Abstract
While the effects and the underlying mechanism of sympathetic stimulation on cardiac Ca handling are relatively well established both in health and disease, the modes of action and mechanisms of parasympathetic modulation are poorly defined. Here we demonstrate that parasympathetic stimulation initiates a novel mode of excitation-contraction (EC) coupling that enhances the efficiency of cardiac SR Ca store utilization. This “efficient” mode of EC coupling involves reciprocal changes in the phosphorylation of RyR2 at Ser-2808 and Ser-2814. Specifically, Ser-2808 phosphorylation was mediated by muscarinic receptor subtype 2 (M2R) and activation of PKG, whereas dephosphorylation of Ser-2814 involved activation of muscarinic receptor subtype 3 (M3R) and decreased ROS-dependent activation of CaMKII. The overall effect of these changes in phosphorylation of RyR2 is an increase in systolic Ca release at the low SR Ca content, and a paradoxical reduction in aberrant Ca leak. Accordingly, cholinergic stimulation of cardiomyocytes isolated from failing hearts improved Ca cycling efficiency by restoring altered RyR2 phosphorylation balance.
Keywords: Calcium, cholinergic stimulation, ryanodine receptors, heart failure, muscarinic receptor, carbachol, reactive oxygen species
Introduction
The function of the heart is regulated by two arms of the autonomic nervous system: the sympathetic and parasympathetic branches.1,2 Whereas the sympathetic arm is responsible for boosting cardiac performance to support the “fight or flight” response, the parasympathetic stimulation adjusts cardiac pump activity to the reduced demands of the “rest and digest” state.3 A pathologic shift of autonomic balance to a dominant sympathetic state is a hallmark of heart failure (HF).2,4–10 Hence, parasympathetic augmentation, and particularly vagal stimulation have been effective means of restoring autonomic balance and alleviating HF.2,6,8,10
Ca-induced Ca release (CICR) from the sarcoplasmic reticulum (SR) via cardiac ryanodine receptors (RyR2s) plays a key role in cardiac excitation-contraction (EC) coupling. For this reason, impaired RyR2 function contributes to cardiac disease, including HF.11–13 The sympathetic regulation of cardiac EC coupling and the resultant contractility has been extensively studied and is relatively well characterized. Specifically, β-AR-mediated phosphorylation of key Ca handling proteins (including L-type Ca channels (Cav1.2), RyR2 and phospholamban (PLB)) results in increased SR Ca accumulation and enhanced CICR leading to improved contractility.14–16 The dependence of CICR on the SR Ca content is highly nonlinear17–19 such that a relatively small elevation in the SR Ca content during β-adrenergic stimulation results in a large increase of Ca release. This is particularly evident in HF where hyperphosphorylation of RyR2 increases RyR2-mediated SR Ca leak thereby resulting in the depletion of the SR Ca store and weakened contractility.11–13
On the other hand, activation of the parasympathetic arm of the autonomic nervous system generally opposes the effects of sympathetic stimulation. However, the mechanism(s) responsible for physiological effects elicited by cholinergic stimulation are poorly defined. For example, the same exponential dependence of CICR on SR Ca content that is evidenced during adrenergic stimulation is expected to result in a drastic reduction of Ca release during cholinergic stimulation, particularly when the SR Ca load is reduced. This raises an important question: in light of the reduced SR Ca content and the highly nonlinear dependence of CICR on SR Ca load, how is an efficient level of EC coupling maintained during rest, when parasympathetic tone is elevated? Furthermore, even less is known about the molecular consequences of parasympathetic stimulation and their effects on cellular Ca handing in the failing heart. This knowledge is required for better understanding of the physiology of the heart in health and disease and is critical for optimization of HF therapies based on parasympathetic augmentation.
In the present study, we hypothesized that cholinergic stimulation enhances the efficacy of cardiac Ca cycling through changes in phosphorylation state of RyR2. We further hypothesized that this parasympathetic modulation of EC coupling is compromised in HF and that the beneficial effects of cholinergic stimulation involve improved utilization of intracellular Ca stores.
Methods
All animal procedures were approved by The Ohio State University Institutional Animal Care and Use Committee and conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996). Please refer to the online supplement for further details.
Electrophysiological recordings
Ca currents in mouse ventricular myocytes were recorded at 0 mV (from holding potential -50 mV) in the absence and presence of carbachol at room temperature in normal Tyrode solution. The pipette solution for voltage clamp experiments contained (mmol/L): 123 CsCl, 20 TEACl, 5 MgATP, 10 NaCl, 1 MgCl2, 0.1 Tris GTP, 10 HEPES, and 0.2 Fluo-4 FF K5-salt (pH 7.2).
Biochemistry Assays
Protein analysis from cardiac samples were performed using western blot. Please refer to the online supplement for further details.
Statistical Analysis
Data are presented as mean ± SEM. The number of cells for Ca/ROS experiments or the number of hearts for western blot experiments are shown as n in the figure legend. For each Ca/ROS imaging experiments, ≥ 3 animals per groups. Statistical analyses were performed using either t test, ANOVA with Tukey’s post hoc test or paired t test. Values of P<0.05 were accepted as statistically significant.
Results
1. Cholinergic stimulation enhances SR Ca store mobilization
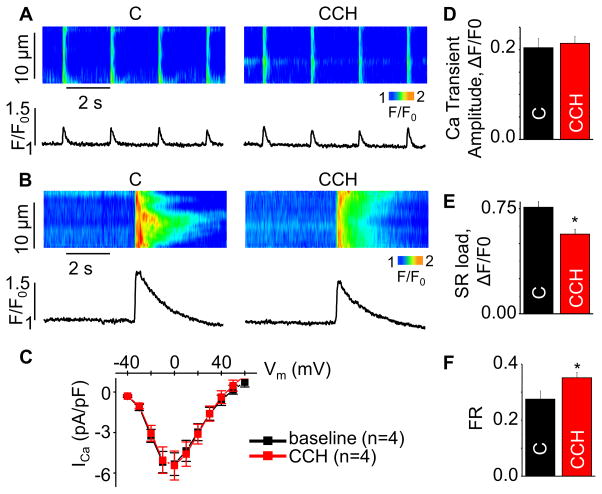
Despite its potentially important therapeutic significance, the impacts of cholinergic stimulation on myocyte Ca handling have not been sufficiently studied. Therefore, we investigated the effects of the cholinergic agonist, carbachol (CCH), on systolic and diastolic SR Ca release in wild type (WT) mouse ventricular myocytes. Exposure of cardiomyocytes (paced at 0.5 Hz) to CCH caused no change in the amplitude of the Ca transient; however, it resulted in a significant decrease in the SR Ca content (Fig 1A–B, D–E). Accordingly, the fraction of stored Ca that was released from the SR during a Ca transient (i.e. fractional release, FR, the ratio of the Ca transient amplitude to total SR Ca content20,21) was increased by CCH (Fig 1F).
Fig. 1. Effects of CCH on Ca handling in WT mouse ventricular myocytes.
Representative line-scan images for (A) Ca transients, (B) SR Ca with or without 10 μmol/L CCH. (C) Voltage dependencies of ICa in baseline (black) and in the presence of CCH (red) (n=4). (D,E,F) Summary data for Ca transient amplitude, caffeine-induced Ca transient amplitude (SR load) and fractional release (FR) (±SEM, n=11–16). *P<0.05 vs control (C). SR Ca content was obtained by application of 20 mmol/L caffeine.
