Evidence implicating obstructive sleep apnea (OSA) in blood pressure elevation is compelling, such that OSA is acknowledged as a risk factor in the major guidelines for prevention and management of hypertension.1,2 Nevertheless, not every patient with OSA will develop comorbid hypertension. Traditional markers of disease severity such as apnea-hypopnea index (AHI) and degree of nocturnal desaturation, as well as coexisting conditions including obesity and old age, have been implicated as determinants of enhanced vulnerability.3 More recently, excessive daytime sleepiness (EDS), a common but not pervasive symptom of OSA, has emerged as a non-conventional indicator for identifying high-risk OSA subjects. However, the available literature on the prognostic role of hypersomnolence for clinical outcomes, including hypertension, is controversial.4,5 This discrepancy may be ascribed to the various approaches used to determine EDS, as suggested by Ren et al6 in this issue of Hypertension.
These investigators examined the association between prevalent hypertension and EDS in a large, well-characterized Chinese population. The sample consisted of referrals to the sleep clinic for suspected OSA, which was assessed by in-laboratory polysomnography (PSG). The use of the gold standard diagnostic method for OSA, in lieu of questionnaires or portable monitors, is a strength of the paper. Another important feature is the dual assessment of sleepiness performed in the study. All subjects completed a self-report measure (Epworth Sleepiness Scale, ESS7) and a sleep laboratory test (Multiple Sleep Latency Test, MSLT8), and were then classified based on the average sleep latency (the time taken to fall asleep) exhibited at the MSLT, as an index of EDS. The MSLT consists of 4 to 5 nap opportunities spread across the day, with the subject monitored continuously by PSG. The average sleep onset latency in all naps is computed to provide a quantitative estimate of somnolence.
After correcting for established risk factors, multivariate analysis showed that those who had OSA and manifested a mean sleep latency at the MSLT of 5 to 8 min or <5 min had 1.95 and 2.11 times higher odds for prevalent hypertension than non-OSA counterparts with an objective sleep latency >8 min. The increased likelihood of hypertension in objectively sleepy OSA patients was evident across sex, age, and BMI groups, indicating that the relation is not modified by traditional risk factors. Importantly, when OSA patients were stratified based on presence or absence of subjective EDS as measured by ESS, the association between objective EDS and hypertension was retained in both strata. Linear trends were noted between blood pressure and MSLT categories, again restricted to the OSA group.
These novel findings suggest that objective sleepiness may be a sensitive, independent indicator of risk of hypertension in OSA population. Its predictive significance irrespective of self-reported or perceived sleepiness deserves further comment.
It is well known that the agreement between subjective and objective methods to ascertain EDS is rather poor. This is assumed to be a reflection of the multifaceted and variable nature of sleepiness, which may not be fully captured by any single instrument. While self-reports such as the ESS estimate sleepiness by targeting cognitive-behavioral components, tests such as the MSLT determine the physiological propensity to fall asleep. The likely relationship between the physiological drive to sleep and MSLT-derived EDS may also account for its closer link to hypertension and other biological markers of risks, as observed by Ren et al and previous research.6,9
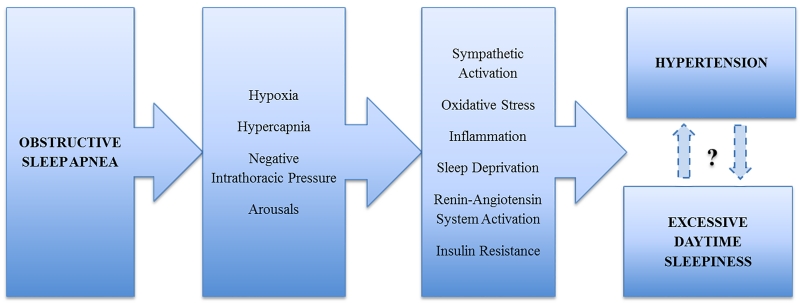
Importantly, the relation between objective EDS and hypertension reported by Ren et al6 was confined to the OSA group, a finding suggesting that there are unique, disease-specific mechanisms linking EDS and high blood pressure in this population. Indeed, the recurrent episodes of hypoxemia, hypercapnia, arousals from sleep, and swings in intrathoracic pressure induced by apneic events activate a cluster of pathogenic responses that, over time, lead to blood pressure elevation and promote daytime hypersomnolence (Figure).3 Whether sleepiness is a marker or mediator of hypertension risk in OSA cannot be definitively answered by this study. However, rather than lying in the causal pathway between OSA and hypertension, EDS is more likely another clinical complication, and, like hypertension, may be a downstream consequence of the sleep disorder. Bearing in mind that both hypertension and EDS are frequent but not ubiquitous manifestations in OSA patients, hypersomnolence may be regarded not only as a mere marker of disease severity, but also as an expression of the individual vulnerability to the adverse effects of OSA.4 Alternatively, the mechanisms that elicit the increase in blood pressure may also be those that contribute to daytime somnolence.
