Abstract
Objective
To examine provincial and regional differences in FPs’ direct access to cancer diagnostic investigations and advice from other specialists regarding investigations and referrals, and to explore FPs’ perceptions about wait times for diagnostic investigations and receipt of results.
Design
A cross-sectional, online survey.
Setting
British Columbia, Manitoba, and Ontario.
Participants
A sample of FPs from participating provinces.
Main outcome measures
Direct FP access to various diagnostic investigations and advice from other specialists regarding investigations and referrals; FPs’ perceptions about wait times for diagnostic investigations ordered directly; and FPs’ perceptions about wait times for results.
Results
A total of 1054 surveys were completed by FPs from British Columbia (n = 229), Manitoba (n = 228), and Ontario (n = 597). Distance from a cancer centre was not significantly associated with direct access to or wait times for diagnostic investigations for most of the investigations studied; however, provincial differences were observed. Family physicians in Manitoba and British Columbia were 30% to 45% less likely to report having direct access to endoscopy and some imaging investigations compared with FPs in Ontario. Family physicians in Manitoba and British Columbia were also at increased odds of waiting longer than 12 weeks for endoscopy investigations and longer than 4 weeks for imaging investigations compared with FPs in Ontario. Most FPs reported wait times of less than 2 weeks for imaging results; however, the proportion of FPs who waited longer than 2 weeks for colonoscopy results ranged from 15% in Ontario to 96% in British Columbia.
Conclusion
Given the disparities observed among provinces, there is an opportunity for provinces to learn from one another to improve direct access to and shorten wait times for diagnostic investigations. This in turn has the potential to shorten the primary care interval for cancer diagnostic assessment.
Résumé
Objectif
Vérifier s’il existe des différences entre les provinces et les régions pour l’accès des MF aux examens diagnostiques et à l’opinion de spécialistes à propos des examens et des demandes de consultation; et consulter les MF sur ce qu’ils pensent des temps d’attente pour les demandes d’examens diagnostiques et pour la réception des résultats.
Type d’étude
Une enquête transversale en ligne.
Contexte
La Colombie-Britannique, le Manitoba et l’Ontario.
Participants
Un échantillon de MF des provinces participantes.
Principaux paramètres à l’étude
L’accès direct des MF à différents examens diagnostiques et à des conseils de la part de spécialistes à propos de l’investigation et des demandes de consultation; l’opinion des médecins sur les temps d’attente pour les examens diagnostiques demandés directement; et leur opinion sur le temps requis pour recevoir les résultats.
Résultats
Au total, 1054 enquêtes ont été complétées, soient 229 de la Colombie-Britannique, 228 du Manitoba et 597 de l’Ontario. Pour la plupart des examens diagnostiques, il n’y avait pas de relation significative entre la distance d’un centre de cancérologie et l’accès direct ou le temps d’attente pour les examens demandés; il y avait toutefois des différences entre les provinces. Par rapport aux MF de l’Ontario, ceux du Manitoba et de la Colombie-Britannique étaient entre 30 % et 45 % moins susceptibles de rapporter qu’ils avaient un accès direct à l’endoscopie et à certains examens d’imagerie. Ils étaient aussi plus susceptibles que ceux de l’Ontario d’attendre plus de 12 semaines pour une endoscopie et plus de 4 semaines pour un examen d’imagerie. La plupart des MF consultés mentionnaient des temps d’attente de moins de 2 semaines pour des résultats d’imagerie; toutefois, la proportion de ceux qui attendaient plus de 2 semaines pour les résultats d’une colonoscopie variait entre 15 % en Ontario et 96 % en Colombie-Britannique.
Conclusion
Compte tenu des disparités interprovinciales observées, il serait opportun que les provinces se consultent afin d’améliorer l’accès direct aux examens diagnostiques et pour raccourcir le temps d’attente pour les résultats. Cela pourrait aussi raccourcir l’intervalle des soins primaires requis pour établir le diagnostic du cancer.
Cancer outcomes differ substantially among Canadian provinces and by urban or rural status.1–5 There is evidence that poorer outcomes might be associated with a longer diagnostic time interval (time between onset of symptoms and diagnosis).6–10 The primary care interval (time between first presentation to primary care and the request for referral) represents a large segment of the diagnostic time interval, and thus it is reasonable to expect that primary care delays might influence outcomes.11 Further, a longer diagnostic interval might also lead to increased patient anxiety and dissatisfaction, and result in inefficiencies in the health care system. There is limited research examining this, especially in the Canadian context; thus the objectives of this study were to examine provincial and regional differences in factors that influence the primary care interval including direct FP access to cancer diagnostic investigations and advice from other specialists, and FPs’ perceptions about wait times for diagnostic investigations and receipt of results.
