Abstract
BACKGROUND
Beta-blockers (BBs) are the mainstay prognostic medication for all stages of chronic heart failure (CHF). There are many classes of BBs, each of which has varying levels of evidence to support its efficacy in CHF. However, most CHF patients have one or more comorbid conditions such as diabetes, renal impairment, and/or atrial fibrillation. Patient enrollment to randomized controlled trials (RCTs) often excludes those with certain comorbidities, particularly if the symptoms are severe. Consequently, the extent to which evidence drawn from RCTs is generalizable to CHF patients has not been well described. Clinical guidelines also underrepresent this point by providing generic advice for all patients. The aim of this review is to examine the evidence to support the use of BBs in CHF patients with common comorbid conditions.
METHODS
We searched MEDLINE, PubMed, and the reference lists of reviews for RCTs, post hoc analyses, systematic reviews, and meta-analyses that report on use of BBs in CHF along with patient demographics and comorbidities.
RESULTS
In total, 38 studies from 28 RCTs were identified, which provided data on six BBs against placebo or head to head with another BB agent in ischemic and nonischemic cardiomyopathies. Several studies explored BBs in older patients. Female patients and non-Caucasian race were underrepresented in trials. End points were cardiovascular hospitalization and mortality. Comorbid diabetes, renal impairment, or atrial fibrillation was detailed; however, no reference to disease spectrum or management goals as a focus could be seen in any of the studies. In this sense, enrollment may have limited more severe grades of these comorbidities.
CONCLUSIONS
RCTs provide authoritative information for a spectrum of CHF presentations that support guidelines. RCTs may provide inadequate information for more heterogeneous CHF patient cohorts. Greater Phase IV research may be needed to fill this gap and inform guidelines for a more global patient population.
Keywords: beta-blockers, chronic heart failure, comorbidity, external validity, review
Introduction
Chronic heart failure (CHF) is independently associated with several comorbid conditions, which in turn are also independent contributors to CHF. From the clinical and research perspectives, CHF and associated comorbidities are often treated as independent pathologies despite increasing evidence of significant overlap. Similarly, randomized controlled trials (RCTs) on CHF often exclude all but the mildest forms of any comorbidity and enroll a restricted patient demographic, which may limit the generalizability of findings to the “real world”.1 This is problematic considering that comorbidities are common in CHF, the presence of which may alter the pathophysiology and response to treatments.2 Changes to CHF guidelines reflect a growing awareness of these issues; however, the efficacy of CHF treatment in comorbid settings remains poorly understood. Phase IV trials, which involve postmarketing surveillance of the uncontrolled treatment of a patient population, are needed to advance understanding of external validity.3–5
In this review, we have focused on patient demographics, diabetes mellitus (DM), chronic renal failure (CRF), and atrial fibrillation in CHF. Pathophysiological variations in responses to treatments are seen for the following factors: doses (eg, statin doses and Asian patients), class of agents used (eg, benefits of nitrates and hydralazine for CHF in African American patients),6 or a particular agent within a class (eg, the lack of effect of bucindolol in African American patients).7 Specifically, in the case of beta-blockers (BBs), there are intrinsic differences in pharmacology due to receptor selectivity, pharmacogenomics, and peripheral vasodilatory capacity. All these points have the potential to influence therapy for different groups of patients or those with a particular comorbidity. These points have been previously well discussed.8–10 The presence of common comorbidities, such as DM and CRF, can affect the size of the treatment effect and, particularly in relation to CRF, the size and safety of the dosing regime.11,12 These points may guide selection of one agent in a class over another or indicate the need for different treatment protocols. For these reasons, it is important that clinical demographics and comorbidities be representative, if not in the RCT but in subsequent postmarketing tools, such as post hoc analyses, systematic reviews, and meta-analyses, or with actual surveillance during Phase IV effectiveness and cost-effectiveness research. In this review, we examine the efficacy of BBs in CHF patients with particular focus on hetergeneous demographic cohorts and comorbid conditions.
Methods
Eligibility criteria
RCTs, as well as reviews/meta-analyses of RCTs, which met the following criteria, were included: (i) head-to-head comparison of a BB with placebo, another BB, or another agent, irrespective of baseline therapy; (ii) a minimum of 50 patients in total; (iii) incidence of death as a reported end point; (iv) incidence of cardiac and noncardiac events as reported end points; (v) clinical follow-up of at least three months; and (vi) peer-reviewed journal publications before December 1, 2015, indexed in MEDLINE.
Data sources and search
We searched for RCTs in MEDLINE (1966–2015), PubMed, EMBASE, Scopus, and Cochrane databases, in addition to hand-searching the bibliographies of identified studies for additional references. We restricted our searches to English language, human studies, clinical trials, and controlled clinical trials. We used the keywords and medical subject headings “heart failure” and “chronic” or “congestive” and “beta-blockers” or “adrenergic beta-antagonists”. To the results of this core search, we added the additional keyword “clinical trials” as the first criterion. From the core search history, secondary searches were conducted, limiting studies to post hoc analyses, meta-analyses, and reviews, with the keywords “diabetes mellitus”, “renal failure”, “atrial fibrillation”, or “race” to identify posttrial studies. We also searched using the individual BB names “bisoprolol”, “bucindolol”, “carvedilol”, “metoprolol”, and nebivolol” in the secondary search.
Study collection and data extraction
The first author performed the scan of articles by title and abstract and then analyzed each of the short-listed studies. We reviewed the methodological quality of the studies using standard criteria.13,14 The first author performed the data extraction, which included the following factors: age, sex, race, follow-up duration, New York Heart Association (NYHA) class, left ventricular ejection fraction (LVEF), DM, CRF, hypertension, ischemia, atrial fibrillation, and all-cause mortality.
Results
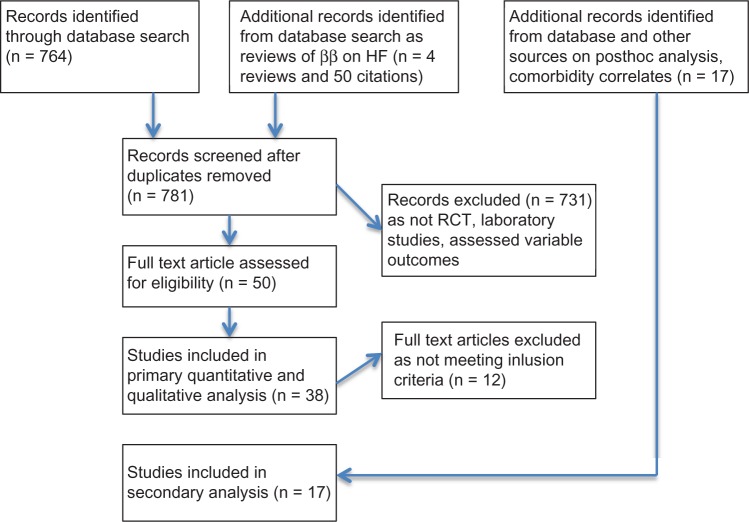
In total, 764 citations, four BB reviews,15–18 and the references in the relevant articles were screened for RCTs meeting the inclusion criteria. From among 50 articles, 38 articles were included19–56 and 12 were excluded as not meeting the highlighted criteria.57–68 Additionally, 20 post hoc analyses, systematic reviews, and meta-analyses were identified in the secondary search and search of references (Table 1).69–87
Table 1.
