Abstract
INTRODUCTION
Amiodarone is often used in the suppression of tachyarrhythmias. One of the more serious adverse effects includes amiodarone pulmonary toxicity (APT). Several pulmonary diseases can manifest including interstitial pneumonitis, organizing pneumonia, acute respiratory distress syndrome, diffuse alveolar hemorrhage, pulmonary nodules or masses, and pleural effusion. Incidence of APT varies from 5–15% and is correlated to dosage, age of the patient, and preexisting lung disease.
DESCRIPTION
A 56-year-old male with a past medical history of coronary artery disease and chronic obstructive pulmonary disease was admitted for a coronary artery bypass graft. Post-operatively, the patient was admitted to the ICU for ventilator management and continued to receive his home dose of amiodarone 400 mg orally twice daily, which he had been taking for the past 3 months. The patient was found to be hypoxemic with a PaO2 52 mmHg and bilateral infiltrates on chest x-ray. Patient also complained of new onset dyspnea. Physical exam found bilateral rhonchi with bibasilar crackles and subcutaneous emphysema along the left anterior chest wall. Daily chest x-rays showed worsening of bilateral interstitial infiltrates and pleural effusions. A chest high-resolution computed tomography on post-operative day 3 showed extensive and severe bilateral ground glass opacities. APT was suspected and amiodarone was discontinued. A course of oral prednisone without antibiotics was initiated, and after one week of treatment the chest film cleared, the PaO2 value normalized and dyspnea resolved.
DISCUSSION
APT occurs via cytotoxic T cells and indirectly by immunological reaction. Typically the lungs manifest a diffuse interstitial pneumonitis with varying degrees of fibrosis. Infiltrates with a ‘ground-glass’ appearance appreciated on HRCT are more definitive than chest x-ray. Pulmonary nodules can be seen, frequently in the upper lobes. These are postulated to be accumulations of amiodarone in areas of previous inflammation. Those undergoing major cardiothoracic surgery are known to be predisposed to APT. Some elements require consideration: a baseline pulmonary function test (PFT) did not exist prior. APT would manifest a restrictive pattern of PFTs. In APT diffusing capacity (DLCO) is generally >20 percent from baseline. A DLCO was not done in this patient. Therefore, not every type of interstitial lung disease could be ruled out. Key features support a clinical diagnosis: (1) new dyspnea, (2) exclusion of lung infection, (3) exclusion of heart failure, (4) new radiographic features, (5) improvement with withdrawal of amiodarone. Our case illustrates consideration of APT in patients who have extensive use of amiodarone and new onset dyspnea.
Keywords: amiodarone, amiodarone induced, pulmonary toxicity, interstitial lung disease, pulmonary fibrosis, ground glass, honeycombing, postcardiac surgery, idiopathic, drug induced
Introduction
Amiodarone is often used in the suppression of tachyarrhythmias.1 One of the more serious adverse effects includes amiodarone pulmonary toxicity (APT).1 Several pulmonary diseases can manifest, including interstitial pneumonitis, organizing pneumonia, acute respiratory distress syndrome, diffuse alveolar hemorrhage, pulmonary nodules or masses, and pleural effusion.1 The incidence of APT varies from 5% to 15% and is correlated with dosage, age of the patient, and preexisting lung disease.2–4 In this report, we describe a patient, who received a home dose of amiodarone, presented with APT status post coronary artery bypass graft (CABG). The patient has given consent for publication of this report.
Case Report
A 56-year-old male with a medical history of coronary artery disease, multivessel disease, and chronic obstructive pulmonary disease (COPD) was admitted for a CABG. Of note, the patient continued to receive his home dose of amiodarone 400 mg orally twice daily, which he had been taking for the past three months for treatment of ventricular tachycardia. On postoperative day 1, he became hypoxemic and a chest X-ray (CXR) was obtained, demonstrating bilateral infiltrates. He reported feeling weak, dyspneic at rest, and warmer than normal. The patient also complained of chest pain related to the CABG incision site and chest tube. He denied any cough or rhinorrhea and reported an improvement in dyspnea on high-flow oxygen. Other relevant medical history included peripheral vascular disease, dyslipidemia, and type II diabetes mellitus (A1c 9.2). Home medications included aspirin, clopidogrel, atorvastatin, amiodarone, and ropinirole. Body mass index (kg/m2) was 26. He underwent a femoral popliteal bypass graft surgery in 2010 with a redo bypass in 2011. The patient has 44 pack-year history of cigarette smoking without history of alcohol or recreational drug abuse.