METHODS
The International Cancer Benchmarking Partnership is a collaboration of 13 jurisdictions: New South Wales and Victoria (Australia); British Columbia, Manitoba, Ontario, and Alberta (Canada); Denmark; Norway; Sweden; and England, Wales, Northern Ireland, and Scotland (United Kingdom). It comprises 5 modules, each examining a different hypothesis to explain observed international differences in cancer survival.1,12 This paper reports the Canadian results of module 3, which examined the role of primary care in cancer diagnosis. Each jurisdiction sought individual research ethics board approval (from the University of Toronto in Ontario; the University of Manitoba and CancerCare Manitoba; and the University of British Columbia). Additional information on the International Cancer Benchmarking Partnership has been published elsewhere.12
Study design and participants
This study used a cross-sectional, online survey of FPs who were invited to participate via postal mail or e-mail between June 11, 2012, and March 31, 2013. Lists of licensed, registered FPs were provided by the respective provincial bodies responsible for the regulation of family medicine in participating provinces. The recruitment strategies are outlined in Table 1. Participants were not eligible if they indicated on the initial eligibility screening question that they were not involved in direct clinical care or they were not an FP. Each jurisdiction aimed to recruit 200 FPs to meet the sample size requirement for the larger international study (which gave a 95% CI of 43% to 57% for an equally distributed response).
Table 1.
Comparison of recruitment strategies
| FACTORS | ONTARIO | MANITOBA | BRITISH COLUMBIA |
|---|---|---|---|
| FP sampling strategy | FPs were sampled from a list of all licensed FPs registered with the College of Physicians and Surgeons of Ontario; all rural physicians were invited owing to small numbers and a simple random sample of urban physicians was drawn | FPs were sampled from a list of all physicians registered with the College of Physicians and Surgeons of Manitoba; FPs were stratified by urban and rural status and an equal no. of invitations was sent to each group | FPs were sampled from lists from the British Columbia College of Family Physicians and the UBC Department of Family Practice |
| No. of FPs invited | 3175 (2400 urban, 945 rural)* | 500 (250 urban, 250 rural) | > 4200 |
| Response rate, % | 19.2 (37.6 rural, 12.0 urban) | 45.6 | 5.5 |
| Method of invitation | Invitation was sent by postal mail | Invitation was sent by postal mail | Invitation was sent by e-mail |
| Date of survey invitations | 2 waves of invitations were mailed: December 3, 2012, and January 30, 2013. | June 11, 2012 | October 24, 2012 |
| Date of survey closure | March 30, 2013 | September 28, 2012 | March 31, 2013 |
| Additional letters or reminders | All physicians were sent notification letters 1 wk before the invitation letter and thank-you letters 2 wk after the invitation letter; 2 reminder letters were sent to nonrespondents | Physicians were sent notification letters before the invitation letters and 2 reminder letters | None |
| Incentives | None | Unconditional $10 coffee card | Participants could win 1 of 3 tablet computers |
UBC—University of British Columbia.
A total of 50 rural and 120 urban FPs were excluded if they had no street address, if the address was a military base, if they lived on reserve, or if they lived in a correctional facility.
Survey content and development
The survey examined FPs’ demographic characteristics; FPs’ direct access to diagnostic investigations and advice from other specialists within 48 hours regarding cancer investigations and referrals; FPs’ perceived wait times for investigations; and practice organization factors. Details of survey development and validation have been previously reported.7,13
Statistical analyses
We used
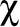 2 tests to examine provincial and regional differences in demographic characteristics, FPs’ direct access to diagnostic investigations and advice from other specialists, and FPs’ perceptions about wait times for diagnostic investigations ordered directly and wait times for the receipt of results. To evaluate regional differences within provinces, distance from a specialist cancer centre was defined using the question, “Do more than half of your patients live more than 40 km from the nearest centre with specialist cancer services?” Family physicians who answered yes were coded as distant; those who answered no were coded as close. To correct for multiple comparisons, false discovery rate P values were calculated and reported.14
2 tests to examine provincial and regional differences in demographic characteristics, FPs’ direct access to diagnostic investigations and advice from other specialists, and FPs’ perceptions about wait times for diagnostic investigations ordered directly and wait times for the receipt of results. To evaluate regional differences within provinces, distance from a specialist cancer centre was defined using the question, “Do more than half of your patients live more than 40 km from the nearest centre with specialist cancer services?” Family physicians who answered yes were coded as distant; those who answered no were coded as close. To correct for multiple comparisons, false discovery rate P values were calculated and reported.14
Logistic regression was conducted to assess associations between FPs’ direct access to diagnostic investigations (yes or no) and ability to obtain advice from other specialists (measured on a 5-point Likert scale and collapsed into 2 response options: strongly agree or agree versus neutral, disagree, or strongly disagree), and the predictors of region and province. Multinomial logistic regression was conducted to examine associations between FPs’ perceptions about wait times for diagnostic investigations ordered directly and for receipt of results, and the predictors of region and province. An a priori decision was made to adjust all models for the country in which participants obtained their medical degrees and the ability to arrange faster access to investigations when there was high suspicion of cancer (access to and wait times for the conduction of investigations models) or systems used to ensure investigations are followed up (receipt of results models). Wait times were collapsed into 3 response categories post hoc, dependent on the distribution of responses (ie, to ensure that the response option categories were informative).
