Abstract
BACKGROUND
Many studies have noted an increase in the number of recognized cases of invasive infections due to Propionibacterium acnes, especially after shoulder replacement surgery. The increase in the number of recognized cases of P. acnes, a nonspore-forming, anaerobic, Gram-positive organism, appears due to both an increase in the number of shoulder operations being performed and more specimens being sent for anaerobic cultures. Nevertheless, the optimal surgical and antibiotic management of P. acnes remains controversial.
METHODS
We tested the susceptibility of 106 P. acnes strains from sterile body sites collected at the Erie County Medical Center between 2012 and 2015, using Etest gradient antibiotic strips.
RESULTS
P. acnes is very susceptible to the penicillins and the first-generation cephalosporins. We noted an association between hemolytic phenotype on Brucella Blood Agar and clindamycin resistance.
CONCLUSIONS
Antimicrobial susceptibility testing of P. acnes should no longer just be confined to the research laboratory but expanded and incorporated into routine microbiological evaluation of P. acnes. This would improve patient care as well as help clarify the relationship between hemolysis and clindamycin resistance.
Keywords: clindamycin, hemolysin, penicillin G, sarcoidosis
Introduction
We and others have noticed an increase in the number of implant-associated infections due to Propionibacterium acnes in our institution, which includes an active Orthopaedic Surgery department, including several orthopedic surgeons performing upper extremity joint replacements.1–3 P. acnes infections have been reported to be more common after shoulder surgery than after surgery at other sites in the body.2 Diagnosis of P. acnes infections can be challenging, because of the difficulty of distinguishing true infections from contamination. The current procedure at our institution is to obtain multiple tissue cultures and to hold the anaerobic cultures for 21 days. When P. acnes infection is identified, however, the optimal treatment is still not been well defined. We previously reported our findings on the antimicrobial susceptibility testing on 28 P. acnes isolates from the shoulder, which have been tested against 10 antibiotics.1 In this study, we sought to extend antimicrobial susceptibility testing to a larger group of isolates.
In the current study, we performed antimicrobial susceptibility testing on 106 P. acnes isolates from sterile body sites and tested these strains against five antibiotics. We noted clindamycin resistance in our collection of strains. In addition, we noted a previously unrecognized association between hemolytic phenotype and clindamycin resistance.
Methods
Bacterial strains
All the P. acnes from sterile body sites identified in the clinical microbiology laboratory at the Erie County Medical Center, Buffalo, NY, between 2012 and 2015 were collected and stored frozen on CryoCare beads (Key Scientific) at −70°C. P. acnes were identified using the Anaerobe ID System (Siemens Healthcare Diagnostics). The samples were deidentified and sent to the laboratory of one of the authors (JKC) for antimicrobial susceptibility testing. We collected 108 P. acnes strains during this period, but 2 strains were nonviable, leaving 106 strains available for testing. The anatomic sites from which the P. acnes strains were isolated are shown in Figure 1. All of the strains included in the present study were new, meaning that they did not include the 28 strains we tested and reported previously.1 Although this study does not need any approval from the Institutional Review Board since these samples were stripped of patient identifiers, Institutional Review Board approval was sought and obtained to investigate if the hemolytic phenotype had any effect on the clinical outcome of infection. That sub-study focused only on the isolates from the shoulder and inpatients treated at our hospital, where outcome could be ascertained from the medical record. This clinical correlative study, which includes information on both the patients and their isolates, will be reported separately.
Figure 1.
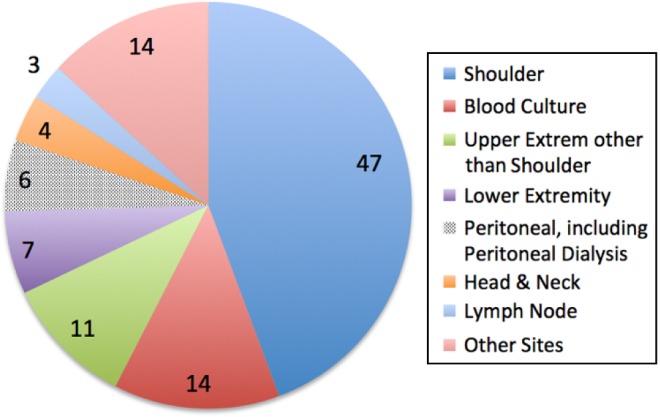
Distribution of the sources of the P. acnes isolates according to anatomical site.