Figure.
Simplified schematic of potential pathogenic mechanisms causing EDS and hypertension in OSA patients.
On the other hand, as the cross-sectional, observational design of this study precludes inferring causality, it cannot be excluded that hypertension may have contributed to the degree of EDS seen in the OSA population. Indeed, hypersomnolence is a recognized, unwelcome side effect of antihypertensive therapy, and mostly related to beta-blocker and alpha2-agonist administration. Since the use of blood pressure lowering medications was not an exclusion criterion, and since hypertension was more prevalent among OSA patients, it is conceivable that the proportion of treated patients was also higher in this group. Hence, the possibility that EDS was at least partially caused or exacerbated by antihypertensive drugs is a legitimate concern. Translating this concept into the clinical setting, the risk of aggravating EDS should be critically considered when treating hypertension in patients with comorbid OSA.
Another therapeutic consideration concerns the blood pressure lowering effects of OSA therapy. Although continuous positive airway pressure (CPAP), the elective treatment for OSA, is effective in reducing blood pressure and may even attenuate risk of incident hypertension, an interaction with the individual degree of EDS has been observed, as benefits of CPAP may be limited in non-sleepy patients.3,4 Although these data are mostly derived from studies determining hypersomnolence by means of questionnaires, they are consistent with the findings of Ren et al6 and supportive of a common pathological background for objective EDS and hypertension. Thus, objectively sleepy patients not only represent a high-risk population but would also, presumably, benefit the most from OSA therapy. A further consideration is the potential implication of sleepiness as a marker of increased risk for understanding the findings of randomized clinical trials on the effects of CPAP intervention. Subjectively sleepy patients are generally excluded from long term outcome studies. Thus, these studies may be investigating effective therapy in a conceivably lower risk group, hence possibly biasing the findings against detecting benefits of intervention.
It should also be pointed out that current guidelines do not mandate MSLT as a diagnostic or follow-up measure for OSA.8 The MSLT is certainly a laborious and lengthy procedure, which requires trained staff and PSG equipment. These technical requirements and the associated financial burden limit greatly its routine applicability. Nevertheless, the clinical significance of MSLT is increasingly recognized and may encourage more extensive use of this test as a risk stratification tool. This would be further justified if longitudinal studies confirm and extend the trajectories seen in this cross-sectional study, with sleep latency at MSLT predicting incident hypertension and/or cardiovascular events beyond traditional risk factors. Cost-benefit concerns may be alleviated by assessing the prognostic value of a shortened version of the test, comprising only 2, or even 1, nap. In this regard, latency with a single nap has been shown to discriminate reasonably well between patients with EDS and no EDS.10 If the predictive ability of a single nap is proven in the context of clinical outcomes, broader applications of the test would add importantly to diagnosis, risk stratification, and therapeutic strategies in OSA patients.
Acknowledgments
Sources of Funding
This work was supported by National Institutes of Health grants R01 HL114676, R01 HL114024, and R01 HL065176 to V.K.S., and American Heart Association grant 16SDG27250156 to N.C.
