Summary
Sickle cell disease (SCD) pain transitions from acute to chronic for unknown reasons. Chronic elevation of the pain neurotransmitter substance P (SP) sensitizes pain nociceptors. We evaluated SP levels in controls and SCD patients during baseline and acute pain and investigated associations between SP and age, gender, pain history, haemolysis and hydroxycarbamide (also termed hydroxyurea) use. Plasma SP levels were measured using enzyme-linked immunosorbent assay. Independent samples t-test compared SP levels between: 1) SCD baseline and controls and 2) SCD baseline and acute pain. Multivariate linear regression determined associations between SP and age, gender, pain history and hydroxycarbamide use. Spearman correlation determined an association between SP and haemolysis. We enrolled 35 African American controls, 25 SCD baseline and 12 SCD pain patients. SCD patients were 7-19 years old. Mean±standard deviation SP level (pg/ml) in SCD baseline was higher than controls (32.4±11.6 vs. 22.9±7.6, P=0.0009). SP in SCD pain was higher than baseline (78.1±43.4 vs. 32.4±11.6, P=0.004). Haemolysis correlated with increased SP: Hb (r=−0.7, P=0.0002), reticulocyte count (r=0.61, P=0.0016), bilirubin (r=0.68, P=0.0216), LDH (r=0.62, P=0.0332), aspartate aminotransferase (r=0.68, P=0.003). Patients taking hydroxycarbamide had increased SP (β=29.2, P=0.007). SP could be a mediator of or marker for pain sensitization in SCD and a biomarker and/or target for novel pain treatment.
Keywords: substance P, sickle cell disease, pain, sensitization, haemolysis
Introduction
Pain causes significant morbidity for patients with sickle cell disease (SCD) and is associated with increased mortality and markedly reduced health-related quality of life. (Brandow et al., 2010; Platt et al., 1991) Severe acute intermittent pain is the norm in childhood; however, as SCD patients age, sickle cell pain becomes chronic with almost 30% of patients reporting daily pain. (Brandow et al., 2010; Brousseau et al. 2010; Panepinto et al., 2005; Smith et al., 2008) Increasing evidence in both murine models and patients with SCD suggests that abnormalities in the peripheral and central nervous systems contribute to the development of chronic pain in SCD that is sustained over time. (Brandow et al., 2015; Darbari et al., 2015; Hillery et al., 2011; Kohli et al., 2010; Vincent et al., 2013) Patients with SCD experience increased pain to thermal (cold, heat) stimuli compared to healthy controls(Brandow et al., 2015; Brandow et al., 2013; Ezenwa et al., 2016; Jacob et al., 2015; O'Leary et al., 2014) and report features of neuropathic pain on validated screening questionnaires. (Brandow et al., 2014; Ezenwa et al., 2016) Collectively, these data illustrate the complex nature of sickle cell pain that probably includes inflammatory, neuropathicand other aetiologies that ultimately drive the pain. Thus, the underlying neurobiology that contributes to the development of acute pain or the transition to chronic pain is complex and not well understood.
Substance P (SP), a neuropeptide encoded by the tachykinin, precursor 1 (TAC1) gene in primary afferent neurons, is a primary pain neurotransmitter. (Harrison & Geppetti 2001; O'Connor et al., 2004; Sacerdote & Levrini 2012) Chronic elevation of SP can sensitize peripheral nociceptors resulting in mechanical and thermal hypersensitivity. Mechanical, cold and heat hypersensitivity occurs in both mice and humans with SCD(Kohli et al., 2010; Vincent et al., 2013; Zappia et al., 2014); however the mechanisms driving this hypersensitivity are not known. In mice with SCD, SP is increased in skin(Kohli et al., 2010), is associated with thermal hypersensitivity, and SP release is associated with activation of mast cells. (Vang et al., 2015; Vincent et al., 2013) Furthermore, SP levels were significantly reduced in these mice after treatment with cromolyn, imatinib or a nociceptin receptor agonist. (Vang et al., 2015; Vincent et al., 2013) Only one published study evaluated SP in humans with SCD (Douglas, 2008; Michaels et al., 1998) and found that SP was elevated at baseline and further increased during acute pain. However this study included predominantly Caucasian controls and patient and disease-related factors that may impact the variability of SP levels in patients with SCD were not evaluated in this study. In humans with pain disorders other than SCD, higher plasma SP levels are associated with pain and disease severity. Specifically, in patients with chronic migraines, SP levels are significantly higher in patients compared to controls and higher SP levels are associated with migraine pain intensity. (Jang et al., 2011) Furthermore, in patients with complex regional pain syndrome who suffer from both acute and chronic pain, SP levels are significantly elevated in the affected and unaffected arm of patients compared to controls. (Schinkel et al., 2006) SP levels are also increased in patients with fibromyalgia and higher levels are associated with increased pain in these patients. (Field et al., 2002; Russell et al., 1994; Schwarz et al., 1999; Stahl, 2009) The chronic widespread pain syndrome seen in patients with fibromyalgia has some overlapping characteristics with pain in SCD patients. (Ramprakash & Fishman 2015; Schlesinger, 2004) Chronic widespread pain is defined as pain that persists for 3 or more months and occurs in both the axial skeleton and two or more contralateral quadrants of the body. (Arnold et al., 2011; Wolfe et al., 1990) Together, these data support the potential role of SP in SCD pain neurobiology.
The primary objective of this study was to evaluate plasma SP levels in patients with SCD in baseline health compared to controls and in patients with SCD during acute vaso-occlusive crisis pain compared to baseline. We hypothesized that patients with SCD in baseline health have significantly higher SP levels compared to healthy African American controls. We also hypothesized that SP levels significantly increase during acute pain in patients with SCD compared to baseline levels. Our secondary objective was to determine patient- and disease-related factors associated with SP levels in patients with SCD. Specifically, we investigated the association between levels of SP and age, gender, prior pain history, markers of haemolysis, and use of hydroxycarbamide (also termed hydroxyurea). We hypothesized older age, female gender, history of more frequent painful events, increased markers of haemolysis and use of hydroxycarbamide would be associated with increased SP levels in patients with SCD.
Methods
Study setting and subjects
This prospective cohort study was conducted between 2013 and 2015. Study subjects included children aged 7-19 years with SCD and healthy African American controls. All SCD patients were recruited during a routine visit to the Wisconsin Sickle Cell Clinic or during hospitalization for acute pain. A single consent was obtained for inclusion in both the baseline health cohort and acute pain cohort. Consent was obtained via one of the following methods: 1) Pre-consented during routine SCD clinic visits. If the child was hospitalized for pain in the future, participation was affirmed at that time. 2) Within 48 h of hospitalization if pre-consent was not obtained. Controls were recruited from siblings, parents/guardians, relatives and friends of patients with SCD. After informed consent, patients and controls were scheduled to return at a different date for baseline testing (baseline health cohort) or were tested during future hospitalizations for acute pain (acute pain cohort).
