Abstract
Arsenicals are highly reactive inorganic and organic derivatives of arsenic. These chemicals are very toxic and produce both acute and chronic tissue damage. Based on these observations, and considering the low cost and simple methods of their bulk syntheses, these agents were thought to be appropriate for chemical warfare. Among these, the most known agent synthesized and weaponized during World War I (WWI) is Lewisite. Exposure to Lewisite causes painful inflammatory and blistering responses in the skin, lung, and eye. These chemicals also manifest systemic tissue injury following their cutaneous exposure. Although largely discontinued after WWI, their stockpiles are still known to exist in the former Soviet Union, Germany, Italy, the United States, and Asia. Thus, their access by terrorists or accidental exposure could be highly dangerous for humans and the environment. This review summarizes studies which describe the biological, pathophysiological, toxicological, and environmental effects of exposure to arsenicals, with a major focus on cutaneous injury. Studies related to the development of novel molecular pathobiology–based antidotes against these agents are also described.
Keywords: arsenicals, vesicants, skin, inflammation, antidotes systemic damage
Introduction
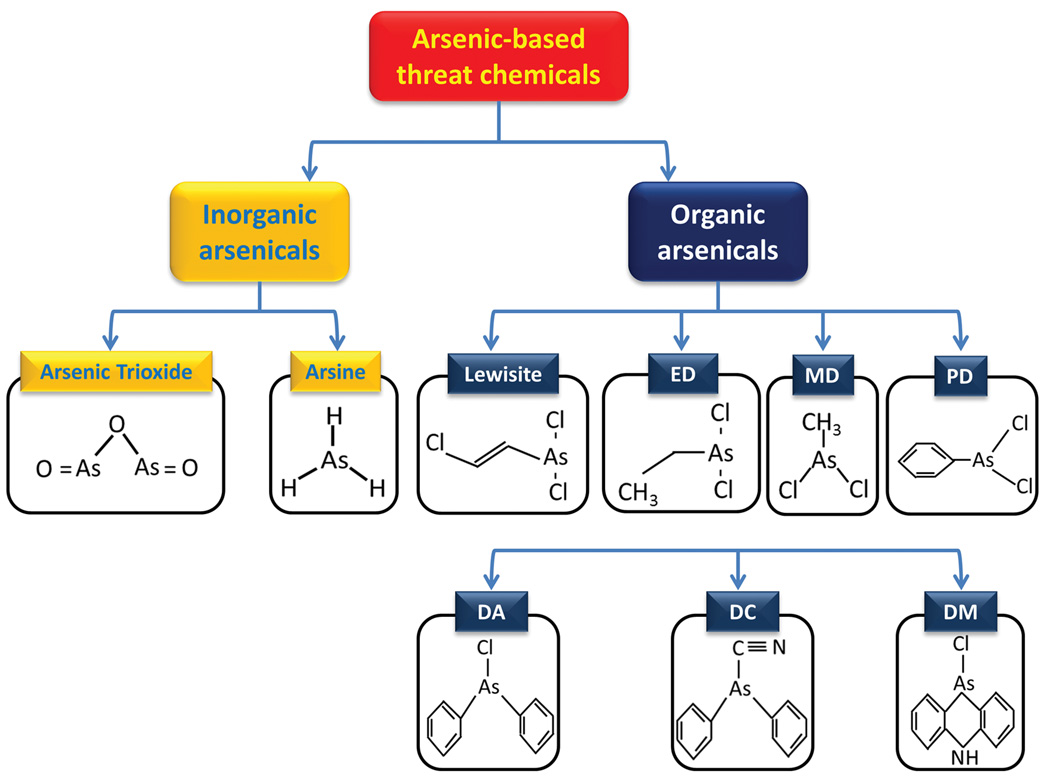
Arsenic is a highly toxic metalloid that occurs in both organic and inorganic chemical forms in oxidation states of −3, 0, +3 (As III) and +5 (As V), of which As III is considered the most toxic. Naturally occurring inorganic arsenicals that may also pose health hazards at least in some geographical areas include arsenate (AsO43−), arsenite (AsO33−), arsenic oxides (As2O3 and As2O5), arsenic sulfide (As2S3) and arsine gas (AsH3).1 Among these inorganic arsenic compounds, all of which are undoubted highly toxic, this review focuses on arsenic trioxide, (As2O3) and arsine, which have been considered industrially important chemicals whose accidental or terrorist activity–related exposure may cause severe damage to human lives and the environment. In addition, this review describes a number of organoarsenic compounds that have been developed as chemical warfare agents (CWAs) in the period including World War I (WWI) and World War II (WWII).2,3 These chemicals are a group of structurally related agents known as vesicants, as their cutaneous exposure could lead to irritation and painful blistering of the skin, eye, or airway mucosa.3,4 Their chemical structures are derived from trichloroarsine or arsenic trichloride (AsCl3), where one or more of the chlorine atoms are replaced by an organic moiety, such as methyl, ethyl, phenyl, or their derivatives.3 Some of the important derivatives that have relevance to CWAs are summarized in Figure 1 and described below. Although these studies have previously been described in the literature, the purpose of this review is to provide summary of this knowledge in one place to facilitate simulating studies in animal models. We have integrated the recently published data with some of the earlier studies to provide comprehensive account of gross tissue, cellular, and molecular damage caused by these chemicals. This is also within the primary interest of the National Institutes of Health (NIH) Countermeasures Against Chemical Terrorism (CounterACT) program to identify novel therapeutic antidotes against CWAs.
Figure 1.
Chemical structures of various arsenicals. ED, ethyldichloroarsine; MD, methyldichloroarsine; PD, phenyldichloroarsine; DA, diphenylchlorarsine; DC, diphenylcyanarsine; DM, diphenylaminechlorarsine.
Although the majority of these agents have been programmed to be destroyed, significant amount of their stockpiles are known to still exist in several countries, putting civilian populations under the potential threat of accident exposure/terrorists attacks. This is particularly evident from the past history of accidents. In 2004, the onset of some nervous system symptoms among the residents of Kamisu, Japan was reported to result from the consumption of well water contaminated with the degradation products of arsenic-based chemical weapons, perhaps Clark-I and Clark-II.5,6 Similarly, in 2002 at a road construction site in Samukawa, Kanagawa, where the Sagami Naval Arsenal was formally located, several hundred beer bottles containing Lewisite were unearthed, and laborers working there were exposed to Lewisite when the first group of bottles was discovered.7 There often appear reports in the news media that, during excavation and construction, WWI/WWII–related arsenals are discovered. Although never reported for arsenicals, other chemical warfare agents have been used by terrorists. In 1995, the Tokyo subway sarin attack killed 12 people, with 50 severely injured and nearly 1000 people suffering from temporary vision problems.8 Recently, the deliberate use of chemical weapons in Syria raised similar concerns worldwide.
Chemical warfare and industrial arsenicals
Lewisite
Lewisite (chlorovinyldichloroarsine) is the most important arsenic-based CWA. Isolated by Lee Lewis in 1918,9 Lewisite was proposed to be used as a CWA in WWI. However, due to the agreement of armistice, the first marine shipment of Lewisite to the European battlefield was destroyed during shipping.10 After that, there is no documented record showing that Lewisite has been applied to the battlefield. Based on the consensus to the disarmament following WWII, lewisite was abandoned and buried by Germany and Japan at various sites in the Mediterranean Sea, the Baltic Sea, Europe, and Haerbaling in Asia.7,11,12 Nevertheless, several countries, including Russia, Germany, Japan, Italy, and the United States, have been known to stockpile significant amounts of Lewisite.12–14 As reported to the Organization for the Prohibition of Chemical Weapons (OPCW) in 2000, there are 6745 tons of stockpiled chemical weapons in the form of Lewisite and 344 tons in the form of mustard/Lewisite mixtures.15 Therefore, it still remains a significant threat to the public via accidental exposure or a terrorist attack.
Purified Lewisite is a colorless, oily liquid at room temperature, whereas the munitions-grade Lewisite is an amber-to-dark-brown liquid with a geranium-like odor.4 It has a low freezing point (−18 °C) and is often mixed with mustard agents to make them more suitable for use in winter climates.4 It can easily penetrate clothing and even latex rubber gloves, rendering protective equipment ineffective. Therefore, under experimental setting, the Guidelines for Managing a Research Development Testing Evaluation (RDTE) Dilute Solution Laboratory are followed, which recommend the use of two pairs of nitrile gloves or one pair of the North® Silver Shield 4H chemical-resistant disposable glove to work with dilute arsenicals when the operator is in the hood. However, this rule may not be applied to other conditions when different protective clothing is required based upon hazard analysis and risk assessment.
