Abstract
Fragile X‐associated disorders are a family of genetic conditions resulting from the partial or complete loss of fragile X mental retardation protein (FMRP). Among these disorders is fragile X syndrome, the most common cause of inherited intellectual disability and autism. FMRP is an RNA‐binding protein involved in the control of local translation, which has pleiotropic effects, in particular on synaptic function. Analysis of the brain FMRP transcriptome has revealed hundreds of potential mRNA targets encoding postsynaptic and presynaptic proteins, including a number of ion channels. FMRP has been confirmed to bind voltage‐gated potassium channels (Kv3.1 and Kv4.2) mRNAs and regulates their expression in somatodendritic compartments of neurons. Recent studies have uncovered a number of additional roles for FMRP besides RNA regulation. FMRP was shown to directly interact with, and modulate, a number of ion channel complexes. The sodium‐activated potassium (Slack) channel was the first ion channel shown to directly interact with FMRP; this interaction alters the single‐channel properties of the Slack channel. FMRP was also shown to interact with the auxiliary β4 subunit of the calcium‐activated potassium (BK) channel; this interaction increases calcium‐dependent activation of the BK channel. More recently, FMRP was shown to directly interact with the voltage‐gated calcium channel, Cav2.2, and reduce its trafficking to the plasma membrane. Studies performed on animal models of fragile X syndrome have revealed links between modifications of ion channel activity and changes in neuronal excitability, suggesting that these modifications could contribute to the phenotypes observed in patients with fragile X‐associated disorders.
Abbreviations
- BK
large conductance Ca2+‐activated potassium channel
- CaV2.2
voltage‐gated calcium channel
- FMR1
fragile X mental retardation 1 gene
- FMRP
fragile X mental retardation protein
- FXS
fragile X syndrome
- FXTAS
fragile X‐associated tremor/ataxia syndrome
- Kv
voltage‐gated potassium channel
- PP2A
protein phosphatase 2A
- S6K
ribosomal protein S6 kinase
- Slack
sodium‐activated potassium channel
The fragile X mental retardation protein (FMRP) is an RNA‐binding protein encoded by the fragile X mental retardation 1 (FMR1) gene located on the chromosome X (Bhakar et al. 2012). A variety of disorders are associated with mutation in the FMR1 gene including fragile X syndrome (FXS) and fragile X‐associated tremor/ataxia syndrome (FXTAS) (Lozano et al. 2014).
FXS is the most common heritable form of intellectual disability and is the leading known monogenic cause for autism spectrum disorders (Bhakar et al. 2012). The FMR1 gene contains an unstable CGG‐repeat in the 5’ untranslated region which is normally 5–44 repeats long. FXS is caused by a CGG expansion of more than 200 repeats (called full mutation) which induces methylation of the gene and leads to the partial or complete absence of FMRP. Rarely, FXS can also be caused by point mutations or deletions (Bassell & Warren, 2008; Myrick et al. 2015). FXS has a prevalence of 1 in 2500–4000 males and 1 in 7000–8000 females. The prevalence of carrier status has been estimated to be up to 1 in 130–250 of females. People with FXS show mild to moderate cognitive dysfunction, attention deficits and hyperactivity, anxiety, autistic behaviours, sensory integration problems (such as hypersensitivity to loud noises, bright lights and heightened tactile sensitivity) and they are often also affected by seizures.
FXTAS is caused by an expansion of 55–200 CGG‐repeats (called premutation) inducing an elevation in FMR1 mRNA transcript levels (Lozano et al. 2014). The leading molecular mechanism proposed for these disorders involves elevated levels of mRNA containing the expanded CGG‐repeats. This is thought to sequester RNA‐binding proteins and as a consequence affect their normal functions (Hagerman & Hagerman, 2013). However, a recent study investigating FMR1 splice variants in brain samples of premutation carriers has shown that mRNA isoforms lacking the C‐terminal of FMRP are the most increased (Pretto et al. 2015). The fact that FMRP C‐terminus contains important functional domains (Bagni & Greenough, 2005; Bassell & Warren, 2008; Ferron et al. 2014) led the authors of the study to suggest that the overexpression of these truncated FMRP isoforms could inhibit FMRP function and contribute to the pathology of premutation disorders. People with the premutation expansions can present with a wide range of clinical phenotypes, from mild cognitive problems during childhood (attention deficit hyperactivity disorder, autism spectrum disorder) to psychiatric disorders in adulthood (anxiety and depression), motor symptoms (tremor, ataxia, muscle weakness and Parkinsonism), neuropathy and chronic pain. FXTAS has a prevalence of 1 in 260–814 males and 1 in 100–260 females indicating that 1 in 3000 men and 1 in 5200 women in the general population will develop symptoms of FXTAS.
