Abstract
Cytopiloyne (CP), a novel polyacetylene compound extracted from B. pilosa, shows a multi-bioactivity, including immunomodulatory and antidiabetes. Here, we investigated the anti-Listeria effect of cytopiloyne in mice by assessing mortality, clearance of L. monocytogenes, and pathology examination. The data presented herein are supplemental to our research article entitled “Cytopiloyne, a polyacetylenic glucoside from Bidens pilosa, acts as a novel anticandidial agent via regulation of macrophages” [1].
Specifications Table
| Subject area | Biology |
| More specific subject area | Infection, immunity, and ethnopharmcology |
| Type of data | Figure, image |
| How data was acquired | Survival rate monitor, microscope, and serial dilution plate method |
| Data format | Analyzed |
| Experimental factors | Control and Cytopiloyne intraperitoneal injections at various concentration |
| Experimental features | Resistance of C57BL/6J mice to Listeria monocytogenes |
| Data source location | Taichung, Taiwan |
| Data accessibility | Data is within this article |
Value of the data
-
•
Provides a mouse animal model for assessing the antimicrobial effect of herb compounds to intracellular pathogens.
-
•
May be valuable for further studies to identify the molecular mechanism of CP involving in the intracellular survival of pathogens.
-
•
May provide a new therapeutic strategy that uses a combination treatment of antimicrobial drugs and edible immunodulatory herbs to improve the efficacy of antimicrobial drug therapy on immunosuppressed patients.
1. Data
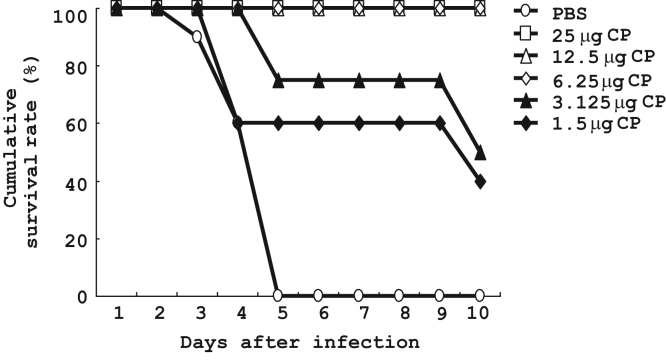
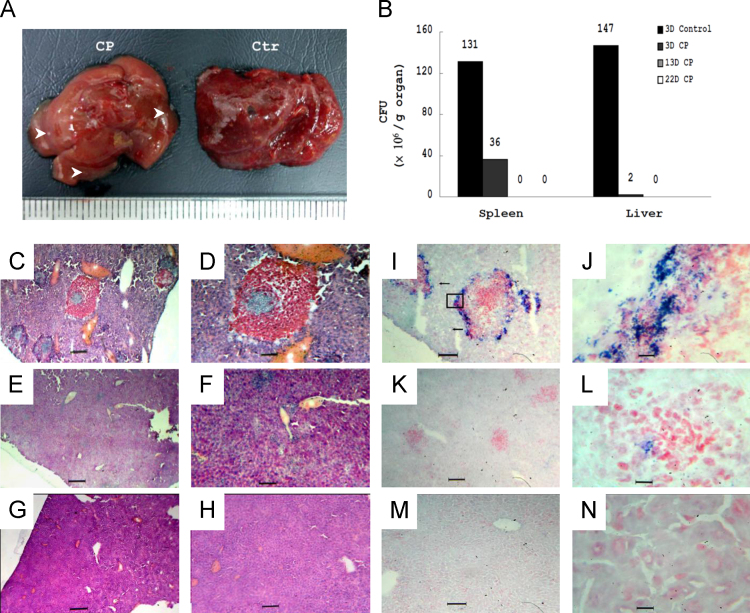
Here, we demonstrated that CP protected mice against Listeria infection by assessing the survival rate (n=10 in each groups; Fig. 1), gross pathology observation (Fig. 2A), the CFU counts in liver and spleen (Fig. 2B), and histopathology observations of frozen liver and spleen sections (Fig. 2C–N).
Fig. 1.
Cytopiloyne (CP) dose-dependently protected mice against L. monocytogenes infection (n=10 in each groups).
Fig. 2.