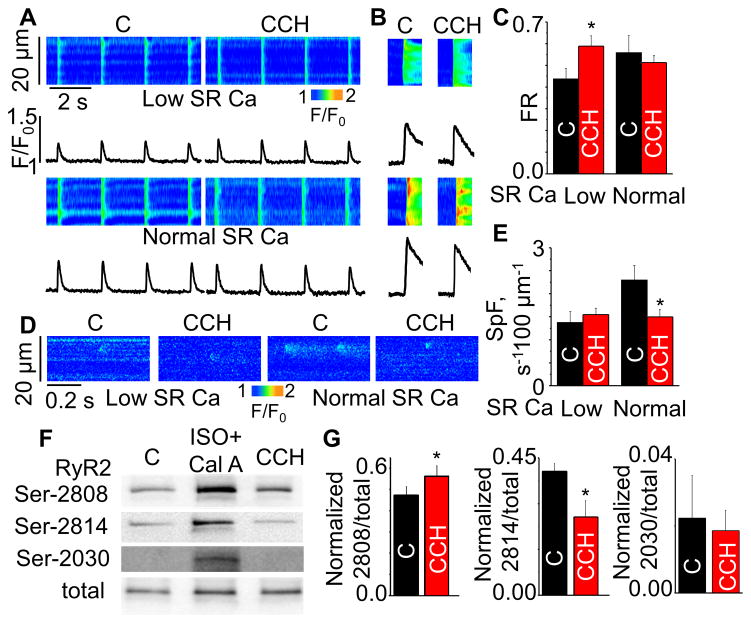
FR is considered to be an index of efficacy of EC coupling during systolic Ca release.17,19 FR is strongly influenced by both L-type Ca current and SR Ca load. To assess the role of potential changes in the L-type Ca current (ICa), we examined the effect of CCH on ICa. Consistent with previous reports,22,23 I-V curves for ICa were similar with and without CCH (Fig. 1C), thus revealing no changes in ICa. Next, we examined the load-dependency of CCH effects on EC coupling by measuring FR in CCH-treated and untreated (control) cells at matched SR Ca loads. To restore the reduced SR Ca content in CCH-treated myocytes toward the baseline (“normal”) level, extracellular Ca was raised from 1 to 2.5 mmol/L. Then the SR Ca contents between the CCH-treated and untreated groups were adjusted by thapsigargin (TG, 60 – 150 nmol/L) at two different levels i.e. normal and low (60% of normal). Consistent with previous reports,17 lowering SR Ca content to 60% of normal markedly depressed FR in the absence of CCH. Strikingly, CCH increased FR at low SR content (Fig. 2A–C). Thus CCH facilitated systolic SR Ca release at low SR Ca content.
Fig. 2. Effects of CCH on Ca handling at different SR Ca contents and RyR2 phosphorylation in WT mouse ventricular myocytes.
Representative line-scan images for (A) Ca transients, (B) SR Ca content and (D) Ca sparks at normal and low (60% of normal) SR Ca loads with or without 10 μmol/L CCH. (C,E) Summary data for fractional release (FR) and spark frequency (SpF) at different SR Ca contents (±SEM, n=4–23). *P<0.05 vs control (C). (F,G) Immunoblots and bar graphs for the effects of CCH on phosphorylation of RyR2 (±SEM, n=4–18). *P<0.05, paired t-test. Cal A (Calyculin A) + ISO produces maximum phosphorylation. SR Ca content was obtained by application of 20 mmol/L caffeine.
Next we assessed the effects of CCH on diastolic SR Ca leak by measuring Ca sparks in the load-matched myocyte groups. Whereas CCH reduced Ca spark frequency under normal SR Ca load, it had no effect in cells with low SR Ca load (Fig. 2D,E). Thus, cholinergic stimulation appears to enhance the efficacy of Ca store utilization by facilitating systolic Ca release at low SR Ca content without enhancing diastolic SR Ca leak.
To test the relevance of results obtained in mice to a large animal model, experiments were carried out in canine ventricular myocytes. In agreement with the effects of CCH on SR Ca release in mouse myocytes, CCH did not promote diastolic Ca leak (Supplemental Fig. S1A,B). Thus cholinergic augmentation of SR Ca store utilization is observed in mouse and canine ventricular myocytes.
2. Cholinergic stimulation increases phosphorylation of Ser-2808 while decreasing that of Ser-2814
Cardiac EC coupling is modulated through changes in the phosphorylation status of the RyR2 at several sites including Ser-2808, Ser-2814 and Ser-2030. To examine the possible role of RyR2 phosphorylation in the observed effects of CCH on Ca handling, we performed western blot analysis using phospho-specific antibodies. Exposure of WT mouse cardiomyocytes to CCH significantly increased RyR2 Ser-2808 phosphorylation while reducing Ser-2814 phosphorylation without affecting the status of Ser-2030 (Fig. 2F,G and Supplemental Fig. S2A). Similar results were obtained in canine cardiac myocytes (Supplemental Fig. S1C,D). The concentration-dependency of CCH effects on RyR2 phosphorylation was also examined in the concentration range of 0.1–10 μmol/L (Supplemental Fig. S2B). These effects were prevented by pretreatment of the cells with the muscarinic receptor antagonist, atropine (ATR) (Supplemental Fig. S2C), suggesting that phosphorylation changes were mediated by activation of muscarinic acetylcholine receptors (mAchRs).
3. The role of changes in RyR2 phosphorylation at Ser-2808 and Ser-2814 in mediating cholinergic effects on SR Ca release
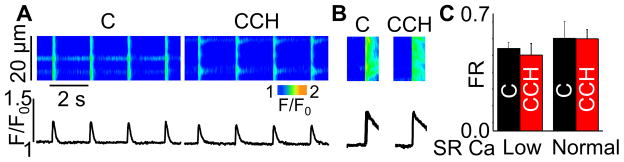
RyR2 functional activity has consistently been demonstrated to be in direct relation with the level of Ser-2814 phosphorylation by CaMKII.24,25 Therefore, while in agreement with reduced Ca spark rate, the observed decrease in Ser-2814 phosphorylation cannot explain the increased fractional Ca release in myocytes exposed to CCH. To investigate the involvement of the RyR2 phosphorylation site Ser-2808 in cholinergic-mediated regulation of SR Ca release, experiments were performed in mice genetically modified to render this site nonphosphorylatable by replacing serine at 2808 with alanine (RyR2-S2808A). Ablation of RyR2 phosphorylation at Ser-2808 prevented the increase in fractional Ca release at low SR Ca load observed in WT myocytes (Fig. 3). Thus RyR2 phosphorylation at Ser-2808 is indeed required for the observed load-dependent facilitation of SR Ca release by CCH. At the same time, inhibiting CaMKII activity with KN-93 had no significant impact on FR (Supplemental Fig. S2D). This result supports the notion that muscarinic modulation of systolic Ca release is independent of CaMKII-dependent phosphorylation of Ser-2814. Taken together, these findings suggest that increased efficiency of Ca store utilization in cardiac cells (i.e. facilitation of systolic release and inhibition of diastolic SR Ca leak) in response to muscarinic stimulation involves reciprocal changes in RyR2 phosphorylation at Ser-2808 and Ser-2814.