Patients with SCD were eligible for the following two cohorts: 1) baseline health cohort and/or 2) acute pain cohort. Controls were only assessed once. Inclusion and exclusion criteria for these cohorts are subsequently outlined. SCD baseline health cohort. Inclusion criteria: 1) aged 7-19 years and 2) SCD (all genotypes). Exclusion criteria: 1) other disease resulting in pain phenotype (i.e., arthritis, chronic regional pain syndrome, etc.), 2) acute pain within 2 weeks of assessment, 3) any analgesics within 24 h of assessment, 4) overt/silent stroke based on history and available neuroimaging and 5) receiving chronic transfusions. SCD acute pain cohort. Inclusion criteria: 1) aged 7-19 years, 2) SCD (all genotypes) and 3) hospitalized for acute pain. Exclusion Criteria: 1) other disease resulting in pain phenotype, 2) overt/silent stroke based on history and available neuroimaging, and 3) receiving chronic transfusions. For the purpose of the study, an acute pain crisis was defined as a hospital admission with a primary diagnosis of pain where the patient was treated with intravenous pain medication. All patients in the acute pain cohort received standard of care for acute pain. Patients that were admitted for febrile episodes (fever ≥101.5 °F) only without pain were excluded. Patients with a haemolytic crisis only, defined as worsening anaemia with compensatory reticulocytosis without pain were also excluded. Controls. Inclusion criteria: 1) aged ≥7 years, 2) African American and 3) healthy. Exclusion Criteria: 1) other disease resulting in pain phenotype and 2) neurological disease (including stroke).
The Institutional Review Board of the Children's Hospital of Wisconsin approved the study. Informed consent was obtained from the parent or legal guardian and assent was obtained from the child when appropriate. Stipends were given for study completion and compensation for transportation to the testing site was provided in the form of a taxi or gas card.
Primary outcome
The primary outcome of the study was plasma SP level (pg/ml) measured in three cohorts: 1) patients with SCD in baseline health (baseline health cohort), 2) patients with SCD during hospitalization for acute vaso-occlusive crisis pain (acute pain cohort) and 3) healthy African American controls. In order to provide supporting evidence for pain sensitization, plasma SP levels were compared between healthy controls and patients with SCD in baseline health. Furthermore, in order to determine the impact of acute pain on SP levels, plasma SP levels were compared between patients with SCD in baseline health and during acute pain.
Measurement of SP levels
Citrated whole blood was collected from each subject, centrifuged within 30 min and platelet poor plasma stored in aliquots at −80°C. Plasma SP levels were determined using a competitive enzyme-linked immunosorbent assay (ELISA) from Cayman Chemical (Ann Arbor, MI). We found evidence for interference and therefore purified the samples using a reverse phase C18 column prior to running the assay. Plasma samples were assayed in duplicate and SP levels are reported in pg/ml.
Covariates
In order to determine factors associated with SP levels, we investigated the following covariates: age, gender, prior pain history, markers of haemolysis and use of hydroxycarbamide. Our rationale for evaluating these covariates include published data that increased pain sensitivity is associated with older age and female gender(Edwards & Fillingim 2001a; Edwards & & Fillingim 2001b; Edwards et al., 2003; Fillingim et al., 2009; Paller et al., 2009; Riley et al., 1998) and history of frequent pain can impact pain perception leading to increased pain sensitivity. (Hogeweg et al., 1995; Sethna et al., 2007; Wollgarten-Hadamek et al., 2009; Zohsel et al., 2008; Zohsel et al., 2006) Furthermore, inflammation and oxidative damage associated with haemolysis(Nur et al., 2011; Rother et al., 2005) can lead to nociceptor sensitization and increased pain sensitivity. (Kim et al., 2004; Linley et al., 2012; Nishio et al., 2013) Finally, hydroxycarbamide is an increasingly used front-line medication to prevent sickle cell-related complications, including pain, and its use can be a marker for more severe disease. Prior pain history was defined as total number of hospitalizations and emergency department visits for pain over the lifetime of a patient as obtained from the medical record and comprehensive SCD clinical database. The history of pain events was adjusted for age. The markers of haemolysis included haemoglobin (Hb), reticulocyte count, total bilirubin, lactate dehydrogenase (LDH) and aspartate aminotransferase (AST). These laboratory values were obtained during baseline health and the mean was taken of 3 individual assessments that were most proximal to the time the SP level was obtained. The use of hydroxycarbamide was determined based on documentation of prescription of the drug in the medical record.
Statistical analysis
Independent samples student's t-test was used to compare SP levels between: 1) SCD baseline health cohort and controls and 2) SCD baseline health cohort and SCD acute pain cohort. Paired t-test was used to compare differences in SP levels between individual patients with SCD that were evaluated at both baseline health and during acute pain. Multivariate linear regression analysis was used to determine the association between SP levels and age, gender, prior pain history and hydroxycarbamide use in patients with SCD. Spearman correlation was performed to determine the association between SP levels and markers of haemolysis in the SCD baseline health cohort. Finally, in order to illustrate the extreme values of SP levels in patients with SCD we determined the proportion of SCD patients that had SP levels one standard deviation above the mean SP level of the control group. Statistical significance was set at a p-value of 0.05. All analyses were conducted with STATA version 11.2 (STATA, College Station, TX). All data were fully accessible to all authors and analyses were done in collaboration with our biostatistical colleagues.
Results
Study population
Twenty-five patients with SCD in baseline health, 12 patients with SCD during acute pain, and 35 African American controls completed the study. Controls were significantly older than SCD patients in the baseline health cohort. There was no difference in the mean age of SCD patients in the baseline health cohort compared to those in the acute pain cohort. Gender between the same comparison groups did not differ. The remainder of the demographic characteristics of the entire study population is shown in Table I.
Table I.
Demographic, clinical, and laboratory characteristics of subjects
| Variable | Controls (n=35) | SCD Baseline (n=25) | SCD Acute Pain (n=12) |
|---|---|---|---|
| Age (years) (Mean ± standard deviation) | 21.5 (12.5)* | 11.8 (3.8)*# | 12.1 (3.5)# |
| Gender: Female | 22 (62.9) | 17 (68%) | 8 (66.7) |
| Genotype | N/A | ||
| HbSS | 17 (68%) | 8 (66.7%) | |
| HbSC | 6 (24%) | 3 (25%) | |
| HbSβ+thal | 2 (8%) | ||
| Other | 1 (8.3%) SO Arab | ||
| Hydroxycarbamide use: yes | N/A | 19 (76%) | 3 (25%) |
| Prior Pain History** (Median, IQR) | N/A | 5 (1-10.5) | 8 (2-12.25) |
| Laboratory values (Median IQR) | N/A | N/A | |
| Hb (g/l) | 97 (86-114) | ||
| Reticulocytes (%) | 7.7 (3.5-10.5) | ||
| Total bilirubin (μmol/l) | 22.2 (13.7-44.5) | ||
| Lactate dehydrogenase (iu/l) | 885.5 (512.3-1281.5) | ||
| Aspartate aminotransferase (u/l) | 46 (35.3-78.3) | ||
IQR, interquartile range; N/A, not applicable; SCD, sickle cell disease.