FMRP is expressed in the nucleus and the cytoplasm, and is part of cytoplasmic RNA granules, where it plays a role in both the trafficking of specific mRNAs to sites of translation, and the stalling of their translation (Bassell & Warren, 2008; Darnell et al. 2011). FMRP has been shown to bind a large number of mRNAs, also called the FMRP transcriptome, and many of them code for proteins involved in neuronal excitability and synaptic transmission (Darnell et al. 2011). In fmr1 knockout mice, the loss of FMRP results in an excessive and unregulated dendritic mRNA translation (Antar et al. 2004; Bassell & Warren, 2008), and an alteration of synapse number and shape (Antar et al. 2006). Consequently, research has concentrated particularly on the dendritic/postsynaptic role of FMRP (Ronesi & Huber, 2008; Krueger & Bear, 2011). However, there is now growing evidence for a presynaptic role of FMRP. Loss of presynaptic FMRP reduces the formation of functional synapses (Hanson & Madison, 2007) and modifies presynaptic protein levels (Liao et al. 2008; Klemmer et al. 2011). Moreover, electron microscopy studies of the ultrastructure of the synapses of CA3 pyramidal neurons onto CA1 pyramidal neurons in the hippocampus of fmr1 knockout mice have revealed an increase in the number of docked vesicles at the active zones compared with control animals (Deng et al. 2011; Klemmer et al. 2011). In central neurons, granules containing FMRP are present in presynaptic terminals and axons and they are mostly prominent during synapse maturation (Christie et al. 2009; Akins et al. 2012). Studies also show a role for FMRP in local protein synthesis in peripheral sensory axons (Price et al. 2006). While fmr1 knockout mice present normal acute nociceptive responses, they show modifications of the chronic responses, both in the peripheral and central nervous system (Price et al. 2007). Heightened tactile sensitivity and self‐injurious behaviour is described in some FXS patients, and this could be linked to dysregulation of nocifensive behaviour (Price et al. 2007).
The analyses of the brain FMRP transcriptome have revealed that, among the mRNA coding for proteins involved in excitability and synaptic transmission, a number of target mRNAs code for ion channels (Brown et al. 2001; Darnell et al. 2011; Brager & Johnston, 2014). Voltage‐gated potassium channels Kv3.1b and Kv4.2 mRNA have been confirmed as targets of FMRP (Darnell et al. 2001, 2011; Gross et al. 2011; Lee et al. 2011). Kv3.1 channels play a critical role in auditory brainstem sound localisation circuits in rodents (Brown & Kaczmarek, 2011). In fmr1 knockout mice, the normal gradient of Kv3.1 in the medial nucleus of the trapezoid body is flattened and the activity‐dependent increase of Kv3.1 expression is abolished damaging encoding and processing of auditory information (Strumbos et al. 2010). In hippocampal neurons, the A‐type potassium channel Kv4.2 is the major potassium channel regulating neuronal excitability, and it has been confirmed that FMRP binds Kv4.2 mRNAs (Gross et al. 2011; Lee et al. 2011). However, the impact of FMRP on Kv4.2 expression is still a matter of debate. Indeed, two studies have investigated the level of Kv4.2 expression in fmr1 knockout mice and their results point towards opposite conclusions: Gross et al. concluded that FMRP acts as a positive regulator of Kv4.2 whereas Lee et al. found that FMRP acts as a repressor of Kv4.2 expression (Gross et al. 2011; Lee et al. 2011). The reason for this discrepancy has not been elucidated but the use of two different mouse strains has been suggested as a possible explanation (Brager & Johnston, 2014).