Footnotes
Disclosures
The Mayo Foundation has received a gift from the Phillips Respironics Foundation for the study of sleep and cardiovascular disease. V.K.S. served as a consultant for Respicardia, ResMed, Sorin Inc., U-Health, Philips, Dane Garvin, Ronda Grey and Glaxo Smith Kline and is working with Mayo Health Solutions and their industry partners on intellectual property related to sleep and cardiovascular disease. No other conflicts are reported.
References
- 1.The Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) of the European Society of Cardiology (ESC) 2013 ESH/ESC Guidelines for the management of arterial hypertension. Eur Heart J. 2013;34:2159–2219. doi: 10.1093/eurheartj/eht151. [DOI] [PubMed] [Google Scholar]
- 2.Calhoun DA, Jones D, Textor S, Goff DC, Murphy TP, Toto RD, White A, Cushman WC, White W, Sica D, Ferdinand K, Giles TD, Falkner B, Carey RM. Resistant hypertension: diagnosis, evaluation, and treatment: a scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Hypertension. 2008;51:1403–1419. doi: 10.1161/HYPERTENSIONAHA.108.189141. [DOI] [PubMed] [Google Scholar]
- 3.Parati G, Lombardi C, Hedner J, Bonsignore MR, Grote L, Tkacova R, Levy P, Riha R, Bassetti C, Narkiewicz K, Mancia G, McNicholas WT. Position paper on the management of patients with obstructive sleep apnea and hypertension: joint recommendations by the European Society of Hypertension, by the European Respiratory Society and by the members of European COST (COoperation in Scientific and Technological research) ACTION B26 on obstructive sleep apnea. J Hypertens. 2012;30:633–646. doi: 10.1097/HJH.0b013e328350e53b. [DOI] [PubMed] [Google Scholar]
- 4.Kapur VK, Resnick HE, Gottlieb DJ, Sleep Heart Health Study Group Sleep disordered breathing and hypertension: does self-reported sleepiness modify the association? Sleep. 2008;31:1127–1132. [PMC free article] [PubMed] [Google Scholar]
- 5.Empana JP, Dauvilliers Y, Dartigues JF, Ritchie K, Gariepy J, Jouven X, Tzourio C, Amouyel P, Besset A, Ducimetiere P. Excessive daytime sleepiness is an independent risk indicator for cardiovascular mortality in community-dwelling elderly. The Three City Study. Stroke. 2009;40:1219–1224. doi: 10.1161/STROKEAHA.108.530824. [DOI] [PubMed] [Google Scholar]
- 6.Ren R, Li Y, Zhang J, Zhou J, Sun Y, Tan L, Li T, Wing YK, Tang X. Obstructive sleep apnea with objective daytime sleepiness is associated with hypertension. Hypertension. 2016 doi: 10.1161/HYPERTENSIONAHA.115.06941. In press. [DOI] [PubMed] [Google Scholar]
- 7.Johns MW. A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale. Sleep. 1991;14:540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 8.Littner MR, Kushida C, Wise M, Davila DG, Morgenthaler T, Lee-Chiong T, Hirshkowitz M, Daniel LL, Bailey D, Berry RB, Kapen S, Kramer M. Practice parameters for clinical use of the Multiple Sleep Latency Test and the Maintenance of Wakefulness Test. Sleep. 2005;28:113–121. doi: 10.1093/sleep/28.1.113. [DOI] [PubMed] [Google Scholar]
- 9.Barcelo A, Barbe F, de la Pena M, Martinez P, Soriano JB, Pierola J, Agusti AGN. Insulin resistance and daytime sleepiness in patients with sleep apnoea. Thorax. 2008;63:946–950. doi: 10.1136/thx.2007.093740. [DOI] [PubMed] [Google Scholar]
- 10.Sunwoo BY, Jackson N, Maislin G, Gurubhagavatula I, George CF, Pack AI. Reliability of a single objective measure in assessing sleepiness. Sleep. 2012;35:149–158. doi: 10.5665/sleep.1606. [DOI] [PMC free article] [PubMed] [Google Scholar]