Cytopiloyne (CP) lowered the CFU counts and severity of lesions in Listeria-infection mice. (A) Gross pathology observation of livers of PBS-(Ctr) and CP-treated mice at 3 days post infection. Diffuse microabscesses were observed in liver of PBS-treated (Ctr) mice, while only focal microabscesses indicated by arrows were noted in CP-treated (CP) mice. (B) CP lowered the CFU counts in liver and spleen (n=3–6 in each groups). (C–H) Histopathology observation (H and E) of livers of PBS-(C and D, at 3 days post infection) and CP-treated (E and F, at 3 days post infection; G and H, at 13 days post infection) mice. C, E, and G: 100×; D, F, and H: 400×. (I–N) Histopathology observation (Gram staining) of livers of PBS-(I and J, at 3 days post infection) and CP-treated (K and L, at 3 days post infection; M and N, at 13 days post infection) mice. I, K, and M: 400×; J, L, and N: 1000×.
2. Experimental design, materials and methods
2.1. Chemicals, cells, and mice
B. pilosa plants were collected from Academia Sinica, Taiwan and authenticated by the Biodiversity Center of Academia Sinica. Cytopiloyne was prepared to 98% purity from whole plant of B. pilosa as previously described [2]. Briefly, Cytopiloyne was isolated on an RP-18 HPLC column by methanol extraction and ethyl acetate partition of whole B. pilosa plants. Structure and purity were confirmed by NMR spectra using a Bruker DMX-500 spectrometer and nuclear magnetic resonance determination, respectively. [2] Listeria monocytogenes (BCRC 15386) was obtained from Bioresource Collection and Research Center (Taiwan). Female 6–8 week-old C57BL/6J mice (National Laboratory Animal Center, Taiwan) were maintained and handled according to the guidelines of Academia Sinica Institutional Animal Care and Utilization Committee.
2.2. Listeria challenge
Six groups of 6 to 8-week-old C57BL/6J mice received intraperitoneal injection of PBS (control) or PBS solution of CP (1.5, 3.125, 6.25, 12.5 and 25 μg/kg BW) three times per week for 2 weeks. After 24 h, mice were intraperitoneally injected with Listeria (2×106 CFU). The animals were then observed every day for determination of mortality [3].
2.3. 2.3. Clearance of Listeria and histopathology examination
As described in Section 2.2, C57BL/6 J mice received PBS (control) or PBS solution of CP (25 μg/kg BW) for 2 weeks and were intraperitoneally injected with Listeria (2×106 CFU). The mice were then killed at 3, 13, and 22 days (if survival) post infection. Their liver and spleen were weighed and cut into 2 parts for CFU determination and histopathological examination. For CFU count, one part of the liver and spleen were homogenized, diluted and plated on BHI agar incubated at 37 °C for 12–16 h. The rest of the organs were frozen for tissue section and then stained by H&E and Gram staining (Hucker–Conn method).
Acknowledgments
This work was supported by the Ministry of Science and Technology of Taiwan (NSC101-2313-B-005-019-and NSC97-2320-B-005-001-MY3).
Footnotes
Supplementary data associated with this article can be found in the online version at http://dx.doi.org/10.1016/j.dib.2016.03.044.
Appendix A. Supplementary material
Supplementary material
References
- 1.C.Y. Chung, W.C. Yang, C.L. Liang, H.Y. Liu, S.K. Lai, C.L.T. Chang, Cytopiloyne, a polyacetylenic glucoside from Bidens pilosa, acts as a novel anticandidial agent via regulation of macrophages, J. Ethnopharmacol. 184 (2016) 72–80. http://ac.els-cdn.com/S0378874116300800/1-s2.0-S0378874116300800-main.pdf?_tid=ce994ce6-f244-11e5-a132-00000aacb360&acdnat=1458881484_034942659a73f7420a5953ade8122e0c [DOI] [PubMed]
- 2.Chang C.L., Chang S.L., Lee Y.M., Chiang Y.M., Chuang D.Y., Kuo H.K. Cytopiloyne, a polyacetylenic glucoside, prevents type 1 diabetes in nonobese diabetic mice. J. Immunol. (Baltim., Md.: 1950) 2007;178(11):6984–6993. doi: 10.4049/jimmunol.178.11.6984. Epub 2007/05/22. PubMed PMID: 17513748. [DOI] [PubMed] [Google Scholar]
- 3.Chang S.L., Chiang Y.M., Chang C.L., Yeh H.H., Shyur L.F., Kuo Y.H. Flavonoids, centaurein and centaureidin, from Bidens pilosa, stimulate IFN-gamma expression. J. Ethnopharmacol. 2007;112(2):232–236. doi: 10.1016/j.jep.2007.03.001. PubMed PMID: 17408892. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material