Materials
Bacteria were grown on the Brucella Blood Agar plates (Anaerobe Systems) under anaerobic conditions using the GasPak EZ Pouch System (BD). Antibiotic susceptibility studies were carried out using the Etest method (bioMérieux). Antibiotics were chosen based on expert recommendation,4 clinical usage patterns for P. acnes infections, and our previous work, which included penicillin G, vancomycin, clindamycin, ciprofloxacin, and cephalothin.
Bacterial growth and culture
P. acnes strains were streaked onto Brucella Blood Agar plates from the CryoCare bead and grown for 48 hours. At this point, the hemolytic phenotype of the strain in the absence of antibiotics was recorded. Strains were considered hemolytic if there was a zone of clearing ≥2 mm around a colony on Brucella Blood Agar. A colony sweep of the growth from this plate was diluted in sterile phosphate-buffered saline and adjusted to a 0.5 McFarland standard using OD600 on a SmartSpec 3000 Spectrophotometer (Bio-Rad). As in our previous study,1 an OD600 of 0.132 was considered equivalent to a 0.5 McFarland standard. Bacteria were subsequently streaked onto Brucella Blood Agar plates using a sterile swab and a crosshatch method to cover the entire plate, and Etest strips were added. All plates were incubated at 37°C for 48–72 hours under anaerobic conditions as described earlier. Plates were examined for the zone of inhibition and the minimum inhibitory concentration (MIC) read from the Etest strips. MIC values for penicillin G and cephalothin were frequently lower than the lowest number of the Etest strip, and these were entered as if they were at the lower limit. MIC50 and MIC90 values were calculated as reported, and the percent of resistant strains was determined for those antibiotics for which interpretive breakpoints have been established by the Clinical Laboratory Standards Institute (CLSI) or by the European Committee for Antimicrobial Susceptibility Testing (EUCAST).
Tests of hemolysin on P. acnes susceptibility to clindamycin
We used the potent hemolysin produced by Aeromonas hydrophila, called aerolysin, to induce hemolysis and determine if hemolysis had any effect on antibiotic susceptibility.5 A. hydrophila is a wild-type isolate from a leech collected from Ellicott Creek, Williamsville, NY, in 2002. Aeromonas was grown overnight in an antibiotic-free Dulbecco’s modified Eagle’s medium at 30°C with 300 rpm shaking, sub-cultured 1:100 into Dulbecco’s Modified Eagle’s Medium, and then the growth was continued at 30°C with 300 rpm for five hours. The bacteria were pelleted by centrifugation at 1600 × g for 10 minutes at 4°C, and the supernatant was collected. The supernatant was concentrated using Millipore Amicon centrifugal concentrators with a 10,000 molecular weight cut-off at 4000 × g for 20 minutes. The concentrated material (retentate) contained the hemolytic material and was used as the hemolysin, while the filtrate (<10 kDa) was used as a negative control. P. acnes was then grown on TSA + 5% sheep blood or on CDC anaerobe agar and tested for susceptibility to clindamycin.
Statistical analysis
Microsoft Excel was used to calculate the MIC50 and MIC90 values using the PERCENTILE.INC (argument, 0.5 and argument, 0.9, respectively) functions. A 2 × 2 contingency table analysis was performed, using GraphPad Prism, to determine the significance of the association between hemolytic phenotype and clindamycin resistance.
Results
Figure 1 shows the body sites from which the 106 P. acnes strains were isolated. A total of 47 strains were isolated from the shoulder joint itself, and another 11 strains were isolated from other locations in the arm, meaning that 57% of the P. acnes strains were from the upper extremity. The remaining strains were isolated from diverse locations, including three P. acnes strains from lymph node biopsies; the latter is interesting in light of the theory that P. acnes might be a trigger for the development of sarcoidosis.6
Table 1 shows the MIC50 and MIC90 values for the 106 P. acnes strains against the five antibiotics we tested. The MIC values for penicillin G and cephalothin were very low, as previously noted in the smaller set of 28 strains. There were no P. acnes strains resistant to vancomycin. A total of 2.8% of strains showed resistance to ciprofloxacin and 8.5% of strains in the overall collection were resistant to clindamycin. We and others have noted clindamycin resistance in P. acnes from sterile body sites, and clindamycin is well described in P. acnes from dermatology collections as well. 7–9
Table 1.