Besides its role as an RNA binding protein and translation modulator, FMRP has recently been shown to directly interact with ion channels. The first ion channel to be identified that interacts with FMRP was the sodium‐activated potassium channel Slack (Brown et al. 2010). In this study, Brown and co‐workers used biochemical techniques and single channel recordings to demonstrate that FMRP directly interacts with the cytoplasmic carboxy‐terminal tail of the Slack channel and increases the channel mean open time (Brown et al. 2010). FMRP has also been shown to interact with endogenous Slack channels and modulate their activity in bag cell neurons of Aplysia (Zhang et al. 2012). Slack channels contribute to the firing patterns of a variety of neurons (Yang et al. 2007; Zhang et al. 2012) and it has been suggested that some of the neuronal defects observed in FXS patients could be linked to the alteration of Slack channel activity (Kim & Kaczmarek, 2014).
A second type of potassium channel has been shown to be modulated by FMRP: the large conductance Ca2+‐activated potassium BK channel (Deng et al. 2013). The modulation of BK channel function by FMRP does not occur directly with the pore‐forming subunits of the BK channel but involves an interaction with the auxiliary β4 subunit. β4 subunits have been described as a negative modulator of BK channels (Brenner et al. 2000; Torres et al. 2007). The proposed mechanism of action is that the binding of FMRP to the auxiliary β4 subunit alters the interaction of β4 subunits with the pore‐forming subunits and consequently reduces its sensitivity to Ca2+ (Deng et al. 2013). BK channels are important regulators of action potential duration by driving both the phases of repolarisation and after‐hyperpolarisation (Bean, 2007). In hippocampal and cortical pyramidal neurons of knockout fmr1 knockout mice, Deng et al. have shown a reduction of BK channel activity that leads to the elongation of the action potential duration and an increase in presynaptic calcium influx (Deng et al. 2013). As a direct consequence, glutamate release and short‐term synaptic plasticity is affected between CA3 and CA1 pyramidal neurons of the hippocampus of fmr1 knockout mice. Interestingly, a recent study has shown that the genetic upregulation of BK channel activity normalises a number of neuronal defects in a mouse model of fragile X syndrome (Deng & Klyachko, 2016). In this latter study, the authors have crossed fmr1 knockout mice with sloβ4 knockout mice (sloβ4 corresponds to kcnmb4 gene that codes for the BK channel auxiliary β4 subunit) to genetically upregulate BK channels in the absence of FMRP and they show that BK single‐channel properties, action potential duration, glutamate release and presynaptic short‐term plasticity in hippocampal pyramidal neurons are similar to those in control animals (Deng & Klyachko, 2016).
In addition to potassium channels, FMRP has also been shown to directly interact with N‐type voltage‐gated calcium channels (Ferron et al. 2014). These channels (CaV2.2) are critical for neurotransmission both in central neurons, particularly early in development, and in the autonomic and sensory nervous system (Hirning et al. 1988; Turner et al. 1993; Catterall & Few, 2008). Thus they are the main mediators of neurotransmission between primary sensory afferent neurons involved in nociception and other sensory modalities, and the spinal cord (Bowersox et al. 1996; Altier et al. 2007). CaV2.2 channels are formed from a main pore‐forming α1 subunit and auxiliary α2δ and β subunits (Dolphin, 2012). FMRP has been shown to interact with the α1 subunit of CaV2.2 channels (Ferron et al. 2014). The interaction with FMRP occurs between two cytoplasmic domains of the CaV2.2 α1 subunit: the cytoplasmic loop between the transmembrane domains II and III and the carboxy terminal tail. These intracellular domains of the CaV2.2 channel are important for the targeting to the presynaptic terminals (Mochida et al. 2003; Szabo et al. 2006; Kaeser et al. 2011) and they have been described to functionally interact with presynaptic proteins (Sheng et al. 1994; Bezprozvanny et al. 1995; Mochida et al. 1996; Maximov et al. 1999; Coppola et al. 2001; Kaeser et al. 2011). In peripheral neurons, the loss of FMRP induces an increase in CaV2.2 channel cell surface expression and an increase in neurotransmitter release (Ferron et al. 2014).