RESULTS
Demographic characteristics of the respondents are presented in Table 2. A total of 1054 surveys were used in the analyses: 229 from British Columbia, 228 from Manitoba, and 597 from Ontario, representing response rates of 5%, 46%, and 19%, respectively. The proportion of distant FPs varied across provinces from 30% in Ontario to 47% in Manitoba. Demographic characteristics of FPs were not significantly different between regions, with the exception of the country in which the medical degree was obtained (a higher percentage of distant FPs and FPs from Manitoba received their degrees outside of Canada). The percentage of FPs who reported that they could not arrange faster access to investigations in the context of high clinical suspicion ranged from 9% (Ontario) to 15% (Manitoba). In all regions, less than 10% of FPs reported having no system for or relying on their patients to ensure follow-up of investigation results.
Table 2.
Demographic characteristics of participants, by province
| CHARACTERISTICS | FULL SAMPLE, N (%) (N = 1054)* | PROVINCIAL COMPARISONS | FDR P VALUES | ||
|---|---|---|---|---|---|
|
| |||||
| ONTARIO, N (%) (N = 597)* | MANITOBA, N (%) (N = 228)* | BRITISH COLUMBIA, N (%) (N = 229)* | |||
| Region | < .001 | ||||
| • Urban | 678 (64.6) | 414 (69.9) | 120 (52.6) | 144 (62.9) | |
| • Rural | 371 (35.4) | 178 (30.1) | 108 (47.4) | 85 (37.1) | |
| Sex | .02 | ||||
| • Male | 544 (51.7) | 306 (51.4) | 135 (59.2) | 103 (45.0) | |
| • Female | 508 (48.3) | 289 (48.6) | 93 (40.8) | 126 (55.0) | |
| Year medical degree was obtained | .002 | ||||
| • Before 1979 | 241 (22.9) | 150 (25.1) | 44 (19.3) | 47 (20.5) | |
| • 1980 to 1999 | 531 (50.4) | 281 (47.1) | 141 (61.8) | 109 (47.6) | |
| • 2000 or later | 282 (26.8) | 166 (27.8) | 43 (18.9) | 73 (31.9) | |
| Country in which medical degree was obtained | < .001 | ||||
| • Canada | 815 (77.3) | 537 (90.0) | 111 (48.7) | 167 (72.9) | |
| • Other | 239 (22.7) | 60 (10.0) | 117 (51.3) | 62 (27.1) | |
| Sole physician in practice | .002 | ||||
| • Yes | 180 (17.1) | 116 (19.5) | 40 (17.5) | 24 (10.5) | |
| • No | 871 (82.9) | 478 (80.5) | 188 (82.5) | 205 (89.5) | |
| Can arrange faster access to tests if suspicion is high | .02 | ||||
| • Strongly disagree or disagree | 118 (11.3) | 51 (8.7) | 34 (14.9) | 33 (14.4) | |
| System used to ensure investigation results are followed up | .30 | ||||
| • No system or rely on patient to follow up | 81 (8.0) | 48 (8.4) | 21 (9.7) | 12 (5.4) | |
| • Follow-up is at my discretion or contact patient only if test result is abnormal | 931 (92.0) | 525 (91.6) | 196 (90.3) | 210 (94.6) | |
FDR—false discovery rate.
Not all respondents answered all questions. Proportions are calculated based on the number of responses to the question.
Family physicians reported similar direct access to diagnostic investigations and advice from other specialists regardless of their distance from a cancer centre, with the exception of magnetic resonance imaging (MRI); 78% of distant FPs reported having direct access compared with 88% of close FPs (P < .001). Additionally, a greater percentage of distant FPs waited less than 2 weeks for computed tomography (CT) compared with close FPs (46% vs 29%, P < .001). Conversely, a greater percentage of close FPs reported waiting less than 2 weeks for ultrasound scans compared with distant FPs (56% vs 45%, P = .003). No significant differences in the wait times for results were observed.
Responses about direct access to diagnostic investigations and advice from other specialists and perceptions about wait times, by province, are outlined in Tables 3 to 5. Compared with FPs in Ontario, FPs in British Columbia were approximately 2.5 times more likely to report having access to advice from other specialists. With respect to direct access to endoscopy investigations, FPs in Manitoba and British Columbia were approximately 30% (British Columbia) and 45% (Manitoba) less likely to report having direct access to upper gastrointestinal endoscopy and colonoscopy. Family physicians in Manitoba were also 30% to 46% less likely to report having direct access to all imaging investigations (x-ray and ultrasound scans, CT, and MRI), and FPs in British Columbia were 27% less likely to report having direct access to MRI (Table 3).
Table 3.
