorder and amygdala responses during social referencing trials and objects associated with fearful expressions in the object test phase. The response within the right (though not the left) amygdala to objects associated with fearful (but not other) expressions was significantly associated with LSAS severity in the participants with social anxiety disorder (Pearson’s r = 0.351, p < 0.05). The amygdala response during social referencing trials was not significantly related to LSAS severity in the participants with social anxiety disorder.
Discussion
The current study compared the neural response to a social referencing task in patients with social anxiety disorder and healthy, matched adults. There were four main findings. First, during the social reference learning phase, patients with social anxiety disorder relative to healthy adults did show the expected enhancement of amygdala activity, though this was not selective for negative expressions but was rather a function of gaze direction towards an object. Second, significant group × social context interactions were further seen within the rostral, dorsomedial and lateral frontal cortices. Third, during the object test phase, behaviorally there was a trend towards the social anxiety disorder group being more likely than the healthy comparison group to want to avoid the objects associated with other individuals’ fearful expressions. Fourth, and in line with this, evidence of face emotion specificity emerged during the object test phase. Specifically, relative to the comparison individuals, the patients with social anxiety disorder showed a significantly increased activation within the bilateral amygdala to the objects previously paired with others’ fearful expressions. In addition, there was a trend towards the patients with social anxiety disorder showing a decreased neural activation relative to the comparison individuals to the objects that had become associated with others’ happy and neutral expressions.
Previous fMRI work finds that patients with social phobia show heightened amygdala and temporal cortical activity to angry (Stein et al. 2002; Straube et al. 2004, 2005; Phan et al. 2006; Evans et al. 2008) and fearful expressions (Blair et al. 2008b, 2011a; though see Stein et al. 2002). The current study extends this work by considering how responses to facial emotions might influence learning. Specifically, patients with social anxiety disorder showed greater amygdala responsiveness than healthy adults in the context of a social referencing situation; this occurred when observing another individual change the focus of their attention to a novel object. Nevertheless, this association was found across emotional expressions rather than only for fearful and angry expressions, as had been expected. This may reflect a lack of power to detect the group × social context × emotion interaction. Alternatively, it may reflect a role for perturbed amygdala function in processing gaze direction information during social referencing (Graham & Labar, 2012). Given this, the current study indicates that patients with social anxiety disorder may be particularly responsive to relevant gaze direction information on faces.
During the social reference learning phase, several other regions apart from the amygdala showed significant group × social context interactions: the rostral, dorsomedial and lateral frontal cortices. This might reflect heightened self-referential processing to these stimuli in the patients with social anxiety disorder. Previous work has shown that this population shows heightened medial frontal cortical responses to self-relevant material (Blair et al. 2008a, 2010, 2011b; Goldin & Gross, 2010). Moreover, self-referential processing has been shown to recruit the cortical midline structure from ventromedial and rostral regions to the posterior cingulate cortex (Amodio & Frith, 2006; Northoff et al. 2006). However, there have been suggestions that regions more rostral to those seen here are particularly critical for self-referential processing (e.g. Mitchell et al. 2006). Certainly, the regions previously implicated in aberrant self-referential processing in patients with social anxiety disorder have been more rostral (Blair et al. 2008a, 2010; Goldin & Gross, 2010). Alternatively, the enhanced activity may represent a heightened attention response to the social referencing trials. Previous studies examining the response to emotional expressions in patients with social anxiety disorder have revealed increased responses within the dorsomedial and lateral frontal cortices (Stein et al. 2002; Amir et al. 2005; Phan et al. 2006; Blair et al. 2008b). Moreover, behavioral work has shown heightened orientation to face information in social phobia (e.g. Gamble & Rapee, 2010; Moriya & Tanno, 2011).