FMRP interaction with CaV2.2 does not affect the biophysical properties of the channel which contrasts with the interaction of FMRP with Slack and BK channels. Another noticeable difference resides in the domain of FMRP that is involved in the interaction with the channel. The amino terminal domain of FMRP is a well‐described platform for protein–protein interactions (Bagni & Greenough, 2005; Ramos et al. 2006; Bassell & Warren, 2008) and this domain interacts with Slack channels and the β4 subunit of BK channels (Brown et al. 2010; Deng et al. 2013). Interestingly, it is the carboxy terminal domain of FMRP that has been shown to interact with voltage‐gated calcium channels (Ferron et al. 2014). The carboxy terminal domain of FMRP is a non‐conserved region in the related FXR1P and FXR2P (Bassell & Warren, 2008) and only two other protein–protein interactions have been described (Menon et al. 2004; Dictenberg et al. 2008). The carboxy terminal domain of FMRP was then suggested to contribute to the specificity of FMRP function (Menon et al. 2004). This idea is supported by a recent study performed on premutation carriers that suggests a potential link between the overexpression of an FMRP mRNA splicing variant lacking the carboxy terminal domain and the pathology of premutation disorders (Pretto et al. 2015).
One can speculate on the function of the direct interaction between FMRP and ion channels. It has been hypothesised that the interaction of an ion channel with part of the biochemical machinery that regulates translation of mRNAs suggests that changes in channel activity may contribute to the regulation of activity‐dependent protein synthesis in neurons (Zhang et al. 2012; Lee et al. 2014). FMRP has been shown to modulate postsynaptic local protein synthesis in dendrites of hippocampal neurons (Muddashetty et al. 2007). FMRP phosphorylation status, controlled by protein phosphatase 2A (PP2A) and ribosomal protein S6 kinase (S6K), determines the switch between translational activation and repression of mRNA targets of FMRP (Narayanan et al. 2007, 2008). Local protein synthesis also occurs in presynaptic terminals (Akins et al. 2009) and PP2A and S6K are expressed in presynaptic terminals (Viquez et al. 2009; Cheng et al. 2011). Moreover, a recent study identified a subset of mRNAs encoding presynaptic proteins as targets of FMRP (Darnell et al. 2011). FMRP has been shown to form protein complexes with CaV2.2 channels in the soma and also in the presynaptic terminals of neurons (Ferron et al. 2014). Therefore, FMRP tethering to the vicinity of CaV2.2 may localise it to sites where local activity‐dependent presynaptic protein synthesis may occur. Moreover, PP2A activity can be modulated by Ca2+ influx through voltage‐gated calcium channels (Ferron et al. 2011), which suggests that presynaptic Ca2+ influx resulting from CaV2.2 channel activation may activate PPA2, which in turn would dephosphorylate FMRP and affect local translation. Determining the mechanisms that control FMRP function will be an important issue for future investigations. Indeed, a study has recently shown that the deletion of S6K1 in fmr1 knockout mice partially corrected the phenotypes associated with FXS (Bhattacharya et al. 2012).
In conclusion, FMRP can regulate ion channel activity (Fig. 1) either by controlling the stability and trafficking of the mRNA encoding particular channels (Kv3.1b and Kv4.2) or by a new and unconventional way, by directly binding to a channel subunit (Slack, BK and CaV2.2 channels). Several other ion channels have been reported to be altered in different parts of the brain of animal models of fragile X syndrome but the mechanisms of regulation have not been identified yet (Brager & Johnston, 2014; Contractor et al. 2015). All those modifications of ion channel expression contribute to the modification of neuronal excitability and could account for the alterations observed in fragile X‐associated disorders (Fig. 1).
Figure 1. Diagram illustrating the interaction between FMRP and ion channels in neurons .
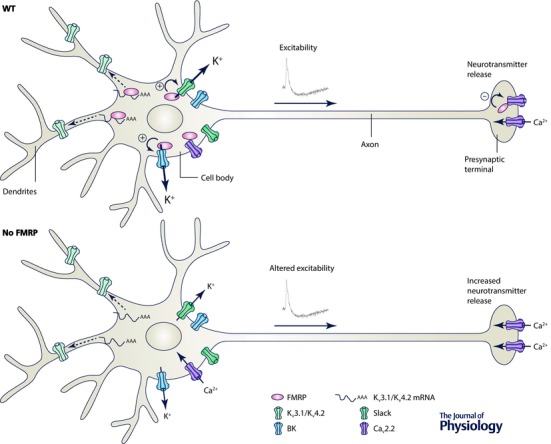
A, in wild‐type neurons (WT), FMRP interacts with voltage‐gated potassium channels (Kv3.1 and Kv4.2) mRNAs and regulates their expression in somatodendritic compartments of neurons. In the soma and presynaptic terminals, FMRP directly interacts with Slack, BK and Cav2.2 channel complexes and regulates their activity. B, in neurons lacking FMRP (no FMRP), in the same way as in models of fragile X syndrome, ion channel expression and activity is modified inducing alteration of excitability and neurotransmitter release.