Cutaneous effects
Lewisite exposure to human skin causes instant burning pain followed by extensive erythema, which occurs within 15–30 min. Vesication accompanying edema may develop later. Large fluid-filled blisters surrounded by minute vesicles occur within 24 h. The severity of skin injury continues to increase up to day 4 and remains stable until day 7, after which recovery of the skin lesions starts, and the healing is often completed after 4 weeks.16 Exposure to Lewisite vapor (0.06–0.33 mg/L) also induces painful blistering, which is maximal around 36–48 h.16
Experiments were conducted on human and pig skins with topically exposed Lewisite carrying radio-arsenic (As74) containing ~10 µcurie of As74/mg of Lewisite. The biopsy specimens of the exposed areas taken 24 h after exposure showed accumulation of As74-labeled Lewisite, mainly in the epidermal compartment as compared with the dermis.17 The hair follicles also accumulate Lewisite to the same level as the epidermis.18 The tissue areas of high accumulation were associated with massive necrosis of the epidermal layer.17
Animal models, including horse, swine, hairless guinea pig, and mice have been used to investigate the pathogenesis of skin lesions.4,13,19,20 Changes in the skin following Lewisite exposure in the majority of these models are generally similar and have been characterized by the early onset of erythema followed by edema of the skin, which is gradually develops a greyish and brownish color that clearly demarcates the involved and normal skin margins. Wound healing process is accompanied by skin regeneration at the edges of the wound. These macroscopic changes are accompanied by temporal pathological observations, such as microvesication, degeneration of the basal cells in the epidermis, infiltration of inflammatory cells, and cell death, in both the epidermis and the dermis.13,19 Generally, the pattern of skin lesion development following Lewisite exposure is similar to that found following mustard agent challenge. However, the skin response to Lewisite exposure is much more rapid, and the overall time course of these manifestations is highly compressed. In Lewisite exposure, epidermal necrosis is accompanied by more extensive edema, inflammation, and vascular thrombosis. On the other hand, the healing process of Lewisite-injured skin is reported to be significantly different than that of mustard-induced skin lesions.13 In the SKH1 hairless mouse model, Nguon et al. showed the progression of Lewisite-induced skin injury in terms of paraclinical (color, transepidermal water loss (TEWL)) and biomechanical measurements and histological and biochemical indices over a period of 21 days to 27 weeks, which is similar to that reported in other models.19 In this regard, the TEWL parameter could be considered the most appropriate index to follow the progression of tissue damage. Histological analysis showed inflammatory cell infiltration and microvesication, which is initiated at day 1 requires at least 21 days for complete wound closure. The temporal molecular changes, including dysregulation of expression of cytokines and their receptors involved in the manifestations of inflammation and increase in pro-matrix metalloproteinases 2/9, were more or less similar to those observed in other models.19 Animal studies did not demonstrate teratogenicity or reproductive effects of Lewisite, at least at the doses tested.14
The toxicity data of Lewisite degradation products is limited. However, because of the rapid hydrolysis of Lewisite to 2-chlorovinyl arsonous acid, the toxic properties of Lewisite in humans may be due in part to its hydrolysis products.21 The U.S. Army reported that Lewisite oxide has vesicant properties.22 The histologic changes of the skin lesions caused by Lewisite oxide are indistinguishable from that of phenyldichloroarsine (PD).23
Chronic effects of single exposure to Lewisite could also manifest. For example, a German soldier who had been accidentally exposed to liquid Lewisite on his lower right leg developed malignant lesions 8 years later, and the exposed area was still ulcerated and diagnosed as Bowen's disease 38 years later.22 Bowen's disease was also diagnosed among workers at a Japanese facility that produced Lewisite.24
Ocular effects
The eye is extremely vulnerable to Lewisite, and ocular exposure may result in permanent blindness if decontamination is not accomplished within 60 s. Recent study was performed in a rabbit model for the screening of effective therapeutics to treat ocular injury and to develop clinically relevant end points. Ocular injury caused by Lewisite resulted in edema of the eyelids, inflammation, massive corneal necrosis, and blindness, which was similar to that reported in humans.25 The single vapor exposure of Lewisite to the eye of New Zealand white rabbits induced clinical ocular lesions, mainly in the cornea. In this model, while a mock-exposed left eye served as a control, the right eye was exposed to Lewisite vapor (0.2 mg/L) for four different time intervals between 2.5 and 10.0 min, and ocular injury progression was recorded up to day 28 or 56 postexposure. A dose-dependent increase in corneal opacity was observed, which could be seen as early as 6 h postexposure, and gradually increased up to day 3. However, corneal ulceration peaked at 1 day. These alterations were associated with neovascularization which could be visualized at day 7, peaked between days 22 and 35, and remained persistent thereafter. Lewisite also caused corneal thickness, iris redness, and redness and swelling of the conjunctiva.25
Systemic effects
Lewisite is a systemic poison and could be lethal following dermal exposure. It is estimated that 37.6 mg/kg Lewisite applied on the skin of an adult man can be fatal within several hours.26 Cutaneous exposure causes extensive damage to the liver, lung, kidney, gall bladder, and bile duct.14 The death caused by Lewisite exposure is known to be due to a condition known as “Lewisite shock,” which occurs as a result of the loss of blood plasma from capillaries damaged by circulating Lewisite.27 However, delayed fatality (within a week) of Lewisite may be attributable to multiorgan damage, including impaired kidney and liver function.27 Inhalation of Lewisite causes a burning pain and irritation throughout the respiratory tract, and severe exposure can cause fatal pulmonary edema, pneumonitis, or respiratory failure. The toxicology data of Lewisite in animal model is summarized in Table 1.
Table1.
Summary of LD50 data for animals exposed to Lewisite.
| Species | Route of exposure | LD50 |
|---|---|---|
| Rat | Inhalation | 166 mg/m3 for 9 min |
| Oral | 50 mg/kg | |
| Dermal | 24 mg/kg | |
| Subcutaneous | 1 mg/kg | |
| Mouse | Inhalation | 190 mg/m3 for 10 min |
| 200 mg/m3 for 10 min | ||
| Dog | Dermal | 15 mg/kg |
| Subcutaneous | 25 mg/kg | |
| Rabbit | Inhalation | 160 mg/m3 for 7.5 min |
| 25 mg/m3 for 60 min | ||
| Dermal | 6 mg/kg | |
| Intravenous | 0.5 mg/kg | |
| Guinea Pigs | Inhalation | 111 mg/m3 for 9 min |
| 8 mg/m3 for 60 min | ||
| Dermal | 12 mg/kg | |
| Subcutaneous | 1 mg/kg | |
| Goats | Inhalation | 12.5 mg/m3 for 100 min |
| Dermal | 15 mg/kg |
Molecular targets
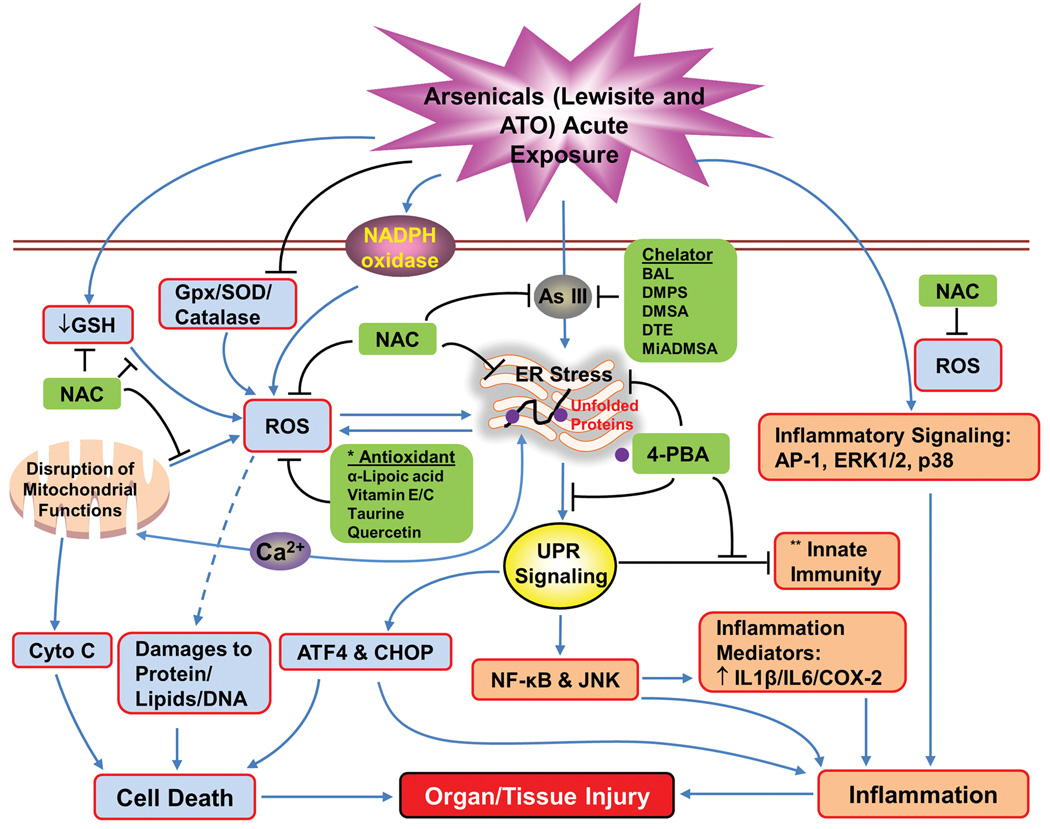
The trivalent arsenic in vesicant agents, including Lewisite and its analogs, may be an important contributor to skin and systemic toxicity.28 Besides targeting the enzyme pyruvate dehydrogenase (PDH) affecting metabolism, other molecular models of action proposed in early investigations for Lewisite include its reactivity with glutathione, leading to loss of protein thiols, loss of calcium homeostasis, oxidative stress, lipid peroxidation, membrane damage, and cell death (Fig. 2).28 While investigating the molecular pathogenesis of Lewisite, we recently found that its exposure to murine skin induces activation of the unfolded protein response (UPR) signaling pathway, and blocking UPR signaling with the chemical chaperone 4-phenylbutyric acid (4-PBA) protected against Lewisite-induced skin damage (Fig. 2). Despite an earlier belief that some of the key toxic effects of arsenical vesicants are due to arsenic, the rapidity with which arsenical vesicants act distinguishes the two biological responses and suggests arsenic-independent mechanisms associated with the exposure to organic arsenicals. Cutaneous exposure to ATO or sodium arsenite does not cause painful inflammation or blistering, which further indicates that the early molecular pathobiology of the two agents (arsenic and arsenicals) could be distinct.