Direct FP access to advice from other specialists and diagnostic investigations, by province
| QUESTION | FULL SAMPLE, N (%) (N=1054)* | PROVINCIAL COMPARISONS | FDR P VALUE | LOGISTIC REGRESSION | ||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| ONTARIO, N (%) (N=597)* | MANITOBA, N (%) (N=228)* | BRITISH COLUMBIA, N (%) (N=229)* | ONTARIO, OR (REFERENCE) | MANITOBA, OR (95% CI) | BRITISH COLUMBIA, OR (95% CI) | |||
| Can you obtain specialist advice in less than 48 h regarding [strongly agree or agree] ... | ||||||||
| • investigations for suspected cancers? | 562 (54.1) | 298 (51.3) | 109 (47.8) | 155 (67.7) | < .001 | 1.00 | 1.05 (0.74–1.49) | 2.44 (1.72–3.46) |
| • potential referrals to secondary care or specialist cancer services for suspected cancer? | 504 (48.5) | 258 (44.3) | 103 (45.2) | 143 (62.5) | < .001 | 1.00 | 1.26 (0.88–1.78) | 2.52 (1.80–3.52) |
| Do you have direct access to [yes] ... | ||||||||
| • all blood tests needed for cancer diagnosis? | 815 (78.4) | 470 (80.6) | 158 (69.3) | 187 (81.7) | .002 | 1.00 | 0.67 (0.46–0.99) | 1.20 (0.80–1.80) |
| • endoscopy? | ||||||||
| -upper GI endoscopy | 162 (15.6) | 114 (19.5) | 31 (13.8) | 17 (7.5) | .0003 | 1.00 | 0.48 (0.29–0.80) | 0.30 (0.17–0.52) |
| -flexible sigmoidoscopy | 163 (16.1) | 103 (18.1) | 30 (13.6) | 30 (13.3) | .2 | 1.00 | 0.62 (0.38–1.02) | 0.68 (0.43–1.07) |
| -colonoscopy | 179 (17.2) | 131 (22.2) | 30 (13.3) | 18 (8.0) | < .001 | 1.00 | 0.45 (0.28–0.74) | 0.29 (0.17–0.49) |
| • imaging? | ||||||||
| -x-ray scan (full body) | 1012 (96.4) | 581 (98.0) | 211 (92.5) | 220 (96.1) | .002 | 1.00 | 0.30 (0.13–0.69) | 0.53 (0.21–1.33) |
| -CT (full body) | 973 (92.9) | 564 (95.1) | 197 (86.4) | 212 (93.8) | .0002 | 1.00 | 0.46 (0.25–0.83) | 0.90 (0.46–1.77) |
| -MRI (full body) | 852 (84.2) | 539 (91.7) | 170 (74.6) | 143 (73.0) | < .001 | 1.00 | 0.37 (0.23–0.58) | 0.27 (0.17–0.42) |
| -ultrasound (full body) | 971 (92.6) | 566 (95.5) | 192 (84.2) | 213 (93.4) | < .001 | 1.00 | 0.31 (0.17–0.56) | 0.74 (0.38–1.45) |
CT—computed tomography, FDR—false discovery rate, GI—gastrointestinal, MRI—magnetic resonance imaging, OR—odds ratio.
Not all respondents answered all questions. Proportions are calculated based on the number of responses to the question.
Table 5.
Family physicians’ perceived wait times for results of diagnostic investigations, by province
| WAIT TIME FOR INVESTIGATION RESULTS | FULL SAMPLE, N (%) (N=1,054)* | PROVINCIAL COMPARISONS | FDR P VALUE | LOGISTIC REGRESSION | ||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| ONTARIO, N (%)(N=597)* | MANITOBA, N (%) (N=228)* | BRITISH COLUMBIA, N (%) (N=229)* | ONTARIO, OR (REFERENCE) | MANITOBA, OR (95% CI) | BRITISH COLUMBIA, OR (95% CI) | |||
| Upper GI endoscopy | .01 | |||||||
| • ≤ 1 wk | 363 (39.9) | 231 (44.9) | 64 (31.5) | 68 (35.1) | 1.00 | 1.00 | 1.00 | |
| • > 1 to 2 wk | 381 (41.8) | 202 (39.3) | 92 (45.3) | 87 (44.9) | 1.00 | 1.63 (1.08–2.48) | 1.48 (1.01–2.17) | |
| • > 2 wk | 167 (18.3) | 81 (15.8) | 47 (23.2) | 39 (20.1) | 1.00 | 2.29 (1.38–3.81) | 1.70 (1.04–2.77) | |
| Flexible sigmoidoscopy | .08 | |||||||
| • ≤ 1 wk | 319 (40.2) | 196 (45.4) | 55 (31.1) | 68 (37.0) | 1.00 | 1.00 | 1.00 | |
| • > 1 to 2 wk | 329 (41.5) | 173 (40.1) | 79 (44.6) | 77 (41.9) | 1.00 | 1.49 (0.96–2.33) | 1.25 (0.84–1.87) | |
| • > 2 wk | 145 (18.3) | 63 (14.6) | 43 (24.3) | 39 (21.2) | 1.00 | 2.49 (1.44–4.28) | 1.82 (1.10–3.03) | |
| Colonoscopy | < .001 | |||||||
| • ≤ 1 wk | 287 (31.8) | 222 (43.2) | 62 (30.7) | 3 (1.6) | 1.00 | 1.00 | 1.00 | |