SP levels in patients with SCD and African American controls
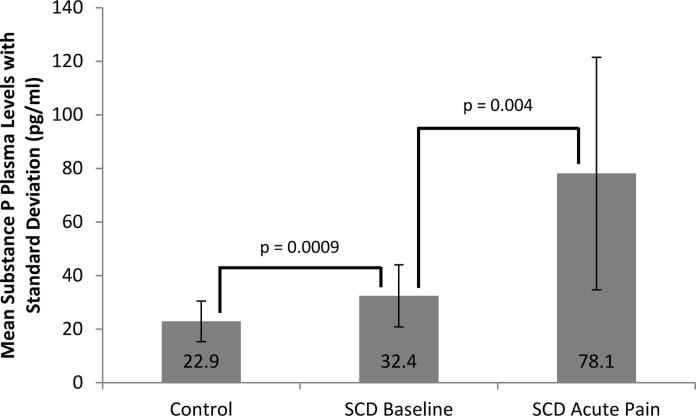
Table II displays the mean (standard deviation; SD) and range of SP levels in controls and patients with SCD during baseline health and during acute pain.
Table II.
Substance P levels in controls and SCD patients at baseline and during acute pain
| Controls (n=35) | SCD Baseline (n=25) | SCD Acute Pain (n=12) | |
|---|---|---|---|
| Mean (SD), pg/ml | 22.9 (7.6) | 32.4 (11.6) | 78.1 (43.4) |
| Range, pg/ml | 12.2 - 38.34 | 16.3 - 60.16 | 34.57 - 152.28 |
SCD, sickle cell disease; SD, standard deviation.
SP levels are significantly elevated in patients with SCD during baseline health compared to healthy African American controls
The mean (SD) SP level of SCD patients in baseline health was almost 50% higher than controls [32.4 (11.6) vs. 22.9 (7.6) pg/ml, P=0.0009] (Table II and Figure 1). When controls were restricted to patients less than 19 years of age (n=21) to better match the cohort of SCD patients in baseline health, the significant elevation of SP levels in patients with SCD persisted [32.4 (11.5) vs. 21 (7.1) pg/ml, P<0.0001].
Figure 1. Differences in substance P Levels between controls, SCD patients in baseline health and SCD patients during acute pain.
Mean plasma substance P levels (pg/ml) were compared using independent samples student's t-test between three cohorts. Substance P levels were significantly higher in sickle cell disease (SCD) patients in baseline health compared to African American controls and were also significantly higher in SCD patients during acute pain compared to SCD patients during baseline health.
SP levels significantly increase in patients with SCD during acute pain
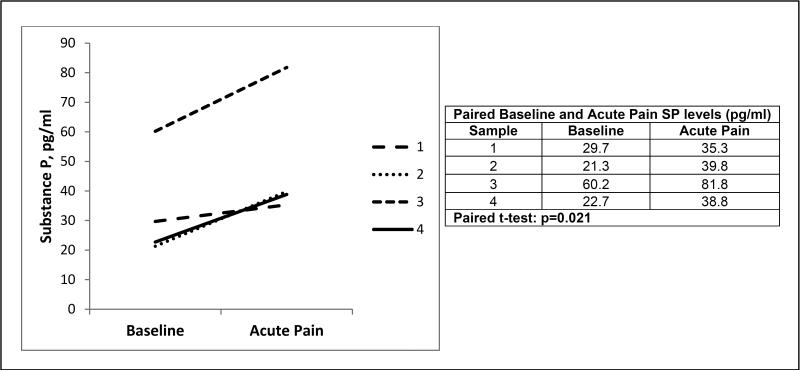
The mean (SD) SP level was 2.4-fold higher in the SCD acute pain cohort compared to the mean SP level in the SCD baseline health cohort [78.1 (43.4) vs. 32.4 (11.6) pg/ml, P=0.004] (Table II and Figure 1). A small cohort of patients with SCD (n=4) had paired plasma samples obtained both at baseline health and during acute pain. Plasma SP levels significantly increased in these paired samples despite this small cohort (Figure 2).
Figure 2. Individual SCD patients with paired substance P levels measured at baseline and during acute pain.
A subset (n=4) of sickle cell disease patients had substance P levels measured both at baseline and during acute pain as represented by the four different lines and in the table. Substance P levels increased during acute pain in each patient and this increase was significant using paired t-test.
In order to illustrate the extreme values of SP levels in patients with SCD, we determined the proportion of SCD patients in the baseline health and acute pain cohorts with SP levels that were one full SD above the mean SP level in the control group as defined in the methods above. (Varni et al., 2003) A SP level of 30.5 pg/ml was one full SD above the mean of the control group. We found 44% (n=11) of patients in the SCD baseline health cohort and 100% (n=12) of patients in the SCD acute pain cohort had SP levels above this value.
Age, gender and prior pain history were not associated with SP levels in our SCD patients
Age and gender were not associated with differences in SP levels in patients with SCD in the baseline health cohort (Table III). Furthermore, prior pain history adjusted for age was not associated with differences in SP levels in patients with SCD in the baseline health cohort (Table III). Similarly, in the SCD acute pain cohort there was no association between SP levels and age (β=1.25, P=0.801), gender (β=−29.22, P=0.41) or prior pain history (β=0.29, P=0.85).
Table III.
Sickle cell disease baseline cohort: associations between substance P levels and age, gender, prior pain history and hydroxycarbamide use (n=25)
| Covariate | β * | P-value |
|---|---|---|
| Age | −0.58 | 0.36 |
| Gender | 0.49 | 0.93 |
| Prior pain history | 0.30 | 0.52 |
| Hydroxycarbamide# | 29.2 | 0.007 |
Multivariable linear regression
reference group is hydroxycarbamide “no”
SP levels in patients with SCD in baseline health were strongly correlated with markers of haemolysis
All markers of haemolysis were significantly correlated with plasma SP levels in the SCD baseline health cohort (Table IV). Importantly, haemoglobin was strongly negatively correlated with SP and all other markers of haemolysis (i.e., reticulocyte count, total bilirubin, LDH, AST) were strongly positively correlated with SP levels (Table IV) providing strong supporting evidence that increased haemolysis is associated with higher levels of SP.
Table IV.
Sickle cell disease baseline cohort: associations between substance P levels and markers of hemolysis
| Laboratory Value | r * | P-value |
|---|---|---|
| Haemoglobin (n=22) | −0.7 | 0.0002 |
| Reticulocyte count (n=22) | 0.61 | 0.0016 |
| Total Bilirubin (n=11) | 0.68 | 0.0216 |
| Lactate dehydrogenase (n=12) | 0.62 | 0.0332 |
| Aspartate aminotransferase (n=17) | 0.68 | 0.003 |
Spearman correlations
Patients taking hydroxycarbamide had significantly higher SP levels compared to those not taking hydroxycarbamide
The majority (n=19, 76%) of patients with SCD in the baseline health cohort were taking hydroxycarbamide. In our regression model compared to the reference group of SCD patients not taking hydroxycarbamide, SCD patients taking the drug had SP levels that were 29.2 pg/ml higher (Table III).
Discussion
We found that the neuropeptide SP is significantly increased in patients with SCD both in baseline health and during an acute pain event. Increased SP levels were also strongly associated with increased markers of haemolysis and the use of hydroxycarbamide.