MICs of 106 P. acnes strains to five antibiotics.
| ANTIBIOTIC | MIC VALUES, µg/mL | CLSIa RESISTANCE BREAKPOINT | EUCASTb RESISTANCE BREAKPOINT | % OF STRAINS RESISTANT | |
|---|---|---|---|---|---|
| MIC50 | MIC90 | ||||
| Penicillin G | 0.016 | 0.032 | ≥2 | ≥0.5 | 0 |
| Cephalothin | 0.094 | 0.094 | –c | –c | – |
| Vancomycin | 0.38 | 0.5 | –c | ≥2 | 0 |
| Ciprofloxacin | 0.25 | 0.5 | –c | ≥1 | 2.8 |
| Clindamycin | 0.047 | 1.5 | ≥8 | ≥4 | 8.5* |
Notes:
Clinical Laboratory Standards Institute.
European Committee on Antimicrobial Susceptibility Testing.
No interpretive standards yet established.
Based on the CLSI breakpoints.
A histogram of the distribution of clindamycin MIC is shown in Figure 2. As seen in Figure 2 (right, red arrow), nine strains of P. acnes had MICs ≥256 µg/mL. In addition to these nine highly resistant strains, there was also a spread among the MICs of the remaining strains (Fig. 2, inset, magnified view).
Figure 2.
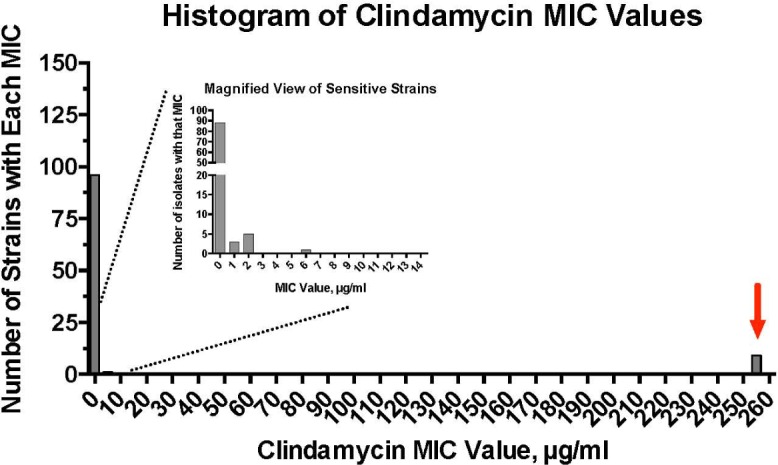
Histogram of the distribution of MICs of 106 P. acnes strains to clindamycin. Inset graph shows a subset of strains with MICs <14 µg/mL.
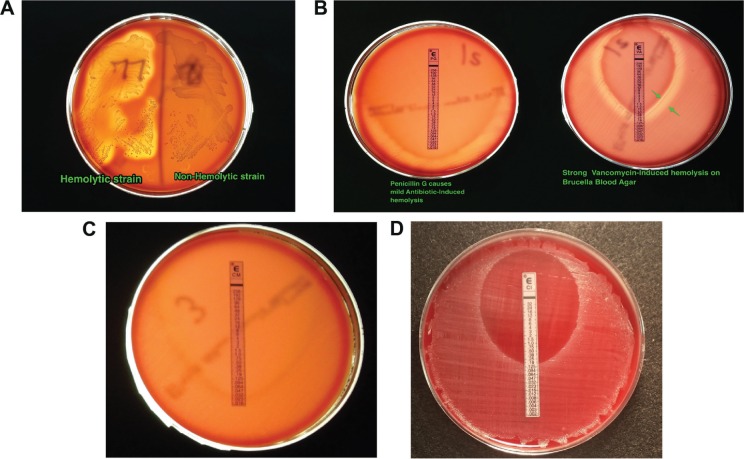
As mentioned in the “Methods” section, we recorded the hemolytic phenotype of the strains along with the MICs of the antibiotics. We previously noted that some strains show hemolysis on Brucella Blood Agar and others do not. This hemolytic phenotype is not observed if CDC Anaerobe Agar is used, even though both include 5% sheep’s blood. Figure 3A shows a P. acnes strain exhibiting the hemolytic phenotype (left) and a nonhemolytic strain (right). The hemolysis seen in strain 77 in Figure 3A is spontaneous, ie, not induced by antibiotic exposure. Some nonhemolytic P. acnes strains show hemolysis just outside the zone of growth inhibition when antibiotics are applied in the form of Etest strips, which we term antibiotic-induced hemolysis. Figure 3B shows one such strain showing mild antibiotic-induced hemolysis around penicillin G (left) and strong hemolysis around vancomycin (Fig. 3B, right, green arrows). Of the 84 nonhemolytic strains, 19 (23%) strains showed vancomycin-induced hemolysis. In contrast, ciprofloxacin and clindamycin never triggered hemolysis in any of our 106 strains (Fig. 3C and D). The significance of hemolysis in P. acnes strains is still being investigated. A previous report from our group indicated that, in the shoulder, hemolytic strains might have a somewhat more aggressive clinical presentation than nonhemolytic strains.10 The significance of antibiotic-induced hemolysis, however, is even less well understood than spontaneous hemolysis. Indeed, to our knowledge, the phenomenon of antibiotic-induced hemolysis has not been previously reported for P. acnes. Vancomycin and the β-lactams are cell wall-acting antibiotics, so we presume that antibiotic-induced cell wall stress may increase the release of hemolysin from inside the P. acnes bacterial cell, just as they aid DNA release.11
Figure 3.