Additional information
Competing interests
None declared.
Funding
This study was supported by a grant from the Medical Research Council (MR/J013285/1) held by Professor Annette C. Dolphin.
Acknowledgements
I thank Professor Annette C. Dolphin for her constructive comments on this review.
Biography
Laurent Ferron did his PhD with Veronique Capuano and Alain Coulombe (CNRS ESA 8078, Le Plessis Robinson, France) and he is currently a postdoctoral fellow with Professor Annette C. Dolphin in the Department of Neuroscience, Physiology and Pharmacology at University College London. His principal research interest is the regulation of voltage‐gated calcium (CaV) channels and he has recently characterised a novel interaction between neuronal CaV channels and the fragile X mental retardation protein and its impact on synaptic transmission.

This review was presented at the symposium “Voltage‐gated calcium channels ‐ from basic mechanisms to disease”, which took place at Physiology 2015, Cardiff, UK between 6–8 July 2015.
References
- Akins MR, Berk‐Rauch HE & Fallon JR (2009). Presynaptic translation: stepping out of the postsynaptic shadow. Front Neural Circuits 3, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akins MR, Leblanc HF, Stackpole EE, Chyung E & Fallon JR (2012). Systematic mapping of fragile X granules in the mouse brain reveals a potential role for presynaptic FMRP in sensorimotor functions. J Comp Neurol 520, 3687–3706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altier C, Dale CS, Kisilevsky AE, Chapman K, Castiglioni AJ, Matthews EA, Evans RM, Dickenson AH, Lipscombe D, Vergnolle N & Zamponi GW (2007). Differential role of N‐type calcium channel splice isoforms in pain. J Neurosci 27, 6363–6373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antar LN, Afroz R, Dictenberg JB, Carroll RC & Bassell GJ (2004). Metabotropic glutamate receptor activation regulates Fragile X mental retardation protein and Fmr1 mRNA localization differentially in dendrites and at synapses. J Neurosci 24, 2648–2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antar LN, Li C, Zhang H, Carroll RC & Bassell GJ (2006). Local functions for FMRP in axon growth cone motility and activity‐dependent regulation of filopodia and spine synapses. Mol Cell Neurosci 32, 37–48. [DOI] [PubMed] [Google Scholar]
- Bagni C & Greenough WT (2005). From mRNP trafficking to spine dysmorphogenesis: the roots of fragile X syndrome. Nat Rev Neurosci 6, 376–387. [DOI] [PubMed] [Google Scholar]
- Bassell GJ & Warren ST (2008). Fragile X syndrome: loss of local mRNA regulation alters synaptic development and function. Neuron 60, 201–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean BP (2007). The action potential in mammalian central neurons. Nat Rev Neurosci 8, 451–465. [DOI] [PubMed] [Google Scholar]
- Bezprozvanny I, Scheller RH & Tsien RW (1995). Functional impact of syntaxin on gating of N‐type and Q‐type calcium channels. Nature 378, 623–626. [DOI] [PubMed] [Google Scholar]
- Bhakar AL, Dolen G & Bear MF (2012). The pathophysiology of fragile X (and what it teaches us about synapses). Annu Rev Neurosci 35, 417–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya A, Kaphzan H, Alvarez‐Dieppa AC, Murphy JP, Pierre P & Klann E (2012). Genetic removal of p70 S6 kinase 1 corrects molecular, synaptic, and behavioral phenotypes in fragile X syndrome mice. Neuron 76, 325–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowersox SS, Gadbois T, Singh T, Pettus M, Wang YX & Luther RR (1996). Selective N‐type neuronal voltage‐sensitive calcium channel blocker, SNX‐111, produces spinal antinociception in rat models of acute, persistent and neuropathic pain. J Pharmacol Exp Ther 279, 1243–1249. [PubMed] [Google Scholar]
- Brager DH & Johnston D (2014). Channelopathies and dendritic dysfunction in fragile X syndrome. Brain Res Bull 103, 11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner R, Jegla TJ, Wickenden A, Liu Y & Aldrich RW (2000). Cloning and functional characterization of novel large conductance calcium‐activated potassium channel β subunits, hKCNMB3 and hKCNMB4. J Biol Chem 275, 6453–6461. [DOI] [PubMed] [Google Scholar]