Key features of RCT’s included in analysis.
| STUDY YEAR REF | ββ COMPARATOR (N) | NYHA EF (%) | MEAN AGE | SEX M (%) | RACE (%) | DM (%) | CRF (%) | HT (%) | ISCH (%) | AF (%) | NOTES |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Strum et al.19 | Atenolol 51 Placebo 49 | II–IV 17 | 52 | 88 | NA | 18 | NA | 30 | 28 | 16 | • Safety and efficacy |
| CIBIS20 | Bisoprolol 320 Placebo 321 | III–IV 25 | 60 | 83 | NA | NA | NA | 5 | 55 | 13 | • Morbidity and mortality |
| CIBIS 1121,22,82 | Bisoprolol 1327 Placebo 1320 | III–IV 27 | 61 | 81 | NA | NA | Group mean | 16 | 50 | 21 | • Morbidity and mortality |
| CIBIS III23 | Bisoprolol (505) Enalapril (505) | II–III 28 | 72 | 68 | NA | 21 | 18 | 66 | 62 | NA | • Safety and efficacy |
| Bristow et al.24 | Bucindolol 105 Placebo 34 | II–III 26 | 54 | 61 | NA | NA | NA | NA | 29 | NA | • Safety and efficacy |
| BEST25 | Bucindolol 1354 Placebo 1354 | III–IV 24 | 60 | 78 | 70 | 36 | NA | 59 | 59 | 11 | • Morbidity and mortality |
| Olsen et al.26 | Carvedilol 36 Placebo 24 | II–III 20 | 52 | 93 | NA | NA | NA | NA | NA | NA | • Hemodynamic |
| ANZHFRCG27,28 | Carvedilol 207 Placebo 208 | I–III 28 | 67 | 80 | NA | 19 | NA | NA | 89 | NA | • Morbidity and mortality and hemodynamic |
| USCHFSG29–31 | Carvediolol 696 Placebo 398 | II–IV 23 | 58 | 77 | 20 | 18–40 | NA | NA | 48 | NA | • Morbidity and Mortality |
| PRECISE32 | Carvedilol 133 Placebo 143 | II–IV 22 | 61 | 73 | NA | 38 | NA | NA | 52 | NA | • Morbidity and mortality |
| MOCHA33 | Carvedilol 261 Placebo 84 | II–IV 23 | 59 | 78 | NA | 34 | NA | NA | 52 | NA | • Morbidity and mortality |
| Sanderson et al.34 | Metoprolol 19 Celiprolol 21 Placebo 10 | II–IV 29 | 62 | 76 | NA | NA | NA | NA | 44 | NA | • Hemodynamic and QOL |
| Sanderson et al.35 | Carvedilol 25 Metoprolol 26 | II–IV 26 | 60 | 78 | NA | NA | NA | 33 | 22 | NA | • Hemodynamic and QOL |
| Metra et al.36 | Carvedilol 75 Metoprolol 75 | II–IV 21 | 57 | 91 | NA | 20 | NA | 26 | 38 | NA | • Hemodynamic and QOL |
| CAPRICORN37 | Carvedilol 975 Placebo 984 | NA 33 | 63 | 74 | NA | 22 | NA | 53 | 100 | NA | • Morbidity and mortality |
| COPERNICUS38 | Carvedilol 1156 Placebo 1133 | II–IV 20 | 63 | 80 | 5 | 26 | Group mean | NA | 68 | NA | • Morbidity and Mortality |
| COMET39 | Carvedilol 1511 Metoprolol 1518 | II–IV 26 | 62 | 80 | 99 | 24 | NA | 37 | 53 | 20 | • Morbidity and mortality |
| CHRISTMAS40 | Carvedilol 142 Metoprolol 163 | I–III 30 | 62 | 90 | 91 | 28 | 22 | NA | 100 | NA | • Response in myocardial hibernation |
| CARMEN41 | Carvedilol 191 Enalapril 190 Both 191 | I–III NA | 62 | 81 | 99 | 14 | Group Mean | 31 | 67 | 18 | • Safety and efficacy |
| Cice et al.42 | Carvedilol 54 Placebo 49 | II–III 26 | 55 | 51 | NA | NA | 100 | NA | 40 | NA | • Morbidity and mortality |
| Anderson et al.43 | Metoprolol 25 Placebo 25 | II–IV 28 | 51 | 66 | NA | NA | NA | NA | NA | NA | • Morbidity and function |
| Waagstein F et al.44 | Metoprolol 194 Placebo 189 | I–IV 22 | 49 | 73 | NA | NA | Group mean | NA | NA | NA | • Morbidity and haemodynamic |
| Fischer et al.45 | Metoprolol 25 Placebo 25 | II–IV 23 | 63 | 96 | NA | 12 | NA | NA | 74 | 16 | • Safety and efficacy |
| Goldstein et al.46 | Metoprolol 42 Placebo 19 | II–IV 27 | NA | 75 | NA | NA | NA | NA | 36 | NA | • Safety and efficacy |
| Kukin et al.47 | Carvedilol 37 Metoprolol 30 | II–IV 19 | 57 | 69 | NA | NA | Group mean | NA | 27 | NA | • Morbidity and efficacy |
| MERIT-HF48–50 | Metoprolol 1990 Placebo 2001 | II–IV 28 | 64 | 78 | 94 | 25 | Group Mean | 44 | 66 | 17 | • Morbidity and mortality |
| RESOLVD51 | Metoprolol 214 Placebo 212 | I–IV 29 | 62 | 82 | 87 | 26 | NA | 36 | 69 | NA | • Morbidity and mortality |
| SENIORS52–55 | Nebivolol Placebo | II–IV 34 | 76 | 73 | NA | 26 | Group Mean | 62 | 68 | 35 | • Morbidity and mortality |
| ENECA56 | Nebivolol 134 Placebo 126 | II–IV 26 | 72 | 99 | 99 | 25 | NA | 57 | 58 | 26 | • Safety and efficacy |
Notes: Age and LVEF are presented as mean value as documented in the studies. Standard errors and placebo group values are not presented. In multiple treatment arms, mean values are averaged. In all cases, this was near-equally matched in the placebo arm. In trials that listed comorbidities such as ischemia either as cause of CHF or as a concomitant condition, we have provided the larger number in the table. ANZHFRCG, Australia/New Zealand Heart Failure Research Collaborative Group; BEST, β-blocker Evaluation of Survival Trial; CAPRICORN, Carvedilol on outcome after myocardial infarction in patients with left-ventricular dysfunction; CARMEN, Carvedilol ACE-Inhibitor Remodelling Mild CHF EvaluatioN; CHRISTMAS, Carvedilol Hibernating Reversible Ischaemia Trial; CIBIS, Cardiac Insufficiency Bisoprolol Trial; COMET, Carvedilol or Metoprolol European Trial; COPERNICUS, Carvedilol Prospective Randomized Cumulative Survival Study; ENECA, Effects of Nebivolol on left ventricular function in Elderly patients with Chronic heart failure; MERIT-HF, Metoprolol Randomized Intervention Trial in Congestive Heart Failure; MOCHA, Multicenter Oral Carvedilol Heart Failure Assessment; PRECISE, Prospective Randomized Evaluation of Carvedilol on Symptoms and Exercise; RESOLVD, the randomized evaluation of strategies for left ventricular dysfunction pilot study; SENIORS, Randomized trial to determine the effect of nebivolol on mortality and cardiovascular hospital admission in elderly patients with heart failure; USCHFSG, US Carvedilol Heart Failure Study Group. Additional data also extracted from references 12–15.
Abbreviations: ACE-I, angiotensin-converting enzyme inhibitor; AF, atrial fibrillation; ββ, beta-blocker; CRF, chronic renal failure; DM, diabetes mellitus; EF, left ventricular ejection fraction; HT, hypertension; Isch, ischemia; M, male; n, number; NA, not available; NYHA, New York Heart Association; QOL, quality of life; Ref, reference.