| • > 1 to 2 wk | 311 (34.5) | 211 (41.1) | 91 (45.1) | 9 (4.8) | 1.00 | 1.65 (1.08–2.51) | 3.27 (0.87–12.31) | |
| • > 2 wk | 304 (33.7) | 81 (15.8) | 49 (24.3) | 174 (95.6) | 1.00 | 2.44 (1.47–4.07) | 171.1 (52.74–555.29) | |
| X-ray scan | .002 | |||||||
| • ≤ 1 wk | 947 (90.5) | 556 (93.9) | 194 (85.8) | 197 (80.0) | 1.00 | 1.00 | 1.00 | |
| • > 1 wk | 100 (9.6) | 36 (6.1) | 32 (14.2) | 32 (14.0) | 1.00 | 2.14 (1.20–3.81) | 2.20 (1.29–3.76) | |
| CT | .003 | |||||||
| • ≤ 1 wk | 741 (71.3) | 447 (76.0) | 149 (66.2) | 145 (64.2) | 1.00 | 1.00 | 1.00 | |
| • > 1 to 2 wk | 255 (25.4) | 126 (21.4) | 63 (28.0) | 66 (29.2) | 1.00 | 1.75 (1.18–2.61) | 1.63 (1.13–2.36) | |
| • > 2 wk | 43 (4.1) | 15 (2.6) | 13 (5.8) | 15 (6.6) | 1.00 | 1.78 (0.75–4.18) | 2.43 (1.12–5.30) | |
| MRI | < .001 | |||||||
| • ≤ 1 wk | 609 (61.3) | 396 (68.0) | 107 (50.7) | 106 (52.7) | 1.00 | 1.00 | 1.00 | |
| • > 1 to 2 wk | 316 (31.8) | 164 (28.2) | 82 (38.9) | 70 (34.8) | 1.00 | 1.95 (1.33–.85) | 1.50 (1.04–2.16) | |
| • > 2 wk | 69 (7.0) | 22 (3.8) | 22 (10.4) | 25 (12.4) | 1.00 | 2.68 (1.34–5.35) | 3.46 (1.84–6.50) | |
| Ultrasound | < .001 | |||||||
| • ≤ 1 wk | 814 (78.3) | 508 (86.3) | 140 (63.1) | 166 (72.5) | 1.00 | 1.00 | 1.00 | |
| • > 1 to 2 wk | 196 (18.9) | 75 (12.7) | 71 (32.0) | 50 (21.8) | 1.00 | 3.80 (2.49–5.80) | 2.00 (1.32 3.04) | |
| • > 2 wk | 30 (2.9) | 6 (1.0) | 11 (5.0) | 13 (5.7) | 1.00 | 4.94 (1.65–14.78) | 5.96 (2.18–16.34) | |
CT—computed tomography, FDR—false discovery rate, GI—gastrointestinal, MRI—magnetic resonance imaging, OR—odds ratio.
Not all respondents answered all questions. Proportions are calculated based on the number of responses to the question.
Overall, FPs in Manitoba and British Columbia were at increased odds of waiting longer for diagnostic investigations to be conducted and results to be received compared with FPs in Ontario. Family physicians in Manitoba and British Columbia were 5 to 10 times more likely to wait longer than 12 weeks for endoscopy (Table 4). Notably, 71% of FPs in British Columbia reported waiting longer than 12 weeks for MRIs compared with 10% in Ontario (odds ratio of 34.71, 95% CI 18.66 to 64.57) (Table 4). Finally, in Manitoba, 61% of FPs waited longer than 4 weeks for ultrasound compared with only 7% in Ontario (odds ratio of 50.20, 95% CI 29.24 to 86.20).
Table 4.
Family physicians’ perceived wait times for diagnostic imaging, by province
| WAIT TIME FOR INVESTIGATION | FULL SAMPLE, N (%) (N=1054)* | PROVINCIAL COMPARISONS | FDR P VALUE | LOGISTIC REGRESSION | ||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| ONTARIO, N (%) (N=597)* | MANITOBA, N (%) (N=228)* | BRITISH COLUMBIA, N (%) (N=229)* | ONTARIO, OR (REFERENCE) | MANITOBA, OR (95% CI) | BRITISH COLUMBIA, OR (95% CI) | |||
| Upper GI endoscopy | < .001 | |||||||
| • ≤ 4 wk | 262 (31.1) | 186 (39.8) | 31 (15.8) | 45 (25.1) | 1.00 | 1.00 | 1.00 | |
| • > 4 to 12 wk | 386 (45.8) | 224 (48.0) | 95 (48.5) | 67 (37.4) | 1.00 | 3.53 (2.12–5.88) | 1.43 (0.92–2.23) | |
| • > 12 wk | 194 (23.0) | 57 (12.2) | 70 (35.7) | 67 (37.4) | 1.00 | 10.15 (5.67–18.17) | 5.58 (3.38–9.21) | |
| Flexible sigmoidoscopy | < .001 | |||||||
| • ≤ 4 wk | 239 (33.6) | 166 (43.9) | 35 (20.5) | 38 (23.5) | 1.00 | 1.00 | 1.00 | |
| • > 4 to 12 wk | 302 (42.5) | 168 (44.4) | 75 (43.9) | 59 (36.4) | 1.00 | 2.83 (1.69–4.74) | 1.75 (1.09–2.82) | |
| • > 12 wk | 170 (23.9) | 44 (11.6) | 61 (35.7) | 65 (40.1) | 1.00 | 8.80 (4.83–16.02) | 7.33 (4.26–12.61) | |
| Colonoscopy | < .001 | |||||||
| • ≤ 4 wk | 213 (25.5) | 156 (33.5) | 24 (12.3) | 33 (18.8) | 1.00 | 1.00 | 1.00 | |