SP is a primary neurotransmitter that transmits pain information to the spinal cord and the central nervous system. (O'Connor et al., 2004) Thus, the significant elevation of SP in patients with SCD during baseline health and the further increase during acute pain supports existing data that the peripheral and/or central nervous system are sensitized in SCD (Brandow et al., 2013; Hillery et al., 2011; Kohli et al., 2010; Vincent et al., 2013)
Furthermore, the knowledge that SP is elevated in baseline health suggests that the levels are chronically increased and thus could contribute to the development of peripheral and/or central nervous system sensitization.
Several factors can activate and/or sensitize nociceptors directly or indirectly at the site of tissue injury and induce the release of SP into the periphery. Once released from peripheral endings of dorsal root ganglion neurons(O'Connor et al., 2004; Sacerdote & Levrini 2012), SP acts at the Neurokinin 1 (NK1) receptor and induces neurogenic inflammation resulting in vasodilation, oedema, and activation of lymphocytes, granulocytes, mast cells and macrophages; all of which have NK1 receptors. (Geppetti et al., 2008; Harrison et al., 2001; Sacerdote et al., 2012) These activated inflammatory cells produce cytokines (i.e., interleukins 1 and 6, tumour necrosis factor) that promote inflammation and pain. In turn, these cytokines sustain the synthesis and release of new SP. Collectively, this cascade of events creates a positive feedback loop and vicious cycle of neurogenic inflammation, pain transmission, peripheral nerve sensitization and development of persistent or chronic neuropathic pain. (Sacerdote et al., 2012; Tang et al., 2007) Therefore, SP has both neuromodulating and immunomodulating effects that could contribute to the development of severe unrelenting pain in SCD. Interestingly, many inflammatory cytokines that are involved in neurogenic inflammation are significantly increased in patients with SCD compared to controls, and increase further during acute vaso-occlusive crisis. (Francis & Haywood 1992; Keikhaei et al., 2013; Pathare et al., 2004; Sarray et al., 2015) Our data support the need for further study into the downstream effects of chronic SP elevation in baseline health and further SP elevation during acute pain. In addition, risk factors and reasons for increased SP levels in patients with SCD require further investigation.
The role of SP in the pathophysiology of SCD pain has been studied in the murine model. Increased levels of SP are present in the skin of SCD mice and SP is associated with thermal hypersensitivity in these mice. (Kohli et al., 2010) SP is also shown to be a mediator of neurogenic inflammation in SCD mice(Vincent et al., 2013) leading to thermal hypersensitivity, suggesting the presence of pain sensitization that is probably sustained over time. Furthermore, the thermal hypersensitivity is mediated by mast cell activation promoting SP release, leading to the positive feedback loop as discussed above. (Vincent et al., 2013) Only one published study evaluated SP levels in humans with SCD. (Michaels et al., 1998) This study included predominantly Caucasian controls and did not evaluate patient or disease-related factors associated with elevated SP levels in patients with SCD. This single study found increased SP levels in SCD patients at baseline compared to controls and SP levels further increased during acute crisis. (Douglas, 2008; Michaels et al., 1998) Our data corroborates these findings and expands these data. First, we included all African American controls in our study. Race-matched controls are imperative in pain studies as data support race and ethnic differences in pain sensitivity with African American people displaying increased pain sensitivity. (Campbell et al., 2005; Edwards et al., 2001; Edwards & Fillingim 1999) In addition, we report novel findings including the strong associations between higher SP levels and increased markers of haemolysis and the use of hydroxycarbamide in patients with SCD.
SP levels were strongly correlated with all markers of haemolysis. We investigated this association because the degree of haemolysis in SCD patients has been associated with a variety of sickle cell-related complications. (Gladwin et al., 2004; Kato et al., 2007; Kato et al., 2006; Rees et al., 2010; Reiter et al., 2002; Taylor et al., 2008) Although prior data supports that increased haemolysis is associated with lower rates of health care encounters for acute pain(Taylor et al., 2008), the role of haemolysis in the development of peripheral and/or central nervous system sensitization is less clear. Data in models other than SCD conceptually support a potential role of the downstream effects of haemolysis in the development of peripheral and/or central nervous system sensitization. Thus, we present a hypothetical mechanism for the relationship we found between haemolysis and SP levels. Specifically, haemolysis is associated with release of cell-free plasma haemoglobin, which in turn causes nitric oxide depletion. (Minneci et al., 2005; Potoka & Gladwin 2015; Rother et al., 2005) Nitric oxide depletion leads to oxidative stress and the development of free radicals and reactive oxygen species. (Rother et al., 2005) Oxidative stress and reactive oxygen species can lead to peripheral and/or central nociceptor sensitization and chronic neuropathic pain. (Kim et al., 2004; Nishio et al., 2013) Chronic nociceptor sensitization can lead to increased release of SP(Kim et al., 2004) and SP can, in turn, cause the release of more reactive oxygen species leading to further nociceptor sensitization. In support of our hypothesis, it has been shown that both SP and reactive oxygen species are elevated in the spinal cords of sickle cell mice and administration of curcumin and Coenzyme Q10 (CoQ10) to these mice led to decreased SP levels and reduced pain sensitivity. (Valverde et al., 2016) These data support the role of reactive oxygen species in nitric oxide depletion secondary to haemolysis in SCD leading to elevation of SP and that release of SP is not necessarily limited to the periphery. In further support of nitric oxide depletion leading to increased SP release, published data reveal that nitric oxide depletion leads to increased activation of mast cells. (Brooks et al., 1999) As outlined above, increased mast cell activation is associated with increased SP levels and markers of peripheral and/or central nervous sensitization in the sickle cell murine model. (Vang et al., 2015; Vincent et al., 2013) Therefore, further investigation into the association between higher SP levels and increased markers of haemolysis in SCD patients is warranted.
The significant increase of SP during acute pain could have implications for the diagnosis of acute pain in patients with SCD. Due to the known role of SP as a primary pain neurotransmitter, SP could potentially serve as a biomarker for pain in SCD patients. To date, a biomarker for SCD acute pain has not been established. A biomarker could be useful for determining the onset of an acute pain event and could assist with determining severity and resolution of an acute pain event. SP could also potentially be used as a screening marker for pain sensitization. A pain biomarker would also be useful as an endpoint in clinical trials targeted at the treatment of pain and for validating other patient-centred outcome measures in patients with SCD. Further longitudinal work is needed to establish SP as a pain biomarker including evaluation of levels post-hospital discharge, longitudinally over time, and investigating the association between SP and chronic pain.