Figure 2.
Flow diagram showing mechanisms by which arsenicals induce inflammation and tissue injury. This diagram also includes our recent findings defining the role of unfolded protein response (UPR) signaling in this intricate mechanism of molecular pathogenesis of tissue injury caused by exposure to arsenicals. *These agents show some efficacy against arsenic-induced tissue damage. **This indicates the effects of arsenic trioxide on macrophages.
Effects on the basement membrane
To understand the mechanism of microblister formation, which is characterized by epidermal–dermal separation, King et al.29 investigated the effects of Lewisite on laminin, a cysteine-rich and highly protease-sensitive adhesive glycoprotein. They tested whether chemical modification of laminin, either directly via chemical alkylation of laminin thiols or indirectly via to Lewisite-induced cytotoxic release of proteases, could contribute to blister formation. However, in this study, no evidence for proteolytic activity against human keratinocyte laminin was identified in the blister fluid. In addition, no evidence for direct chemical modification of laminin by Lewisite was seen, as 36% of the free thiol groups in human keratinocyte laminin immunoprecipitates were potentially available for reaction with alkylating agents.29 Therefore, the exact molecular mechanisms by which these arsenicals cause rapid cutaneous blisters remain to be defined. In our laboratory, we focus on defining the effects of Lewisite on water/glycerin transport, which is regulated by proteins known as aquaporins. These studies are promising but are still in progress.
Microarray analysis
To further identify genes directly involved in vesication, the transcriptional profile of Lewisite was evaluated using microarrays containing 7075 sequence-verified human cDNAs. Screening of mRNA from human epidermal keratinocytes treated with Lewisite (200 µM) for 2 h identified a large number of differentially expressed genes. Apoptotic transcripts were clearly evident in Lewisite-mediated injury.30 However, the exact molecular mechanism underlying massive tissue damage remain largely undefined. We recently observed that UPR signaling and mitochondrial membrane damage together underlie the extensive epidermal damage following arsenic exposure (Fig. 2). This mechanism may also at least in part underlie what is observed in Lewisite challenge to murine skin.
Other arsenicals CWAs
Other dichloroarsine-containing arsenicals include ethyldichloroarsine (ED), methyldichloroarsine (MD), and PD, which exhibit more or less similar potential to induce skin blistering, as shown in Figure 1. Additional Lewisite analogs include phenylarsine oxide (PAO), Lewisite oxide (also known as (trans)chlorovinylarsine oxide), Lewisite iodide (also known as (trans)chlorovinyldiiodoarsine), and diiodophenylarsine.23 These agents are known to produce skin lesions histologically similar to those caused by exposure to PD.23 Similarly, phenylarsenic compounds, which are also listed as CWAs, include diphenylchlorarsine (DA), diphenylcyanarsine (DC), and diphenylaminechlorarsine (DM, also known as Adamsite) (Fig. 1), PD, and arsine oil (a mixture of arsenic trichloride, PD, DA, and triphenylarsine).31 DA, DC, and Adamsite are classified as sternutatory agents because they can cause extreme sneezing, coughing, and vomiting.32
Toxicology
MD, ED, and PD are moderate- to fast-acting blistering agents that are capable of producing casualties within minutes of exposure. Inhalation, skin contact, or eye contact to these agents may produce immediate irritation.28 Skin exposure to liquid or vapor may lead to the development of blistering within 32 h. Both inhalation and skin absorption can induce systemic poisoning in 4 h.33 MD was known to be used in battles by the German military in 1917.28 MD can penetrate rubber, rendering protective measures ineffective.33 Inhalation of MD may cause “dry-land drowning,” in which the lungs are flooded with water and the victim dies of a combination of blood poisoning and asphyxiation effects.34 MD (50 mg) may produce skin irritation, but its higher concentrations may lead to systemic damage. The lethal dose in humans through inhalation is between 1000 mg and 4000 mg.33 ED may produce immediate irritation when inhaled or ingested or upon skin or eye contact. Exposure of ED vapor to the eyes can cause permanent corneal damage leading to blindness.33 Similar to MD, inhalation of ED causes pulmonary edema or dry-land drowning. Damage to the lung tissue is permanent in survivors, predisposing them to secondary infection and tumor development.28 A dose of 900–4000 mg through inhalation is lethal in the average human. Doses as low as 5 mg and 50 mg cause irritation upon exposure to eye and skin, respectively. As little as 500 uL can produce blistering.33 The presence of an ethyl group in ED is considered to be the structural basis for systemic damage to bone marrow and to the digestive and endocrine systems.28 PD is mainly a emetic agent, but it is also capable of inducing skin blistering with less severity than Lewisite and ED. It is also capable of penetrating rubber and may persist in the environment from 48 h to 7 days.33 The lethal dose of PD is estimated to be 900–2700 mg or 2600 mg·min/m3 through inhalation.35 As little as 5–15 mg can produce severe nausea and vomiting leading to incapacitation.33 Although the skin toxicity of PD is less severe than other arsenicals in this category, skin contact with a larger dose can also induce blisters in 4–32 h. Systemic poisoning through ingestion, skin absorption, or inhalation could develop within 12–32 h after exposure. PD may produce severe irritation to eyes after contact, and high doses may result in blindness.33
DA, DC, and DM are sternutator agents that cause extreme sneezing, coughing, and vomiting. These agents act by producing acute inflammation of the upper respiratory tract, the nasal accessory sinuses, and the eyes.32 In 2004, onset of some nervous system symptoms was reported to be due to consumption of well water contaminated with diphenylarsinic acid (DPAA) and phenylarsonic acid (PAA) in Kamisu, Japan, which were possibly derived from DA and DC.5,6 This accident signifies the importance of understanding the molecular pathogenesis associated with the exposure to these chemicals.
Molecular targets
The mechanisms of dichloroarsine-containing arsenicals (ED, MD, PD), sternutatory agents (DA, DC, DM), and additional Lewisite analogs (PAO, Lewisite oxide, Lewisite iodide, and diiodophenylarsine) have not been clearly defined. However, some of these agents are also believed to work by targeting sulfhydryl group–containing enzymes, as reported for Lewisite. This mechanism of enzyme inhibition interferes with key functions of metabolic processes, leading to disruption of the cell structure.36 In addition, arsenic (III) released from these agents may play some role in the delayed toxicity of these agents.36
Arsenic trioxide
Arsenic trioxide (ATO), in contrast to other arsenic-based CWAs, is not a vesicant. ATO is both acutely toxic and carcinogenic. In case of suicidal and homicidal use, oral ingestion is the most important route of exposure and may lead to significant tissue and organ damage and often death.37–39 The lethal dose for ingested ATO is 70–180 mg in humans.38 Symptoms usually develop within minutes to hours of exposure, and death may occur around 24 h to 4 days after exposure, depending on the quantities systemically absorbed. The immediate acute effects following very-high-dose arsenic poisoning manifest with symptoms of gastrointestinal, cardiovascular, and nervous system toxicity. Gastroenteritis is characterized by nausea, vomiting, abdominal pain, severe watery diarrhea, and a garlic odor of the breath and stool. These symptoms are soon followed by dehydration and hypotension. Death is usually due to cardiovascular collapse and hypovolemic shock. Symptoms of nervous system induce encephalopathy (delirium, coma, seizures, and others) and peripheral neuropathy. In some cases, acute arsenic poisoning may also induce kidney injury (proteinuria, hematuria, acute tubular necrosis, and anuria) and acute respiratory distress syndrome. Symptoms that may manifest following acute arsenic poisoning include hepatitis (within 1 week), pancytopenia (within 1 week), dermatologic lesions (patchy alopecia, diffuse pruritic macular rash, herpetic-like ulcers in the mouth), respiratory effects (dry, hacking cough), and Mees lines after 30 days. Less severe arsenic poisoning may lead to persistent gastroenteritis and mild hypotension, along with a metallic taste and irritated mucous membranes that can mimic pharyngitis.37 Asymmetrical sensorimotor polyneuropathy is one of the most prominent symptoms that can develop after both acute poisoning and chronic exposure. It often mimics Guillain-Barré syndrome, with similar electromyographic findings.40
Chronic exposure to inorganic arsenic is known to induce disorders in multiple organ systems including cancers (skin, lung, bladder, and kidney), skin lesions, cardiovascular disease, neurological defects, type 2 diabetes, gastrointestinal disease, liver disease, kidney disease, reproductive disorders, and others. These manifestations and their molecular mechanisms have been thoroughly reviewed in our and others’ review articles.41–45
Molecular targets
Disruption of cellular metabolism
It is well known that trivalent arsenic can avidly bind to proteins or molecules that are rich in sulfhydryl groups. This binding of arsenic to a protein may alter conformation, resulting in loss-of-function effects. It may lead to the inhibition of critical enzymes involved in cellular respiration, which ultimately causes depletion in cellular energy production and cell death. One of the most important enzymes in energy metabolism targeted by a majority of arsenicals is PDH. Arsenic inhibits the PDH complex by binding to its dithiol-containing cofactor lipoic acid to form a stable six-member ring structure (cyclic thioarsenite complexes). Besides PDH, lipoic acid is a known cofactor for pyruvate oxidase, 2-oxoglutarate oxidase, and aldehyde dehydrogenase, which are all required for respiration.26 Inhibition of the PDH complex by arsenic blocks the citric acid cycle and ultimately decreases the production of ATP, resulting in cell damage and death.46 Other studies suggest that production of reactive oxygen species (ROS) may also contribute to the inhibition of these enzymes.46 Similarly, arsenic interferes with gluconeogenesis and glucose uptake and glutathione metabolism by binding to other thiol group–rich proteins.46 Initially, however it may affect the recruitment of conformationally altered protein to other proteins and DNA. These mechanisms have been the basis for the toxicity of many arsenicals, including ATO and other arsenic-based CWAs.46