General description
Six BBs with varying cardiac specificity and extracardiac properties were tested in the studies identified. Two BBs were found to have established vasodilatory properties. The majority of the studies compared BBs to placebo, with one larger study comparing two established BBs head to head. Nearly all studies recruited CHF cases ranging from mild to severe, with representation of the entire spectrum of illness within each study. In many cases, the enrollment to NYHA class tended to be better than the corresponding EFs. Most studies addressed one or more of the following themes: the effects of BB with baseline heart failure (HF) either mild or severe; safety, efficacy, and optimal methods for deriving clinical benefit; utility in ischemic and nonischemic etiologies; and benefits for older CHF cohorts. The mean age of participants generally ranged between 50 years and 70 years, and no strict criteria were placed on excluding older participants. The SENIORS study, however, specifically enrolled patients older than 70 years of age, achieving a cohort mean age of 76 ± 4.7 years.52 Post hoc data were also provided by MERIT-HF.87 Females were generally underrepresented, with only four studies enrolling at least one-third of female. Seven studies described patient ethnicity, five of which predominantly enrolled Caucasians. In the BEST study, 627 (23%) of 2,708 patients were African American, 143 (6%) Hispanic, and 42 (2%) listed as other. In this demographically diverse cohort, the study objectives were not achieved.25 A smaller study with the same agent, which did not disclose racial demography, however, demonstrated safety and efficacy.24
Etiology and comorbidities
Ischemic cardiomyopathy as etiology, coronary artery disease as comorbidity, and revascularization strategies were listed in 26 studies. In more than half the studies, at least 50% of participants had ischemic heart disease. More than two-thirds of trials reported hypertension as the etiology or a comorbidity of CHF. We explore three specific comorbidities in greater detail.
Diabetes
Eighteen trials listed concomitant diabetes. In most cases, the number of diabetics was between 18% and 40%. In the BEST trial, 36% of participants were diabetic. This study also provided greater details on the comorbidity, with 964 (35.5%) of 2,708 participants diabetic, 398 (15%) on insulin, and 313 (12%) with end-organ complications. In a meta-analysis of seven trials, wherein 1,411 (25%) of 5,757 participants had diabetes, Bell et al showed evidence for benefit with carvedilol in all groups, specifically, a reduction in relative risk (RR) of 28% (95% confidence interval [CI]: 3%–46%; P = 0.03] in diabetics.69 Haas et al compared four different BBs from six studies and noted similar prognostic benefits for treated diabetics (RR: 0.84; 95% CI: 0.73–0.96; P = 0.011); however, the magnitude of benefit was less compared to that in nondiabetics, (RR: 0.72; 95% CI: 0.65–0.79; P < 0.001).70 Finally, post hoc analyses in MERIT-HF showed a 76% higher risk of hospitalizations for HF; however, a mortality benefit from metoprolol was only seen when the data were pooled with those from the CIBIS II and COPERNICUS trials, due to the small sample size.71,72
Renal impairment
Renal function and renal impairment were presented in a limited number of studies, mostly presenting data as mean serum creatinine. In the CIBIS III trial, 182 (18%) of 1,010 of participants were reported as having a history of renal disease. Mean serum creatinine was 101 µmol/L, and estimated glomerular filtration rate (eGFR) was not detailed. Post hoc analysis of the SENIORS trial showed that eGFR was strongly associated with outcomes and that nebivolol was equally efficacious across low-, middle-, or high-eGFR (<55.5, 55.5–72.8, or >72.8 mL/min/1.73 m2) tertiles. In the low eGFR group to the high, the primary outcome (all-cause mortality or cardiovascular hospitalization) was reported in 282 (40%) of 704 patients (hazard ratio [HR]: 0.81; 95% CI: 0.64–1.03; P = 0.087), 218/704 (31%) patients (HR: 0.83; 95% CI: 0.63–1.08; P = 0.164), and 202/704 (29%) patients (HR: 0.93; 95% CI: 0.70–1.22; P = 0.597). There were higher rates of drug discontinuation due to bradycardia in the moderate-eGFR group.54 In a post hoc analysis of CIBIS II trial, 849/2,647 (32%) participants who had eGFR <60 mL/min were more likely to die or be hospitalized (RR: 0.66; 95% CI: 0.5–0.88). All participants showed equal benefit with bisoprolol. Treatment withdrawals were higher, as was mortality, with reducing renal function, more so the 63/2,584 patients with eGFR <30 mL/min, with HR = 0.59 (95% CI: 0.30–1.18), compared to 0.68 (95% CI: 0.56–0.83) for patients with eGFR >30 mL/min.73 In a post hoc analysis of MERIT-HF, 493/3,965 (12%) were identified with low eGFR <45 mL/min, 976/3,965 (25%) had moderate eGFR 45–60 mL/min, and 2,496/3,965 (63%) had high eGFR. Metoprolol had differing efficacy levels against placebo across eGFR tertiles, whereby the mortality and HF hospitalization risk were the greatest in the low-eGFR group (HR: 0.44; 95% CI: 0.31–0.63; P = 0.0001) compared to the high-eGFR group (HR: 0.75; 95% CI: 0.62–0.92; P = 0.05).74 Finally, in an eight-trial meta-analysis, Badve et al reported findings that also supported the benefit of BBs in terms of reducing risk of all-cause (RR: 0.72; 95% CI: 0.64–0.80) and cardiovascular mortality (RR: 0.66; 95% CI: 0.49–0.89), but with increased risk of bradycardia (RR: 4.92; 95% CI: 3.20–7.55) and hypotension (RR: 5.08; 95% CI: 3.48–7.41).75
Rate and rhythm
Atrial fibrillation was reported in 10 studies, with rates of approximately 10%–20% of participants. With an older cohort, in the SENIORS and ENECA trials, between 25% and 35% had atrial fibrillation at baseline.59,60 Kotecha et al extracted individual patient data from 10 RCTs, where 3,066/18,254 (17%) had atrial fibrillation at baseline. Crude mortality rates were higher with atrial fibrillation (633/3,064; 21%) compared to 2,237/13,945 (16%) for sinus rhythm after mean follow-up of 1.5 years (SD: 1.1 years). BB treatment did not lead to a significant mortality benefit in atrial fibrillation (RR: 0.97; 95% CI: 0.83–1.14; P = 0.73) as with sinus rhythm (RR: 0.73; 95% CI: 0.67–0.80; P < 0.01). The authors also did not find benefit across subgroups of age, sex, LVEF, NYHA class, heart rate, and baseline medical therapy.76 In an older meta-analysis, benefits of BB in preventing atrial fibrillation were highlighted77; however, in known atrial fibrillation and HF, a four study meta-analysis,78 and a post hoc analysis from CIBIS II22 supported the findings of Kotecha et al For heart rates, McAlister et al performed a meta-analysis that showed that for every 5 beats/min reduction, there was a concomitant 18% reduction (95% CI: 6%–29%) in risk of death.79
Discussion
RCTs are the gold standard for delivering the foundations of therapy. Post hoc analyses, systematic reviews, and meta-analyses are postmarketing tools that help refine or make sense of the collective evidence. All the small and large RCTs using BBs in CHF have answered the question of safety and efficacy very well. Studies have set out to enroll cohorts with a good spectrum of illness severity, as detailed in the NYHA class and mean LVEF. To control for confounders, studies may have controlled the heterogeneity of the other demographic and comorbid variables. Why is this important? Guidelines are shaped around the findings of large RCTs, and appear to suggest that findings from these homogeneous studies apply equally to heterogeneous “real-world” patients. This may, in fact, be the case, although examples are presenting that a broader perspective may be needed.