| • > 4 to 12 wk | 373 (44.6) | 239 (51.3) | 85 (43.6) | 49 (27.8) | 1.00 | 2.93 (1.68–5.12) | 1.08 (0.66–1.79) | |
| • > 12 wk | 251 (30.0) | 71 (15.2) | 86 (44.1) | 94 (53.4) | 1.00 | 10.79 (5.93–19.62) | 7.23 (4.36–12.01) | |
| X-ray scan | .40 | |||||||
| • ≤ 2 wk | 1037 (99.6) | 589 (99.8) | 223 (99.1) | 225 (99.6) | NA | NA | NA | |
| • > 2 wk | 4 (0.4) | 1 (0.2) | 2 (0.9) | 1 (0.4) | NA | NA | NA | |
| CT | < .001 | |||||||
| • ≤ 2 wk | 360 (34.9) | 246 (41.9) | 61 (27.4) | 53 (24.0) | 1.00 | 1.00 | 1.00 | |
| • > 2 to 4 wk | 374 (36.3) | 228 (38.8) | 65 (29.2) | 81 (36.7) | 1.00 | 1.48 (0.95–2.30) | 1.83 (1.22–2.75) | |
| • > 4 wk | 297 (28.8) | 113 (19.3) | 97 (43.5) | 87 (39.4) | 1.00 | 4.78 (3.04–7.52) | 4.06 (2.64–6.24) | |
| MRI | < .001 | |||||||
| • ≤ 4 wk | 255 (26.1) | 214 (37.2) | 26 (12.3) | 15 (7.9) | 1.00 | 1.00 | 1.00 | |
| • > 4 to 12 wk | 461 (47.2) | 303 (52.6) | 118 (55.9) | 40 (21.1) | 1.00 | 4.13 (2.46–6.91) | 2.09 (1.11–3.91) | |
| • > 12 wk | 261 (26.7) | 59 (10.2) | 67 (31.8) | 135 (71.1) | 1.00 | 11.09 (6.10–20.16) | 34.71 (18.66–64.57) | |
| Ultrasound | < .001 | |||||||
| • ≤ 2 wk | 542 (52.3) | 436 (74.3) | 41 (18.4) | 65 (28.8) | 1.00 | 1.00 | 1.00 | |
| • > 2 to 4 wk | 234 (22.6) | 111 (18.9) | 45 (20.2) | 78 (34.5) | 1.00 | 5.45 (3.20–9.29) | 5.26 (3.50–7.92) | |
| • > 4 wk | 260 (25.1) | 40 (6.8) | 137 (61.4) | 83 (36.7) | 1.00 | 50.20 (29.24–86.20) | 15.77 (9.80–25.40) | |
CT—computed tomography, FDR—false discovery rate, GI—gastrointestinal, MRI—magnetic resonance imaging, NA—not applicable, OR—odds ratio.
Not all respondents answered all questions. Proportions are calculated based on the number of responses to the question.
DISCUSSION
A high proportion (84% to 96%) of all FPs reported having direct access to imaging, but access to endoscopy was low (16% to 17%). Although most FPs reported receiving the results of diagnostic investigations within 2 weeks (with the exception of colonoscopy), approximately a quarter of FPs reported waiting longer than 12 weeks for endoscopy investigations to be conducted, and waiting longer than 4 weeks for imaging investigations to be conducted, indicating that FPs self-reported having better access to imaging than to endoscopy. This might influence FP behaviour and the speed of diagnostic workup of patients. While it has been previously shown that access to diagnostic investigations was not associated with FP readiness to investigate or refer,7 access might influence which investigations to order. This might result in investigations that are less likely to definitively confirm cancer, thereby increasing patient anxiety and the diagnostic interval. Indeed, a randomized control study in Denmark found that patients of FPs with direct access to CT scans had shorter diagnostic intervals.15
This study did not find patterns in differences by distance from a centre with specialist cancer services; however, provincial differences were observed. A higher percentage of FPs in British Columbia reported having access to advice from other specialists compared with FPs in Ontario and Manitoba. This could be attributed to the Rapid Access to Consultative Expertise initiative in British Columbia, where FPs have access to real-time specialist advice via a telephone line. Conversely, FPs in British Columbia and Manitoba were at decreased odds of having direct access to upper gastrointestinal endoscopy and colonoscopy, and FPs in Manitoba were at decreased odds of having direct access to all imaging investigations compared with FPs in Ontario.