Induction of ER stress
Recent findings suggest that trivalent arsenic binding to proteins may lead to accumulation of unfolded proteins in the endoplasmic reticulum (ER) in cells, which ultimately induces ER stress and the activation of the UPR signaling pathway,47–49 UPR signaling is considered important in the pathogenesis of a broad spectrum of human diseases where the underlying mechanisms are believed to involve induction of apoptosis and onset of inflammatory responses.50 Our earlier studies suggest that ATO treatment in macrophages activates UPR signaling, which ultimately results in the suppression of innate immune functions. More importantly, treatment with the chemical chaperone 4-PBA suppressed the induction of UPR while simultaneously restoring several macrophage functions (Fig. 2).48 In murine skin, subchronic administration of arsenic induces ROS-dependent activation of the UPR signaling pathway associated with a mild cutaneous inflammatory response. These responses are associated with increased production of various mediators of inflammation. However, we still lack an in-depth understanding of the molecular mechanism underlying increased production of these mediators. UPR signaling is known to regulate NF-κB and JNK. These proteins are clearly linked to enhanced inflammatory responses.50 Consistently, these alterations have been found to be diminished by the treatment with antioxidant N-acetylcysteine (NAC) (Fig. 2).47
Oxidative stress
ATO-mediated generation of ROS/reactive nitrogen species (RNS) may be another important toxicity-mediating factor.41 Although the specific mechanism has not yet been fully defined, we and others have shown that arsenic can induce the production of superoxide radical, peroxyl radical, hydroxyl radical, nitric oxide, hydrogen peroxide, dimethyl arsenic peroxyl radicals, and dimethylarsinic radical by activating various pathways, of which NADPH oxidase activation is considered to be of critical importance (Fig. 2).43–45,51 Excessive ROS production overwhelms the endogenous antioxidant mechanism and may lead to extensive damage to cellular membranes, proteins, and DNA and, ultimately, to cell death.45 Interestingly, our studies have shown that ROS and UPR signaling are also closely interrelated, suggesting the involvement of ER in mediating arsenic-induced ROS production and vice versa (Fig. 2).47,48 In addition, our earlier studies showed that mitochondria contribute to the arsenic-induced ROS production and DNA damage. Arsenic is known to damage mitochondria, thus leading to release of superoxide anion and the formation of RNS under certain experimental settings.52,53 It is likely that the extent and severity of tissue damage may depend on the robustness of ROS production accompanied by the simultaneous activation of various ROS-generating mechanisms (Fig. 2).
Disruption of signal transduction
Arsenic has a broad impact on the dysregulation of many cell signaling pathways, since the sulfhydryl (SH) moieties are known to exist in more than 200 proteins.42 In this regard, a number of pathways that are important in the regulation of inflammation, proliferation, and apoptosis are affected. These include NF-κB, EGF/VEGF, MAPK, HIF, p53, AP1, and PI3K/AKT, among others.42,45 Recently, our lab found that subacute arsenic treatment in SKH-1 hairless mice disrupts the Hippo signaling pathway, leading to the disruption of tight/adherens junctions.47 Yap nuclear translocation and transcription of Yap target genes, including Gli2, which encodes an important transcription factor in Sonic hedgehog signaling, are considered important in various delayed cutaneous manifestations, including the skin cancers.54,55
Arsine gas
Arsine is colorless, non-irritating gas with a garlic-like order at concentrations of 0.5 ppm or above.57 It is the most toxic form of inorganic arsenic and the inhalation of small amounts may be fatal.28 In humans, inhalation of 250 ppm (800 mg/m3) of arsine gas is instantly lethal. Exposures of 25–50 ppm (80–160 mg/m3) for 30 min are lethal, and 10 ppm (32 mg/m3) is lethal after longer exposures.58 Although arsine has never been used as a chemical weapon, it is identified as a potential toxic agent of industrial importance or an agent of biochemical warfare.59 Accidental exposure to arsine gas is rare and usually occurs in occupational settings. Exposure often happens when arsenic-containing crude ores or metals are treated with acid.59 Since arsine is used for the synthesis of many semiconducting materials, stockpiles of arsine in different industrial facilities are another significant concern. The toxicity of arsine gas is quite different from that of other arsenicals. Arsine is a powerful hemolytic poison in both acute and chronic exposures.60,61 The primary target of its toxicity is the blood and kidneys, but the nervous system, digestive system, liver, heart, and lungs may also be affected.59 Because there are no symptoms at the time of exposure, persons who have been exposed to arsine gas are often unaware.59 Hemolysis is followed by bloody urine (usually 4–6 h after exposure) and jaundice (12–48 h).58 The metabolites that are responsible for its hemolytic effects are believed to be the oxidized products of arsine, arsenic dihydride intermediates, and elemental arsenic.59 Renal failure is often considered the cause of death following arsine poisoning.59 It may be attributed to clogging of the renal pathway by large numbers of dead red blood cells and hemoglobin released following hemolysis.59 The mechanism of action has not yet been defined.28,59
Molecular targets
Arsine gas preferentially binds to hemoglobin once it enters red blood cells. The mechanism of action of acute arsine poisoning has not been fully defined. However, it is known to be oxidized to trivalent arsenic in vivo and thus may exert its toxicity through targeting sulfhydryl groups and facilitating the hemolysis of red blood cells.62 Moreover, arsine-mediated uncoupling of oxidative phosphorylation may also induce massive damage to red blood cells via adduct formation with oxyhemoglobin. An oxidative mechanism involving the generation of hydrogen peroxide may also be involved.28,63,64 Arsine may act on sodium–potassium pumps, thus producing cell swelling and hemolysis.63 As described for organic arsenicals, arsine also depletes reduced glutathione, although the mechanism has not been defined.28 Other studies showed that arsine causes a nonspecific disruption of ion gradients, leading to cell membrane instability.62 These studies indicate the complexity of toxic manifestations, which may cause certain difficulties in the development of its effective antidotes.
Potential terror threats and risk of accidental exposure
Being fast-acting and highly toxic chemicals, arsenicals represent simple weapons that could cause mass casualties if exposed to the general public. Possible terror threats involving arsenic-based CWAs include the deliberate release of illegally obtained or manufactured arsenicals, the release of purchased or stolen industrial arsenicals, and attacks on chemical manufacturing plants, stockpile sites, or transport vehicles.70
ATO is listed as a threat chemical by the NIH CounterACT program. ATO is the precursor for the synthesis of many of the inorganic and organic arsenicals. ATO is an important and widely used industrial material. More than 50,000 tons of ATO are produced globally each year.71,72 China is the largest producer of ATO in the world and exports ATO to the United States and other countries.71 Thus, large quantities of ATO are transported to various storage sites all over the world and may present potential targets for terror attacks. In addition, release of ATO from transportation and storage facilities may also be possible as a result of industrial accidents or natural disasters. Given the fact that ATO is the main precursor for the production of many other arsenicals, including organoarsenical compounds,71 terrorists may also take advantage of it to generate other arsenic-based threat agents.
Accidental exposure to lethal dose of ATO is currently less common than what was reported 50–100 years ago. However, acute ATO intoxication still exists owing to medical therapy as well as intentional poisoning in homicides and suicides.38 Accidental ingestion of ATO-containing substances appears to be most common among children, but also occurs occasionally in the older age group. Most acute poisonings are fatal, though some poisonings end in recovery.39
In clinical medicine, ATO has been approved by the U.S. Food and Drug Administration (FDA) to treat acute promyelocytic leukemia.73 In some patients, its toxic manifestations have been noticed. Therefore, this patient population, although small in number, may be used to develop antidotes, particularly ones repurposed from FDA-approved agents for other diseases for eventual FDA approval.
Environmental residues and degradation products
Potential pathways determining the fate of CWAs in the environment include volatilization, sorption, hydrolysis, photolysis, and microbial degradation.21 Some of the arsenic-containing chemical warfare degradation products (CWDPs) are listed in Table 2.10,36 The ultraviolet (UV) absorption spectrum of Lewisite suggests that some photodegradation may take place in the atmosphere.21 Hydrolysis of Lewisite occurs in both the aqueous phase and the gas phase. Although it is slightly soluble in water, hydrolysis of Lewisite is rapid and is essentially 100% in solution. Therefore, it was thought that the water-soluble hydrolysis product, 2-chlorovinyl arsonous acid might be the mediator of Lewisite-related toxic effects.21 Hydrolysis of Lewisite may also occur once it is absorbed following its exposure. In tissues, most of the 2-chlorovinyl arsonous acid forms adducts with cysteine-rich proteins, whereas unbound 2-chlorovinyl arsonous acid is rapidly cleared through the renal pathway within 12 h postexposure.74
Table2.