Fig. 3. Ablation of the RyR2 Ser-2808 phosphorylation site prevents a CCH-dependent increase in fractional release (FR).
(A,B) Representative line-scan images (top) and time-dependent profiles (bottom) for Ca transients and SR Ca content at low (60%) SR Ca loads under control conditions and in the presence of 10 μmol/L CCH. (C) Summary data for FR in S2808A cells at normal and low SR Ca contents (±SEM, n=6–11). SR Ca content was obtained by application of 20 mmol/L caffeine.
4. M2R and M3R mediate two divergent roles of cholinomimetics
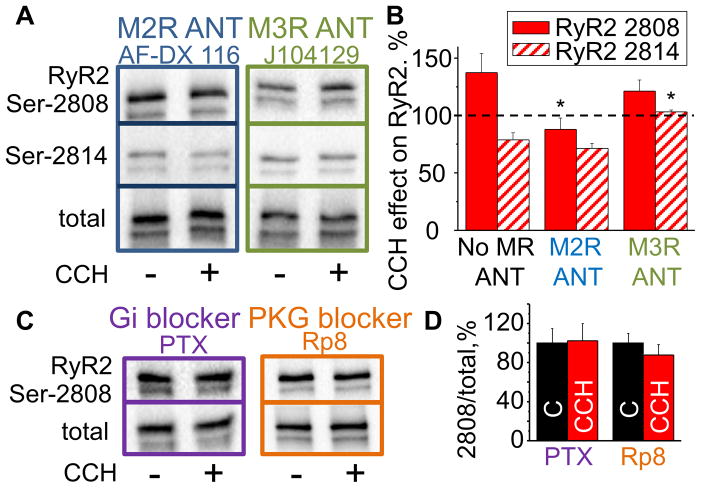
Cardiac myocytes predominantly express the muscarinic receptor (MR) subtype 2 (M2R); however, the receptor subtype 3 (M3R) is also present.26,27 Accordingly, selective inhibitors of the aforementioned MR subtypes (AF-DX 116 (specific for M2R), J 104129 fumarate (specific for M3R))27,28 were used to examine the role of these receptor subtypes in mediating RyR2 phosphorylation in response to cholinergic stimulation. Inhibition of M2R (but not inhibition of M3R) prevented changes in RyR2 phosphorylation at Ser-2808 in mouse myocytes (Fig. 4A,B). Moreover, to further confirm the involvement of M2R, we inhibited Gi protein with pertussis toxin (PTX). Application of PTX also prevented phosphorylation changes at Ser-2808 (Fig. 4C,D). Notably, blocking M2R by AF-DX 116 had no effects on CCH-dependent dephosphorylation on Ser-2814. Instead, the M3R antagonist, J 104129 fumarate, successfully inhibited Ser-2814 dephosphorylation by CCH, indicating that the effects of CCH exerted on RyR2 Ser-2808 and Ser-2814 are mediated via distinct MR subtypes.
Fig. 4. Type 2 and 3 muscarinic receptor subtypes mediate CCH-dependent alterations in RyR2 phosphorylation at Ser-2808 and Ser-2814.
Representative western blots (A,C) and the average effects of CCH on RyR2 Ser-2808 and Ser-2814 phosphorylation (B,D) in the presence of M2R (AF-DX 116) and M3R (J104129) antagonists, PTX (inhibitor of Gi protein) and Rp8 (PKG inhibitor) (±SEM, n=4–7). *P<0.05 vs No MR ANT. ANT, antagonist.
5. Downstream mediators of cholinergically-promoted modulation in SR Ca release
Several different protein kinases have been shown to be able to phosphorylate RyR2 at Ser-2808 in vitro, including PKA, PKG, CaMKII and PKC.29–34 Therefore, we applied specific pharmacological inhibitors of these kinases to test their roles on muscarinic-dependent phosphorylation of Ser-2808 (e.g. H89, Rp-8-Br-PET-cGMPS (Rp8), KN-93, and Bisindolylmaleimide I (BIS I), respectively). CCH-dependent phosphorylation of Ser-2808 was prevented by inhibition of PKG (Fig. 4C, D) but not by inhibition of PKA, PKC or CaMKII (Supplemental Fig. S3A). Ser-2808 have been reported to exhibit a robust baseline phosphorylation independent of PKA and CaMKII.29,35 Similarly, PKG and PKC inhibition failed to reduce Ser-2808 phosphorylation at baseline condition (Supplemental Fig. S3B). Collectively, these results suggest involvement of the M2R/Gi/PKG pathway in the cholinergic effects on Ser-2808. On the other hand, RyR2 Ser-2814 has been shown to be the primary CaMKII phosphorylation site. Therefore, we examined whether CCH-dependent dephosphorylation of Ser-2814 is associated with changes in CaMKII activity. CaMKII activity was indeed decreased in the presence of CCH suggesting that the decreased phosphorylation of Ser-2814 is attributable to reduced CaMKII activation (Supplemental Fig. S3C).
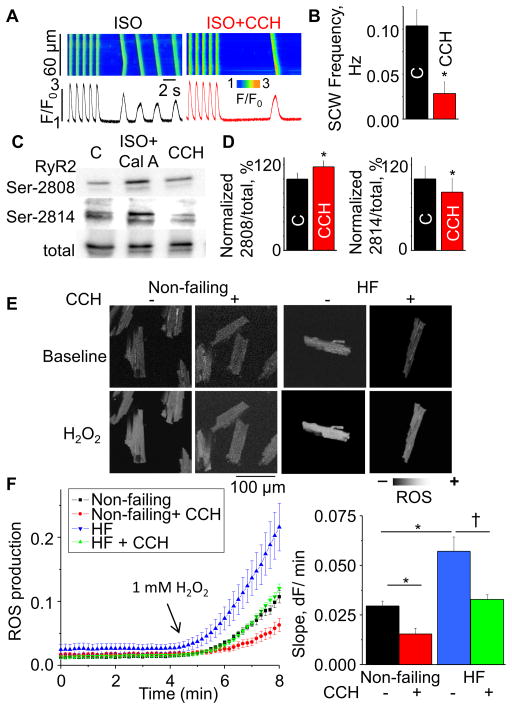
6. Cholinergic modulation of Ca cycling in failing canine hearts
To examine the possible beneficial effects of cholinergic stimulation on Ca handling in myocytes from failing hearts, we measured spontaneous Ca release and RyR2 phosphorylation levels with or without CCH in failing myocytes using a well-characterized canine model of chronic HF.36–40 Consistent with our previous studies,39,40 failing myocytes exhibited spontaneous Ca waves in the presence of 10 nmol/L isoproterenol (ISO). CCH decreased the frequency of these waves (Fig. 5 A,B). Also consistent with previous results,39,40 RyR2 phosphorylation at Ser-2814 (but not at Ser-2808) was elevated in failing cardiomyocytes relative to non-failing ones. Treatment with CCH increased phosphorylation of RyR2 Ser-2808 but reversed hyper-phosphorylation of RyR2 Ser-2814 (Fig. 5C, D). Studies from our group and others have shown HF is associated with elevated ROS production and increased CaMKII activity.38,39,41 Oxidative stress promotes constitutively active CaMKII through methionine oxidation.42,43 To examine whether cholinergically-medicated decreases in Ser-2814 phosphorylation are attributable to decreased ROS production, we measured the rate of ROS generation in response to H2O2 using the ROS-specific fluorescent indicator, CM-H2DCFDA, in non-failing and failing myocytes. Consistent with previous reports, failing myocytes showed a higher rate of ROS generation than non-failing cells (Fig 5E,F). Notably, CCH reduced ROS in both the non-failing and failing groups. Thus, reduction of ROS generation by cholinergic modulation may contribute to the beneficial effects of vagal stimulation therapy by reducing CaMKII-dependent phosphorylation of RyR2 at Ser-2814.