Family physicians in British Columbia and Manitoba were at increased odds of waiting longer for diagnostic investigations and results compared with FPs in Ontario. This is consistent with previous research examining wait times for medical imaging. The Canadian Institute for Health Information has reported that the 50th percentile for MRI and CT wait times was longer in Manitoba compared with Ontario (99 vs 36 days for MRI and 18 vs 7 days for CT); British Columbia wait times were not reported.16
Limitations
This study has several limitations. First, the question, “Do more than half of your patients live more than 40 km from the nearest centre with specialist cancer services?” was used as a proxy for examining regional differences, as it was not possible to geographically locate FP practice address in all provinces. Consequently, it is possible that misclassification occurred, which would bias the results toward the null hypothesis. However, geocoding was conducted in Ontario according to the population centre definition from the 2011 Canadian census, and the agreement between urban or rural status and distance from the nearest centre with specialist cancer services was 81.3% (κ = 0.56, 95% CI 0.49 to 0.64, P < .001). Second, the regression analyses suffered from low power. Nevertheless, the magnitude of the odds ratios and the statistical significance indicate that the associations observed are clinically meaningful and real. Third, the results of this study are self-reported and are thus subject to recall bias. Further, because the data are self-reported, FP access to investigations and wait times are based on FP perception. Fourth, the external validity of this study is compromised owing to low response rates. Moreover, our recruitment methodology was dependent on jurisdictional feasibility, and thus response rates (and likely the study populations) varied substantially by province. Fifth, external validity was also compromised because rural FPs were oversampled in Manitoba and Ontario. Although greater regional representation can be interpreted as a study strength, it is conceivable that access and wait times might differ by urban or rural status.
Conclusion
Despite these limitations, this study adds to the literature by examining regional and provincial differences in Canada with respect to factors that influence the primary care and diagnostic intervals for cancer. Statistically significant provincial differences in wait times and FPs’ direct access to diagnostic investigations were observed. Although urban and rural differences could not be directly examined, using distance from a cancer centre as a proxy, the provincial differences observed do not appear to be attributed to other regional differences. Consequently, this study demonstrates that there is considerable opportunity for provinces to learn from one another’s strengths and weaknesses. Although it is unknown whether the provincial differences observed influence outcomes, we do know that the diagnostic interval has been shown to influence patient outcomes. Given that the primary care interval is a large segment of the diagnostic interval, not only does a longer primary care interval potentially result in poorer outcomes, affecting survival and stage progression, it also likely increases patient anxiety and dissatisfaction, and might lead to inefficiencies in the health care system. Thus it is important to identify factors within primary care that might decrease the time to cancer diagnosis.
EDITOR’S KEY POINTS
Previous research has demonstrated that the diagnostic interval (the time between onset of symptoms and diagnosis) influences cancer outcomes. Because the primary care interval represents a large segment of the diagnostic interval, this study aimed to examine primary care factors that might influence the diagnostic interval and in turn affect cancer outcomes.
Considerable provincial differences were observed. A higher percentage of FPs in British Columbia reported having access to advice from other specialists within 48 hours compared with FPs in Ontario and Manitoba, and FPs in British Columbia and Manitoba were at increased odds of waiting longer for diagnostic investigations and results compared with FPs in Ontario.
Family physicians self-reported having better access to imaging than to endoscopy. Although the survey did not examine FP behaviour, this differential access has the potential to influence which investigations FPs order and consequently the speed of diagnostic workup of patients. It could result in investigations that are less likely to definitively confirm cancer, thereby increasing patient anxiety and the diagnostic interval.
POINTS DE REPÈRE DU RÉDACTEUR
Des études antérieures ont montré que l’intervalle diagnostique (la période entre le début des symptômes et le diagnostic) influence les issues d’un cancer. Comme l’intervalle pour les premiers soins représente une portion considérable de l’intervalle diagnostique, cette étude voulait déterminer si certains facteurs liés aux soins primaires pouvaient influencer l’intervalle diagnostique et ainsi affecter les issues d’un cancer.
D’importantes différences ont été observées entre les provinces. Par rapport aux MF du Manitoba et de l’Ontario, un plus fort pourcentage de ceux de la Colombie-Britannique ont déclaré avoir accès à des conseils d’autres spécialistes dans les 48 heures; par ailleurs, ceux de Colombie Britannique et du Manitoba étaient plus susceptibles d’attendre plus longtemps que ceux de l’Ontario pour les examens diagnostiques et pour les résultats.
Les médecins de famille ont déclaré avoir un meilleur accès à l’imagerie qu’à l’endoscopie. Même si l’enquête ne vérifiait pas le comportement des MF, une telle différence pourrait influencer le choix des examens demandés et donc, la rapidité avec laquelle le diagnostic est établi. Le médecin pourrait donc demander des examens qui sont moins susceptibles de confirmer la présence d’un cancer, augmentant ainsi l’anxiété du patient et l’intervalle diagnostique.
Footnotes
This article has been peer reviewed.
Cet article a fait l’objet d’une révision par des pairs.
Contributors
All authors contributed to the concept and design of the study; data gathering, analysis, and interpretation; and preparing the manuscript for submission.