While we did not observe group differences to emotion during the social reference learning phase, there were emotion-specific group differences during the object test phase. Relative to the healthy comparison group, the patients with social anxiety disorder showed significantly greater neural activation to the stimuli that had become associated with others’ fearful expressions and a trend towards significantly decreased neural responses to the objects that had become associated with others’ happy and neutral expressions. Previous work has examined aversive conditioning in patients with social phobia (Schneider et al. 1999; Hermann et al. 2002; Veit et al. 2002). This work has reported heightened conditioning as well as greater amygdala responses whilst associating neutral facial expressions with aversive stimuli (Schneider et al. 1999). The current study extends this work by showing heightened association formation as a function of observational learning/social referencing (Klinnert et al. 1986; Mineka & Cook, 1993) – at least with respect to fearful expressions and the amygdala response. Indeed, responses within the right amygdala to objects associated with fearful expressions were significantly associated with LSAS severity in the patients with social anxiety disorder. Such findings are particularly interesting in the context of observational learning models of anxiety/phobia generation (see Muris et al. 1996; Mineka & Zinbarg, 2006; Fisak & Grills-Taquechel, 2007). In addition, the current findings are interesting in that they suggest that there is reduced observational learning when the valence is positive (or neutral).
Three caveats should be considered with respect to the current study. First, while, as predicted, the patients with social anxiety disorder showed increased amygdala responses to objects associated with fearful expressions, they did not show increased amygdala responses to objects associated with angry expressions. This might reflect a type II error. Alternatively, it might reflect the possibility that angry expressions play less of a role in communications of an object’s/action’s valence and more of a role in initiating immediate response control; stopping the current act (Blair, 2003). As such, the amygdala would not show increased responses to objects associated with angry expressions because the amygdala is less involved in emotional learning on the basis of angry expressions. Second, we assessed the subjects’ attitudes towards the objects associated with other people’s reactions by asking them whether they would approach or avoid the object. While this revealed group differences in the predicted direction they were only trend-level findings. Future work might consider potentially more sensitive techniques such as graded response options; i.e. a five-button choice scaling for ‘like’ to ‘dislike’. Third, the groups significantly differed in their depression severity as indexed by the IDS. One way to deal with this potential confound would be to use IDS as a covariate. The problem with this approach is that it may result in co-varying out a phenomenon related to the pathophysiology of social anxiety disorder; if severity of social anxiety disorder increases the risk for feelings of depression (LSAS and IDS scores were significantly correlated), the pathophysiology of social anxiety disorder would inevitably be associated with depression severity in these patients. An ideal scenario would be to include a third group of participants – those with diagnoses of major depressive disorder (or at least a group who did not differ on IDS scores with the participants with social anxiety disorder). However, that was not possible here. As such, we cannot be certain that the pathophysiology here relates to social anxiety disorder rather than depression severity. However, slightly mitigating this concern it should be noted that follow-up analyses did reveal that IDS was not a significant covariate for any of the follow-up analyses on the functional regions of interest. In addition, the right amygdala response to objects associated with fearful expressions was not significantly associated with IDS score in the patients with social anxiety disorder.
In summary, we found that during social reference learning, patients with social anxiety disorder showed increased neural activation relative to healthy individuals in a number of regions, including the amygdala as well as the rostral, dorsomedial and lateral frontal and parietal cortices. In addition, following social reference learning, the patients with social anxiety disorder showed emotion-specific abnormalities, with increased neural activation to objects that had become associated with other individuals’ fear, and a trend towards decreased responses to objects that had become associated with other individuals’ happy and neutral expressions. These results are strongly supportive of an important role of social referencing in the development and/or maintenance of social anxiety disorder and suggest that this mechanism be the focus of future biomarker development.
Acknowledgments
This research was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Mental Health. Further, the authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Footnotes
Supplementary material
The supplementary material for this article can be found at http://dx.doi.org/10.1017/S0033291716001537
Declaration of Interest
None.
The authors have no financial disclosures to report.