Our data could have implications for the development of novel treatments for sickle cell pain. The significant elevation of SP during acute pain suggests that decreasing SP production or SP receptor (NK1) blockade could be novel treatment approaches(Douglas, 2008). SP receptor antagonists (NK1 receptor antagonists) improve pain sensitivity measures in animal models. (Dionne et al., 1998; Greenwood-Van Meerveld et al., 2014; Zheng et al., 2013) A single study in mice with SCD found that indirect decrease in SP production led to improved pain sensitivity. Although not targeted directly at the SP receptor, the compound used in this study targeted the Nociceptin Receptor and ameliorated mast cell activation and led to decreased SP release and improved pain. (Vang et al., 2015) In addition, imatinib, a mast cell inhibitor, has also been shown to reduce SP levels and pain in sickle cell mice. (Vincent et al., 2013) These data further support the consideration for human trials of compounds targeted at SP as novel SCD pain treatments. Existing therapies that mediate SP release could also have a role in SCD. Gabapentin can reverse SP-induced thermal hypersensitivity in a mouse model of pain. (Partridge et al., 1998) In patients with rheumatoid arthritis, etanercept resulted in decreased serum SP levels and pain with the probable mechanism of interrupting the interplay between SP and neurogenic inflammation. (Origuchi et al., 2011) In conclusion, our data support further investigation of drugs that cause SP receptor blockade or decrease SP production as novel treatments for SCD pain.
Contrary to our hypothesis, prior pain history was not associated with increased levels of SP. The reason for this in unclear, however, hospitalizations and emergency department visits are a poor marker for the amount of pain that patients with SCD experience. (Dampier et al., 2002; Smith et al., 2008; Wang, 2002) Measures of healthcare utilization also do not phenotype the type of pain (i.e., neuropathic vs. non-neuropathic) that patients are suffering from. Thus, the conclusion that SP levels are not associated with pain history should be interpreted with caution. Chronic elevation of SP may be a better marker of chronic pain (Lisowska et al., 2015) that is often managed at home and this was not assessed in our study.
Interestingly, patients in the baseline health cohort that were taking hydroxycarbamide had significantly higher levels of SP compared to patients not taking the drug. These data suggest that SP may be a marker for more severe SCD and more frequent pain as criteria for initiating hydroxycarbamide in this cohort included frequent pain that occurred both at home and in the hospital. Furthermore, since this was a cross-sectional study, we do not know what the deleterious effects that chronically elevated SP have on patients with SCD. Thus, a longitudinal study would be important to determine the impact of chronically elevated SP levels on the development of disease manifestations in patients with SCD.
Limitations
Our study is limited in that the majority of SCD patients were not sampled at both baseline and during acute pain. Due the intermittent nature of acute pain exacerbations in SCD patients, it was not feasible to obtain only paired samples although the small subset analysis we completed of the paired samples confirmed our findings from the larger independent cohort analyses. Furthermore, from our data it is unclear if the elevation of SP is a cause or a result of SCD pathology. Longitudinal work needs to be done to obtain supporting data to answer this question. The limitations of using health care utilization as a marker for prior pain were discussed in detail above. Our controls were significantly older than our patients, however, our analyses revealed age had no impact on SP levels thus this age difference is unlikely to have affected our results. Furthermore, our subset analysis that restricted controls to ≤19 years of age to match our SCD patients revealed the significant elevation in SP levels persisted. The origin of the increased release of SP in the plasma is not currently known. We measured SP in the plasma as this was the most feasible place to measure this neuropeptide in humans, however, future work should include investigating the primary origin of SP release to better understand the pathophysiology. Finally, we were unable to determine the effect of opioids on levels of SP during acute pain as it would be unethical to conduct this study without the administration of opioids to patients with SCD suffering from acute pain.
Conclusions
Significantly elevated SP levels in patients with SCD during baseline health compared to controls suggest that SP may be a mediator of, or marker for, pain sensitization. SP levels further increase during acute pain suggesting SP also plays a role in mediating or marking an acute pain event. The downstream effects of chronically increased levels of SP on the development and propagation of pain in patients with SCD warrant further investigation. SP levels were strongly correlated with all markers of haemolysis. The reason for this strong relationship warrants further investigation. Ultimately, SP could differentiate those at risk for pain sensitization and could be used as a novel biomarker for acute pain and resolution of a painful event. Ultimately, compounds aimed at decreasing SP levels may be a target of novel treatments for SCD pain.
Acknowledgments
We acknowledge all of the patients and controls that participated in the study. This work was supported by grants from the American Society of Hematology (AMB), National Institutes of Health National Heart, Lung, and Blood Institute 1K23 HL114636-01A1 (AMB) and the Midwest Athletes Against Childhood Cancer and Blood Diseases Fund (AMB).
Footnotes
Conflicts of Interest: The authors declare no competing financial interests.
Contribution: A.M.B. designed the research, performed research, analysed data, and wrote the manuscript. N.J.W. conducted SP assays, analysed SP data and critically reviewed and edited the manuscript. M.D. conducted statistical analysis of data and critically reviewed and edited the manuscript. R.G.H. conducted statistical analysis of data and critically reviewed and edited the manuscript. C.A.H. analysed data and critically reviewed and edited the manuscript. C.L.S. analysed data and critically reviewed and edited the manuscript. J.A.P. designed the research and critically reviewed and edited the manuscript.
Disclosure of Conflicts of Interest: The authors declare no conflicts of interests.
References