Female sex and race have not received good representation in any RCT. Post hoc analysis from MERIT-HF and pooling of results with CIBIS II and COPERNICUS show similar survival in women and men.80,81 The BEST trial, with greater participant heterogeneity, showed significant differences in baseline clinical and laboratory characteristics, such as younger age, black race, higher nonischemic etiology, higher heart rate and left bundle branch block, and lower plasma noradrenaline levels, where the prognostic predictive variables also varied in magnitude between females and males.82 In another post hoc analysis using the BEST study population, the authors achieved conformity with the CIBIS-II and MERIT-HF trials, including matching of the racial demographics, and were able to show that bucindolol treatment was associated with significantly lower mortality, raising the possibility of subgroup differences in responses to BBs.15 Possible explanations include difference in disease pathophysiology, different baseline noradrenaline levels, functional polymorphisms of β-adrenergic receptors, or more advanced disease at baseline, ie, higher risk. However, for carvedilol, in the USCHF trials, 217/1,004 African American patients and a further 121 from the COPERNICUS trial showed significant mortality benefits.7,83 The African American Heart Failure Trial (AHeFT) has highlighted the benefits of vasodilatory therapy in this group.88 Whether BBs as a class or intrinsic differences between agents have effects on diverse communities must be the focus of future Phase IV research.89,90
BB therapy is underutilized for both HF and diabetes, as well as the latter’s complication such as nephropathy, despite evidence of sympathetic system overactivity, partly due to historical concerns of tolerability, adverse hemodynamic and metabolic effects, and lack of selectivity of BBs. Heterogeneity within the same class of BBs perhaps poses the greatest challenge in its use. Arguably, vasodilating BBs such as carvedilol and nebivolol offer the opportunity to study whether theoretical benefits translate to real-world improvements in metabolic profiles and renal function as they reduce insulin resistance and do not adversely interfere with blood glucose control.77,91 Haas et al noted that 24.6% of diabetic subjects from six studies using bisoprolol, metoprolol, and carvedilol (ANZHFRCG, BEST, CIBIS-II, COPERNICUS, MERIT-HF, and USCHFSG) appeared to derive benefits from BB therapy, although the magnitude of benefit was less than that achieved in non-DM subjects.78 In the MERIT-HF study, diabetics had a 76% higher risk of hospitalization, and this risk was significantly reduced with metoprolol, although considering the small sample size, a mortality benefit was not shown.22,79 However, with carvedilol, a seven-study meta-analysis, wherein 25% of 5,757 patients had diabetes, showed similar survival benefits between the different subclasses.80 In the COMET study, which compared carvedilol and metoprolol, the diabetics, who comprised 24% of the patients, did not reach statistical significance.79,86 Published RCTs have not conclusively addressed the question of whether a BB class is superior in CHF with comorbid DM, and this remains an area for Phase IV trials.
Autonomous sympathetic overactivity and susceptibility to iatrogenic peripheral vasoconstriction are several considerations in cardiorenal syndrome. In the latter, first-generation BBs, such as propranolol, which modulate at both β1 and β2 receptors, pose the greatest risk to renal function by reducing cardiac output, and unopposed activation of α1-induced reflex increased sympathetic activity and peripheral resistance.92 Even second-generation BBs such as metoprolol with smaller affinity for β2 receptors can increase renal vascular resistance, although not conclusively shown to alter renal function.93 Similar to diabetics, higher grades of renal impairment were a limiting factor for enrollment.12 Data from MERIT-HF and CIBIS-II trials showed equal efficacy in reducing mortality and hospitalization with eGFR <45 mL/min and >60 mL/min for metoprolol and across all strata for bisoprolol, although, numerically, all-cause mortality did not improve with eGFR <60 mL/min.84,85 Third-generation or vasodilatory BBs, however, increase cardiac output and renal blood flow while reducing renal vascular resistance. Post hoc analysis from SENIORS, trialling the vasodilatory BB nebivolol demonstrated that the relative benefits were similar in patients with and without renal impairment, perhaps highlighting a greater benefit in absolute terms.2,54 Pooled data of 4,217 patients from the CAPRICORN and COPERNICUS trials showed significantly improved outcomes for patients with eGFR between 45 mL/min and 60 mL/min, but not with eGFR <45 mL/min.85 However, in a trial with 114 hemodialysis-dependent patients, there was a statistically significant mortality benefit with carvedilol treatment.42 There is uniformity in the consensus that all grades of renal impairment require BB therapy; however, the optimal agent when faced with metabolic and cardiorenal syndromes awaits dedicated research. Even more importantly, in the Acute Decompensated Heart Failure National Registry (ADHERE) database, 63.6% of admitted patients were classified as having at least moderate renal impairment, which in turn predicted other comorbid risks,12 flagging this area as needing greater focus.
The question of rate has a clear consensus, whereby lower rates do translate to better outcomes.79 The dose or the agents are vehicles in delivering this target; however, as all trials have used the maximal tolerated dose as a study criterion, this still remains the target in clinical settings. A rhythm-based strategy in atrial fibrillation and HF is less clear. The main pharmacological therapies that are used in maintaining sinus rhythm are either contraindicated as with flecainide, not proven with sotalol, or have long-term toxicity concerns with amiodarone. Between 10% and 35% of trial participants have comorbid atrial fibrillation, whereby the most recent 10-study meta-analysis could not demonstrate an outcome benefit with BBs.76 Interestingly, in seven studies with 11,952 patients, BBs significantly reduced the incidence of atrial fibrillation from 39 to 28 per 1,000 patient-years, a 27% reduction of the RR (95% CI: 14–38; P < 0.001).77 Using a strategy of catheter ablation restored and maintained sinus rhythm, with concomitant improvement of cardiac function, cardiac dimensions and quality of life.94,95 A randomized study of 1,376 patients, which excluded persistent atrial fibrillation and used cardioversion with amiodarone, sotalol, or dofetilide to maintain sinus rhythm, did not show significantly different results relative to the rate-controlled arm.96 The finding from these, albeit relatively small, trials showing discrepancies in outcomes between the strategies, compared to the larger study, does again raise the question of external validity and application of results. Again, population-level posttrial studies must be used to monitor or even trial such strategies when there are no controls on the patients.
In summation, post hoc analysis has provided some insights into the interaction between BB treatments in HF for race and comorbid conditions. The results suggest that the RR reduction could be greater for those with more advanced disease compared to those on placebo. The results, however, do not provide any pathophysiological insights into the potential mechanism of benefit. They also do not provide insight into the additional benefits of using a particular class of BB with a theoretical benefit against a particular comorbidity, eg, vasodilatory BB in diabetics or renal impairment. What can be done? While there are robust postmarketing publications in the form of post hoc analyses, clinical reviews, or meta-analyses, actual postmarketing surveillance or Phase IV trials on effectiveness and cost-effectiveness appear to be less well conducted. Greater examples of such studies should be conducted. The most feasible option for this would be using the prospective database from treating centers that would collect data from all treated patients. The more difficult part would be finding a method that allows these findings to sit alongside RCTs and thus be factored into guidelines relevant for a more global HF community and patient demography.
Conclusion
A consistent process – from establishing safety and efficacy, to evaluating morbidity and mortality outcomes and posttrial subgroup or pooled analysis – has been established in the comparison of BB or placebo for systolic HF. Gaps exist in the recruitment of more heterogeneous patient cohorts, showing features such as ethinic diversity, comorbid conditions of diabetes, renal impairment, or sicker patients. With the need to remove confounders and maintain strong internal and external validity in trial design, the potential for Phase IV studies, however, to expand on the validity does not appear to be met. The assessment of efficacy and cost-effectiveness is also lacking. Future researchers and drug companies should continue to focus on delivering all levels of randomized controlled studies. Research groups or institutes and health systems should give greater emphasis on building robust Phase IV studies to better understand the safety, efficacy, and cost-effectiveness of proven therapies initially used in a controlled group, at the population level. Clinical guidelines should similarly reflect potential problems with data from a homogeneous cohort for what is now a more global population being treated.
Figure 1.
Search strategy.
Abbreviations: ββ, beta-blocker; RCT, randomized controlled trial.
Abbreviations
- ANZHFRCG
Australia/New Zealand Heart Failure Research Collaborative Group
- BEST
β-blocker Evaluation of Survival Trial
- CAPRICORN
Carvedilol on outcome after myocardial infarction in patients with left-ventricular dysfunction
- CARMEN
Carvedilol ACE-Inhibitor Remodelling Mild CHF EvaluatioN
- CHF
Chronic Heart Failure
- CHRISTMAS
Carvedilol Hibernating Reversible Ischaemia Trial
- CIBIS
Cardiac Insufficiency Bisoprolol Trial
- COMET
Carvedilol or Metoprolol European Trial
- COPERNICUS
Carvedilol Prospective Randomized Cumulative Survival Study
- ENECA
Effects of Nebivolol on left ventricular function in Elderly patients with Chronic heart failure
- MERIT-HF
Metoprolol Randomized Intervention Trial in Congestive Heart Failure
- MOCHA
Multicenter Oral Carvedilol Heart Failure Assessment
- PRECISE
Prospective Randomized Evaluation of Carvedilol on Symptoms and Exercise
- RESOLVD
the randomized evaluation of strategies for left ventricular dysfunction pilot study
- SENIORS
Randomized trial to determine the effect of nebivolol on mortality and cardiovascular hospital admission in elderly patients with heart failure
- USCHFSG
US Carvedilol Heart Failure Study Grou
Footnotes
ACADEMIC EDITOR: Thomas E. Vanhecke, Editor in Chief
PEER REVIEW: Six peer reviewers contributed to the peer review report. Reviewers’ reports totalled 1,459 words, excluding any confidential comments to the academic editor.