Fig. 5. Effects of CCH on Ca handling, RyR2 phosphorylation and ROS production in HF canine myocytes.
Confocal Ca recordings (A) before and after application of CCH in the presence of 10 nmol/L ISO in field-stimulated failing cells (2Hz). (B) Summary bar graph for spontaneous Ca wave frequency (SCW) (±SEM, n=21–25). *P<0.05 vs control. Immunoblots (C) and pooled data (D) showing effect of CCH on RyR2 phosphorylation (±SEM, n=3). *P<0.05, paired t-test. (E) Representative images of ROS generation measured by using the ROS-sensitive dye, CM-H2DCFDA, for non-failing and failing myocytes under baseline condition and in the presence of 1 mmol/L H2O2. (F, left) Averaged traces of ROS production from each group. (F, right) Pooled data for ROS accumulation rates obtained from the slopes in the presence of 1 mmol/L H2O2 (±SEM, n=5–8). Data pointes were normalized to maximum fluorescence signal produced by 10 mmol/L H2O2. * P < 0.05 vs non-failing control. †P < 0.05 vs failing control.
Discussion
These data suggest a novel “efficiency” mode of cardiac EC coupling which enhances the utilization of intracellular Ca stores during the “rest and digest” state predominated by the parasympathetic tone. This efficient utilization of Ca store involves reciprocal changes in phosphorylation of RyR2 at Ser-2808 and Ser-2814, which in turn increases systolic release at low SR Ca content while inhibiting aberrant SR Ca leak. Such reciprocal alteration of RyR2 phosphorylation proved to be beneficial in failing hearts where, cholinergic/muscarinic stimulation improved the efficiency of Ca cycling by reducing pathologic SR Ca leak while preserving enhanced systolic release.
Efficiency mode in myocyte Ca cycling during cholinergic stimulation
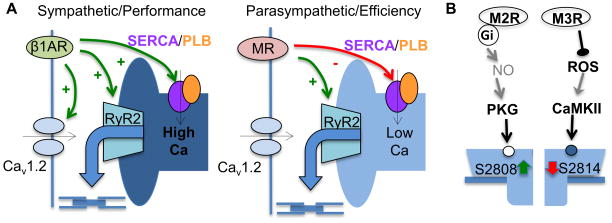
Sympathetic modulation of the cardiac muscle has been extensively studied and is known to involve adrenergically-mediated phosphorylation of key EC proteins, including PLB, Cav1.2 and RyR2.14–16 This multiprotein response facilitates trans-sarcolemmal and SR Ca fluxes to increase the chronotropic and inotropic states of the heart (Fig. 6A). In contrast, cholinergic stimulation is generally thought to act by slowing cardiac Ca cycling via activation of muscarinic receptors; however, the specific mechanisms of the parasympathetic response remain to be defined. Here we show that in ventricular myocytes, muscarinic receptor stimulation initiates a distinct mode of EC coupling that operates at reduced intra-SR Ca levels characteristic of resting state. However, the fraction of SR Ca release increases upon muscarinic receptor stimulation without affecting L-type Ca current (Fig. 1 and 2). Therefore, in contrast to the performance-oriented mode of EC coupling during sympathetic stimulation, the cholinergic “efficiency” mode is poised to attain maximal Ca release at minimal SR Ca load thereby minimizing the energy costs of Ca cycling. Previously, it has been shown that the dependency of SR Ca release on the SR Ca content is highly nonlinear with release failing when SR Ca content falls below 60% of normal.17,44 Our results show that SR Ca release is profoundly influenced by the cholinergic activation which makes Ca release more efficient at reduced SR Ca content.
Fig. 6.
Ca cycling during sympathetic and parasympathetic dominance (A) and molecular mechanisms and pathways (B) involved in the regulation of reciprocal phosphorylation/dephosphorylation of RyR2 at Ser-2808 and Ser-2814.
Commonly, interventions that enhance RyR2 functional activity (such as CaMKII phosphorylation or caffeine) are expected to facilitate both systolic and diastolic release when the load is kept constant.24,45 Notably, CCH failed to increase Ca spark frequency at various SR Ca loads, despite improving systolic Ca release (Fig. 2A–E and Supplemental Fig. S1A,B). Thus cholinergic stimulation facilitates systolic Ca release without promoting energetically wasteful diastolic Ca release, suggesting the increased utilization of intracellular Ca stores during parasympathetic dominance.
Reciprocal modulation of RyR2 via changes in phosphorylation at 2808 and 2814
Increased phosphorylation of RyR2 Ser-2808 and Ser-2814 have been implicated in adrenergically-induced elevation of systolic and diastolic SR Ca release.24,46–48 However, little is known about the consequences of parasympathetic stimulation aside from the general notion that the effects oppose those of the sympathetic stimulation. Consistent with this antagonistic phenotype, cholinergic stimulation decreased RyR2 Ser-2814 phosphorylation along with diastolic Ca leak (Fig. 2, 5 and Supplemental Fig. S1). However, Ser-2808 phosphorylation in both mouse and canine preparations was increased rather than decreased during cholinergic stimulation. Notably, increased Ser-2808 phosphorylation was associated with enhanced efficiency of EC coupling (increased fractional Ca release) at reduced SR Ca load (Fig. 1, 2 and 3). Thus during cholinergic stimulation, reciprocal changes in phosphorylation at Ser-2808 and Ser-2814 confers a new mode of “smart” regulation of SR Ca release. Mainly, cholinergic stimulation results in an enhanced utilization of SR Ca stores through increased systolic Ca release at low SR Ca loads while reducing aberrant diastolic SR Ca leak at the same time.