Impurities and degradation products associated with arsenic-containing CWAs.
| Process | Product | Formula | Synonym |
|---|---|---|---|
| Hydrolysis of Lewisite | 2-Chlorovinyl arsonous acid | C2H4AsClO2 | CVAA |
| Hydrolysis or dehydration of CVAA | 2-Chlorovinyl arsenous oxide | C2H2ClAsO | Lewisite oxide |
| Oxidation of Lewisite or Lewisite oxide | 2-Chlorovinyl arsonic acid | C2H4AsClO3 | |
| Ozone oxidation of Lewisite | 2-Chloroarsonous formaldehyde | HCOAsCl2 | |
| Polymer of Lewisite oxide | Lewisite oxide polymer | (C2H2ClAsO)n | |
| Combustion product or impurity of Lewisite | Arsenic trichloride | AsCl3 | |
| Combustion product of Lewisite | Arsenic trioxide | As2O3 | |
| Combustion product of Lewisite | Arsenic oxychloride | AsOCl | |
| Impurity of Lewisite | Bis (2-chlorovinyl)chloroarsine | C4H4AsCI3 | Lewisite 2 |
| Impurity of Lewisite | Tris (2-chlorovinyl)arsine | C6H6AsCI3 | Lewisite 3 |
| Breakdown product of DA and DC | Bis (diphenylarsine) oxide | C24H20As2O | BDPAO |
| Hydrolysis of bis(diphenylarsine)oxide | Diphenylarsinic acid | C12H11AsO2 | DPAA |
| Hydrolysis of PD or degradation of DPAA or Oxidation of PAO |
Phenylarsonic acid | C6H5AsO(OH)2 | PAA |
| Hydrolysis of PD | Phenylarsine oxide | C6H5AsO | PAO |
| Oxidation of triphenylarsine | Triphenylarsine oxide | (C6H5)3AsO | TPO |
Dehydration of 2-chlorovinyl arsonous acid results in the formation of Lewisite oxide (2-chlorovinyl arsenous oxide) and polymerized Lewisite oxide products, which are normally insoluble but are still considered to be highly toxic.21,75 In basic pH, the trans-Lewisite isomer is readily cleaved by the hydroxyl ion, generating acetylene and sodium arsenite at low temperatures. On the other hand, cis-Lewisite requires higher temperatures (~ 40 °C) under the similar basic pH environment to produce vinyl chloride, sodium arsenite, and acetylene.21
In soil, Lewisite undergoes rapid volatilization or conversion to Lewisite oxide in the presence of moisture.75 The low water solubility of Lewisite oxide indicates intermediate persistence in the moist soil.14 Both Lewisite and Lewisite oxide can be slowly oxidized to 2-chlorovinylaronic acid.75 Microbial degradation of Lewisite in soil include epoxidation of C=C bond and reductive dehalogenation and dehydrohalogenation, which results in the production of toxic metabolites because of the epoxy bond and arsine group.26,76 Conversion of Lewisite to inorganic arsenic in soil has also been described.75 Although Lewisite does not bioaccumulate in food chains, its degradation products can be detected in rice, wine and marine food.34
In the polluted troposphere, ozone (O3) is recognized as one of the key players involved in photochemical air pollution. In polluted atmosphere, O3 is known to react with some important classes of organic chemicals, specifically containing unsaturated double bonds, such as those present in Lewisite.11,77 Therefore, a recent publication defining gas-phase reactions of O3 with Lewisite was considered important for understanding the environmental impact assessment of the process of excavating and destroying abandoned CWAs. It is believed that Lewisite emitted into the atmosphere from the dumped unexploded chemical ordnance will interact with the atmospheric O3. It is recently suggested that the environmental behavior of Lewisite could also be influenced by oxidation reactions with photo-oxidants in the atmosphere. In this regard, the atmospheric lifetime of this agent will be determined by its ability to react with •OH radical, •NO3 radical, and O3. On the basis of this hypothesis, recent studies described the kinetics of reaction of O3 with Lewisite. These results revealed that ozonolysis could be an important approach for Lewisite elimination as compared to other oxidants such as •OH radicals in the atmosphere. These results also provide evidence that ionization potential of halogenated alkene derivatives with different substituents could be a useful determinant in further evaluating of rate constants for reactions of O3 with haloalkenes.77
The degradation pathways of other organoarsenicals and their environmental chemistry are not fully defined. However, phenylarsenic compounds are known to be degraded via hydrolysis and oxidation in the environment.36,78 Hass et al. showed that DA and DC (also known as CLARK I and CLARK II, respectively) are hydrolyzed to diphenyl arsine hydroxide, which can be further converted to bis(diphenylarsine) oxide (BDPAO). BDPAO can be hydrolyzed to DPAA, which was found as one of the principal contaminants in drinking water in Kamisu, Japan.6 ADAMSITE could be hydrolyzed to bis(diphenylaminearsine) oxide, which is further oxidized to diphenylamine arsenic acid. PD is known to be hydrolyzed to phenylarsine oxide (PAO), followed by oxidation to PAA. Triphenylarsine (TPA) can be oxidized to triphenylarsine oxide (TPAO).78 Unfortunately, these degradation products could be more toxic than inorganic arsenic.79 Environmental bacteria may also play an important role in the biodegradation of insoluble organoarsenic CWAs to form soluble organoarsenic compounds in the contaminated soil. Kohler et al. reported that bacterial degradation of various diphenyl and triphenyl organoarsenic CWAs is a slow process and is largely independent of temperature.76 Regardless of the fate of these organoarsenic-based CWAs, the ultimate degradation product, particularly inorganic arsenic, will continue to contaminate the environment following their disposal. This process could be better understood from the exposure of the general population to arsenic through air, food, and water.42,45
Arsine is a strong reducing agent and can easily be transformed into other oxidized arsenic forms like As III and As V.28 In humans and animals, arsine is metabolized into trivalent arsenic, as well as pentavalent arsenic, followed by methylation and excretion.59,80,81
Countermeasures
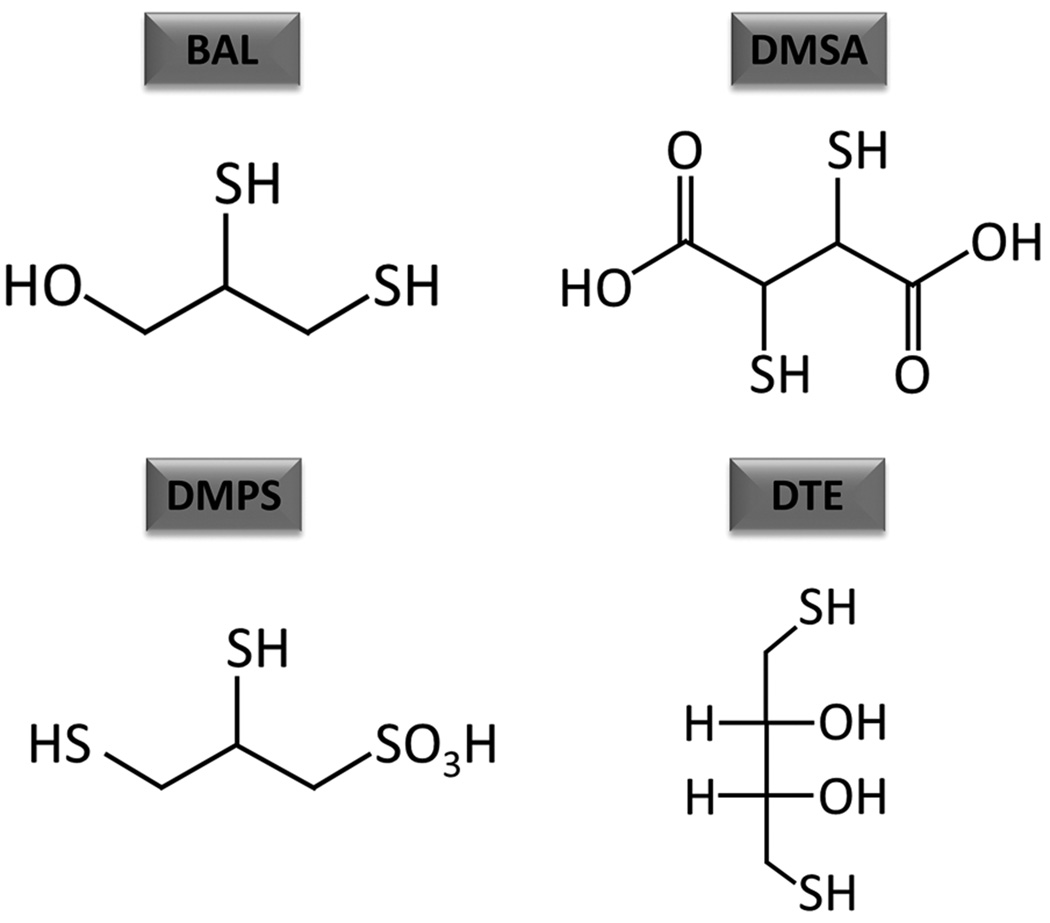
Currently, the only known effective FDA-approved medical countermeasure against arsenical-induced toxicity is 2,3-dimercapto-1-propanol, also known as British anti-Lewisite (BAL). The mechanism by which it is known to act involves chelation of trivalent arsenic. The vicinal thiol groups in BAL bind to arsenic released from Lewisite as a result of its hydrolysis in the body tissues. Thus, it was thought to facilitate arsenic excretion out of the body and thus prevent arsenic-induced damage to tissues.82 BAL ointment was also shown to be effective to some extent in reducing the severity of skin or eye lesions only if it was administered topically soon after Lewisite exposure.70 However, diminution of arsenicals’ systemic poisoning requires intramuscular injection of BAL in oil (10%), which is highly painful and has limited efficacy.83 Moreover, BAL manifests many side effects, some of which could be so severe to mandate the discontinuation of its use.83 Water-soluble analogs of BAL, including meso-2,3-dimercaptosuccinic acid (DMSA), 2,3-dimercapto-1-propanesulfonic acid (DMPS), and 2,3-dithioerythritol (DTE), have been developed (Fig. 3).84,85 In some experimental studies, these agents showed some efficiency with fewer side effects. However, their topical administration remains significantly less potent. In this regard, Mouret et al., using an SKH-1 murine model, showed that both BAL and DMSA are effective. These authors demonstrated that the protection against cutaneous damage by Lewisite could be alleviated by these agents if they are topically applied. However, dimercapto-chelating agents are not found effective via subcutaneous administration 1 h after Lewisite exposure83. BAL was consistently more effective than DMSA in reducing Lewisite-induced skin injury.83 Kehe et al. employed human skin keratinocytes and showed significant alterations in energy metabolism following Lewisite treatment. DMPS and DMSA were found effective in this in vitro experimental setting to restore Lewisite-mediated alterations in glucose consumption, lactate formation, and lactate dehydrogenase leakage.86 To enhance the tissue uptake of DMSA, a larger number of esters of DMSA have been developed as novel chelators for arsenic. Among these, monoisoamyl ester of DMSA (MiADMSA) was the most effective in reducing arsenic levels and manifested the least toxicity.45 Recently, a nanoformulation of this agent has also been developed.87 However, its therapeutic effects have not been tested against Lewisite-mediated toxicity in specific animal models.