Hemolysis and antibiotic-induced hemolysis among P. acnes strains on Brucella Blood Agar. (A) Comparison of a hemolytic strain and a nonhemolytic P. acnes strain on the same Petri dish. (B) Appearance of strains showing weak antibiotic-induced hemolysis and strong antibiotic-induced hemolysis in P. acnes. (C) Absence of antibiotic-induced hemolysis surrounding a clindamycin Etest strip, which is photographed using transmitted light on a light box. (D) Absence of antibiotic-induced hemolysis surrounding a ciprofloxacin E-test strip, which is photographed using reflected light.
While compiling the susceptibility results in Table 1, one of the authors (TEW) noted that there appeared a correlation between the spontaneous hemolysis phenotype and clindamycin resistance. Tables 2A and 2B show our observations about clindamycin resistance and hemolysis. As shown in Table 2A, the clindamycin MIC50 did not differ between hemolytic and nonhemolytic strains. The MIC90, however, rose dramatically from 0.75 µg/mL in the nonhemolytic strains to 256 µg/mL in the hemolytic strains. Table 2B shows the same data as Table 2A, but rearranged in the form of a 2 × 2 contingency table. The relationship between hemolysis and clindamycin resistance was significant in this chi-square analysis (P < 0.0001). As another way of presenting the data, only 1.2% of the nonhemolytic strains were resistant to clindamycin, versus 36% of the hemolytic strains. Once again, to our knowledge, the association between hemolysis and clindamycin resistance has not been previously reported. Cercenado et al12 reported, in contrast, that strains of enterococci that were b-hemolytic were more sensitive to high-dose ampicillin than nonhemolytic strains.
Table 2A.
Correlation between hemolysis and clindamycin resistance: effect of hemolytic phenotype on clindamycin MICs (microgram per milliliter).
| NON-HEMOLYTIC STRAINS, n = 84 | HEMOLYTIC STRAINS, n = 22 | |||
|---|---|---|---|---|
| MIC50 | MIC90 | MIC50 | MIC90 | |
| Clindamycin | 0.047 | 0.75 | 0.047 | 256 |
Table 2B.
Correlation between hemolysis and clindamycin resistance: contingency table of clindamycin susceptibility vs hemolytic phenotype.
| CLINDA SUSCEPTIBLE | CLINDA RESISTANT | ROW TOTALS | |
|---|---|---|---|
| Non-Hemolytic strains | 83 | 1 | 84 |
| Hemolytic strains | 14 | 8 | 22 |
| Column totals | 97 | 9 | |
| 106 |
Notes: The relationship between hemolysis and clindamycin resistance was significant at P < 0.0001 by Fisher’s exact test and also by the chi-square test. The sites of isolation of the nine clindamycin-resistant strains were as follows: four from shoulder, one from blood, one from axilla, one from palate, one from scrotal abscess, and one from other.
Abbreviation: Clinda, clindamycin.