Upon physical examination, the patient was found to be febrile to 38.4 °C and normotensive with a pulse oximetry of 94% on high-flow nasal cannula. The cardiac examination revealed a regular rate and rhythm with normal heart sounds, and the pulmonary examination was notable for bilateral rhonchi and bibasilar crackles with normal Anterior-poster (AP) diameter. He had a chest tube present anteriorly located below the xiphoid process. Subcutaneous emphysema was noted along the anterior left chest wall. A midline incision was also present at the site of the CABG. The dressing was clean, dry, and intact. Examination of the extremities showed no peripheral edema, with minimal venous stasis changes noted on the lower extremities. The remainder of the examination was unremarkable.
Upon review of the laboratory findings, he was noted to have a PaO2 of 52 mmHg. Daily portable CXRs demonstrated worsening of his bilateral interstitial infiltrates and pleural effusions. A high-resolution chest CT was obtained on postoperative day 3, which showed extensive and severe bilateral ground glass opacities. The differential diagnosis considered at the time included pneumonia, acute decompensated heart failure, or COPD exacerbation. He did have leukocytosis with left shift but without bandemia; this was thought to be due to administration of IV steroids for suspected COPD exacerbation and reactive response to stress from the CABG procedure. An adequate sputum sample could not be obtained for culture. There was a suspicion of APT as a contributing factor of his new-onset dyspnea based on the imaging and clinical features. A Naranjo Scale score of six was assessed, suggestive of probable APT. Amiodarone was discontinued, and he was started on oral prednisone without antibiotics. One week after initiation of the treatment, CXR of the patient demonstrated resolution of the bilateral interstitial infiltrates and pleural effusions. The PaO2 on ABG normalized, and his dyspnea resolved.
Discussion
Amiodarone is an antiarrhythmic agent often used to manage supraventricular and ventricular arrhythmias.1 It is an iodinated benzofuran compound, which has a high affinity for several organs including the lung tissue. It has a large side effect profile with APT being one of the most life-threatening side effects. Risk factors for developing APT include a high cumulative dose (>400 mg/day), a duration of therapy exceeding two months, increased patient age, preexisting lung disease, thoracic or nonthoracic surgery, and pulmonary angiography.1 The highest risk appears to occur in patients with abnormally low pretreatment pulmonary diffusing capacity of the lungs for carbon monoxide (DLCO) values.
The pathogenesis of APT is not completely understood. It has been implicated that a direct toxic injury to type II pneumocytes and lung parenchyma and an indirect immunologic reaction in genetically predisposed patients are the two major mechanisms of action.5 Different forms of pulmonary disease, including interstitial pneumonitis, organizing pneumonia, acute respiratory distress syndrome, diffuse alveolar hemorrhage, pulmonary nodules and solitary masses, and pleural effusion, can occur due to amiodarone.1 Typically, the lungs manifest a diffuse interstitial pneumonitis with varying degrees of fibrosis. As shown in Figure 1, infiltrates with a ground glass appearance can be detected on high-resolution computed tomography (HRCT) in our patient, along with septal thickening, honeycombing, traction bronchiectasis, and pulmonary nodules.1,6,7 These nodules are frequently located in the upper lobes, which have been postulated to be accumulations of amiodarone in areas of previous inflammation. HRCT can also demonstrate bilateral, asymmetric, hyperdense changes in the lungs and also in the liver and spleen due to the accumulation of the iodinated drug within macrophages.6 HRCT is a more definitive imaging modality than a chest radiograph, which can show multiple, peripheral areas of dense airspace opacities, characterized as focal or diffuse reticular or ground glass.8
Figure 1.
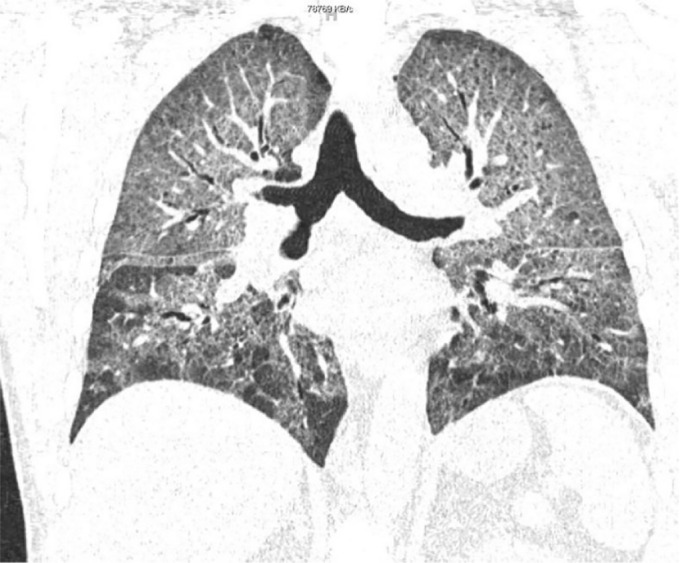
HRCT showing extensive and severe bilateral ground glass opacities. There is mild pleural thickening seen within the major fissures bilaterally and scattered lucencies seen within the chest wall extending to the bilateral axilla.