Figure-3.
Chemical structures of various antidotes against arsenic toxicity. BAL, British anti-Lewisite; DMSA, meso-2,3-dimercaptosuccinic acid; DMPS, 2,3-dimercapto-1-propanesulfonic acid; DTE, 2,3-dithioerythritol.
Hypothermia treatment has been shown to reduce Lewisite-induced skin injury and cutaneous pain. However, the mechanism underlying hypothermia therapy remains unclear.88 In male hairless guinea pigs, therapeutic cooling of Lewisite-exposed skin afforded dramatic protection against injury. Interestingly, cooling was also shown to increase the therapeutic window in which drugs were effective against vesicant agents.88 To support these animal studies, Sawyer and Nelson demonstrated a significant delay in Lewisite cytotoxicity in cells in culture when maintained at 25 °C. However, these effects were reversed when cells returned to 37 °C.88 When employing isolated perfused porcine skin flap, Lewisite exposure led to the formation of gross blisters. The location and characterization of epidermal–dermal junction separation and the time course of lesion production paralleled the description of Lewisite-induced lesions in humans, suggesting that porcine skin flap may provide an early and relevant in vitro model with which to study mechanisms of chemical vesication and arsenical toxicity.89 Nonetheless, this model has a number of limitations, including lack of inflammatory cell infiltration.
With regard to inorganic arsenicals, such as arsine gas, no effective antidote is currently available. Treatment for accidental exposure to arsine remains largely supportive and may include transfusions and dialysis in severe cases.59 These studies warrant immediate attention to develop better agents with high efficacy and low toxicity. BAL is also effective in controlling toxic manifestations of other inorganic arsenic chemical compounds. In this regard, use of a number of antidotes has also been proposed to reduce the toxicity of arsenic. These agents include α-lipoic acid, vitamins E and C, taurine, and quercetin.45 However, demonstration of their effectiveness never progressed beyond the preclinical murine model setting. Moreover, these agents have not been tested against arsenical vesicants. We recently found that Lewisite induces ROS-dependent activation of UPR signaling in exposed skin. Thus, treatment with the antioxidant NAC and the chemical chaperone, 4-PBA showed remarkable effects. These agents can decrease UPR signaling and may provide a mechanism-based intervention against the progression of skin injuries. However, it is difficult to predict at this stage whether chemical chaperone treatment will also be effective against arsine toxicity.
Conclusions and future prospects
On the basis of the published literature described in this review article, the potential use of CWAs during accidental or intentional exposure against civilian population cannot be ignored. Thus, arsenicals and other similar CWAs are still considered as important threats and warrant further investigations to define the mechanism of action of these agents. Existing FDA-approved antidotes, particularly BAL and DMSA, have not shown promising effects against multiple arsenicals and are also associated with several severe side effects, which discourage their administration. Therefore, there is a need to develop molecular target–based therapy. We recently described the possibility of using chemical chaperones as potent antidotes against these highly toxic agents. These are most the novel class of antidotes ever described in the literature. We are trying to expand these early observations. In addition, we strongly feel that these agents should be formulated as creams or auto-injectors for immediate use without significant help of medical personnel at the exposure site. Our lab in the Dermatology Department at the University of Alabama at Birmingham is working with dedication in this area.
Acknowledgments
This work is supported by U01 NSO95678 to M.A.
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Eric Wildfang, S.M.H.a.H.V.A. Arsenic. In: Rudolfs DJK, Zalups K, editors. Molecular Biology and Toxicology of Metals. London and New York: Taylor & Francis; 2000. [Google Scholar]
- 2.Haas R, Krippendorf A. Determination of chemical warfare agents in soil and material samples: Gas chromatographic analysis of phenylarsenic compounds (sternutators) (1st communication) Environ Sci Pollut Res Int. 1997;4:123–124. doi: 10.1007/BF02986314. [DOI] [PubMed] [Google Scholar]
- 3.Flora SJS. arsenicals: toxicity, their use as chemical warfare agents, and possible remedial measures. In: Gupta RC, editor. Handbook of Toxicology of Chemical Warefare Agents. London: Acdemic Press; 2015. pp. 171–191. [Google Scholar]
- 4.Timothy C, Marrs RLM. Organic Arsenicals. Chemical Warfare Agents: Toxicology and Treatment, Second Edition. 2007:467–475. [Google Scholar]
- 5.Hagiwara K, Inui T, Koike Y, et al. Determination of diphenylarsinic acid, phenylarsonic acid and inorganic arsenic in drinking water by graphite-furnace atomic-absorption spectrometry after simultaneous separation and preconcentration with solid-phase extraction disks. Anal Sci. 2013;29:1153–1158. doi: 10.2116/analsci.29.1153. [DOI] [PubMed] [Google Scholar]
- 6.Ishii K, Tamaoka A, Otsuka F, et al. Diphenylarsinic acid poisoning from chemical weapons in Kamisu, Japan. Ann Neurol. 2004;56:741–745. doi: 10.1002/ana.20290. [DOI] [PubMed] [Google Scholar]
- 7.Hanaoka S, Nomura K, Wada T. Determination of mustard and lewisite related compounds in abandoned chemical weapons (Yellow shells) from sources in China and Japan. J Chromatogr A. 2006;1101:268–277. doi: 10.1016/j.chroma.2005.10.028. [DOI] [PubMed] [Google Scholar]
- 8.Murder on the metro. Nature. 1995;374:392. doi: 10.1038/374392a0. [DOI] [PubMed] [Google Scholar]
- 9.Haas R. Determination of chemical warfare agents: Gas chromatographic analysis of ethylarsine dichloride by derivatization with dithiols (3rd Communication) Environ Sci Pollut Res Int. 1998;5:63–64. doi: 10.1007/BF02986387. [DOI] [PubMed] [Google Scholar]
- 10.Marrs TT, Maynard RL, Sidell F. Chemical Warfare Agents: Toxicology and Treatment. John Wiley & Sons; 2007. [Google Scholar]
- 11.Wang H, Zhang Y, Guo X, et al. Kinetic and products study of the gas-phase reaction of Lewisite with ozone under atmospheric conditions. J Environ Sci (China) 2016;40:3–9. doi: 10.1016/j.jes.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 12.Henriksson J, Johannisson A, Bergqvist PA, et al. The toxicity of organoarsenic-based warfare agents: in vitro and in vivo studies. Arch Environ Contam Toxicol. 1996;30:213–219. doi: 10.1007/BF00215800. [DOI] [PubMed] [Google Scholar]
- 13.Rice P, Brown RF. The development of Lewisite vapour induced lesions in the domestic, white pig. Int J Exp Pathol. 1999;80:59–67. doi: 10.1046/j.1365-2613.1999.00099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Watson AP, Griffin GD. Toxicity of vesicant agents scheduled for destruction by the Chemical Stockpile Disposal Program. Environ Health Perspect. 1992;98:259–280. doi: 10.1289/ehp.9298259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bismuth C, Borron SW, Baud FJ, et al. Chemical weapons: documented use and compounds on the horizon. Toxicology letters. 2004;149:11–18. doi: 10.1016/j.toxlet.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 16.Ware GW. Reviews of Environmental Contamination and Toxicology. New York: Springer-Verlag; 1989. [Google Scholar]
- 17.Axelrod DJ, Hamilton JG. Radio-Autographic Studies of the Distribution of Lewisite and Mustard Gas in Skin and Eye Tissues. Am J Pathol. 1947;23:389–411. [PMC free article] [PubMed] [Google Scholar]
- 18.Ferguson RL, Silver SD. A method for the visual demonstration of lewisite in skin. Am J Clin Pathol. 1947;17:37. [PubMed] [Google Scholar]
- 19.Nguon N, Clery-Barraud C, Vallet V, et al. Time course of lewisite-induced skin lesions and inflammatory response in the SKH-1 hairless mouse model. Wound Repair Regen. 2014;22:272–280. doi: 10.1111/wrr.12147. [DOI] [PubMed] [Google Scholar]
- 20.Chilcott RP, Brown RF, Rice P. Non-invasive quantification of skin injury resulting from exposure to sulphur mustard and Lewisite vapours. Burns. 2000;26:245–250. doi: 10.1016/s0305-4179(99)00129-1. [DOI] [PubMed] [Google Scholar]