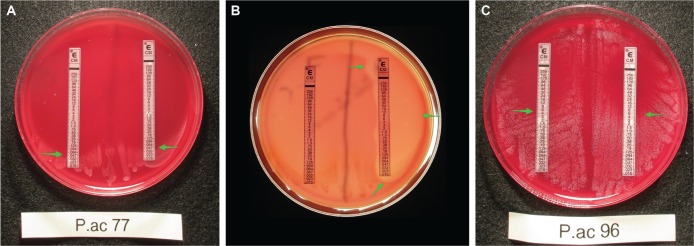
Although it seemed unlikely, we tested the idea that hemolysis itself might be affecting the susceptibility of the P. acnes strains to clindamycin. To do this, we used the potent hemolysin aerolysin from A. hydrophila.5 Unlike P. acnes strains, aerolysin is active on all blood agars, not just Brucella Blood Agar. Figure 4A shows that there was no difference in the clindamycin MIC in the presence of the inactive Aeromonas filtrate (<10 kDa fraction; left), versus the MIC in the presence of the active aerolysin (right, MIC = 0.047 µg/mL for both). Figure 4B shows the same blood agar plate as in Figure 4A, which when photographed via transmitted light showed the area of hemolysis where the aerolysin had been applied (green arrows). Figure 4C shows that a more resistant P. acnes strain also showed no change in the clindamycin MIC of 6 µg/mL when hemolysis was induced (Fig. 4C, right, received the active aerolysin). Similar results were obtained using a third P. acnes strain and with vancomycin as well as clindamycin (data not shown). Therefore, we concluded that adding an exogenous hemolysin to P. acnes did not change the susceptibility of the bacteria to clindamycin and therefore cannot be considered an explanation for the findings of Tables 2A and 2B.
Figure 4.
Lack of effect of induced hemolysis on clindamycin susceptibility in P. acnes. Aerolysin, a potent hemolysin from Aeromonas hydrophila bacteria, was used to induce hemolysis on agars not permissive for P. acnes hemolysis. (A) P. acnes strain 77 on TSA + 5% sheep’s blood, testing the clindamycin MIC in the absence (left) or presence (right) of aerolysin. (B) The same plate photographed via transmitted light on a light box shows the hemolysis induced by the aerolysin. Bacterial growth is not well seen in transmitted light conditions in (B). (C) Lack of effect of aerolysin on the clindamycin MIC of a moderately resistant P. acnes strain. Arrows indicate the MIC of 6 µg/mL.
Discussion
This study began as a straightforward antimicrobial susceptibility testing project. Indeed, our new collection of 106 strains from sterile body sites and invasive infections is one of the largest such collections reported on so far and confirms our previous work highlighting the high activity of penicillin G and cephalothin against P. acnes. Amoxicillin, ceftriaxone, and ertapenem also showed good activity against P. acnes in our previous report.1 In our experience, however, based on referrals we have received from other hospitals and clinics, treatment of P. acnes infections in North America tends to be dominated by the use of IV vancomycin and clindamycin, and clindamycin continues to be mentioned as a good choice for P. acnes prosthetic joint infections.13 The problem is, of course, that for P. acnes, like for other anaerobes, antimicrobial susceptibility testing is considered as a research laboratory procedure and is not offered in clinical laboratories.
While doing the antibiotic susceptibility testing, however, we made several other observations that we found intriguing. Our first observation is that a minority of P. acnes strains are strongly hemolytic (Fig. 3A). Second, some strains that are not hemolytic can be induced to show hemolysis in the presence of antibiotics, especially vancomycin. Third, hemolytic strains show an increased frequency of clindamycin resistance (Tables 2A and 2B).
The presence of a hemolysin gene in P. acnes was noted by Brüggemann et al14 in their report of the first genome sequence of P. acnes, in 2004, and confirmed by many other laboratories since then. The number of P. acnes strains with sequenced genomes is up to 115 at the time of this submission, according to GenBank http://www.ncbi.nlm.nih.gov/genome/genomes/1140. Although no P. acnes gene is annotated specifically as a clindamycin-resistant locus, P. acnes contains multiple genes annotated as multidrug ABC ATP-binding transporters: http://www.ncbi.nlm.nih.gov/genome/proteins/1140?genome_assembly_id=170630. In addition, some of the many variations of erm genes, which encode resistance to erythromycin in P. acnes, also provide cross-resistance to clindamycin.15 Although there are many possible molecular explanations for clindamycin resistance in P. acnes, the exact genes responsible for clindamycin resistance have not been identified, and therefore, the reason for the apparent linkage between hemolysis and clindamycin resistance that we observed remains unknown at this time.