Pathology is the gold standard for diagnosing APT; however, a lung biopsy is not necessary in every patient.9,10 The histopathologic findings of APT include lipid laden macrophages in airspaces, nonspecific interstitial pneumonitis, type II pneumocyte hyperplasia, interstitial edema, and fibrosis.11
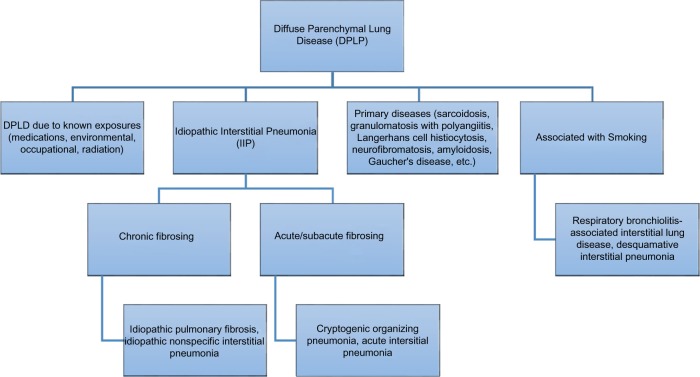
Pulmonary function tests (PFTs) typically show a restrictive pattern with reduced forced vital capacity and total lung capacity (approximately ≥15% from baseline) in addition to a reduced diffusing capacity (DLCO; generally >20% decrease from baseline).9 For our patient, a baseline PFT did not exist prior and a DLCO was not done. Therefore, not every type of interstitial lung disease (ILD) could be ruled out, and our differential for our patient accordingly included lung damage due to occupational or environmental agents, other drug-induced pulmonary toxicities, radiation-induced lung injury, and idiopathic causes. Occupational or environmental agents were less likely to be involved in the pathogenesis of our patient’s lung disease as he was not found to be exposed to known dusts, gases, or chemicals.12 A thorough history of the patient’s past medication use and radiation exposure is less likely to be a factor as he had not been exposed to other known drugs found to be a cause of ILD and had not been previously treated with therapeutic irradiation.13–15 A review of systems consistent with polymyositis/dermatomyositis, rheumatoid arthritis, systemic lupus erythematosus, scleroderma, and mixed connective tissue disease was negative. The patient denied any family history of systemic diseases and inherited disease associated with ILD, such as tuberous sclerosis, neurofibromatosis, familial hypocalciuric hypercalcemia, Hermansky–Pudlak syndrome, or Gaucher’s disease (Fig. 2).16
Figure 2.
How to sort between various forms of diffuse parenchymal lung disease.
In addition to the understanding that those undergoing major cardiothoracic surgery are predisposed to APT, the following key features in our patient support a clinical diagnosis: (1) new-onset dyspnea, (2) new radiographic features, (3) improvement with withdrawal of amiodarone, (4) exclusion of lung infection, and (5) exclusion of heart failure.10 The sudden onset of a nonproductive cough and dyspnea tends to occur within 6–12 months of starting amiodarone, which is also characteristic of APT.10 Other presenting symptoms include fever, weight loss, pleuritic chest pain, and lethargy. There is no single diagnostic test pathognomonic for APT, and diagnosis is often one of exclusion and is made by the presence of a constellation of clinical, laboratory, and radiographic findings. Our case illustrates consideration of APT in patients who have had extensive use of amiodarone and new-onset dyspnea following a cardiac procedure.
Conclusion
Amiodarone toxicity should be considered in patients who have extensive use of amiodarone with progressive or acute respiratory symptoms. It is relevant to remember that those patients with preexisting lung disease, those who use supplemental oxygen therapy, and those who are postcardiac surgery patients all require evaluation for APT. Baseline chest radiograph and PFTs should be obtained prior to initiation of amiodarone.10,17 APT is a diagnosis of exclusion and is made on the basis of clinical, laboratory, and radiographic findings. Prompt discontinuation of the drug and early aggressive corticosteroid therapy should be initiated once the diagnosis is determined.10
Supplementary Material
Supplementary Table 1. Recent cases that describe amiodarone pulmonary toxicity, in order of date of publication.
Acknowledgments
This case and abstract publication were also a part of the American Thoracic Society International Conference in 2016.
Abbreviations
- AP
anterior-posterior
- APT
amiodarone pulmonary toxicity
- CABG
coronary artery bypass graft
- COPD
chronic obstructive pulmonary disease
- CXR
chest X-ray
- DLCO
diffusing capacity of the lungs for carbon monoxide
- HRCT
high-resolution computed tomography
- ILD
interstitial lung disease
- PFT
pulmonary function test
Footnotes
ACADEMIC EDITOR: Athavale Nandkishor, Editor in Chief
PEER REVIEW: Three peer reviewers contributed to the peer review report. Reviewers’ reports totaled 195 words, excluding any confidential comments to the academic editor.