References
- Aktar E, Majdandzic M, de Vente W, Bogels SM. The interplay between expressed parental anxiety and infant behavioural inhibition predicts infant avoidance in a social referencing paradigm. Journal of Child Psychology and Psychiatry. 2013;54:144–156. doi: 10.1111/j.1469-7610.2012.02601.x. [DOI] [PubMed] [Google Scholar]
- Amir N, Klumpp H, Elias J, Bedwell JS, Yanasak N, Miller LS. Increased activation of the anterior cingulate cortex during processing of disgust faces in individuals with social phobia. Biological Psychiatry. 2005;57:975–981. doi: 10.1016/j.biopsych.2005.01.044. [DOI] [PubMed] [Google Scholar]
- Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nature Reviews Neuroscience. 2006;7:268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Blair KS, Geraci M, Devido G, McCaffrey D, Gang C, Vythilingam M, Ng P, Hollon NG, Jones M, Blair RJR, Pine DS. Neural response to self- and other referential praise and criticism in generalized social phobia. Archives of General Psychiatry. 2008a;65:1176–1184. doi: 10.1001/archpsyc.65.10.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair KS, Geraci M, Hollon N, Otero M, DeVido J, Majestic C, Jacobs M, Blair RJ, Pine DS. Social norm processing in adult social phobia: atypically increased ventromedial frontal cortex responsiveness to unintentional (embarrassing) transgressions. American Journal of Psychiatry. 2010;167:1526–1532. doi: 10.1176/appi.ajp.2010.09121797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair KS, Geraci M, Korelitz K, Otero M, Towbin K, Ernst M, Leibenluft E, Blair RJ, Pine DS. The pathology of social phobia is independent of developmental changes in face processing. American Journal of Psychiatry. 2011a;168:1202–1209. doi: 10.1176/appi.ajp.2011.10121740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair KS, Geraci M, Otero M, Majestic C, Odenheimer S, Jacobs M, Blair RJ, Pine DS. Atypical modulation of medial prefrontal cortex to self-referential comments in generalized social phobia. Psychiatry Research. 2011b;193:38–45. doi: 10.1016/j.pscychresns.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair KS, Shaywitz J, Smith BW, Rhodes R, Geraci M, Jones M, McCaffrey D, Vythilingam M, Finger E, Mondillo K, Jacobs M, Charney D, Blair RJR, Drevets WC, Pine DS. Response to emotional expressions in generalized social phobia (GSP) and generalized anxiety disorder (GAD): evidence for separate disorders. American Journal of Psychiatry. 2008b;165:1193–1202. doi: 10.1176/appi.ajp.2008.07071060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair RJR. Facial expressions, their communicatory functions and neuro-cognitive substrates. Philosophical Transactions of the Royal Society of London Biological Sciences. 2003;358:561–572. doi: 10.1098/rstb.2002.1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Ekman P, Friesen WV. Pictures of Facial Affect. Palo Alto, CA: Consulting Psychologists Press; 1976. [Google Scholar]
- Evans KC, Wright CI, Wedig MM, Gold AL, Pollack MH, Rauch SL. A functional MRI study of amygdala responses to angry schematic faces in social anxiety disorder. Depression and Anxiety. 2008;25:496–505. doi: 10.1002/da.20347. [DOI] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV. Washington, DC: American Psychiatric Press; 1997. [Google Scholar]
- Fisak B, Jr, Grills-Taquechel AE. Parental modeling, reinforcement, and information transfer: risk factors in the development of child anxiety? Clinical Child and Family Psychology Review. 2007;10:213–231. doi: 10.1007/s10567-007-0020-x. [DOI] [PubMed] [Google Scholar]
- Gamble AL, Rapee RM. The time-course of attention to emotional faces in social phobia. Journal of Behavior Therapy and Experimental Psychiatry. 2010;41:39–44. doi: 10.1016/j.jbtep.2009.08.008. [DOI] [PubMed] [Google Scholar]
- Goldin PR, Gross JJ. Effects of mindfulness-based stress reduction (MBSR) on emotion regulation in social anxiety disorder. Emotion. 2010;10:83–91. doi: 10.1037/a0018441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham R, Labar KS. Neurocognitive mechanisms of gaze-expression interactions in face processing and social attention. Neuropsychologia. 2012;50:553–566. doi: 10.1016/j.neuropsychologia.2012.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann C, Ziegler S, Birbaumer N, Flor H. Psychophysiological and subjective indicators of aversive pavlovian conditioning in generalized social phobia. Biological Psychiatry. 2002;52:328–337. doi: 10.1016/s0006-3223(02)01385-9. [DOI] [PubMed] [Google Scholar]