In terms of clinical applications, our work continues to show that antimicrobial resistance testing of P. acnes should be done more commonly, or even routinely, especially in large teaching and referral hospitals. This is often viewed as cost prohibitive, because such testing is labor intensive and requires skilled personnel, while the trend in laboratory medicine these days is to emphasize automation, high throughput, and cost containment, often summarized by the phrase lean microbiology. This means that for now, most clinicians must lean on anaerobic susceptibility reports from the research laboratory and unfortunately cannot expect to receive such data on their own individual patients. In addition, clinical laboratories should be encouraged to report if a P. acnes strain is hemolytic, because currently the hemolytic phenotype is not routinely included on any microbiology reports. We believe that the use of clindamycin in treating P. acnes infections may be overemphasized in the literature. Based on our experience in this study, clindamycin might need to be avoided in P. acnes strains that are hemolytic, given the high (36%) resistance in that subpopulation of P. acnes. Our data also call into question the efficacy of using clindamycin as prophylaxis for shoulder surgery as well. b-Lactam antibiotics, such as first-generation cephalosporins, penicillin G, amoxicillin, ceftriaxone, and ertapenem, have strong activity against P. acnes in vitro.16–18 Oral therapy for P. acnes may even be possible but needs more testing in biofilm models of infection and also in vivo. The use of combination therapy with rifampin also needs to be better defined.18,19
Acknowledgments
We thank Jacqueline E. Broome for excellent technical support and the Clinical Microbiology Laboratories at the Erie County Medical Center and Kaleida Health for collecting P. acnes strains. We thank Sarah R. Burke for collecting the leeches from which the A. hydrophila strain was isolated.
Footnotes
ACADEMIC EDITOR: Douglas MacPherson, Editor in Chief
PEER REVIEW: Three peer reviewers contributed to the peer review report. Reviewers’ reports totaled 1931 words, excluding any confidential comments to the academic editor.
FUNDING: This research was funded by the Department of Orthopaedics, University at Buffalo. The authors confirm that the funder had no influence over the study design, content of the article, or selection of this journal.
COMPETING INTERESTS: TRD discloses a research grant and consulting fees from Zimmer-Biomet, outside the work presented here. Other authors disclose no potential conflicts of interest.
Paper subject to independent expert single-blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Performed the vast majority of the antibiotic susceptibility testing and analyzed the MIC data: TEW. Did some of the hemolysis assays and helped edit the article: KKB. Taught anaerobic bacteriology techniques, created the graphs and figures, and wrote the first draft and revised drafts of the article: JKC. Conceived of the experiments, helped select the antibiotics to be tested, and made revisions: TRD. All authors reviewed and approved the final article.
REFERENCES
- 1.Crane JK, Hohman DW, Nodzo SR, Duquin TR. Antimicrobial susceptibility of Propionibacterium acnes isolates from shoulder surgery. Antimicrob Agents Chemother. 2013;57:3424–3426. doi: 10.1128/AAC.00463-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levy PY, Fenollar F, Stein A, et al. Propionibacterium acnes postoperative shoulder arthritis: an emerging clinical entity. Clin Infect Dis. 2008;46:1884–1886. doi: 10.1086/588477. [DOI] [PubMed] [Google Scholar]
- 3.Wang B, Toye B, Desjardins M, Lapner P, Lee C. A 7-year retrospective review from 2005 to 2011 of Propionibacterium acnes shoulder infections in Ottawa, Ontario, Canada. Diag Microbiol Infect Dis. 2013;75:195–199. doi: 10.1016/j.diagmicrobio.2012.10.018. [DOI] [PubMed] [Google Scholar]