- Arnold LM, Clauw DJ, McCarberg BH. Improving the recognition and diagnosis of fibromyalgia. Mayo Clin Proc. 2011;86(5):457–464. doi: 10.4065/mcp.2010.0738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandow AM, Brousseau DC, Pajewski NM, Panepinto JA. Vaso-occlusive painful events in sickle cell disease: impact on child well-being. Pediatr Blood Cancer. 2010;54(1):92–97. doi: 10.1002/pbc.22222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandow AM, Stucky CL, Hillery CA, Hoffmann RG, Panepinto JA. Patients with sickle cell disease have increased sensitivity to cold and heat. Am J Hematol. 2013;88(1):37–43. doi: 10.1002/ajh.23341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandow AM, Farley RA, Panepinto JA. Neuropathic pain in patients with sickle cell disease. Pediatr Blood Cancer. 2014;61(3):512–517. doi: 10.1002/pbc.24838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandow AM, Farley RA, Panepinto JA. Early insights into the neurobiology of pain in sickle cell disease: A systematic review of the literature. Pediatr Blood Cancer. 2015;62(9):1501–1511. doi: 10.1002/pbc.25574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks AC, Whelan CJ, Purcell WM. Reactive oxygen species generation and histamine release by activated mast cells: modulation by nitric oxide synthase inhibition. British journal of pharmacology. 1999;128(3):585–590. doi: 10.1038/sj.bjp.0702838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brousseau DC, Owens PL, Mosso AL, Panepinto JA, Steiner CA. Acute care utilization and rehospitalizations for sickle cell disease. JAMA. 2010;303(13):1288–1294. doi: 10.1001/jama.2010.378. [DOI] [PubMed] [Google Scholar]
- Campbell CM, Edwards RR, Fillingim RB. Ethnic differences in responses to multiple experimental pain stimuli. Pain. 2005;113(1-2):20–26. doi: 10.1016/j.pain.2004.08.013. [DOI] [PubMed] [Google Scholar]
- Dampier C, Ely E, Brodecki D, O'Neal P. Home management of pain in sickle cell disease: a daily diary study in children and adolescents. J Pediatr Hematol Oncol. 2002;24(8):643–647. doi: 10.1097/00043426-200211000-00008. [DOI] [PubMed] [Google Scholar]
- Darbari DS, Hampson JP, Ichesco E, Kadom N, Vezina G, Evangelou I, Clauw DJ, Taylor JG, Harris RE. Frequency of Hospitalizations for Pain and Association With Altered Brain Network Connectivity in Sickle Cell Disease. J Pain. 2015;16(11):1077–1086. doi: 10.1016/j.jpain.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dionne RA, Max MB, Gordon SM, Parada S, Sang C, Gracely RH, Sethna NF, MacLean DB. The substance P receptor antagonist CP-99,994 reduces acute postoperative pain. Clinical pharmacology and therapeutics. 1998;64(5):562–568. doi: 10.1016/S0009-9236(98)90140-0. [DOI] [PubMed] [Google Scholar]
- Douglas SD. Elevated plasma substance P in sickle cell disease and vaso-occlusive crisis. Medical hypotheses. 2008;70(6):1229. doi: 10.1016/j.mehy.2007.12.013. [DOI] [PubMed] [Google Scholar]
- Edwards CL, Fillingim RB, Keefe F. Race, ethnicity and pain. Pain. 2001;94(2):133–137. doi: 10.1016/S0304-3959(01)00408-0. [DOI] [PubMed] [Google Scholar]
- Edwards RR, Fillingim RB. Ethnic differences in thermal pain responses. Psychosom Med. 1999;61(3):346–354. doi: 10.1097/00006842-199905000-00014. [DOI] [PubMed] [Google Scholar]
- Edwards RR, Fillingim RB. Age-associated differences in responses to noxious stimuli. J Gerontol A Biol Sci Med Sci. 2001a;56(3):M180–185. doi: 10.1093/gerona/56.3.m180. [DOI] [PubMed] [Google Scholar]
- Edwards RR, Fillingim RB. Effects of age on temporal summation and habituation of thermal pain: clinical relevance in healthy older and younger adults. J Pain. 2001b;2(6):307–317. doi: 10.1054/jpai.2001.25525. [DOI] [PubMed] [Google Scholar]
- Edwards RR, Fillingim RB, Ness TJ. Age-related differences in endogenous pain modulation: a comparison of diffuse noxious inhibitory controls in healthy older and younger adults. Pain. 2003;101(1-2):155–165. doi: 10.1016/s0304-3959(02)00324-x. [DOI] [PubMed] [Google Scholar]
- Ezenwa MO, Molokie RE, Wang ZJ, Yao Y, Suarez ML, Pullum C, Schlaeger JM, Fillingim RB, Wilkie DJ. Safety and Utility of Quantitative Sensory Testing among Adults with Sickle Cell Disease: Indicators of Neuropathic Pain? Pain practice : the official journal of World Institute of Pain. 2016;16(3):282–293. doi: 10.1111/papr.12279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field T, Diego M, Cullen C, Hernandez-Reif M, Sunshine W, Douglas S. Fibromyalgia pain and substance P decrease and sleep improves after massage therapy. Journal of clinical rheumatology : practical reports on rheumatic & musculoskeletal diseases. 2002;8(2):72–76. doi: 10.1097/00124743-200204000-00002. [DOI] [PubMed] [Google Scholar]
- Fillingim RB, King CD, Ribeiro-Dasilva MC, Rahim-Williams B, Riley JL., 3rd Sex, gender, and pain: a review of recent clinical and experimental findings. J Pain. 2009;10(5):447–485. doi: 10.1016/j.jpain.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis RB, Jr., Haywood LJ. Elevated immunoreactive tumor necrosis factor and interleukin-1 in sickle cell disease. J Natl Med Assoc. 1992;84(7):611–615. [PMC free article] [PubMed] [Google Scholar]
- Geppetti P, Nassini R, Materazzi S, Benemei S. The concept of neurogenic inflammation. BJU international. 2008;101(Suppl 3):2–6. doi: 10.1111/j.1464-410X.2008.07493.x. [DOI] [PubMed] [Google Scholar]
- Gladwin MT, Sachdev V, Jison ML, Shizukuda Y, Plehn JF, Minter K, Brown B, Coles WA, Nichols JS, Ernst I, Hunter LA, Blackwelder WC, Schechter AN, Rodgers GP, Castro O, Ognibene FP. Pulmonary hypertension as a risk factor for death in patients with sickle cell disease. N Engl J Med. 2004;350(9):886–895. doi: 10.1056/NEJMoa035477. [DOI] [PubMed] [Google Scholar]
- Greenwood-Van Meerveld B, Mohammadi E, Tyler K, Pietra C, Bee LA, Dickenson A. Synergistic effect of 5-hydroxytryptamine 3 and neurokinin 1 receptor antagonism in rodent models of somatic and visceral pain. The Journal of pharmacology and experimental therapeutics. 2014;351(1):146–152. doi: 10.1124/jpet.114.216028. [DOI] [PubMed] [Google Scholar]
- Harrison S, Geppetti P. Substance p. The international journal of biochemistry & cell biology. 2001;33(6):555–576. doi: 10.1016/s1357-2725(01)00031-0. [DOI] [PubMed] [Google Scholar]
- Hillery CA, Kerstein PC, Vilceanu D, Barabas ME, Retherford D, Brandow AM, Wandersee NJ, Stucky CL. Transient receptor potential vanilloid 1 mediates pain in mice with severe sickle cell disease. Blood. 2011;118(12):3376–3383. doi: 10.1182/blood-2010-12-327429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogeweg JA, Kuis W, Huygen AC, de Jong-de vos van Steenwijk C, Bernards AT, Oostendorp RA, Helders PJ. The pain threshold in juvenile chronic arthritis. Br J Rheumatol. 1995;34(1):61–67. doi: 10.1093/rheumatology/34.1.61. [DOI] [PubMed] [Google Scholar]