FUNDING: Authors disclose no external funding sources.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE). Provenance: the authors were invited to submit this paper.
Disclosure
All co-authors have won independent and governmental research funding. Several members provide counsel to pharmaceuticals. None pose a conflict of interest for this review.
Author Contributions
Conceived and designed the experiments: PI. Analyzed the data: PI. Wrote the first draft of the manuscript: PI. Contributed to the writing of the manuscript: PI, ST, MJ, MT, DH, JDH. Agree with manuscript results and conclusions: PI, ST, MJ, MT, DH, JDH. Jointly developed the structure and arguments for the paper: PI, ST, MJ, MT, DH, JDH. Made critical revisions and approved final version: PI, ST, MJ, MT, DH, JDH. All authors reviewed and approved of the final manuscript.
REFERENCES
- 1.Niederseer D, Thaler CW, Niederseer M, Niebauer J. Mismatch between heart failure in clinical trials and the real world. Int J Cardiol. 2013;168:1859–65. doi: 10.1016/j.ijcard.2012.12.069. [DOI] [PubMed] [Google Scholar]
- 2.Iyngkaran P, Majoni W, Cass A, et al. Northern territory perspectives on heart failure with comorbidities – understanding trial validity and exploring collaborative opportunities to broaden the evidence base. Heart Lung Circ. 2015;24(6):536–43. doi: 10.1016/j.hlc.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 3.Iyngkaran P, Beneby GS. Toward phase 4 trials in heart failure: a social and corporate responsibility of the medical profession. World J Methodol. 2015;5(4):179–84. doi: 10.5662/wjm.v5.i4.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iyngkaran P, Liew D, McDonald P, et al. Phase 4 research in heart failure – where are we and what should be done? Curr Cardiol Rev. 2016 Jun 6; doi: 10.2174/1573403X12666160606121458. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iyngkaran P, Thomas M. Bedside-to-bench translational research for chronic heart failure: creating an agenda for clients who do not meet trial enrollment criteria. Clin Med Insights Cardiol. 2015;9(suppl 1):121–32. doi: 10.4137/CMC.S18737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taylor AL, Ziesche S, Yancy C, et al. African-American Heart Failure Trial Investigators Combination of isosorb. Combination of isosorbide dinitrate and hydralazine in blacks with heart failure. N Engl J Med. 2004;351(20):2049–57. doi: 10.1056/NEJMoa042934. [DOI] [PubMed] [Google Scholar]
- 7.Yancy CW, Laskar S, Eichhorn E. The use of beta-adrenergic receptor antagonists in the treatment of African Americans with heart failure. Congest Heart Fail. 2004;10(1):34–7. doi: 10.1111/j.1527-5299.2004.02024.x. [DOI] [PubMed] [Google Scholar]
- 8.Lymperopoulos A, Rengo G, Koch WJ. Adrenergic nervous system in heart failure: pathophysiology and therapy. Circ Res. 2013;113:739–53. doi: 10.1161/CIRCRESAHA.113.300308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Capote LA, Perez RM, Lymperopoulos A. GPCR signaling and cardiac function. Eur J Pharm. 2015;763:143–8. doi: 10.1016/j.ejphar.2015.05.019. [DOI] [PubMed] [Google Scholar]
- 10.Lymperopoulos A, Garcia D, Walkett K. Pharmacogenetics of cardiac inotropy. Pharmacogenomics. 2014;15(14):1807–21. doi: 10.2217/pgs.14.120. [DOI] [PubMed] [Google Scholar]
- 11.Baliga V, Sapsford R. Diabetes mellitus and heart failure – an overview of epidemiology and management. Diab Vasc Dis Res. 2009;6(3):164–71. doi: 10.1177/1479164109338773. [DOI] [PubMed] [Google Scholar]
- 12.Iyngkaran P, Thomas M, Majoni W, Anavekar N, Ronco C. Comorbid heart failure and renal impairment – epidemiology and management. Cardiorenal Med. 2012;2(4):281–97. doi: 10.1159/000342487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 15.Domanski MJ, Krause-Steinrauf H, Massie BM, et al. BEST Investigators A comparative analysis of the results from 4 trials of beta-blocker therapy for heart failure: BEST, CIBIS-II, MERIT-HF, and COPERNICUS. J Card Fail. 2003;9(5):354–63. doi: 10.1054/s1071-9164(03)00133-7. [DOI] [PubMed] [Google Scholar]
- 16.Flannery G, Gehrig-Mills R, Billah B, Krum H. Analysis of randomized controlled trials on the effect of magnitude of heart rate reduction on clinical outcomes in patients with systolic chronic heart failure receiving beta-blockers. Am J Cardiol. 2008;101:865–9. doi: 10.1016/j.amjcard.2007.11.023. [DOI] [PubMed] [Google Scholar]
- 17.Chatterjee S, Biondi-Zoccai G, Abbate A, et al. Benefits of B-blockers in patients with heart failure and reduced ejection fraction: network meta-analysis. BMJ. 2013;346:f55. doi: 10.1136/bmj.f55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kotecha D, Manzano L, Altman DG, et al. Beta-Blockers in Heart Failure Collaborative Group Individual patient data meta-analysis of beta-blockers in heart failure: rationale and design. Syst Rev. 2013;2:7. doi: 10.1186/2046-4053-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sturm B, Pacher R, Strametz-Juranek J, Berger R, Frey B, Stanek B. Effect of beta 1 blockade with atenolol on progression of heart failure in patients pre-treated with high-dose enalapril. Eur J Heart Fail. 2000;2:407–12. doi: 10.1016/s1388-9842(00)00120-3. [DOI] [PubMed] [Google Scholar]
- 20.CIBIS Investigators Committees A randomised trial of beta-blockade in heart failure. The Cardiac Insufficiency Bisoprolol Study (CIBIS) Circulation. 1994;90:1765–73. doi: 10.1161/01.cir.90.4.1765. [DOI] [PubMed] [Google Scholar]
- 21.CIBIS II Investigators and Committees The cardiac insufficiency bisoprolol study II (CIBIS II): a randomised trial. Lancet. 1999;353:9–13. [PubMed] [Google Scholar]
- 22.Lechat P, Hulot J, Escolano S, et al. on behalf of the CIBIS II Investigators Heart rate and cardiac rhythm relationships with bisoprolol benefit in chronic heart failure in CIBIS II trial. Circulation. 2001;103:1428–33. doi: 10.1161/01.cir.103.10.1428. [DOI] [PubMed] [Google Scholar]
- 23.Willenheimer R, van Veldhuisen D, Silke B, et al. CIBIS III Investigators Effect on survival and hospitalization of initiating treatment for chronic heart failure with bisoprolol followed by enalapril, as compared with the opposite sequence. Results of the randomised cardiac insufficiency bisoprolol study (CIBIS) III. Circulation. 2005;112:2426–35. doi: 10.1161/CIRCULATIONAHA.105.582320. [DOI] [PubMed] [Google Scholar]
- 24.Bristow MR, O’Connell JB, Gilbert EM, et al. Dose-response of chronic beta-blocker treatment in heart failure from either idiopathic dilated or ischemic cardiomyopathy. Bucindolol investigators. Circulation. 1994;89:1632–42. doi: 10.1161/01.cir.89.4.1632. [DOI] [PubMed] [Google Scholar]
- 25.Beta-Blocker Evaluation of Survival Trial Investigators A trial of the beta-blocker bucindolol in patients with advanced chronic heart failure. N Engl J Med. 2001;344:1659–67. doi: 10.1056/NEJM200105313442202. [DOI] [PubMed] [Google Scholar]