- 21.Munro NB, Talmage SS, Griffin GD, et al. The sources, fate, and toxicity of chemical warfare agent degradation products. Environ Health Perspect. 1999;107:933–974. doi: 10.1289/ehp.99107933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goldman M, Dacre JC. Lewisite: its chemistry, toxicology, and biological effects. Rev Environ Contam Toxicol. 1989;110:75–115. doi: 10.1007/978-1-4684-7092-5_2. [DOI] [PubMed] [Google Scholar]
- 23.McGown EL, Ravenswaay Tv, Damlao CR, et al. Histologic changes caused by application of lewisite analogs to mouse skin and human skin xenografts. [Accessed July 27, 2016];1985 http://www.dtic.mil/dtic/tr/fulltext/u2/a159554.pdf. [Google Scholar]
- 24.Nishimura Y, Iwamoto H, Ishikawa N, et al. Long-term pulmonary complications of chemical weapons exposure in former poison gas factory workers. Inhal Toxicol. 2016;28:343–348. doi: 10.3109/08958378.2016.1173133. [DOI] [PubMed] [Google Scholar]
- 25.Tewari-Singh N, Croutch CR, Tuttle R, et al. Clinical progression of ocular injury following arsenical vesicant lewisite exposure. Cutan Ocul Toxicol. 2016 doi: 10.3109/15569527.2015.1127255. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Agents, S.C.R.D.S.C.W., B.E.S. Toxicology, D.E.L. Studies, et al. Review of the U.S. Army's Health Risk Assessments for Oral Exposure to Six Chemical-Warfare Agents. Washington, D.C: National Academies Press; 1999. Appendix F Health Risk Assessment for Lewisite. [PubMed] [Google Scholar]
- 27.Cullumbine H. Treatment of lewisite shock with sodium salt solutions. Br Med J. 1946;1:607. [PubMed] [Google Scholar]
- 28.Flora SSJ. Arsenicals: Toxicity, their Use as Chemical Warfare Agents, and Possible Remedial Measures. In: Gupta RC, editor. Handbook of Toxicology of Chemical Warfare Agents. 2015. pp. 171–191. [Google Scholar]
- 29.King JR, Peters BP, Monteiro-Riviere NA. Laminin in the cutaneous basement membrane as a potential target in lewisite vesication. Toxicol Appl Pharmacol. 1994;126:164–173. doi: 10.1006/taap.1994.1103. [DOI] [PubMed] [Google Scholar]
- 30.Platteborze PL. The transcriptional effects of the vesicants lewisite and sulfur mustard on human epidermal keratinocytes. Toxicol Mech Methods. 2005;15:185–192. doi: 10.1080/15376520590945603. [DOI] [PubMed] [Google Scholar]
- 31.Schmidt TC, Less M, Haas R, et al. Gas chromatographic determination of aromatic amines in water samples after solid-phase extraction and derivatization with iodine. I. Derivatization. J Chromatogr A. 1998;810:161–172. doi: 10.1016/s0021-9673(98)00233-7. [DOI] [PubMed] [Google Scholar]
- 32.Army, U.S.D.o.t., N.A.T. Organization, U.S.N. Department, et al. NATO Handbook on the Medical Aspects of NBC Defensive Operations AMedP-6 (B) Depts. of the Army, the Navy, and the Air Force; 1996. [Google Scholar]
- 33.Ledgard J. [Accessed July 27, 2016];A Laboratory History of Chemical Warfare Agents. 2006 http://www.lulu.com/shop/jared-ledgard/a-laboratory-history-of-chemical-warfare-agents/paperback/product-590198.html. [Google Scholar]
- 34.Pitten F-A, Müller G, König P, et al. Risk assessment of a former military base contaminated with organoarsenic-based warfare agents: uptake of arsenic by terrestrial plants. Science of the total environment. 1999;226:237–245. doi: 10.1016/s0048-9697(98)00400-8. [DOI] [PubMed] [Google Scholar]
- 35.Aydoğdu UF. Technological Dimensions of Defence Against Terrorism. IOS Press; 2013. [Google Scholar]
- 36.Kroening KK, Easter RN, Richardson DD, et al. Analysis of Chemical Warfare Degradation Products. Wiley; 2011. [Google Scholar]
- 37.Goldman RH. Arsenic exposure and poisoning. [Accessed April 6, 2016];2015 Sep 8; 2015. http://www.uptodate.com/contents/arsenic-exposure-and-poisoning. [Google Scholar]
- 38.Ratnaike RN. Acute and chronic arsenic toxicity. Postgrad Med J. 2003;79:391–396. doi: 10.1136/pmj.79.933.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tournel G, Houssaye C, Humbert L, et al. Acute arsenic poisoning: clinical, toxicological, histopathological, and forensic features. J Forensic Sci. 2011;56(Suppl 1):S275–S279. doi: 10.1111/j.1556-4029.2010.01581.x. [DOI] [PubMed] [Google Scholar]
- 40.Heyman A, Pfeiffer JB, Jr, Willett RW, et al. Peripheral neuropathy caused by arsenical intoxication; a study of 41 cases with observations on the effects of BAL (2, 3, dimercapto-propanol) N Engl J Med. 1956;254:401–409. doi: 10.1056/NEJM195603012540901. [DOI] [PubMed] [Google Scholar]
- 41.Hunt KM, Srivastava RK, Athar M. Cutaneous Toxicology of Arsenic. Handbook of Arsenic Toxicology. 2014:301. [Google Scholar]
- 42.Hunt KM, Srivastava RK, Elmets CA, et al. The mechanistic basis of arsenicosis: pathogenesis of skin cancer. Cancer Lett. 2014;354:211–219. doi: 10.1016/j.canlet.2014.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jomova K, Jenisova Z, Feszterova M, et al. Arsenic: toxicity, oxidative stress and human disease. J Appl Toxicol. 2011;31:95–107. doi: 10.1002/jat.1649. [DOI] [PubMed] [Google Scholar]
- 44.Ellinsworth DC. Arsenic, reactive oxygen, and endothelial dysfunction. J Pharmacol Exp Ther. 2015;353:458–464. doi: 10.1124/jpet.115.223289. [DOI] [PubMed] [Google Scholar]
- 45.Flora SJ. Arsenic-induced oxidative stress and its reversibility. Free Radic Biol Med. 2011;51:257–281. doi: 10.1016/j.freeradbiomed.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 46.Shen S, Li XF, Cullen WR, et al. Arsenic binding to proteins. Chem Rev. 2013;113:7769–7792. doi: 10.1021/cr300015c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li C, Xu J, Li F, et al. Unfolded protein response signaling and MAP kinase pathways underlie pathogenesis of arsenic-induced cutaneous inflammation. Cancer Prev Res (Phila) 2011;4:2101–2109. doi: 10.1158/1940-6207.CAPR-11-0343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Srivastava RK, Li C, Chaudhary SC, et al. Unfolded protein response (UPR) signaling regulates arsenic trioxide-mediated macrophage innate immune function disruption. Toxicol Appl Pharmacol. 2013;272:879–887. doi: 10.1016/j.taap.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Oh RS, Pan WC, Yalcin A, et al. Functional RNA interference (RNAi) screen identifies system A neutral amino acid transporter 2 (SNAT2) as a mediator of arsenic-induced endoplasmic reticulum stress. J Biol Chem. 2012;287:6025–6034. doi: 10.1074/jbc.M111.311217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang K, Kaufman RJ. From endoplasmic-reticulum stress to the inflammatory response. Nature. 2008;454:455–462. doi: 10.1038/nature07203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Valko M, Rhodes CJ, Moncol J, et al. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact. 2006;160:1–40. doi: 10.1016/j.cbi.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 52.Liu SX, Davidson MM, Tang X, et al. Mitochondrial damage mediates genotoxicity of arsenic in mammalian cells. Cancer Res. 2005;65:3236–3242. doi: 10.1158/0008-5472.CAN-05-0424. [DOI] [PubMed] [Google Scholar]
- 53.Liu SX, Athar M, Lippai I, et al. Induction of oxyradicals by arsenic: implication for mechanism of genotoxicity. Proc Natl Acad Sci U S A. 2001;98:1643–1648. doi: 10.1073/pnas.031482998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li C, Srivastava RK, Elmets CA, et al. Arsenic-induced cutaneous hyperplastic lesions are associated with the dysregulation of Yap, a Hippo signaling-related protein. Biochem Biophys Res Commun. 2013;438:607–612. doi: 10.1016/j.bbrc.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 55.Li B, Giambelli C, Tang B, et al. Arsenic Attenuates GLI Signaling, Increasing or Decreasing its Transcriptional Program in a Context-Dependent Manner. Mol Pharmacol. 2016;89:226–232. doi: 10.1124/mol.115.100867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nishimoto Y, Yamakido M, Ishioka S, et al. Epidemiological studies of lung cancer in Japanese mustard gas workers. Princess Takamatsu Symp. 1987;18:95–101. [PubMed] [Google Scholar]
- 57.Medical Management Guidelines for Arsine (AsH3) [Accessed May 31, 2016];2014 Oct 21; 2014. http://www.atsdr.cdc.gov/MMG/MMG.asp?id=1199&tid=278.