- Jacob E, Chan VW, Hodge C, Zeltzer L, Zurakowski D, Sethna NF. Sensory and Thermal Quantitative Testing in Children With Sickle Cell Disease. J Pediatr Hematol Oncol. 2015;37(3):185–189. doi: 10.1097/MPH.0000000000000214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang MU, Park JW, Kho HS, Chung SC, Chung JW. Plasma and saliva levels of nerve growth factor and neuropeptides in chronic migraine patients. Oral diseases. 2011;17(2):187–193. doi: 10.1111/j.1601-0825.2010.01717.x. [DOI] [PubMed] [Google Scholar]
- Kato GJ, McGowan V, Machado RF, Little JA, Taylor Jt, Morris CR, Nichols JS, Wang X, Poljakovic M, Morris SM, Gladwin MT. Lactate dehydrogenase as a biomarker of hemolysis-associated nitric oxide resistance, priapism, leg ulceration, pulmonary hypertension, and death in patients with sickle cell disease. Blood. 2006;107(6):2279–2285. doi: 10.1182/blood-2005-06-2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato GJ, Gladwin MT, Steinberg MH. Deconstructing sickle cell disease: reappraisal of the role of hemolysis in the development of clinical subphenotypes. Blood Rev. 2007;21(1):37–47. doi: 10.1016/j.blre.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keikhaei B, Mohseni AR, Norouzirad R, Alinejadi M, Ghanbari S, Shiravi F, Solgi G. Altered levels of pro-inflammatory cytokines in sickle cell disease patients during vaso-occlusive crises and the steady state condition. European cytokine network. 2013;24(1):45–52. doi: 10.1684/ecn.2013.0328. [DOI] [PubMed] [Google Scholar]
- Kim HK, Park SK, Zhou JL, Taglialatela G, Chung K, Coggeshall RE, Chung JM. Reactive oxygen species (ROS) play an important role in a rat model of neuropathic pain. Pain. 2004;111(1-2):116–124. doi: 10.1016/j.pain.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Kohli DR, Li Y, Khasabov SG, Gupta P, Kehl LJ, Ericson ME, Nguyen J, Gupta V, Hebbel RP, Simone DA, Gupta K. Pain-related behaviors and neurochemical alterations in mice expressing sickle hemoglobin: modulation by cannabinoids. Blood. 2010;116(3):456–465. doi: 10.1182/blood-2010-01-260372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linley JE, Ooi L, Pettinger L, Kirton H, Boyle JP, Peers C, Gamper N. Reactive oxygen species are second messengers of neurokinin signaling in peripheral sensory neurons. Proc Natl Acad Sci U S A. 2012;109(24):E1578–1586. doi: 10.1073/pnas.1201544109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisowska B, Lisowski A, Siewruk K. Substance P and Chronic Pain in Patients with Chronic Inflammation of Connective Tissue. PLoS One. 2015;10(10):e0139206. doi: 10.1371/journal.pone.0139206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaels LA, Ohene-Frempong K, Zhao H, Douglas SD. Serum levels of substance P are elevated in patients with sickle cell disease and increase further during vaso-occlusive crisis. Blood. 1998;92(9):3148–3151. [PubMed] [Google Scholar]
- Minneci PC, Deans KJ, Zhi H, Yuen PS, Star RA, Banks SM, Schechter AN, Natanson C, Gladwin MT, Solomon SB. Hemolysis-associated endothelial dysfunction mediated by accelerated NO inactivation by decompartmentalized oxyhemoglobin. J Clin Invest. 2005;115(12):3409–3417. doi: 10.1172/JCI25040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishio N, Taniguchi W, Sugimura YK, Takiguchi N, Yamanaka M, Kiyoyuki Y, Yamada H, Miyazaki N, Yoshida M, Nakatsuka T. Reactive oxygen species enhance excitatory synaptic transmission in rat spinal dorsal horn neurons by activating TRPA1 and TRPV1 channels. Neuroscience. 2013;247:201–212. doi: 10.1016/j.neuroscience.2013.05.023. [DOI] [PubMed] [Google Scholar]
- Nur E, Biemond BJ, Otten HM, Brandjes DP, Schnog JJ. Oxidative stress in sickle cell disease; pathophysiology and potential implications for disease management. Am J Hematol. 2011;86(6):484–489. doi: 10.1002/ajh.22012. [DOI] [PubMed] [Google Scholar]
- O'Connor TM, O'Connell J, O'Brien DI, Goode T, Bredin CP, Shanahan F. The role of substance P in inflammatory disease. Journal of cellular physiology. 2004;201(2):167–180. doi: 10.1002/jcp.20061. [DOI] [PubMed] [Google Scholar]
- O'Leary JD, Crawford MW, Odame I, Shorten GD, McGrath PA. Thermal pain and sensory processing in children with sickle cell disease. Clin J Pain. 2014;30(3):244–250. doi: 10.1097/AJP.0b013e318292a38e. [DOI] [PubMed] [Google Scholar]
- Origuchi T, Iwamoto N, Kawashiri SY, Fujikawa K, Aramaki T, Tamai M, Arima K, Nakamura H, Yamasaki S, Ida H, Kawakami A, Ueki Y, Matsuoka N, Nakashima M, Mizokami A, Kawabe Y, Mine M, Fukuda T, Eguchi K. Reduction in serum levels of substance P in patients with rheumatoid arthritis by etanercept, a tumor necrosis factor inhibitor. Modern rheumatology / the Japan Rheumatism Association. 2011;21(3):244–250. doi: 10.1007/s10165-010-0384-5. [DOI] [PubMed] [Google Scholar]
- Paller CJ, Campbell CM, Edwards RR, Dobs AS. Sex-based differences in pain perception and treatment. Pain Med. 2009;10(2):289–299. doi: 10.1111/j.1526-4637.2008.00558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panepinto JA, Brousseau DC, Hillery CA, Scott JP. Variation in hospitalizations and hospital length of stay in children with vaso-occlusive crises in sickle cell disease. Pediatr Blood Cancer. 2005;44(2):182–186. doi: 10.1002/pbc.20180. [DOI] [PubMed] [Google Scholar]
- Partridge BJ, Chaplan SR, Sakamoto E, Yaksh TL. Characterization of the effects of gabapentin and 3-isobutyl-gamma-aminobutyric acid on substance P-induced thermal hyperalgesia. Anesthesiology. 1998;88(1):196–205. doi: 10.1097/00000542-199801000-00028. [DOI] [PubMed] [Google Scholar]
- Pathare A, Al Kindi S, Alnaqdy AA, Daar S, Knox-Macaulay H, Dennison D. Cytokine profile of sickle cell disease in Oman. Am J Hematol. 2004;77(4):323–328. doi: 10.1002/ajh.20196. [DOI] [PubMed] [Google Scholar]
- Platt OS, Thorington BD, Brambilla DJ, Milner PF, Rosse WF, Vichinsky E, Kinney TR. Pain in sickle cell disease. Rates and risk factors. N Engl J Med. 1991;325(1):11–16. doi: 10.1056/NEJM199107043250103. [DOI] [PubMed] [Google Scholar]
- Potoka KP, Gladwin MT. Vasculopathy and pulmonary hypertension in sickle cell disease. American journal of physiology. Lung cellular and molecular physiology. 2015;308(4):L314–324. doi: 10.1152/ajplung.00252.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramprakash S, Fishman D. Juvenile fibromyalgia in an adolescent patient with sickle cell disease presenting with chronic pain. BMJ case reports 2015. 2015 doi: 10.1136/bcr-2015-211850. ( http://www.ncbi.nlm.nih.gov/pubmed/26430233) [DOI] [PMC free article] [PubMed]