- Hooker CI, Germine LT, D’Esposito M. Amygdala response to facial expressions reflects emotional learning. Journal of Neuroscience. 2006;26:8915–8922. doi: 10.1523/JNEUROSCI.3048-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon D, Kim S, Chetana M, Jo D, Ruley HE, Lin SY, Rabah D, Kinet JP, Shin HS. Observational fear learning involves affective pain system and Cav1.2 Ca2+ channels in ACC. Nature Neuroscience. 2010;13:482–488. doi: 10.1038/nn.2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinnert MD, Emde RN, Butterfield P, Campos JJ. Social referencing: the infant’s use of emotional signals from a friendly adult with mother present. Developmental Psychology. 1986;22:427–432. [Google Scholar]
- Meffert H, Brislin SJ, White SF, Blair JR. Prediction errors to emotional expressions: the roles of the amygdala in social referencing. Social Cognitive and Affective Neuroscience. 2015;10:537–544. doi: 10.1093/scan/nsu085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineka S, Cook M. Mechanisms involved in the observational conditioning of fear. Journal of Experimental Psychology: General. 1993;122:23–38. doi: 10.1037//0096-3445.122.1.23. [DOI] [PubMed] [Google Scholar]
- Mineka S, Zinbarg R. A contemporary learning theory perspective on the etiology of anxiety disorders: it’s not what you thought it was. American Psychology. 2006;61:10–26. doi: 10.1037/0003-066X.61.1.10. [DOI] [PubMed] [Google Scholar]
- Mitchell JP, Macrae CN, Banaji MR. Dissociable medial prefrontal contributions to judgments of similar and dissimilar others. Neuron. 2006;50:655–663. doi: 10.1016/j.neuron.2006.03.040. [DOI] [PubMed] [Google Scholar]
- Moriya J, Tanno Y. The time course of attentional disengagement from angry faces in social anxiety. Journal of Behavior Therapy and Experimental Psychiatry. 2011;42:122–128. doi: 10.1016/j.jbtep.2010.08.001. [DOI] [PubMed] [Google Scholar]
- Muris P, Steerneman P, Merckelbach H, Meesters C. The role of parental tearfulness and modeling in children’s fear. Behavior Research and Therapy. 1996;34:265–268. doi: 10.1016/0005-7967(95)00067-4. [DOI] [PubMed] [Google Scholar]
- Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H, Panksepp J. Self-referential processing in our brain – a meta-analysis of imaging studies on the self. Neurolmage. 2006;31:440–457. doi: 10.1016/j.neuroimage.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Phan KL, Fitzgerald DA, Nathan PJ, Tancer ME. Association between amygdala hyperactivity to harsh faces and severity of social anxiety in generalized social phobia. Biological Psychiatry. 2006;59:424–429. doi: 10.1016/j.biopsych.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Schneider F, Weiss U, Kessler C, Muller-Gartner HW, Posse S, Salloum JB, Grodd W, Himmelmann F, Gaebel W, Birbaumer N. Subcortical correlates of differential classical conditioning of aversive emotional reactions in social phobia. Biological Psychiatry. 1999;45:863–871. doi: 10.1016/s0006-3223(98)00269-8. [DOI] [PubMed] [Google Scholar]
- Stein MB, Goldin PR, Sareen J, Zorrilla LT, Brown GG. Increased amygdala activation to angry and contemptuous faces in generalized social phobia. Archives of General Psychiatry. 2002;59:1027–1034. doi: 10.1001/archpsyc.59.11.1027. [DOI] [PubMed] [Google Scholar]
- Straube T, Kolassa IT, Glauer M, Mentzel HJ, Miltner WH. Effect of task conditions on brain responses to threatening faces in social phobics: an event-related functional magnetic resonance imaging study. Biological Psychiatry. 2004;56:921–930. doi: 10.1016/j.biopsych.2004.09.024. [DOI] [PubMed] [Google Scholar]
- Straube T, Mentzel HJ, Miltner WH. Common and distinct brain activation to threat and safety signals in social phobia. Neuropsychobiology. 2005;52:163–168. doi: 10.1159/000087987. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar Stereotaxic Atlas of the Human Brain. Stuttgart: Thieme; 1988. [Google Scholar]
- Veit R, Flor H, Erb M, Hermann C, Lotze M, Grodd W, Birbaumer N. Brain circuits involved in emotional learning in antisocial behavior and social phobia in humans. Neuroscience Letters. 2002;328:233–236. doi: 10.1016/s0304-3940(02)00519-0. [DOI] [PubMed] [Google Scholar]