- Brown MR & Kaczmarek LK (2011). Potassium channel modulation and auditory processing. Hear Res 279, 32–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MR, Kronengold J, Gazula VR, Chen Y, Strumbos JG, Sigworth FJ, Navaratnam D & Kaczmarek LK (2010). Fragile X mental retardation protein controls gating of the sodium‐activated potassium channel Slack. Nat Neurosci 13, 819–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown V, Jin P, Ceman S, Darnell JC, O'Donnell WT, Tenenbaum SA, Jin X, Feng Y, Wilkinson KD, Keene JD, Darnell RB & Warren ST (2001). Microarray identification of FMRP‐associated brain mRNAs and altered mRNA translational profiles in fragile X syndrome. Cell 107, 477–487. [DOI] [PubMed] [Google Scholar]
- Catterall WA & Few AP (2008). Calcium channel regulation and presynaptic plasticity. Neuron 59, 882–901. [DOI] [PubMed] [Google Scholar]
- Cheng L, Locke C & Davis GW (2011). S6 kinase localizes to the presynaptic active zone and functions with PDK1 to control synapse development. J Cell Biol 194, 921–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie SB, Akins MR, Schwob JE & Fallon JR (2009). The FXG: a presynaptic fragile X granule expressed in a subset of developing brain circuits. J Neurosci 29, 1514–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contractor A, Klyachko VA & Portera‐Cailliau C (2015). Altered neuronal and circuit excitability in fragile X syndrome. Neuron 87, 699–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppola T, Magnin‐Luthi S, Perret‐Menoud V, Gattesco S, Schiavo G & Regazzi R (2001). Direct interaction of the Rab3 effector RIM with Ca2+ channels, SNAP‐25, and synaptotagmin. J Biol Chem 276, 32756–32762. [DOI] [PubMed] [Google Scholar]
- Darnell JC, Jensen KB, Jin P, Brown V, Warren ST & Darnell RB (2001). Fragile X mental retardation protein targets G quartet mRNAs important for neuronal function. Cell 107, 489–499. [DOI] [PubMed] [Google Scholar]
- Darnell JC, Van Driesche SJ, Zhang C, Hung KY, Mele A, Fraser CE, Stone EF, Chen C, Fak JJ, Chi SW, Licatalosi DD, Richter JD & Darnell RB (2011). FMRP stalls ribosomal translocation on mRNAs linked to synaptic function and autism. Cell 146, 247–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng PY & Klyachko VA (2016). Genetic upregulation of BK channel activity normalizes multiple synaptic and circuit defects in a mouse model of fragile X syndrome. J Physiol 594, 83–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng PY, Rotman Z, Blundon JA, Cho Y, Cui J, Cavalli V, Zakharenko SS & Klyachko VA (2013). FMRP regulates neurotransmitter release and synaptic information transmission by modulating action potential duration via BK channels. Neuron 77, 696–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng PY, Sojka D & Klyachko VA (2011). Abnormal presynaptic short‐term plasticity and information processing in a mouse model of fragile X syndrome. J Neurosci 31, 10971–10982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dictenberg JB, Swanger SA, Antar LN, Singer RH & Bassell GJ (2008). A direct role for FMRP in activity‐dependent dendritic mRNA transport links filopodial‐spine morphogenesis to fragile X syndrome. Dev Cell 14, 926–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolphin AC (2012). Calcium channel auxiliary α2δ and β subunits: trafficking and one step beyond. Nat Rev Neurosci 13, 542–555. [DOI] [PubMed] [Google Scholar]