- Rees DC, Williams TN, Gladwin MT. Sickle-cell disease. Lancet. 2010;376(9757):2018–2031. doi: 10.1016/S0140-6736(10)61029-X. [DOI] [PubMed] [Google Scholar]
- Reiter CD, Wang X, Tanus-Santos JE, Hogg N, Cannon RO, 3rd, Schechter AN, Gladwin MT. Cell-free hemoglobin limits nitric oxide bioavailability in sickle-cell disease. Nat Med. 2002;8(12):1383–1389. doi: 10.1038/nm1202-799. [DOI] [PubMed] [Google Scholar]
- Riley JL, 3rd, Robinson ME, Wise EA, Myers CD, Fillingim RB. Sex differences in the perception of noxious experimental stimuli: a meta-analysis. Pain. 1998;74(2-3):181–187. doi: 10.1016/s0304-3959(97)00199-1. [DOI] [PubMed] [Google Scholar]
- Rother RP, Bell L, Hillmen P, Gladwin MT. The clinical sequelae of intravascular hemolysis and extracellular plasma hemoglobin: a novel mechanism of human disease. JAMA. 2005;293(13):1653–1662. doi: 10.1001/jama.293.13.1653. [DOI] [PubMed] [Google Scholar]
- Russell IJ, Orr MD, Littman B, Vipraio GA, Alboukrek D, Michalek JE, Lopez Y, MacKillip F. Elevated cerebrospinal fluid levels of substance P in patients with the fibromyalgia syndrome. Arthritis Rheum. 1994;37(11):1593–1601. doi: 10.1002/art.1780371106. [DOI] [PubMed] [Google Scholar]
- Sacerdote P, Levrini L. Peripheral mechanisms of dental pain: the role of substance P. Mediators of inflammation. 2012;2012:951920. doi: 10.1155/2012/951920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarray S, Saleh LR, Lisa Saldanha F, Al-Habboubi HH, Mahdi N, Almawi WY. Serum IL- 6, IL-10, and TNFalpha levels in pediatric sickle cell disease patients during vasoocclusive crisis and steady state condition. Cytokine. 2015;72(1):43–47. doi: 10.1016/j.cyto.2014.11.030. [DOI] [PubMed] [Google Scholar]
- Schinkel C, Gaertner A, Zaspel J, Zedler S, Faist E, Schuermann M. Inflammatory mediators are altered in the acute phase of posttraumatic complex regional pain syndrome. Clin J Pain. 2006;22(3):235–239. doi: 10.1097/01.ajp.0000169669.70523.f0. [DOI] [PubMed] [Google Scholar]
- Schlesinger N. Clues to pathogenesis of fibromyalgia in patients with sickle cell disease. J Rheumatol. 2004;31(3):598–600. [PubMed] [Google Scholar]
- Schwarz MJ, Spath M, Muller-Bardorff H, Pongratz DE, Bondy B, Ackenheil M. Relationship of substance P, 5-hydroxyindole acetic acid and tryptophan in serum of fibromyalgia patients. Neurosci Lett. 1999;259(3):196–198. doi: 10.1016/s0304-3940(98)00937-9. [DOI] [PubMed] [Google Scholar]
- Sethna NF, Meier PM, Zurakowski D, Berde CB. Cutaneous sensory abnormalities in children and adolescents with complex regional pain syndromes. Pain. 2007;131(1-2):153–161. doi: 10.1016/j.pain.2006.12.028. [DOI] [PubMed] [Google Scholar]
- Smith WR, Penberthy LT, Bovbjerg VE, McClish DK, Roberts JD, Dahman B, Aisiku IP, Levenson JL, Roseff SD. Daily assessment of pain in adults with sickle cell disease. Ann Intern Med. 2008;148(2):94–101. doi: 10.7326/0003-4819-148-2-200801150-00004. [DOI] [PubMed] [Google Scholar]
- Stahl SM. Fibromyalgia--pathways and neurotransmitters. Human psychopharmacology. 2009;24(Suppl 1):S11–17. doi: 10.1002/hup.1029. [DOI] [PubMed] [Google Scholar]
- Tang HB, Li YS, Arihiro K, Nakata Y. Activation of the neurokinin-1 receptor by substance P triggers the release of substance P from cultured adult rat dorsal root ganglion neurons. Molecular pain. 2007;3:42. doi: 10.1186/1744-8069-3-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JGt, Nolan VG, Mendelsohn L, Kato GJ, Gladwin MT, Steinberg MH. Chronic hyper-hemolysis in sickle cell anemia: association of vascular complications and mortality with less frequent vasoocclusive pain. PLoS One. 2008;3(5):e2095. doi: 10.1371/journal.pone.0002095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valverde Y, Benson B, Gupta M, Gupta K. Spinal glial activation and oxidative stress are alleviated by treatment with curcumin or coenzyme Q in sickle mice. Haematologica. 2016;101(2):e44–47. doi: 10.3324/haematol.2015.137489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vang D, Paul JA, Nguyen J, Tran H, Vincent L, Yasuda D, Zaveri NT, Gupta K. Small- molecule nociceptin receptor agonist ameliorates mast cell activation and pain in sickle mice. Haematologica. 2015;100(12):1517–1525. doi: 10.3324/haematol.2015.128736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varni JW, Burwinkle TM, Seid M, Skarr D. The PedsQL 4.0 as a pediatric population health measure: feasibility, reliability, and validity. Ambulatory pediatrics : the official journal of the Ambulatory Pediatric Association. 2003;3(6):329–341. doi: 10.1367/1539-4409(2003)003<0329:tpaapp>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Vincent L, Vang D, Nguyen J, Gupta M, Luk K, Ericson ME, Simone DA, Gupta K. Mast cell activation contributes to sickle cell pathobiology and pain in mice. Blood. 2013;122(11):1853–1862. doi: 10.1182/blood-2013-04-498105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang WC. Pain at home in sickle cell disease: an underrecognized problem. Journal of Pediatric Hematology/Oncology. 2002;24(8):610–612. doi: 10.1097/00043426-200211000-00003. [DOI] [PubMed] [Google Scholar]
- Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, Tugwell P, Campbell SM, Abeles M, Clark P. The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum. 1990;33(2):160–172. doi: 10.1002/art.1780330203. [DOI] [PubMed] [Google Scholar]
- Wollgarten-Hadamek I, Hohmeister J, Demirakca S, Zohsel K, Flor H, Hermann C. Do burn injuries during infancy affect pain and sensory sensitivity in later childhood? Pain. 2009;141(1-2):165–172. doi: 10.1016/j.pain.2008.11.008. [DOI] [PubMed] [Google Scholar]
- Zappia KJ, Garrison SR, Hillery CA, Stucky CL. Cold hypersensitivity increases with age in mice with sickle cell disease. Pain. 2014;155(12):2476–2485. doi: 10.1016/j.pain.2014.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng XF, Li B, Zhang YH, Yang YH, Meng XY, Jiang SD, Jiang LS. Blockade of substance P receptor attenuates osteoporotic pain, but not bone loss, in ovariectomized mice. Menopause. 2013;20(10):1074–1083. doi: 10.1097/GME.0b013e31828837a6. [DOI] [PubMed] [Google Scholar]
- Zohsel K, Hohmeister J, Oelkers-Ax R, Flor H, Hermann C. Quantitative sensory testing in children with migraine: preliminary evidence for enhanced sensitivity to painful stimuli especially in girls. Pain. 2006;123(1-2):10–18. doi: 10.1016/j.pain.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Zohsel K, Hohmeister J, Flor H, Hermann C. Somatic pain sensitivity in children with recurrent abdominal pain. Am J Gastroenterol. 2008;103(6):1517–1523. doi: 10.1111/j.1572-0241.2008.01911.x. [DOI] [PubMed] [Google Scholar]