We further delineated signaling cascades mediating the aforementioned effects of cholinergic stimulation (Fig. 6B). Cardiac myocytes predominantly express the MR subtypes 2 (M2R) and 3 (M3R).26,27 Whereas M2R is coupled to Gi that inhibits adenylate cyclase and PKA but causes NO-dependent stimulation of PKG,23,49 M3R interacts with Gq involved in activation of PKC.26 Recently M3R stimulation has been also shown to decrease ROS-mediated CaMKII activation.50 Here we found that during cholinergic stimulation, these two muscarinic pathways (i.e. M2R and M3R) converged to increase SR Ca release efficiency through reciprocal changes in RyR2 phosphorylation. Specifically, our results demonstrated that RyR2 Ser-2808 phosphorylation is mediated by M2R through activation of PKG; whereas dephosphorylation of RyR2 Ser-2814 appeared to be caused by activation of M3R and the resultant reduction in ROS-dependent CaMKII activity. The precise mechanisms of this composite RyR2 modulation at the subcellular and molecular level remain to be defined, however. One possibility is that this modulatory response is an inherent property of an individual RyR2 channel. Consistent with this possibility, PKA-phosphorylation has been shown to increase the peak transient response while accelerating the rate of decay to a steady level (i.e. adaptation) of single RyR2s during rapid and sustained elevations of Ca.51 This in turn suggests that transient and steady phases of RyR2 activity can be differentially regulated in individual RyR2 channels. Alternatively, the effects could be mediated by the two phosphorylation pathways acting on two distinct pools of RyR2 channels in cardiomyocytes. Consistent with this possibility, cardiomyocytes have been shown to contain two sets of release sites with different response rate and predisposition to CaMKII phosphorylation (i.e. fast, CaMKII dependent and slow, CaMKII independent).52 As to the increase in fractional SR Ca release in response to CCH, it could be attributed to increased sensitivity of RyR2 to luminal Ca and reduced threshold for Ca release termination. Consistent with this possibility, Ullrich et al.53 previously demonstrated that ablation of the Ser-2808 phosphorylation site blunts the increase in RyR2 activity observed in PKA phosphorylated RyR2 channels in response to an increase in luminal Ca. Further studies are required to establish the role(s) of these mechanisms in physiological modulation of cardiac EC coupling.
Regarding the influence of cholinergic agonists on myocyte Ca handling and contractility, previous studies have yielded controversial results demonstrating stimulatory, biphasic or inhibitory effects.54–57 This variability in results could be attributed to the complex nature of muscarinic regulation of SR Ca release demonstrated here, including reciprocal changes in RyR2 phosphorylation mediated by two different MR subtypes.
Restoration of compromised Ca cycling as a basis for vagal stimulation therapy for HF
HF is characterized by altered autonomic balance, impaired functional performance and a compromised energetic state.4 Specifically, patients with HF exhibit sympathetic dominance,2,5,7,58 a result of sympathetic overdrive and attendant reductions of parasympathetic tone. Reduced vagal tone in HF appears to be mainly due to abnormal presynaptic (i.e. ganglionic) mechanisms.59 Changes in MR subtypes have been also reported, although the specific alterations remain controversial.28,60 One of the main consequences of the sympathetic dominance which is characteristic of HF is increased RyR2-mediated diastolic Ca leak.37,46,61 This leak has been variably attributed to RyR2 hyper-phosphorylation either at Ser-2808 or Ser-2814. Given the intrinsic antagonistic relationships between sympathetic and parasympathetic influences on the heart, it is reasonable to suggest that cholinergic stimulation would oppose the detrimental effects of increased sympathetic stimulation on Ca handling. Indeed a body of experimental evidence suggests that vagal nerve stimulation (VNS) may be a viable option for management of HF2,4,6,62 (although see63). However, surprisingly, only limited information is available regarding the effects of cholinergic stimulation on HF-dependent changes in Ca handling. Here we determined that cholinergic stimulation in myocytes reduced SR Ca leak while increasing systolic SR Ca release by decreasing RyR2 phosphorylation at Ser-2814 and increasing it at Ser-2808, respectively. These findings are consistent with a role for CaMKII phosphorylation in HF pathology, and implicate dephosphorylation as a potential mechanism underlying the beneficial effects of parasympathetic stimulation on SR Ca cycling and contractility.64 Notably, the improved Ca handling in failing myocytes was associated with a reduction in ROS coupled with reduced CaMKII activity. Based on previous reports, the reduction of ROS during cholinergic modulation could be a result of increased reducing-enzyme(s) activity or inhibition of ROS generation through NADPH oxidase, xanthine oxidase and mitochondria.65–67
Perspectives
It is to be noted that VNS in cardiac disease is usually applied chronically (i.e. days-months) rather than in an acute manner,68,69 although, recently, even brief stimulation of the vagal nerve has been reported to exert beneficial effects on cardiac performance in a rat model of ischemic HF70 and has been shown to prevent cardiac remodeling in post MI rabbits71. Our study demonstrating the mode of action of muscarinic activation on myocyte Ca handling is consistent with, and provides insights into, the beneficial effects of short-term vagal stimulation. In particular, our results suggest that the therapeutic impact of cholinergic stimulation may involve enhanced efficiency of the SR Ca store utilization and inhibition of arrhythmogenic diastolic Ca release in failing cardiomyocytes. Since HF is associated with chronic alteration in the autonomic balance2,4,5,7, it is reasonable to assume that long-term VNS will provide more substantial and lasting therapeutic effects than brief stimulation. While our study, which employs direct application of cholinomimetics to isolated myocytes, mimics the cellular effects of vagal nerve stimulation that is often employed in vivo, it is important to acknowledge that pleiotropic effects68 may also contribute to the therapeutic benefits of VNS. Therefore, further studies are necessary to define the systemic, cellular and molecular mechanisms of salutary effects of VNS with an ultimate goal of optimization of this potentially promising therapeutic modality.
Supplementary Material
Novelty and Significance.
1) What Is New
Here we demonstrate a previously unrecognized mechanism by which parasympathetic stimulation enhances the utilization of SR Ca stores through reciprocal changes in the phosphorylation of RyR2 via distinct muscarinic receptor subtypes. By showing improved Ca handling in failing myocytes following acute parasympathetic stimulation, we reveal a potential mechanism underlying the beneficial effects of vagal stimulation therapy.
2) What Is Relevant?
The beating heart is regulated by the two branches of the autonomic nervous system, the sympathetic and parasympathetic branches. Certain pathologies, such as heart failure, are marked by an imbalance between the two branches, where the sympathetic overdrive is coupled to reduced parasympathetic tone. Interestingly, vagal nerve stimulation has become a promising therapeutic option for heart failure; however, there is very little mechanistic insight for the success of this therapeutic modality. Thus our study shows a new mode of cardiac regulation and lays a foundation for optimization of therapeutic vagal nerve stimulation.
Summary
The beneficial effect of parasympathetic augmentation on cardiac Ca handling in HF appears to involve restoration of normal phosphorylation balance and improved functional performance of RyR2, which in part may be a result of activation of PKG and decreased CaMKII activity.
Acknowledgments
Source(s) of Funding
This work was supported by the National Institutes of Health (RO1 HL074045 and HL063043 to S.G.), the Russian Science Foundation (N15-15-20008 to I.V.K. and S.G.), and American Heart Association (Postdoctoral Fellowship from Great Rivers Affiliate 16POST27540007 to H.T.H.).