- Ferron L, Nieto‐Rostro M, Cassidy JS & Dolphin AC (2014). Fragile X mental retardation protein controls synaptic vesicle exocytosis by modulating N‐type calcium channel density. Nat Commun 5, 3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferron L, Ruchon Y, Renaud JF & Capuano V (2011). T‐type Ca2+ signalling regulates aldosterone‐induced CREB activation and cell death through PP2A activation in neonatal cardiomyocytes. Cardiovasc Res 90, 105–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross C, Yao X, Pong DL, Jeromin A & Bassell GJ (2011). Fragile X mental retardation protein regulates protein expression and mRNA translation of the potassium channel Kv4.2. J Neurosci 31, 5693–5698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagerman R & Hagerman P (2013). Advances in clinical and molecular understanding of the FMR1 premutation and fragile X‐associated tremor/ataxia syndrome. Lancet Neurol 12, 786–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson JE & Madison DV (2007). Presynaptic Fmr1 genotype influences the degree of synaptic connectivity in a mosaic mouse model of fragile X syndrome. J Neurosci 27, 4014–4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirning LD, Fox AP, McCleskey EW, Olivera BM, Thayer SA, Miller RJ & Tsien RW (1988). Dominant role of N‐type Ca2+ channels in evoked release of norepinephrine from sympathetic neurons. Science 239, 57–61. [DOI] [PubMed] [Google Scholar]
- Kaeser PS, Deng L, Wang Y, Dulubova I, Liu X, Rizo J & Sudhof TC (2011). RIM proteins tether Ca2+ channels to presynaptic active zones via a direct PDZ‐domain interaction. Cell 144, 282–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim GE & Kaczmarek LK (2014). Emerging role of the KCNT1 Slack channel in intellectual disability. Front Cell Neurosci 8, 209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemmer P, Meredith RM, Holmgren CD, Klychnikov OI, Stahl‐Zeng J, Loos M, van der Schors RC, Wortel J, de Wit H, Spijker S, Rotaru DC, Mansvelder HD, Smit AB & Li KW (2011). Proteomics, ultrastructure, and physiology of hippocampal synapses in a fragile X syndrome mouse model reveal presynaptic phenotype. J Biol Chem 286, 25495–25504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger DD & Bear MF (2011). Toward fulfilling the promise of molecular medicine in fragile X syndrome. Annu Rev Med 62, 411–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A, Fakler B, Kaczmarek LK & Isom LL (2014). More than a pore: ion channel signaling complexes. J Neurosci 34, 15159–15169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HY, Ge WP, Huang W, He Y, Wang GX, Rowson‐Baldwin A, Smith SJ, Jan YN & Jan LY (2011). Bidirectional regulation of dendritic voltage‐gated potassium channels by the fragile X mental retardation protein. Neuron 72, 630–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao L, Park SK, Xu T, Vanderklish P & Yates JR III (2008). Quantitative proteomic analysis of primary neurons reveals diverse changes in synaptic protein content in fmr1 knockout mice. Proc Natl Acad Sci USA 105, 15281–15286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano R, Rosero CA & Hagerman RJ (2014). Fragile X spectrum disorders. Intractable Rare Dis Res 3, 134–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maximov A, Sudhof TC & Bezprozvanny I (1999). Association of neuronal calcium channels with modular adaptor proteins. J Biol Chem 274, 24453–24456. [DOI] [PubMed] [Google Scholar]
- Menon RP, Gibson TJ & Pastore A (2004). The C terminus of fragile X mental retardation protein interacts with the multi‐domain Ran‐binding protein in the microtubule‐organising centre. J Mol Biol 343, 43–53. [DOI] [PubMed] [Google Scholar]
- Mochida S, Sheng ZH, Baker C, Kobayashi H & Catterall WA (1996). Inhibition of neurotransmission by peptides containing the synaptic protein interaction site of N‐type Ca2+ channels. Neuron 17, 781–788. [DOI] [PubMed] [Google Scholar]
- Mochida S, Westenbroek RE, Yokoyama CT, Zhong H, Myers SJ, Scheuer T, Itoh K & Catterall WA (2003). Requirement for the synaptic protein interaction site for reconstitution of synaptic transmission by P/Q‐type calcium channels. Proc Natl Acad Sci USA 100, 2819–2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muddashetty RS, Kelic S, Gross C, Xu M & Bassell GJ (2007). Dysregulated metabotropic glutamate receptor‐dependent translation of AMPA receptor and postsynaptic density‐95 mRNAs at synapses in a mouse model of fragile X syndrome. J Neurosci 27, 5338–5348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myrick LK, Deng PY, Hashimoto