- 4.Aubin GG, Portillo ME, Trampuz A, Corvec S. Propionibacterium acnes, an emerging pathogen: from acne to implant-infections, from phylotype to resistance. Médecine et Maladies Infectieuses. 2014;44:241–250. doi: 10.1016/j.medmal.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 5.Fujii Y, Nomuar T, Yokoyama R, Shinoda S, Okamoto K. Studies on the mechanism of action of the aerolysin-like hemolysin of Aeromonas sobria in stimulating T84 cells to produce cyclic AMP. Infect Immun. 2003;71:1557–1560. doi: 10.1128/IAI.71.3.1557-1560.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ichikawa H, Kataoka M, Hiramatsu J, et al. Quantitative analysis of propionibacterial DNA in bronchoalveolar lavage cells from patients with sarcoidosis. Sarcoidosis Vasculitis Diffuse Lung Dis. 2008;25:15–20. [PubMed] [Google Scholar]
- 7.Gonzalez R, Welsh O, Ocampo J, et al. In vitro antimicrobial susceptibility of Propionibacterium acnes isolated from acne patients in northern Mexico. Int J Dermatol. 2010;49:1003–1007. doi: 10.1111/j.1365-4632.2010.04506.x. [DOI] [PubMed] [Google Scholar]
- 8.Oprica C, Nord CE, ESCMID Study Group on Antimicrobial Resistance in Anaerobic Bacteria European surveillance study on the antibiotic susceptibility of Propionibacterium acnes. Clin Microbiol Infect. 2005;11:204–213. doi: 10.1111/j.1469-0691.2004.01055.x. [DOI] [PubMed] [Google Scholar]
- 9.Song M, Seo S-H, Ko H-C, et al. Antibiotic susceptibility of Propionibacterium acnes isolated from acne vulgaris in Korea. J Dermatol. 2011;38:667–673. doi: 10.1111/j.1346-8138.2010.01109.x. [DOI] [PubMed] [Google Scholar]
- 10.Nodzo S, Hohman D, Crane J, Duquin T. Hemolysis as a clinical marker for Propionibacterium acnes orthopedic infection. Am J Orthop. 2014;43:E93–E97. [PubMed] [Google Scholar]
- 11.Perry AL, Worthington T, Hilton AC, Lambert PA, Stirling AJ, Elliott TSJ. Analysis of clinical isolates of Propionibacterium acnes by optimised RAPD. FEMS Microbiol Lett. 2003;228:51–55. doi: 10.1016/S0378-1097(03)00720-1. [DOI] [PubMed] [Google Scholar]
- 12.Cercenado E, Eliopoulos GM, Wennersten CB, Moellering RC. Influence of high-level gentamicin resistance and beta-hemolysis on susceptibility of enterococci to the bactericidal activities of ampicillin and vancomycin. Antimicrob Agents Chemother. 1992;36:2526–2528. doi: 10.1128/aac.36.11.2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shah NB, Tande AJ, Patel R, Berbari EF. Anaerobic prosthetic joint infection. Anaerobe. 2015;36:1–8. doi: 10.1016/j.anaerobe.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 14.Brüggemann H, Henne A, Hoster F, et al. The complete genome sequence of Propionibacterium acnes, a commensal of human skin. Science. 2004;305:671–673. doi: 10.1126/science.1100330. [DOI] [PubMed] [Google Scholar]
- 15.El-Mahdy TS, Abdalla S, El-Domany R, Mohamed MS, Ross JI, Snelling AM. Detection of a new erm(X)-mediated antibiotic resistance in Egyptian cutaneous propionibacteria. Anaerobe. 2010;16:376–379. doi: 10.1016/j.anaerobe.2010.06.003. http://dx.doi.org/10.1016/j.anaerobe.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 16.Bayston R, Ashraf W, Jacobs A, Goosen J. Treatment of Prosthetic Joint Infections Due to Propionibacterium. Vol. 87. Oxon, England: Taylor & Francis Ltd; 2016. pp. 318–319. [Google Scholar]
- 17.Corvec S, Aubin GG, Bayston R, Ashraf W. Which is the best treatment for prosthetic joint infections due to Propionibacterium acnes: need for further biofilm in vitro and experimental foreign-body in vivo studies? Acta Orthop. 2016;87:318–319. doi: 10.3109/17453674.2016.1162037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jacobs AME, Van Hooff ML, Meis JF, Vos F, Goosen JHM. Treatment of prosthetic joint infections due to Propionibacterium. Acta Orthop. 2016;87:60–66. doi: 10.3109/17453674.2015.1094613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Furustrand Tafin U, Corvec S, Betrisey B, Zimmerli W, Trampuz A. Role of Rifampin against Propionibacterium acnes biofilm in vitro and in an experimental foreign-body infection model. Antimicrob Agents Chemother. 2012;56:1885–1891. doi: 10.1128/AAC.05552-11. [DOI] [PMC free article] [PubMed] [Google Scholar]