- 58.Chemical Terrorism Fact Sheet Blood Agents - Arsines (Arsenic Hydride, AsH3) Louis University School of Public Health; 2002. [Google Scholar]
- 59.Pullen-James S, Woods SE. Occupational arsine gas exposure. J Natl Med Assoc. 2006;98:1998–2001. [PMC free article] [PubMed] [Google Scholar]
- 60.Hocken AG, Bradshaw G. Arsine poisoning. Br J Ind Med. 1970;27:56–60. doi: 10.1136/oem.27.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Risk M, Fuortes L. Chronic arsenicalism suspected from arsine exposure: a case report and literature review. Vet Hum Toxicol. 1991;33:590–595. [PubMed] [Google Scholar]
- 62.Rael LT, Ayala-Fierro F, Carter DE. The effects of sulfur, thiol, and thiol inhibitor compounds on arsine-induced toxicity in the human erythrocyte membrane. Toxicol Sci. 2000;55:468–477. doi: 10.1093/toxsci/55.2.468. [DOI] [PubMed] [Google Scholar]
- 63.Pakulska D, Czerczak S. Hazardous effects of arsine: a short review. Int J Occup Med Environ Health. 2006;19:36–44. doi: 10.2478/v10001-006-0003-z. [DOI] [PubMed] [Google Scholar]
- 64.Yamauchi T, Yamano Y, Yamanaka K, et al. Possible production of arsenic hemoglobin adducts via exposure to arsine. J Occup Health. 2015;57:161–168. doi: 10.1539/joh.14-0148-OA. [DOI] [PubMed] [Google Scholar]
- 65.Krause H, Grussendorf EI. [Therapy of morbus Bowen with 5-fluoro-uracil ointment (author's transl)] Derm Beruf Umwelt. 1979;27:176–178. [PubMed] [Google Scholar]
- 66.Acute Exposure Guideline Levels for Selected Airborne Chemicals. Vol. 15. Washington (DC): National Academies Press; 2013. Lewisite Acute Exposure Guideline Levels. [PubMed] [Google Scholar]
- 67.Cameron GR, Carleton HM, Short RH. Pathological changes induced by lewisite and allied compounds. J Pathol Bacteriol. 1946;58:411–422. doi: 10.1002/path.1700580311. [DOI] [PubMed] [Google Scholar]
- 68.Olajos EJ, Morgan EW, Renne RA, et al. Acute inhalation toxicity of neutralized chemical agent identification sets (CAIS) containing agent in chloroform. J Appl Toxicol. 1998;18:363–371. doi: 10.1002/(sici)1099-1263(1998090)18:5<363::aid-jat521>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 69.Harrison HE, Ordway & NK, et al. Poisoning from inhalation of the vapors of lewisite and phenyldichlorarsine; its pathology in the dog and treatment with 2,3-dimercaptopropanol (BAL) J Pharmacol Exp Ther. 1946;87:76–80. [PubMed] [Google Scholar]
- 70.NIH Strategic Plan and Research Agenda for Medical Countermeasures Against Chemical Threats. [Accessed March 31, 2016];2007 https://www.niaid.nih.gov/topics/BiodefenseRelated/ChemicalCountermeasures/Documents/nihstrategicplanchem.pdf.
- 71.Sabina C, Grund KH Hans Uwe Wolf. Ullmann's Encyclopedia of Industrial Chemistry. John Wiley and Sons; 2008. Arsenic and Arsenic Compounds; pp. 199–237. [Google Scholar]
- 72.Emsley J. The Elements of Murder: A History of Poison. OUP Oxford; 2006. [Google Scholar]
- 73.Lo-Coco F, Avvisati G, Vignetti M, et al. Retinoic acid and arsenic trioxide for acute promyelocytic leukemia. N Engl J Med. 2013;369:111–121. doi: 10.1056/NEJMoa1300874. [DOI] [PubMed] [Google Scholar]
- 74.Noort D, Benschop HP, Black RM. Biomonitoring of exposure to chemical warfare agents: a review. Toxicol Appl Pharmacol. 2002;184:116–126. [PubMed] [Google Scholar]
- 75.Rosenblatt DH, Miller TA, Dacre JC, et al. Problem definition studies on potential environmental pollutants. II. Physical, chemical, toxicological, and biological properties of 16 substances. (DTIC Document) [Google Scholar]
- 76.Köhler M, Hofmann K, Völsgen F, et al. Bacterial release of arsenic ions and organoarsenic compounds from soil contaminated by chemical warfare agents. Chemosphere. 2001;42:425–429. doi: 10.1016/s0045-6535(00)00060-6. [DOI] [PubMed] [Google Scholar]
- 77.Zhang W, Sun H, Chen W, et al. Mechanistic and kinetic study on the reaction of ozone and trans-2-chlorovinyldichloroarsine. Chemosphere. 2016;150:329–340. doi: 10.1016/j.chemosphere.2016.01.115. [DOI] [PubMed] [Google Scholar]
- 78.Haas R, Schmidt TC, Steinbach K, et al. Chromatographic determination of phenylarsenic compounds. Fresenius' journal of analytical chemistry. 1998;361:313–318. [Google Scholar]
- 79.Kroening KK, Solivio MJV, Garcia-Lopez M, et al. Cytotoxicity of arsenic-containing chemical warfare agent degradation products with metallomic approaches for metabolite analysis. Metallomics. 2009;1:59–66. [Google Scholar]
- 80.Apostoli P, Alessio L, Romeo L, et al. Metabolism of arsenic after acute occupational arsine intoxication. Journal of toxicology and environmental health. 1997;52:331–342. doi: 10.1080/00984109708984068. [DOI] [PubMed] [Google Scholar]
- 81.Carter DE, Aposhian HV, Gandolfi AJ. The metabolism of inorganic arsenic oxides, gallium arsenide, and arsine: a toxicochemical review. Toxicol Appl Pharmacol. 2003;193:309–334. doi: 10.1016/j.taap.2003.07.009. [DOI] [PubMed] [Google Scholar]
- 82.Peters RA, Stocken LA, Thompson R. British anti-lewisite (BAL) Nature. 1945;156:616. doi: 10.1038/156616a0. [DOI] [PubMed] [Google Scholar]
- 83.Mouret S, Wartelle J, Emorine S, et al. Topical efficacy of dimercapto-chelating agents against lewisite-induced skin lesions in SKH-1 hairless mice. Toxicol Appl Pharmacol. 2013;272:291–298. doi: 10.1016/j.taap.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 84.Boyd VL, Harbell JW, O'Connor RJ, et al. 2, 3-Dithioerythritol, a possible new arsenic antidote. Chemical research in toxicology. 1989;2:301–306. doi: 10.1021/tx00011a006. [DOI] [PubMed] [Google Scholar]
- 85.Delnomdedieu M, Basti MM, Otvos JD, et al. Transfer of arsenite from glutathione to dithiols: a model of interaction. Chemical research in toxicology. 1993;6:598–602. doi: 10.1021/tx00035a002. [DOI] [PubMed] [Google Scholar]
- 86.Kehe K, Flohe S, Krebs G, et al. Effects of Lewisite on cell membrane integrity and energy metabolism in human keratinocytes and SCL II cells. Toxicology. 2001;163:137–144. doi: 10.1016/s0300-483x(01)00389-4. [DOI] [PubMed] [Google Scholar]
- 87.Yadav A, Mathur R, Samim M, et al. Nanoencapsulation of DMSA monoester for better therapeutic efficacy of the chelating agent against arsenic toxicity. Nanomedicine (Lond) 2014;9:465–481. doi: 10.2217/NNM.13.17. [DOI] [PubMed] [Google Scholar]
- 88.Nelson P, Hancock JR, Sawyer TW. Therapeutic effects of hypothermia on Lewisite toxicity. Toxicology. 2006;222:8–16. doi: 10.1016/j.tox.2005.12.026. [DOI] [PubMed] [Google Scholar]
- 89.King JR, Riviere JE, Monteiro-Riviere NA. Characterization of lewisite toxicity in isolated perfused skin. Toxicol Appl Pharmacol. 1992;116:189–201. doi: 10.1016/0041-008x(92)90298-7. [DOI] [PubMed] [Google Scholar]