Competing interests
None declared
References
- 1.Coleman MP, Forman D, Bryant H, Butler J, Rachet B, Maringe C, et al. Cancer survival in Australia, Canada, Denmark, Norway, Sweden, and the UK, 1995–2007 (the International Cancer Benchmarking Partnership): an analysis of population-based cancer registry data. Lancet. 2011;377(9760):127–38. doi: 10.1016/S0140-6736(10)62231-3. Epub 2010 Dec 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hallet J, Law CH, Karanicolas PJ, Saskin R, Liu N, Singh S. Rural-urban disparities in incidence and outcomes of neuroendocrine tumors: a population-based analysis of 6271 cases. Cancer. 2015;121(13):2214–21. doi: 10.1002/cncr.29338. Epub 2015 Mar 30. [DOI] [PubMed] [Google Scholar]
- 3.Leung J, McKenzie S, Martin J, McLaughlin D. Effect of rurality on screening for breast cancer: a systematic review and meta-analysis comparing mammography. Rural Remote Health. 2014;14(2):2730. Epub 2014 Jun 23. [PubMed] [Google Scholar]
- 4.Obertova Z, Brown C, Holmes M, Lawrenson R. Prostate cancer incidence and mortality in rural men—a systematic review of the literature. Rural Remote Health. 2012;12(2):2039. Epub 2012 May 21. [PubMed] [Google Scholar]
- 5.Torabi M, Green C, Nugent Z, Mahmud S, Demers A, Griffith J, et al. Geographical variation and factors associated with colorectal cancer mortality in a universal health care system. Can J Gastroenterol Hepatol. 2014;28(4):191–7. doi: 10.1155/2014/707420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Torring ML, Frydenberg M, Hansen RP, Olesen F, Vedsted P. Evidence of increasing mortality with longer diagnostic intervals for five common cancers: a cohort study in primary care. Eur J Cancer. 2013;49(9):2187–98. doi: 10.1016/j.ejca.2013.01.025. Epub 2013 Feb 27. [DOI] [PubMed] [Google Scholar]
- 7.Rose PW, Rubin G, Perera-Salazar R, Almberg SS, Barisic A, Dawes M, et al. Explaining variation in cancer survival between 11 jurisdictions in the International Cancer Benchmarking Partnership: a primary care vignette survey. BMJ Open. 2015;5(5):e007212. doi: 10.1136/bmjopen-2014-007212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Richards MA, Westcombe AM, Love SB, Littlejohns P, Ramirez AJ. Influence of delay on survival in patients with breast cancer: a systematic review. Lancet. 1999;353(9159):1119–26. doi: 10.1016/s0140-6736(99)02143-1. [DOI] [PubMed] [Google Scholar]
- 9.Neal RD, Tharmanathan P, France B, Din NU, Cotton S, Fallon-Ferguson J, et al. Is increased time to diagnosis and treatment in symptomatic cancer associated with poorer outcomes? Systematic review. Br J Cancer. 2015;112(Suppl 1):S92–107. doi: 10.1038/bjc.2015.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neal RD. Do diagnostic delays in cancer matter? Br J Cancer. 2009;101(Suppl 2):S9–12. doi: 10.1038/sj.bjc.6605384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weller D, Vedsted P, Rubin G, Walter FM, Emery J, Scott S, et al. The Aarhus statement: improving design and reporting of studies on early cancer diagnosis. Br J Cancer. 2012;106(7):1262–7. doi: 10.1038/bjc.2012.68. Epub 2012 Mar 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Butler J, Foot C, Bomb M, Hiom S, Coleman M, Bryant H, et al. The International Cancer Benchmarking Partnership: an international collaboration to inform cancer policy in Australia, Canada, Denmark, Norway, Sweden and the United Kingdom. Health Policy. 2013;112(1–2):148–55. doi: 10.1016/j.healthpol.2013.03.021. Epub 2013 May 18. [DOI] [PubMed] [Google Scholar]
- 13.Rose PW, Hamilton W, Aldersey K, Barisic A, Dawes M, Foot C, et al. Development of a survey instrument to investigate the primary care factors related to differences in cancer diagnosis between international jurisdictions. BMC Fam Pract. 2014;15:122. doi: 10.1186/1471-2296-15-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Storey JD, Taylor JE, Siegmund D. Strong control, conservative point estimation and simultaneous conservative consistency of false discovery rates: a unified approach. J R Stat Soc Series B Stat Methodol. 2004;66(1):187–205. [Google Scholar]
- 15.Guldbrandt LM, Fenger-Gron M, Rasmussen TR, Rasmussen F, Meldgaard P, Vedsted P. The effect of direct access to CT scan in early lung cancer detection: an unblinded, cluster-randomised trial. BMC Cancer. 2015;15:934. doi: 10.1186/s12885-015-1941-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Canadian Institute for Health Information. Wait times for priority procedures in Canada, 2016. Ottawa, ON: Canadian Institute for Health Information; 2016. Available from: https://secure.cihi.ca/free_products/wait_time_report2016_en.pdf. Accessed 2016 Apr 14. [Google Scholar]