- 26.Olsen SL, Gilbert EM, Renlund DG, Taylor DO, Yanowitz FD, Bristow MR. Carvedilol improves left ventricular function and symptoms in chronic heart failure: a double-blind randomized study. J Am Coll Cardiol. 1995;25:1225–31. doi: 10.1016/0735-1097(95)00012-S. [DOI] [PubMed] [Google Scholar]
- 27.Australia/New Zealand Heart Failure Research Collaborative Group Effects of carvedilol, a vasodilator-B-blocker, in patients with congestive heart failure due to ischemic heart disease. Circulation. 1995;92:212–8. [PubMed] [Google Scholar]
- 28.Australia/New Zealand Heart Failure Research Collaborative Group Randomised, placebo-controlled trial of carvedilol in patients with congestive heart failure due to ischaemic heart disease. Lancet. 1997;349:375–80. [PubMed] [Google Scholar]
- 29.Packer M, Bristow MR, Cohn JN, et al. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. U.S. Carvedilol Heart Failure Study Group. N Engl J Med. 1996;334:1349–55. doi: 10.1056/NEJM199605233342101. [DOI] [PubMed] [Google Scholar]
- 30.Colucci WS, Packer M, Bristow MR, et al. Carvedilol inhibits clinical progression in patients with mild symptoms of heart failure. US Carvedilol Heart Failure Study Group. Circulation. 1996;94:2800–6. doi: 10.1161/01.cir.94.11.2800. [DOI] [PubMed] [Google Scholar]
- 31.Cohn JN, Fowler MB, Bristow MR, et al. Safety and efficacy of carvedilol in severe heart failure. The U.S. Carvedilol Heart Failure Study Group. J Card Fail. 1997;3:173–9. doi: 10.1016/s1071-9164(97)90013-0. [DOI] [PubMed] [Google Scholar]
- 32.Packer M, Colucci WS, Sackner-Bernstein JD, et al. for the PRECISE Study Group Double-blind, placebo-controlled study of the effects of carvedilol in patients with moderate to severe heart failure – The PRECISE Trial. Prospective randomized evaluation of carvedilol on symptoms and exercise. Circulation. 1996;94:2793–9. doi: 10.1161/01.cir.94.11.2793. [DOI] [PubMed] [Google Scholar]
- 33.Bristow MR, Gilbert EM, Abraham WT, et al. for the MOCHA Investigators Carvedilol produces dose-related improvements in left ventricular function and survival in subjects with chronic heart failure. Circulation. 1996;94:2807–16. doi: 10.1161/01.cir.94.11.2807. [DOI] [PubMed] [Google Scholar]
- 34.Sanderson JE, Chan SKW, Yu CM, et al. Beta blockers in heart failure: a comparison of a vasodilating beta blocker with metoprolol. Heart. 1998;79:86–92. doi: 10.1136/hrt.79.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sanderson JE, Chan SKW, Yip G, et al. Beta-blockade in heart failure: a comparison of carvedilol with metoprolol. J Am Coll Cardiol. 1999;34:1522–8. doi: 10.1016/s0735-1097(99)00367-8. [DOI] [PubMed] [Google Scholar]
- 36.Metra M, Giubbini R, Nodari S, Boldi E, Modena MG, Dei Cas L. Differential effects of beta-blockers in patients with heart failure: a prospective, randomized, double-blind comparison of the long-term effects of metoprolol versus carvedilol. Circulation. 2000;102:546–51. doi: 10.1161/01.cir.102.5.546. [DOI] [PubMed] [Google Scholar]
- 37.Dargie HJ. Effect of carvedilol on outcome after myocardial infarction in patients with left-ventricular dysfunction: the CAPRICORN randomised trial. Lancet. 2001;357(9266):1385–90. doi: 10.1016/s0140-6736(00)04560-8. [DOI] [PubMed] [Google Scholar]
- 38.Packer M, Coats A, Fowler M, et al. Carvedilol Prospective Randomized Cumulative Survival Study Group Effect of carvedilol on survival in severe chronic heart failure (COPERNICUS) N Engl J Med. 2001;344:1651–8. doi: 10.1056/NEJM200105313442201. [DOI] [PubMed] [Google Scholar]
- 39.Poole-Wilson P, Swedberg K, Cleland J, et al. Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the Carvedilol or Metoprolol European Trial (COMET): randomised controlled trial. Lancet. 2003;362:7–13. doi: 10.1016/S0140-6736(03)13800-7. [DOI] [PubMed] [Google Scholar]
- 40.Cleland JGF, Pennell DJ, Ray SG, et al. on behalf of the Carvedilol Hibernating Reversible Ischaemia Trial: Marker Of Success Investigators Myocardial viability as a determinant of the ejection fraction response to carvedilol in patients with heart failure (CHRISTMAS trial): randomised controlled trial. Lancet. 2003;362:14–21. doi: 10.1016/s0140-6736(03)13801-9. [DOI] [PubMed] [Google Scholar]
- 41.Komajda M, Lutiger B, Madeira H, et al. CARMEN Investigators and Co-Ordinators Tolerability of carvedilol and ACE-Inhibition in mild heart failure. Results of CARMEN (Carvedilol ACE-Inhibitor Remodelling Mild CHF EvaluatioN) Eur J Heart Fail. 2004;6:467–75. doi: 10.1016/j.ejheart.2003.12.019. [DOI] [PubMed] [Google Scholar]
- 42.Cice G, Ferrara L, D’Andrea A, et al. Carvedilol increases two-year survival in dialysis patients with dilated cardiomyopathy: a prospective, placebo-controlled trial. J Am Coll Cardiol. 2003;41:1438–44. doi: 10.1016/s0735-1097(03)00241-9. [DOI] [PubMed] [Google Scholar]
- 43.Anderson JL, Lutz JR, Gilbert EM, et al. A randomized trial of low-dose beta-blockade therapy for idiopathic dilated cardiomyopathy. Am J Cardiol. 1985;55:471–5. doi: 10.1016/0002-9149(85)90396-0. [DOI] [PubMed] [Google Scholar]
- 44.Waagstein F, Bristow MR, Swedberg K, et al. Beneficial effects of metoprolol in idiopathic dilated cardiomyopathy. Metoprolol in dilated cardiomyopathy (MDC) Trial Study Group. Lancet. 1993;342:1441–6. doi: 10.1016/0140-6736(93)92930-r. [DOI] [PubMed] [Google Scholar]
- 45.Fisher ML, Gottlieb SS, Plotnick GD, et al. Beneficial effects of metoprolol in heart failure associated with coronary artery disease: a randomized trial. J Am Coll Cardiol. 1994;23:943–50. doi: 10.1016/0735-1097(94)90641-6. [DOI] [PubMed] [Google Scholar]
- 46.Goldstein S, Kennedy HL, Hall C, et al. Metoprolol CR/XL in patients with heart failure: a pilot study examining the tolerability, safety and effect on left ventricular ejection fraction. Am Heart J. 1999;138:1158–65. doi: 10.1016/s0002-8703(99)70083-9. [DOI] [PubMed] [Google Scholar]
- 47.Kukin ML, Kalman J, Charney RH, et al. Prospective, randomized comparison of effect of long-term treatment with metoprolol or carvedilol on symptoms, exercise, ejection fraction, and oxidative stress in heart failure. Circulation. 1999;99:2645–51. doi: 10.1161/01.cir.99.20.2645. [DOI] [PubMed] [Google Scholar]
- 48.MERIT Investigators Effect of metoprolol CR/XL in chronic heart failure: metoprolol CR/XL randomised intervention trial in congestive heart failure (MERIT-HF) Lancet. 1999;353:2001–7. [PubMed] [Google Scholar]
- 49.Wikstrand J, Hjalmarson A, Waagstein F, et al. MERIT-HF Study Group Dose of metoprolol CR/XL and clinical outcomes in patients with heart failure: analysis of the experience in metoprolol CR/XL randomized intervention trial in chronic heart failure (MERIT-HF) J Am Coll Cardiol. 2002;40:491–8. doi: 10.1016/s0735-1097(02)01970-8. [DOI] [PubMed] [Google Scholar]