FUNDING: Authors disclose no external funding sources.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Wrote the first draft of the manuscript: AJS. Contributed to the writing of the manuscript: AJS, NKS, ND, VL, JD. Made critical revisions and approved final version: AJS. All authors reviewed and approved of the final manuscript.
REFERENCES
- 1.Wolkove N, Baltzan M. Amiodarone pulmonary toxicity. Can Respir J. 2009;16:43. doi: 10.1155/2009/282540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dusman RE, Stanton MS, Miles WM, et al. Clinical features of amiodarone-induced pulmonary toxicity. Circulation. 1990;82:51–9. doi: 10.1161/01.cir.82.1.51. [DOI] [PubMed] [Google Scholar]
- 3.Jackevicius CA, Tom A, Essebag V, et al. Population-level incidence and risk factors for pulmonary toxicity associated with amiodarone. Am J Cardiol. 2011;108(5):705–10. doi: 10.1016/j.amjcard.2011.04.024. [DOI] [PubMed] [Google Scholar]
- 4.Schwaiblmair M, Berghaus T, Haeckel T, Wagner T, von Scheidt W. Amiodarone-induced pulmonary toxicity: an under-recognized and severe adverse effect? Clin Res Cardiol. 2010;99(11):693–700. doi: 10.1007/s00392-010-0181-3. [DOI] [PubMed] [Google Scholar]
- 5.Martin WJ, II, Rosenow EC., III Amiodarone pulmonary toxicity. Recognition and pathogenesis (Part 2) Chest. 1988;93:1242. doi: 10.1378/chest.93.6.1242. [DOI] [PubMed] [Google Scholar]
- 6.Ott MC, Khoor A, Leventhal JP, Paterick TE, Burger CD. Pulmonary toxicity in patients receiving low-dose amiodarone. Chest. 2003;123:646. doi: 10.1378/chest.123.2.646. [DOI] [PubMed] [Google Scholar]
- 7.Hudzik B, Polonski L. Amiodarone-induced pulmonary toxicity. CMAJ. 2012;184:E819. doi: 10.1503/cmaj.111763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuhlman JE, Teigen C, Ren H, Hruban RH, Hutchins GM, Fishman EK. Amiodarone pulmonary toxicity: CT findings in symptomatic patients. Radiology. 1990;177:121. doi: 10.1148/radiology.177.1.2399310. [DOI] [PubMed] [Google Scholar]
- 9.Camus P. Interstitial lung disease from drugs, biologics, and radiation. In: Schwartz MI, King TE Jr, editors. Interstitial Lung Disease. 5th ed. Shelton, CT: People’s Medical Publishing House; 2011. p. 637. [Google Scholar]
- 10.Goldschlager N, Epstein AE, Naccarelli GV, et al. A practical guide for clinicians who treat patients with amiodarone: 2007. Heart Rhythm. 2007;4:1250. doi: 10.1016/j.hrthm.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 11.Bedrossian CW, Warren CJ, Ohar J, Bhan R. Amiodarone pulmonary toxicity: cytopathology, ultrastructure, and immunocytochemistry. Ann Diagn Pathol. 1997;1:47. doi: 10.1016/s1092-9134(97)80008-1. [DOI] [PubMed] [Google Scholar]
- 12.Glazer CS, Newman LS. Occupational interstitial lung disease. Clin Chest Med. 2004;25:467. doi: 10.1016/j.ccm.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 13.Camus P, Bonniaud P, Fanton A, Camus C, Baudaun N, Foucher P. Drug-induced and iatrogenic infiltrative lung disease. Clin Chest Med. 2004;25:479. doi: 10.1016/j.ccm.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 14.Camus P. Drug-induced interstitial lung disease. In: King TE Jr, Schwarz MI, editors. Interstitial Lung Disease. 4th ed. Hamilton, ON: B.C. Decker; 2003. p. 485. [Google Scholar]
- 15.Fernández AB, Karas RH, Alsheikh-Ali AA, Thompson PD. Statins and interstitial lung disease: a systematic review of the literature and of food and drug administration adverse event reports. Chest. 2008;134:824. doi: 10.1378/chest.08-0943. [DOI] [PubMed] [Google Scholar]
- 16.Garcia CK, Raghu G. Inherited interstitial lung disease. Clin Chest Med. 2004;25:421. doi: 10.1016/j.ccm.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 17.Vassallo P, Trohman RG. Prescribing amiodarone: an evidence-based review of clinical indications. JAMA. 2007;298:1312. doi: 10.1001/jama.298.11.1312. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. Recent cases that describe amiodarone pulmonary toxicity, in order of date of publication.