Footnotes
Conflict(s) of Interest/Disclosure(s)
None
References
- 1.Levy MN. Sympathetic-parasympathetic interactions in the heart. Circ Res. 1971;29:437–445. doi: 10.1161/01.res.29.5.437. [DOI] [PubMed] [Google Scholar]
- 2.Singh JP, Kandala J, Camm AJ. Non-pharmacological modulation of the autonomic tone to treat heart failure. Eur Heart J. 2014;35:77–85. doi: 10.1093/eurheartj/eht436. [DOI] [PubMed] [Google Scholar]
- 3.Higgins CB, Vatner SF, Braunwald E. Parasympathetic Control of the Heart. Pharmacol Rev. 1973;25:119–155. [PubMed] [Google Scholar]
- 4.Olshansky B, Sabbah HN, Hauptman PJ, Colucci WS. Parasympathetic Nervous System and Heart Failure Pathophysiology and Potential Implications for Therapy. Circulation. 2008;118:863–871. doi: 10.1161/CIRCULATIONAHA.107.760405. [DOI] [PubMed] [Google Scholar]
- 5.Bibevski S, Dunlap ME. Evidence for impaired vagus nerve activity in heart failure. Heart Fail Rev. 2011;16:129–135. doi: 10.1007/s10741-010-9190-6. [DOI] [PubMed] [Google Scholar]
- 6.De Ferrari GM, Schwartz PJ. Vagus nerve stimulation: from pre-clinical to clinical application: challenges and future directions. Heart Fail Rev. 2011;16:195–203. doi: 10.1007/s10741-010-9216-0. [DOI] [PubMed] [Google Scholar]
- 7.Kishi T. Heart failure as an autonomic nervous system dysfunction. J Cardiol. 2012;59:117–122. doi: 10.1016/j.jjcc.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 8.Kobayashi M, Massiello A, Karimov JH, Van Wagoner DR, Fukamachi K. Cardiac autonomic nerve stimulation in the treatment of heart failure. Ann Thorac Surg. 2013;96:339–345. doi: 10.1016/j.athoracsur.2012.12.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.He X, Zhao M, Bi X, Sun L, Yu X, Zhao M, Zang W. Novel strategies and underlying protective mechanisms of modulation of vagal activity in cardiovascular diseases. Br J Pharmacol. 2015;172:5489–5500. doi: 10.1111/bph.13010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Ferrari GM. Vagal stimulation in heart failure. J Cardiovasc Transl Res. 2014;7:310–320. doi: 10.1007/s12265-014-9540-1. [DOI] [PubMed] [Google Scholar]
- 11.Hasenfuss G, Pieske B. Calcium Cycling in Congestive Heart Failure. J Mol Cell Cardiol. 2002;34:951–969. doi: 10.1006/jmcc.2002.2037. [DOI] [PubMed] [Google Scholar]
- 12.Bers DM. Altered Cardiac Myocyte Ca Regulation In Heart Failure. Physiology. 2006;21:380–387. doi: 10.1152/physiol.00019.2006. [DOI] [PubMed] [Google Scholar]
- 13.Belevych AE, Radwański PB, Carnes CA, Györke S. “Ryanopathy”: causes and manifestations of RyR2 dysfunction in heart failure. Cardiovasc Res. 2013;98:240–247. doi: 10.1093/cvr/cvt024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Song L-S, Wang S-Q, Xiao R-P, Spurgeon H, Lakatta EG, Cheng H. β-Adrenergic Stimulation Synchronizes Intracellular Ca2+ Release During Excitation-Contraction Coupling in Cardiac Myocytes. Circ Res. 2001;88:794–801. doi: 10.1161/hh0801.090461. [DOI] [PubMed] [Google Scholar]
- 15.Bers DM. Calcium Cycling and Signaling in Cardiac Myocytes. Annu Rev Physiol. 2008;70:23–49. doi: 10.1146/annurev.physiol.70.113006.100455. [DOI] [PubMed] [Google Scholar]
- 16.Fearnley CJ, Roderick HL, Bootman MD. Calcium Signaling in Cardiac Myocytes. Cold Spring Harb Perspect Biol. 2011;3:a004242. doi: 10.1101/cshperspect.a004242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bassani JW, Yuan W, Bers DM. Fractional SR Ca release is regulated by trigger Ca and SR Ca content in cardiac myocytes. Am J Physiol. 1995;268:C1313–1319. doi: 10.1152/ajpcell.1995.268.5.C1313. [DOI] [PubMed] [Google Scholar]
- 18.Eisner DA, Trafford AW, Díaz ME, Overend CL, O’Neill SC. The control of Ca release from the cardiac sarcoplasmic reticulum: regulation versus autoregulation. Cardiovasc Res. 1998;38:589–604. doi: 10.1016/s0008-6363(98)00062-5. [DOI] [PubMed] [Google Scholar]
- 19.Bers DM. Excitation-Contraction Coupling and Cardiac Contractile Force. Springer; 2001. [Google Scholar]
- 20.Knollmann BC, Chopra N, Hlaing T, Akin B, Yang T, Ettensohn K, Knollmann BEC, Horton KD, Weissman NJ, Holinstat I, Zhang W, Roden DM, Jones LR, Franzini-Armstrong C, Pfeifer K. Casq2 deletion causes sarcoplasmic reticulum volume increase, premature Ca2+ release, and catecholaminergic polymorphic ventricular tachycardia. J Clin Invest. 2006;116:2510–2520. doi: 10.1172/JCI29128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ho H-T, Stevens SCW, Terentyeva R, Carnes CA, Terentyev D, Györke S. Arrhythmogenic adverse effects of cardiac glycosides are mediated by redox modification of ryanodine receptors. J Physiol. 2011;589:4697–4708. doi: 10.1113/jphysiol.2011.210005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McDonald TF, Pelzer S, Trautwein W, Pelzer DJ. Regulation and modulation of calcium channels in cardiac, skeletal, and smooth muscle cells. Physiol Rev. 1994;74:365–507. doi: 10.1152/physrev.1994.74.2.365. [DOI] [PubMed] [Google Scholar]
- 23.Harvey RD, Belevych AE. Muscarinic regulation of cardiac ion channels. Br J Pharmacol. 2003;139:1074–1084. doi: 10.1038/sj.bjp.0705338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Curran J, Hinton MJ, Ríos E, Bers DM, Shannon TR. Beta-adrenergic enhancement of sarcoplasmic reticulum calcium leak in cardiac myocytes is mediated by calcium/calmodulin-dependent protein kinase. Circ Res. 2007;100:391–398. doi: 10.1161/01.RES.0000258172.74570.e6. [DOI] [PubMed] [Google Scholar]
- 25.Valdivia HH. Ryanodine Receptor Phosphorylation and Heart Failure Phasing Out S2808 and “Criminalizing” S2814. Circ Res. 2012;110:1398–1402. doi: 10.1161/CIRCRESAHA.112.270876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dhein S, van Koppen CJ, Brodde OE. Muscarinic receptors in the mammalian heart. Pharmacol Res Off J Ital Pharmacol Soc. 2001;44:161–182. doi: 10.1006/phrs.2001.0835. [DOI] [PubMed] [Google Scholar]