- 50.Hjalmarson A, Goldstein S, Fagerberg B, et al. Effects of controlled-release metoprolol on total mortality, hospitalizaions, and well-being in patients with heart failure: the Metoprolol CR/XL Randomized Intervention Trial in congestive heart failure (MERIT-HF). MERIT-HF Study Group. JAMA. 2000;283:1295–302. doi: 10.1001/jama.283.10.1295. [DOI] [PubMed] [Google Scholar]
- 51.The RESOLVD Investigators Effects of metoprolol CR in patients with ischemic and dilated cardiomyopathy: the randomized evaluation of strategies for left ventricular dysfunction pilot study. Circulation. 2000;101:378–84. doi: 10.1161/01.cir.101.4.378. [DOI] [PubMed] [Google Scholar]
- 52.Flather M, Shibata M, Coats A, et al. Randomized trial to determine the effect of nebivolol on mortality and cardiovascular hospital admission in elderly patients with heart failure (SENIORS) Eur Heart J. 2005;26:215–25. doi: 10.1093/eurheartj/ehi115. [DOI] [PubMed] [Google Scholar]
- 53.Dobre D, van Veldhuisen DJ, Mordenti G, et al. SENIORS Investigators Tolerability and dose-related effects of nebivolol in elderly patients with heart failure: data from the study of the effects of nebivolol intervention on outcomes and rehospitalisation in seniors with heart failure (SENIORS) trial. Am Heart J. 2007;154:109–15. doi: 10.1016/j.ahj.2007.03.025. [DOI] [PubMed] [Google Scholar]
- 54.Cohen-Solal A, Kotecha D, van Veldhuisen DJ, et al. SENIORS Investigators Efficacy and safety of nebivolol in elderly heart failure patients with impaired renal function: insights from the SENIORS trial. Eur J Heart Fail. 2009;11(9):872–80. doi: 10.1093/eurjhf/hfp104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.van Veldhuisen DJ, Cohen-Solal A, Bohm M, et al. Beta-blockade with nebivolol in elderly heart failure patients with impaired and preserved left ventricular ejection fraction: data from SENIORS (study of effects of nebivolol intervention on outcomes and rehospitalization in seniors with heart failure) J Am Coll Cardiol. 2009;53:2150–8. doi: 10.1016/j.jacc.2009.02.046. [DOI] [PubMed] [Google Scholar]
- 56.Edes I, Gasior Z, Wita K. Effects of nebivolol on left ventricular function in elderly patients with chronic heart failure: results of the ENECA study. Eur J Heart Fail. 2005;7:631–9. doi: 10.1016/j.ejheart.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 57.Pollock SG, Lystash J, Tedesco C, Craddock G, Smucker ML. Usefulness of bucindolol in congestive heart failure. Am J Cardiol. 1990;66:603–7. doi: 10.1016/0002-9149(90)90488-m. [DOI] [PubMed] [Google Scholar]
- 58.Gilbert EM, Anderson JL, Deitchman D, et al. Long-term B-blocker vasodilator therapy improves cardiac function in idiopathic dilated cardiomyopathy: a double-blind, randomized study of bucindolol versus placebo. Am J Med. 1990;88:223–9. doi: 10.1016/0002-9343(90)90146-5. [DOI] [PubMed] [Google Scholar]
- 59.Woodley S, Gilbert E, Anderson J, et al. Beta- blockade with bucindolol in heart failure caused by ischemic versus idiopathic dilated cardiomyopathy. Circulation. 1991;84:2426–41. doi: 10.1161/01.cir.84.6.2426. [DOI] [PubMed] [Google Scholar]
- 60.Metra M, Nardi M, Giubbini R, Dei Cas L. Effects of short-and long-term carvedilol administration on rest and exercise hemodynamic variables, exercise capacity and clinical conditions in patients with idiopathic dilated cardiomyopathy. J Am Coll Cardiol. 1994;24:1678–87. doi: 10.1016/0735-1097(94)90174-0. [DOI] [PubMed] [Google Scholar]
- 61.Krum H, Sackner-Bernstein JD, Goldsmith RL, et al. Double-blind, placebo-controlled study of the long-term efficacy of carvedilol in patients with severe chronic heart failure. Circulation. 1995;92:1499–506. doi: 10.1161/01.cir.92.6.1499. [DOI] [PubMed] [Google Scholar]
- 62.Guazzi M, Agostoni P, Matturri M, Pontone G, Guazzi MD. Pulmonary function, cardiac function, and exercise capacity in a follow-up of patients with congestive heart failure treated with carvedilol. Am Heart J. 1999;138:460–7. doi: 10.1016/s0002-8703(99)70148-1. [DOI] [PubMed] [Google Scholar]
- 63.Di Lenarda A, Sabbadini G, Salvatore L, et al. Long-term effects of carvedilol in idiopathic dilated cardiomyopathy with persistent left ventricular dysfunction despite chronic metoprolol. J Am Coll Cardiol. 1999;33:1926–34. doi: 10.1016/s0735-1097(99)00134-5. [DOI] [PubMed] [Google Scholar]
- 64.Engelmeier RS, O’Connell JB, Walsh R, Rad N, Scanlon PJ, Gunnar RM. Improvement in symptoms and exercise tolerance by metoprolol in patients with dilated cardiomyopathy: a double-blind, randomised, placebo-controlled trial. Circulation. 1985;72:536–46. doi: 10.1161/01.cir.72.3.536. [DOI] [PubMed] [Google Scholar]
- 65.Eichhorn EJ, Heesch CM, Barnett JH, et al. Effect of metoprolol on myocardial function and energetics in patients with non-ischemic dilated cardiomyopathy: a randomized, double-blind, placebo-controlled study. J Am Coll Cardiol. 1994;24:1310–20. doi: 10.1016/0735-1097(94)90114-7. [DOI] [PubMed] [Google Scholar]
- 66.Groenning BA, Nilsson JC, Sondergaard L, Fritz-Hansen T, Larsson HB, Hildebrandt PR. Anti remodeling effects on the left ventricle during beta-blockade with metoprolol in the treatment of chronic heart failure. J Am Coll Cardiol. 2000;36:2072–80. doi: 10.1016/s0735-1097(00)01006-8. [DOI] [PubMed] [Google Scholar]
- 67.Kukin ML, Mannino MM, Freudenberger RS, Kalman J, Buchholz-Varley C, Ocampo O. Hemodynamic comparison of twice daily metoprolol tartrate with once daily metoprolol succinate in congestive heart failure. J Am Coll Cardiol. 2000;35:45–50. doi: 10.1016/s0735-1097(99)00504-5. [DOI] [PubMed] [Google Scholar]
- 68.Wisenbaugh T, Katz I, Davis J, et al. Long-term (3-month) effects of a new beta-blocker (nebivolol) on cardiac performance in dilated cardiomyopathy. J Am Coll Cardiol. 1993;21:1094–100. doi: 10.1016/0735-1097(93)90230-x. [DOI] [PubMed] [Google Scholar]
- 69.Haas SJ, Vos T, Gilbert RE, Krum H. Are beta-blockers as efficacious in patients with diabetes mellitus as in patients without diabetes mellitus who have chronic heart failure? A meta-analysis of large-scale clinical trials. Am Heart J. 2003;146:848–53. doi: 10.1016/S0002-8703(03)00403-4. [DOI] [PubMed] [Google Scholar]
- 70.Deedwania PC, Giles TD, Klibaner M, et al. Efficacy, safety and tolerability of metoprolol CR/XL in patients with diabetes and chronic heart failure: experiences from MERIT-HF. Am Heart J. 2005;149:159–67. doi: 10.1016/j.ahj.2004.05.056. [DOI] [PubMed] [Google Scholar]