H, Oh YM, Cho Y, Poidevin MJ, Suhl JA, Visootsak J, Cavalli V, Jin P, Cheng X, Warren ST & Klyachko VA (2015). Independent role for presynaptic FMRP revealed by an FMR1 missense mutation associated with intellectual disability and seizures. Proc Natl Acad Sci USA 112, 949–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan U, Nalavadi V, Nakamoto M, Pallas DC, Ceman S, Bassell GJ & Warren ST (2007). FMRP phosphorylation reveals an immediate‐early signaling pathway triggered by group I mGluR and mediated by PP2A. J Neurosci 27, 14349–14357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan U, Nalavadi V, Nakamoto M, Thomas G, Ceman S, Bassell GJ & Warren ST (2008). S6K1 phosphorylates and regulates fragile X mental retardation protein (FMRP) with the neuronal protein synthesis‐dependent mammalian target of rapamycin (mTOR) signaling cascade. J Biol Chem 283, 18478–18482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pretto DI, Eid JS, Yrigollen CM, Tang HT, Loomis EW, Raske C, Durbin‐Johnson B, Hagerman PJ & Tassone F (2015). Differential increases of specific FMR1 mRNA isoforms in premutation carriers. J Med Genet 52, 42–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price TJ, Flores CM, Cervero F & Hargreaves KM (2006). The RNA binding and transport proteins staufen and fragile X mental retardation protein are expressed by rat primary afferent neurons and localize to peripheral and central axons. Neuroscience 141, 2107–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price TJ, Rashid MH, Millecamps M, Sanoja R, Entrena JM & Cervero F (2007). Decreased nociceptive sensitization in mice lacking the fragile X mental retardation protein: role of mGluR1/5 and mTOR. J Neurosci 27, 13958–13967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos A, Hollingworth D, Adinolfi S, Castets M, Kelly G, Frenkiel TA, Bardoni B & Pastore A (2006). The structure of the N‐terminal domain of the fragile X mental retardation protein: a platform for protein‐protein interaction. Structure 14, 21–31. [DOI] [PubMed] [Google Scholar]
- Ronesi JA & Huber KM (2008). Metabotropic glutamate receptors and fragile X mental retardation protein: partners in translational regulation at the synapse. Sci Signal 1, e6. [DOI] [PubMed] [Google Scholar]
- Sheng ZH, Rettig J, Takahashi M & Catterall WA (1994). Identification of a syntaxin‐binding site on N‐type calcium channels. Neuron 13, 1303–1313. [DOI] [PubMed] [Google Scholar]
- Strumbos JG, Brown MR, Kronengold J, Polley DB & Kaczmarek LK (2010). Fragile X mental retardation protein is required for rapid experience‐dependent regulation of the potassium channel Kv3.1b. J Neurosci 30, 10263–10271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo Z, Obermair GJ, Cooper CB, Zamponi GW & Flucher BE (2006). Role of the synprint site in presynaptic targeting of the calcium channel CaV2.2 in hippocampal neurons. Eur J Neurosci 24, 709–718. [DOI] [PubMed] [Google Scholar]
- Torres YP, Morera FJ, Carvacho I & Latorre R (2007). A marriage of convenience: β‐subunits and voltage‐dependent K+ channels. J Biol Chem 282, 24485–24489. [DOI] [PubMed] [Google Scholar]
- Turner TJ, Adams ME & Dunlap K (1993). Multiple Ca2+ channel types coexist to regulate synaptosomal neurotransmitter release. Proc Natl Acad Sci USA 90, 9518–9522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viquez NM, Fuger P, Valakh V, Daniels RW, Rasse TM & DiAntonio A (2009). PP2A and GSK‐3β act antagonistically to regulate active zone development. J Neurosci 29, 11484–11494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B, Desai R & Kaczmarek LK (2007). Slack and Slick KNa channels regulate the accuracy of timing of auditory neurons. J Neurosci 27, 2617–2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Brown MR, Hyland C, Chen Y, Kronengold J, Fleming MR, Kohn AB, Moroz LL & Kaczmarek LK (2012). Regulation of neuronal excitability by interaction of fragile X mental retardation protein with Slack potassium channels. J Neurosci 32, 15318–15327. [DOI] [PMC free article] [PubMed] [Google Scholar]