- 27.Wang Z, Shi H, Wang H. Functional M3 muscarinic acetylcholine receptors in mammalian hearts. Br J Pharmacol. 2004;142:395–408. doi: 10.1038/sj.bjp.0705787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DeMazumder D, Kass DA, O’Rourke B, Tomaselli GF. Cardiac Resynchronization Therapy Restores Sympathovagal Balance in the Failing Heart by Differential Remodeling of Cholinergic Signaling. Circ Res. 2015;116:1691–1699. doi: 10.1161/CIRCRESAHA.116.305268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xiao B, Zhong G, Obayashi M, Yang D, Chen K, Walsh MP, Shimoni Y, Cheng H, ter Keurs H, Chen SRW. Ser-2030, but not Ser-2808, is the major phosphorylation site in cardiac ryanodine receptors responding to protein kinase A activation upon β-adrenergic stimulation in normal and failing hearts. Biochem J. 2006;396:7–16. doi: 10.1042/BJ20060116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anderson ME, Brown JH, Bers DM. CaMKII in myocardial hypertrophy and heart failure. J Mol Cell Cardiol. 2011;51:468–473. doi: 10.1016/j.yjmcc.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dobrev D, Wehrens XHT. Role of RyR2 phosphorylation in heart failure and arrhythmias: Controversies around ryanodine receptor phosphorylation in cardiac disease. Circ Res. 2014;114:1311–1319. doi: 10.1161/CIRCRESAHA.114.300568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Swaminathan PD, Purohit A, Hund TJ, Anderson ME. Calmodulin-Dependent Protein Kinase II: Linking Heart Failure and Arrhythmias. Circ Res. 2012;110:1661–1677. doi: 10.1161/CIRCRESAHA.111.243956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takasago T, Imagawa T, Furukawa K, Ogurusu T, Shigekawa M. Regulation of the cardiac ryanodine receptor by protein kinase-dependent phosphorylation. J Biochem (Tokyo) 1991;109:163–170. doi: 10.1093/oxfordjournals.jbchem.a123339. [DOI] [PubMed] [Google Scholar]
- 34.Hohenegger M, Suko J. Phosphorylation of the purified cardiac ryanodine receptor by exogenous and endogenous protein kinases. Biochem J. 1993;296:303–308. doi: 10.1042/bj2960303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huke S, Bers DM. Ryanodine receptor phosphorylation at Serine 2030, 2808 and 2814 in rat cardiomyocytes. Biochem Biophys Res Commun. 2008;376:80–85. doi: 10.1016/j.bbrc.2008.08.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kubalova Z, Terentyev D, Viatchenko-Karpinski S, Nishijima Y, Györke I, Terentyeva R, da Cuñha DNQ, Sridhar A, Feldman DS, Hamlin RL, Carnes CA, Györke S. Abnormal intrastore calcium signaling in chronic heart failure. Proc Natl Acad Sci U S A. 2005;102:14104–14109. doi: 10.1073/pnas.0504298102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Belevych A, Kubalova Z, Terentyev D, Hamlin RL, Carnes CA, Györke S. Enhanced ryanodine receptor-mediated calcium leak determines reduced sarcoplasmic reticulum calcium content in chronic canine heart failure. Biophys J. 2007;93:4083–4092. doi: 10.1529/biophysj.107.114546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Terentyev D, Györke I, Belevych AE, Terentyeva R, Sridhar A, Nishijima Y, de Blanco EC, Khanna S, Sen CK, Cardounel AJ, Carnes CA, Györke S. Redox Modification of Ryanodine Receptors Contributes to Sarcoplasmic Reticulum Ca2+ Leak in Chronic Heart Failure. Circ Res. 2008;103:1466–1472. doi: 10.1161/CIRCRESAHA.108.184457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Belevych AE, Terentyev D, Terentyeva R, Nishijima Y, Sridhar A, Hamlin RL, Carnes CA, Györke S. The relationship between arrhythmogenesis and impaired contractility in heart failure: role of altered ryanodine receptor function. Cardiovasc Res. 2011;90:493–502. doi: 10.1093/cvr/cvr025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Belevych AE, Sansom SE, Terentyeva R, Ho H-T, Nishijima Y, Martin MM, Jindal HK, Rochira JA, Kunitomo Y, Abdellatif M, Carnes CA, Elton TS, Györke S, Terentyev D. MicroRNA-1 and -133 Increase Arrhythmogenesis in Heart Failure by Dissociating Phosphatase Activity from RyR2 Complex. PLoS ONE. 2011;6:e28324. doi: 10.1371/journal.pone.0028324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Erickson JR, He BJ, Grumbach IM, Anderson ME. CaMKII in the Cardiovascular System: Sensing Redox States. Physiol Rev. 2011;91:889–915. doi: 10.1152/physrev.00018.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Erickson JR, Joiner MA, Guan X, Kutschke W, Yang J, Oddis CV, Bartlett RK, Lowe JS, O’Donnell SE, Aykin-Burns N, Zimmerman MC, Zimmerman K, Ham A-JL, Weiss RM, Spitz DR, Shea MA, Colbran RJ, Mohler PJ, Anderson ME. A Dynamic Pathway for Calcium-Independent Activation of CaMKII by Methionine Oxidation. Cell. 2008;133:462–474. doi: 10.1016/j.cell.2008.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ho H-T, Liu B, Snyder JS, Lou Q, Brundage EA, Velez-Cortes F, Wang H, Ziolo MT, Anderson ME, Sen CK, Wehrens XHT, Fedorov VV, Biesiadecki BJ, Hund TJ, Györke S. Ryanodine receptor phosphorylation by oxidized CaMKII contributes to the cardiotoxic effects of cardiac glycosides. Cardiovasc Res. 2014;101:165–174. doi: 10.1093/cvr/cvt233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shannon TR, Ginsburg KS, Bers DM. Potentiation of fractional sarcoplasmic reticulum calcium release by total and free intra-sarcoplasmic reticulum calcium concentration. Biophys J. 2000;78:334–343. doi: 10.1016/S0006-3495(00)76596-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Trafford AW, Sibbring GC, Díaz ME, Eisner DA. The effects of low concentrations of caffeine on spontaneous Ca release in isolated rat ventricular myocytes. Cell Calcium. 2000;28:269–276. doi: 10.1054/ceca.2000.0156. [DOI] [PubMed] [Google Scholar]
- 46.Wehrens XHT, Lehnart SE, Reiken S, Vest JA, Wronska A, Marks AR. Ryanodine receptor/calcium release channel PKA phosphorylation: A critical mediator of heart failure progression. Proc Natl Acad Sci U S A. 2006;103:511–518. doi: 10.1073/pnas.0510113103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grimm M, Brown JH. β-Adrenergic receptor signaling in the heart: Role of CaMKII. J Mol Cell Cardiol. 2010;48:322–330. doi: 10.1016/j.yjmcc.2009.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ogrodnik J, Niggli E. Increased Ca(2+) leak and spatiotemporal coherence of Ca(2+) release in cardiomyocytes during beta-adrenergic stimulation. J Physiol. 2010;588:225–242. doi: 10.1113/jphysiol.2009.181800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Massion PB, Feron O, Dessy C, Balligand J-L. Nitric Oxide and Cardiac Function Ten Years After, and Continuing. Circ Res. 2003;93:388–398. doi: 10.1161/01.RES.0000088351.58510.21. [DOI] [PubMed] [Google Scholar]