- 71.De Freitas O, Lenz O, Fornoni A, Materson BJ. The use of metoprolol CR/XL in the treatment of patients with diabetes and chronic heart failure. Vasc Health Risk Manag. 2006;2(2):139–44. doi: 10.2147/vhrm.2006.2.2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bell DSH, Lukas MA, Holdbrook FK, Fowler MB. The effect of carvedilol on mortality risk in heart failure patients with diabetes: results of a meta-analysis. Curr Med Res Opin. 2006;22(2):287–96. doi: 10.1185/030079906X80459. [DOI] [PubMed] [Google Scholar]
- 73.Erdmann E, Lechat P, Verkenne P, Wiemann H. Results from post-hoc analyses of the CIBIS II trial: effect of bisoprolol in high-risk patient groups with chronic heart failure. Eur J Heart Fail. 2001;3(4):469–79. doi: 10.1016/s1388-9842(01)00174-x. [DOI] [PubMed] [Google Scholar]
- 74.Ghali JK, Wikstrand J, Van Veldhuisen DJ, et al. MERIT-HS Study Group The influence of renal function on clinical outcome and response to beta-blockade in systolic heart failure: insights from Metoprolol CR/XL randomized intervention trial in chronic HF (MERIT-HF) J Card Fail. 2009;15:310–8. doi: 10.1016/j.cardfail.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 75.Badve SV, Roberts MA, Hawley CM, et al. Effects of beta-adrenergic antagonists in patients with chronic kidney disease: a systematic review and meta-analysis. J Am Coll Cardiol. 2011;58(11):1152–61. doi: 10.1016/j.jacc.2011.04.041. [DOI] [PubMed] [Google Scholar]
- 76.Kotecha D, Holmes J, Krum H, et al. Beta-Blockers in Heart Failure Collaborative Group Efficacy of β blockers in patients with heart failure plus atrial fibrillation: an individual-patient data meta-analysis. Lancet. 2014;384(9961):2235–43. doi: 10.1016/S0140-6736(14)61373-8. [DOI] [PubMed] [Google Scholar]
- 77.Nasr IA, Bouzamondo A, Hulot JS, Dubourg O, Le Heuzey JY, Lechat P. Prevention of atrial fibrillation onset by beta-blocker treatment in heart failure: a meta-analysis. Eur Heart J. 2007;28(4):457–62. doi: 10.1093/eurheartj/ehl484. [DOI] [PubMed] [Google Scholar]
- 78.Rienstra M, Damman K, Mulder BA, Van Gelder IC, McMurray JJ, Van Veldhuisen DJ. Beta-blockers and outcome in heart failure and atrial fibrillation: a meta-analysis. JACC Heart Fail. 2013;1(1):21–8. doi: 10.1016/j.jchf.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 79.McAlister FA, Wiebe N, Ezekowitz JA, Leung AA, Armstrong PW. Meta-analysis: beta-blocker dose, heart rate reduction, and death in patients with heart failure. Ann Intern Med. 2009;150:784–94. doi: 10.7326/0003-4819-150-11-200906020-00006. [DOI] [PubMed] [Google Scholar]
- 80.Leizorovicz A, Lechat P, Cucherat M, Bugnard F. Bisoprolol for the treatment of chronic heart failure: a meta-analysis on individual data of two placebo-controlled studies–CIBIS and CIBIS II Cardiac Insufficiency Bisoprolol Study. Am Heart J. 2002;143:301–7. doi: 10.1067/mhj.2002.120768. [DOI] [PubMed] [Google Scholar]
- 81.Ghali JK, Pina IL, Gottlieb SS, Deedwania PC, Wikstrand JC, Group M-HS. Metoprolol CR/XL in female patients with heart failure: analysis of the experience in metoprolol extended-release randomized intervention trial in heart failure (MERIT-HF) Circulation. 2002;105:1585–91. doi: 10.1161/01.cir.0000012546.20194.33. [DOI] [PubMed] [Google Scholar]
- 82.Ghali JK, Krause-Steinrauf HJ, Adams KF, et al. Gender differences in advanced heart failure: insights from the BEST study. J Am Coll Cardiol. 2003;42:2128–34. doi: 10.1016/j.jacc.2003.05.012. [DOI] [PubMed] [Google Scholar]
- 83.Yancy CW, Fowler MB, Colucci WE, et al. for the Carvedilol Heart Failure Study Group Race and the response to adrenergic blockade with carvedilol in patients with chronic heart failure. N Engl J Med. 2001;344:1358–65. doi: 10.1056/NEJM200105033441803. [DOI] [PubMed] [Google Scholar]
- 84.Castagno D, Jhund PS, McMurray JJ, et al. Improved survival with bisoprolol in patients with heart failure and renal impairment: an analysis of the cardiac insufficiency bisoprolol study II (CIBIS-II) trial. Eur J Heart Fail. 2010;12:607–16. doi: 10.1093/eurjhf/hfq038. [DOI] [PubMed] [Google Scholar]
- 85.Wali RK, Iyengar M, Beck GJ, et al. Efficacy and safety of carvedilol in treatment of heart failure with chronic kidney disease: a meta-analysis of randomized trials. Circ Heart Fail. 2011;4:18–26. doi: 10.1161/CIRCHEARTFAILURE.109.932558. [DOI] [PubMed] [Google Scholar]
- 86.Torp-Pedersen C, Metra M, Charlesworth A, et al. COMET Investigators Effects of metoprolol and carvedilol on pre-existing and new onset diabetes in patients with chronic heart failure: data from the carvedilol or metoprolol European trial (COMET) Heart. 2007;93(8):968–73. doi: 10.1136/hrt.2006.092379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Deedwania PC, Gottlieb S, Ghali JK, Waagstein F, Wikstrand JC, MERIT-HF Study Group Efficacy, safety and tolerability of beta-adrenergic blockade with metoprolol CR/XL in elderly patients with heart failure. [Erratum appears in Eur Heart J. 2004 Nov;25(21):1968] Eur Heart J. 2004;25(15):1300–9. doi: 10.1016/j.ehj.2004.05.022. [DOI] [PubMed] [Google Scholar]
- 88.Yancy CW. Heart failure in African Americans. Am J Cardiol. 2005;96(suppl):3i–12i. doi: 10.1016/j.amjcard.2005.07.028. [DOI] [PubMed] [Google Scholar]
- 89.Fonarow GC. A review of evidence-based β-blockers in special populations with heart failure. Rev Cardiovasc Med. 2008;9(2):84–95. [PubMed] [Google Scholar]
- 90.Abraham WT, Massie BM, Lukas MA, et al. COHERE Participant Physicians Tolerability, safety, and efficacy of beta-blockade in black patients with heart failure in the community setting: insights from a large prospective beta-blocker registry. Congest Heart Fail. 2007;13(1):16–21. doi: 10.1111/j.1527-5299.2007.888111.x. [DOI] [PubMed] [Google Scholar]
- 91.Lukas MA. Beta Blockade in diabetic heart failure. Heart Fail Clin. 2006;2:89–99. doi: 10.1016/j.hfc.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 92.Iyngkaran P, Anavekar N, Majoni W, Thomas MC. The role and management of sympathetic overactivity in cardiovascular and renal complications of diabetes. Diabetes Metab. 2013;39(4):290–8. doi: 10.1016/j.diabet.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 93.Bakris GL, Hart P, Ritz E. Beta-blockers in chronic kidney disease. Kid Int. 2006;70:1905–13. doi: 10.1038/sj.ki.5001835. [DOI] [PubMed] [Google Scholar]
- 94.Hsu LF, Jaïs P, Sanders P, et al. Catheter ablation for atrial fibrillation in congestive heart failure. N Engl J Med. 2004;351(23):2373–83. doi: 10.1056/NEJMoa041018. [DOI] [PubMed] [Google Scholar]
- 95.Khan MN, Jais P, Cummings J, et al. Pulmonary-vein isolation for atrial fibrillation in patients with heart failure. N Engl J Med. 2008;359:1778–85. doi: 10.1056/NEJMoa0708234. [DOI] [PubMed] [Google Scholar]
- 96.Roy D, Talajic M, Nattel S, et al. Rhythm control versus rate control for atrial fibrillation and heart failure. N Engl J Med. 2008;358:2667–77. doi: 10.1056/NEJMoa0708789. [DOI] [PubMed] [Google Scholar]