- 50.Lu X-Z, Bi X-Y, He X, Zhao M, Xu M, Yu X-J, Zhao Z-H, Zang W-J. Activation of M3 cholinoceptors attenuates vascular injury after ischaemia/reperfusion by inhibiting the Ca2+/calmodulin-dependent protein kinase II pathway. Br J Pharmacol. 2015;172:5619–5633. doi: 10.1111/bph.13183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Valdivia HH, Kaplan JH, Ellis-Davies GC, Lederer WJ. Rapid adaptation of cardiac ryanodine receptors: modulation by Mg2+ and phosphorylation. Science. 1995;267:1997–2000. doi: 10.1126/science.7701323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dries E, Bito V, Lenaerts I, Antoons G, Sipido KR, Macquaide N. Selective modulation of coupled ryanodine receptors during microdomain activation of calcium/calmodulin-dependent kinase II in the dyadic cleft. Circ Res. 2013;113:1242–1252. doi: 10.1161/CIRCRESAHA.113.301896. [DOI] [PubMed] [Google Scholar]
- 53.Ullrich ND, Valdivia HH, Niggli E. PKA phosphorylation of cardiac ryanodine receptor modulates SR luminal Ca2+ sensitivity. J Mol Cell Cardiol. 2012;53:33–42. doi: 10.1016/j.yjmcc.2012.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McMorn SO, Harrison SM, Zang WJ, Yu XJ, Boyett MR. A direct negative inotropic effect of acetylcholine on rat ventricular myocytes. Am J Physiol. 1993;265:H1393–1400. doi: 10.1152/ajpheart.1993.265.4.H1393. [DOI] [PubMed] [Google Scholar]
- 55.Protas L, Shen J-B, Pappano AJ. Carbachol Increases Contractions and Intracellular Ca++ Transients in Guinea Pig Ventricular Myocytes. J Pharmacol Exp Ther. 1998;284:66–74. [PubMed] [Google Scholar]
- 56.Yang J-M, Chung K-T, Yang S-T, Yang S-N. Muscarinic activation causes biphasic inotropic response and decreases cellular Na+ activity in canine cardiac purkinje fibers. J Biomed Sci. 1999;6:176–182. doi: 10.1007/BF02255901. [DOI] [PubMed] [Google Scholar]
- 57.Hara Y, Ike A, Tanida R, Okada M, Yamawaki H. Involvement of cyclooxygenase-2 in carbachol-induced positive inotropic response in mouse isolated left atrium. J Pharmacol Exp Ther. 2009;331:808–815. doi: 10.1124/jpet.109.156992. [DOI] [PubMed] [Google Scholar]
- 58.Azevedo ER, Parker JD. Parasympathetic control of cardiac sympathetic activity: normal ventricular function versus congestive heart failure. Circulation. 1999;100:274–279. doi: 10.1161/01.cir.100.3.274. [DOI] [PubMed] [Google Scholar]
- 59.Bibevski S, Dunlap ME. Ganglionic mechanisms contribute to diminished vagal control in heart failure. Circulation. 1999;99:2958–2963. doi: 10.1161/01.cir.99.22.2958. [DOI] [PubMed] [Google Scholar]
- 60.Shi H, Wang H, Li D, Nattel S, Wang Z. Differential alterations of receptor densities of three muscarinic acetylcholine receptor subtypes and current densities of the corresponding K+ channels in canine atria with atrial fibrillation induced by experimental congestive heart failure. Cell Physiol Biochem Int J Exp Cell Physiol Biochem Pharmacol. 2004;14:31–40. doi: 10.1159/000076924. [DOI] [PubMed] [Google Scholar]
- 61.Ai X, Curran JW, Shannon TR, Bers DM, Pogwizd SM. Ca2+/calmodulin-dependent protein kinase modulates cardiac ryanodine receptor phosphorylation and sarcoplasmic reticulum Ca2+ leak in heart failure. Circ Res. 2005;97:1314–1322. doi: 10.1161/01.RES.0000194329.41863.89. [DOI] [PubMed] [Google Scholar]
- 62.Klein HU, Ferrari GMD. Vagus nerve stimulation: A new approach to reduce heart failure. Cardiol J. 2010;17:638–644. [PubMed] [Google Scholar]
- 63.Zannad F, De Ferrari GM, Tuinenburg AE, et al. Chronic vagal stimulation for the treatment of low ejection fraction heart failure: results of the neural cardiac therapy for heart failure (NECTAR-HF) randomized controlled trial. Eur Heart J. 36:425–433. doi: 10.1093/eurheartj/ehu345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sossalla S, Fluschnik N, Schotola H, Ort KR, Neef S, Schulte T, Wittköpper K, Renner A, Schmitto JD, Gummert J, El-Armouche A, Hasenfuss G, Maier LS. Inhibition of elevated Ca2+/calmodulin-dependent protein kinase II improves contractility in human failing myocardium. Circ Res. 2010;107:1150–1161. doi: 10.1161/CIRCRESAHA.110.220418. [DOI] [PubMed] [Google Scholar]
- 65.Kong S-S, Liu J-J, Yu X-J, Lu Y, Zang W-J. Protection against Ischemia-Induced Oxidative Stress Conferred by Vagal Stimulation in the Rat Heart: Involvement of the AMPK-PKC Pathway. Int J Mol Sci. 2012;13:14311–14325. doi: 10.3390/ijms131114311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Miao Y, Zhou J, Zhao M, Liu J, Sun L, Yu X, He X, Pan X, Zang W. Acetylcholine Attenuates Hypoxia/Reoxygenation-Induced Mitochondrial and Cytosolic ROS Formation in H9c2 Cells via M2 Acetylcholine Receptor. Cell Physiol Biochem. 2013;31:189–198. doi: 10.1159/000343360. [DOI] [PubMed] [Google Scholar]
- 67.Sun L, Zang W-J, Wang H, Zhao M, Yu X-J, He X, Miao Y, Zhou J. Acetylcholine promotes ROS detoxification against hypoxia/reoxygenation-induced oxidative stress through FoxO3a/PGC-1α dependent superoxide dismutase. Cell Physiol Biochem Int J Exp Cell Physiol Biochem Pharmacol. 2014;34:1614–1625. doi: 10.1159/000366364. [DOI] [PubMed] [Google Scholar]
- 68.Zhang Y, Popovic ZB, Bibevski S, Fakhry I, Sica DA, Van Wagoner DR, Mazgalev TN. Chronic vagus nerve stimulation improves autonomic control and attenuates systemic inflammation and heart failure progression in a canine high-rate pacing model. Circ Heart Fail. 2009;2:692–699. doi: 10.1161/CIRCHEARTFAILURE.109.873968. [DOI] [PubMed] [Google Scholar]
- 69.Hamann JJ, Ruble SB, Stolen C, Wang M, Gupta RC, Rastogi S, Sabbah HN. Vagus nerve stimulation improves left ventricular function in a canine model of chronic heart failure. Eur J Heart Fail. 2013;15:1319–1326. doi: 10.1093/eurjhf/hft118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li Y, Xuan Y-H, Liu S-S, Dong J, Luo J-Y, Sun Z-J. Shortterm vagal nerve stimulation improves left ventricular function following chronic heart failure in rats. Mol Med Rep. 2015;12:1709–1716. doi: 10.3892/mmr.2015.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Uemura K, Zheng C, Li M, Kawada T, Sugimachi M. Early short-term vagal nerve stimulation attenuates cardiac remodeling after reperfused myocardial infarction. J Card Fail. 2010;16:689–699. doi: 10.1016/j.cardfail.2010.03.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.